Abstract
Introduction
Various methods are available to measure hippocampal atrophy rate. We compared methods to predict Alzheimer's dementia.
Methods
Participants with brain imaging at ages 69 and 73 years were identified from a previous study. Simple manual measures and computationally automated volumetry were performed. Receiver operating characteristics assessed the predictive ability of each method at baseline and on logit regression analysis of two serial scans.
Results
Ten of 149 participants developed Alzheimer's dementia and had lower baseline volumes (3647 vs. 4194 mm3P = .002), rates of volume loss (−126 vs. −36 mm3/y; P = .001), and rates of loss in hippocampal fraction (−8.55 vs. −2.35 x 10−5/y; P = .001). Baseline volume with a rate of change gave the highest area under the curve value of 0.96.
Discussion
Automated volumetry measuring hippocampal size at age 69 years and subsequent rate of change predicts Alzheimer's dementia development.
Keywords: Hippocampal volume, Hippocampal atrophy rate, Alzheimer's disease, Automated volumetry, Scheltens' score, Rounding ratio
1. Introduction
Hippocampal size can be assessed in a variety of ways, with differing amounts of manual operator input and processing time. Previously described methods include visual assessment [1], [2], linear measurements [1], [3], manual volumetry [3], automated volumetry [2], and signal intensity based scoring [4]. We are interested in methods that could be incorporated into routine clinical practice, including automated methods.
In 1992, Scheltens et al [1] published their semiquantitative visual rating score for hippocampal atrophy. This initial publication showed correlations between a five-point visual scale, linear measurements, age, and the presence or absence of Alzheimer's dementia (AD). Over the next 20 years, Scheltens' score has been correlated with neuropsychology rating scales, pathological scoring, and future progression from mild cognitive impairment (MCI) to clinical AD [5].
In 2007 and 2012, Adachi et al [3], [6] published ratios of hippocampal height and width to calculate a rounding ratio (RR) in subjects with and without AD. The RR correlated with manually outlined hippocampal volumes (Spearman's ρ = 0.42), AD and Mini-Mental State Examination (MMSE). Hippocampi had a reduced width with increasing RR, changing the coronal cross section from elongated to rounded. This measure has received little attention in the literature and requires validation.
FreeSurfer is established open-source software for automated brain segmentation and readily produces volume measurements [7].
The individuals in our study are from a large, well-characterized population-based cohort who were normal at recruitment and followed up with two brain magnetic resonance imaging (MRI) scans 4 years apart and with AD outcomes recorded. This sample provides unique opportunities to define imaging thresholds for the risk of developing dementia in the general population, including in people who are not yet symptomatic. In this study, we will compare measurement techniques and find if adding a second scan improves our ability to predict who is developing AD. We are using the area under the curve (AUC) of the receiver operating characteristic (ROC) to compare the techniques [8]. Values of AUC range from 1.00 which is a perfect test down to 0.50 which represents no discriminating ability. AUC values of 0.80 to 0.90 are regarded as good and 0.90 to 1.00 as excellent. We will present the risk of AD graphically as a continuum with baseline size and rate of change as contributing factors.
2. Methods
2.1. Subjects
A subset of the Aberdeen Birth Cohort 1936 (ABC36) who had undergone two MRI scans was identified. ABC36 participants were recruited from participants of the Scottish Mental Health Survey of 1947, which was conducted by the Scottish Council for Research and Education (SCRE). All participants were born in 1936, and almost all (N = 75,211) schoolchildren in Scotland were studied.
The SCRE allowed the University of Aberdeen to have access to these data. A total of 2620 of the schoolchildren had been tested in Aberdeen City. Nine hundred thirty-four participants were found to be alive and living in Aberdeen using the Community Health Index and other identifiers. Six hundred sixty-four had Community Health Index numbers and were approached via their primary care physicians. The local Family Doctors' Research Committee requested that each prospective participant's family physician should sign a written invitation letter and that those who were recently bereaved or those with a life-threatening illness should be excluded. The family doctors were asked to invite potential participants if they were not known to have dementia and were living independently in the community. Of 647 individuals who were eligible, 506 agreed to take part in a follow-up study of brain aging and health and 498 provided a minimum data set of consent, clinical history, vital signs, MMSE, and Raven's Progressive Matrices. These 498 participants were recruited between 1999 and 2003. Written informed consent as approved by the Local Research Ethics Committee was obtained by a trained research nurse for assessment, follow-up, inspection of medical records, and sharing of materials with noncommercial collaborators. Participants with an MMSE of less than 24 on initial screening were excluded.
Beginning in 2004, a target number of 250 participants were selected for an MRI study. The database was first scrutinized to remove those with known contraindications to MRI and/or who were in treatment for serious illness. Participants were invited sequentially in the order in which they had entered the study earlier.
Two hundred forty-nine participants agreed and were scanned at an age of approximately 69 years. By the age of about 73 years, there had been an attrition of 67 participants and 166 of the remainder agreed to a repeat MRI.
The details of the cohort are available from Whalley et al [9].
2.2. Imaging protocol
For consistent, directly comparable imaging acquisitions, all participants at all time points were scanned in the same 1.5-T Genesis Signa NVi MRI scanner (General Electric, Milwaukee, WI, USA). T1 spoiled gradient recalled (SPGR) gradient echo images were acquired in the transverse plane with a 20 or 22 ms repetition time, 6.0 ms echo time, 35° flip angle, 256 × 224 matrix, field of view 24 × 18 cm, 1 × 1 mm in-plane resolution, 1.6 mm slice thickness, and no slice gap. The clinical teams involved in dementia diagnosis did not have access to these research MRI scans.
2.3. Hippocampal size measurements
Manual and automated hippocampal measurements reported in this study are the mean of the left and right hippocampus. mm−3 values are not corrected for total intracranial volume.
2.3.1. Manual measurements
Hippocampal rounding measurements and Scheltens' hippocampal atrophy scoring were performed by a single trained rater with an agreed technique after an initial period of double reading. The rater was blinded to the cognitive function scores and dementia outcomes.
The rater manually realigned coronal oblique images from the volumetric T1 series using a commercially available viewer. First, left-right rotational realignment was performed along the head-foot axis. A sagittal plane was positioned to demonstrate one of the hippocampi. The sloping anteroposterior axis of the hippocampus was noted on this sagittal image, and a coronal plane was angled perpendicular to this. Images showing the realignment process are available in the Supplementary Fig. 1.
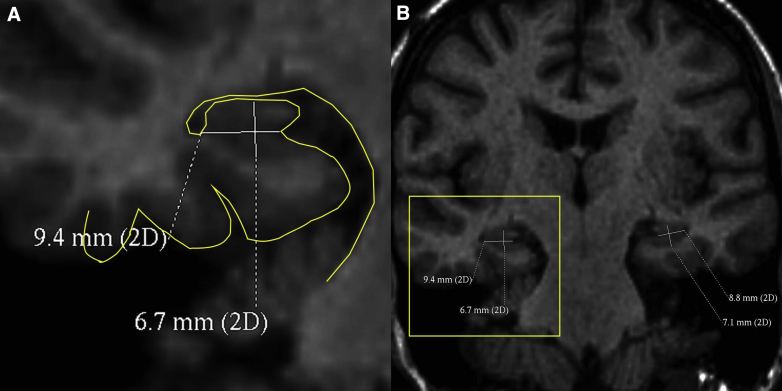
For manual hippocampal rounding, the realigned coronal plane was positioned at the body of the hippocampus in an area without superior digitations and where hippocampal detail was best visualized. Hippocampal width was taken from the hippocampal sulcus medially to the temporal horn of the lateral ventricle (as in Adachi et al [6]). Hippocampal height was measured at ∼90° to this. For consistency, all hippocampal height measurements included the alveus because the alveus was inconsistently discernible as a discrete structure. Hippocampal height measurements included the gray matter of the subiculum. A sample of 20 double-read scans gave intra-class correlation coeffecients (ICCs) of 0.78 for linear measurements and 0.07 for RR. For each scan, the RR was expressed as the mean of both sides' height÷width. An example measurement is shown in Fig. 1.
Fig. 1.
Manual measurements for hippocampal rounding. (A) A magnified image with an outline of the CSF-soft tissue boundary traced for clarity. (B) The size and position of the zoomed area. Abbreviation: CSF, cerebrospinal fluid.
Scheltens' hippocampal atrophy score [1] was performed on a single, angled coronal section where the pons, trigeminal nerves, and third ventricle were visible. The interobserver variability of Scheltens' score is known from previous studies to have a κ value of 0.44 to 0.51 [10].
2.3.2. Automated measurements
The volumetric analysis was performed using the FreeSurfer software package (version 4.5.0) (http://surfer.nmr.mgh.harvard.edu/). The brain imaging preprocessing steps include motion correction, affine transformation to Talairach image space, nonuniform intensity normalization for intensity inhomogeneity correction, and removal of nonbrain tissues [11], [12], [13].
The hippocampus is among a number of subcortical structures segmented to obtain their volume. The segmentation is based on the voxel's location in the volume, the neighboring voxels' tissue classes, and the intensity value in each voxel. This automatic labeling procedure is comparable in accuracy to manual labeling [14] assuring the quality of the procedure. In addition, the obtained results are reliable when comparing the current method with several different automatic segmentation procedures [15]. The ICC for this method applied to the hippocampus is known to be 0.96 to 0.98 [16].
The data follow the longitudinal stream processing creating an unbiased template from both time points for each subject and subsequently extracting the volumes from each time point [16].
Left and right hippocampal volumes (mm3) were extracted, and the average of left and right hippocampal volumes are reported in this study. Because we are comparing rates of change, each person's baseline hippocampal volume served as a denominator and therefore normalization to total intracranial volume was not required.
2.4. AD follow-up
Interviews designed to meet International Classification of Diseases 10th Revision or Diagnostic and Statistical Manual of Mental Disorders, 4th edition, criteria were conducted by a clinical academic with research expertise in dementia whenever dementia seemed likely.
The possibility of a dementia diagnosis was screened by reviewing the following clinical resources approximately 11 years after the baseline MRI: (1) electronic clinical records for dementia codes, (2) attendances at Old Age Psychiatry memory clinics, and (3) clinical referrals for imaging with a key word of “dementia”. Clinical case files were examined, and the year of AD diagnosis was recorded.
Owing to the known propensity of AD to affect the hippocampus early in its clinical course, patients with non-AD were classified into the no Alzheimer's group in this study. It is known that other dementia types also cause hippocampal atrophy; therefore, an analysis with all dementia types combined is presented in the Supplementary Material.
Dementia ascertainment in this cohort is an ongoing process which is updated over time. This study uses the latest census date of September 2015.
2.5. Statistical analysis
R, version 3.2.3, was used for statistical analysis, which is documented and freely available [17]. Included add-on packages were used as needed, such as eeptools [18] which provided the “age_calc” function for accurately calculating a participant's age in fractional years. Independent-sample t tests were used to assess significance between groups that had progressed to Alzheimer's versus those that did not. ROCs were calculated with the pROC package [19]. Confidence intervals were calculated with the default options in pROC, including the DeLong [20] method with 2000 bootstraps. General linear modeling was used to incorporate a rate of change and interaction between sex and volume to baseline measurements. The “predict” function estimated values from the general linear models for ROC values and to produce an easily usable, graphical illustration of AD risk.
3. Results
3.1. Imaging measures and participant demographics
One hundred forty-nine (80 male, 69 female) participants were able to comply with imaging procedures and had 3DT1 imaging data available at both MRI attendances. The MRI scans were acquired between April 2004 and December 2009.
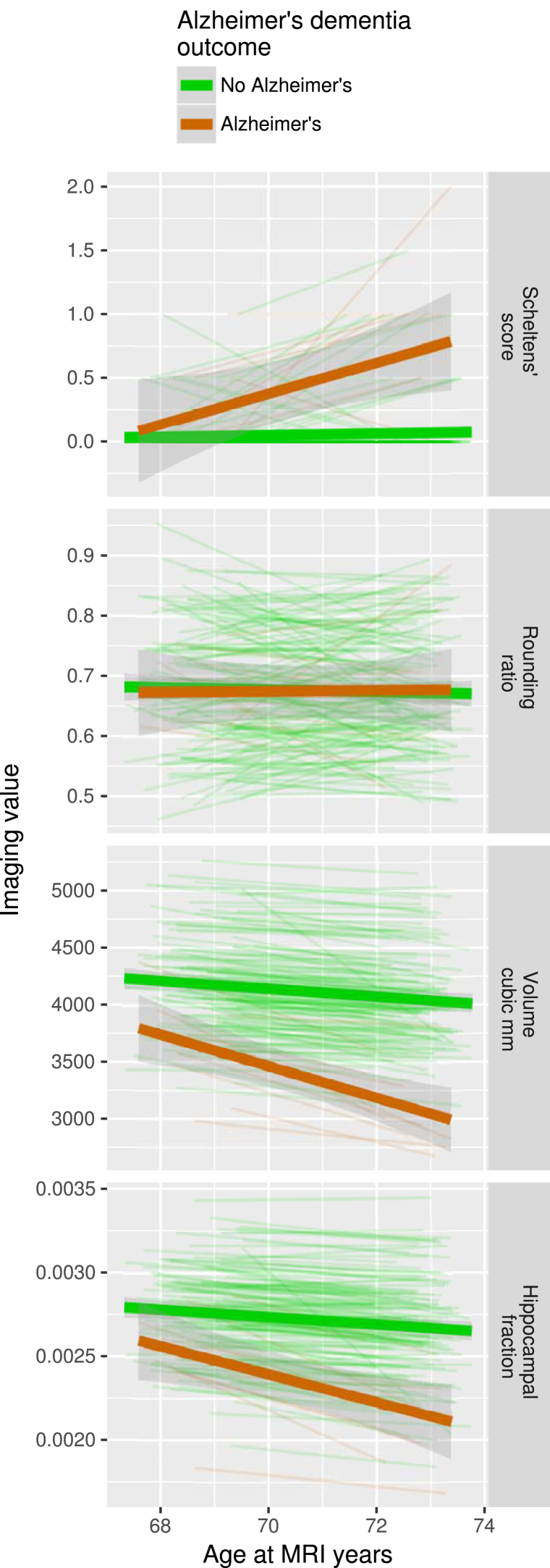
The mean age at the first MRI scan was 68.6 years (SD 0.6 years, range 67.3–69.9 years). The second scan was performed at a mean age of 72.8 years (SD 0.5 years, range 71.4–73.8 years). Graphs of the imaging measures over time are shown in Fig. 2.
Fig. 2.
Hippocampal size over time. Each thin line represents one of the 149 participants. Participants who developed AD are marked with red lines and the other participants are marked with green lines. The time interval between each participant's scans varies; therefore, the rate of change is more appropriate for comparison than absolute differences in hippocampal size. Thick lines are linear model trend lines, in red for the Alzheimer's group and green for the other participants. Top panel: Scheltens' score; 127 participants have a score of zero at both of their scans, and therefore most of the individual participant lines are overlapping along the zero level. Second panel: Rounding ratio. Third panel: Hippocampal volume. Bottom panel: Hippocampal fraction.
There are significant Pearson's correlations between Scheltens' score and hippocampal volume (n = 298, r = −0.342, P < .001 unadjusted), and Scheltens' score and hippocampal fraction (n = 298, r = −0.349, P < .001 unadjusted). RR fails to correlate significantly with hippocampal volume (n = 298, r = 0.009, P = .875 unadjusted) or hippocampal fraction (n = 298, r = 0.034, P = .558 unadjusted).
3.1.1. Male and female differences
Unpaired t tests reveal no significant differences between men (n = 80) and women (n = 69) in Scheltens' scores or RRs at both age points (unadjusted P values .055 to .665).
Men have larger hippocampal volumes than women at age 69 years (4257 mm3 vs 4041 mm3, P = .002 unadjusted) and at age 73 years (4064 mm3 vs 3889 mm3, P = .023 unadjusted).
Men have smaller hippocampal fractions than women at age 69 years (0.265% vs 0.287% average of left hippocampus and right hippocampus, P < .001 unadjusted) and at age 73 years (0.252% vs 0.277%, P < .001 unadjusted).
The rate of change in hippocampal size does not differ significantly between men and women for any of the measurement methods.
3.2. Dementia outcomes
Twelve participants were diagnosed with a dementia syndrome from 2002 to 2011 (one Parkinson's disease dementia, one vascular dementia, one mixed dementia, and nine AD). Of the nine diagnosed with AD, four were male and five were female. Five participants were diagnosed with AD between their first and second MRI scans. Two participants were diagnosed with AD in the year of their second MRI scan in 2008. Two participants were diagnosed with AD after their second MRI scan. The one female diagnosed with mixed dementia in 2006 was classified as a case because she had a mixture of AD (the disease under investigation) and vascular dementia.
3.3. Rates of change and AD
Participants who develop AD have significantly faster losses of hippocampal volume and hippocampal fraction compared with those who do not develop AD (Table 1). The AD patients lose 3.42% (SD 1.59%) volume per year compared with 0.85% (SD 0.85%) volume for controls. Rates of change produce superior AUC values compared with baseline for volume and for hippocampal fraction.
Table 1.
Rates of change in imaging parameters compared with the development of AD
| Imaging parameter | Whole group, n = 149 (mean ± SD) | No AD, n = 139 (mean ± SD) | AD, n = 10 (mean ± SD) | Unadjusted 2-sample t test P-value between AD and no-AD groups | ROC AUC for identifying those with AD (95% CI) |
|---|---|---|---|---|---|
| Scheltens' score at age 68.6 ± 0.6 y | 0.05 ± 0.18 | 0.04 ± 0.16 | 0.25 ± 0.35 | .089 | 0.67 (0.51–0.83) |
| Scheltens' score at age 72.8 ± 0.5 y | 0.11 ± 0.33 | 0.08 ± 0.25 | 0.65 ± 0.67 | .023 | 0.76 (0.60–0.93) |
| ΔScheltens' score/Δt, arbitrary units/y | 0.02 ± 0.07 | 0.010 ± 0.057 | 0.092 ± 0.179 | .162 | 0.67 (0.46–0.87) |
| Rounding ratio at age 68.6 ± 0.6 y | 0.66 ± 0.10 | 0.68 ± 0.11 | 0.68 ± 0.06 | .819 | 0.52 (0.39–0.65) |
| Rounding ratio at age 72.8 ± 0.5 y | 0.67 ± 010 | 0.67 ± 0.10 | 0.67 ± 0.11 | .857 | 0.53 (0.32–0.74) |
| ΔRounding ratio/Δt, no units/y | −0.98 ± 20.6 ×10−3 | −0.81 ± 20.0 ×10−3 | −3.42 ± 28.7 ×10−3 | .783 | 0.61 (0.42–0.80) |
| Volume mm3 at age 68.6 ± 0.6 y | 4157 ± 433 | 4194 ± 413 | 3647 ± 407 | .002** | 0.84 (0.70–0.98) |
| Volume mm3 at age 72.8 ± 0.5 y | 3983 ± 476 | 4046 ± 418 | 3116 ± 385 | <.001**** | 0.95 (0.89–1.00) |
| ΔVolume/Δt, mm3/y | −41.7 ± 46.3 | −35.6 ± 38.5 | −126.1 ± 63.8 | .001** | 0.90 (0.78–1.00) |
| Hippocampal fraction at age 68.6 ± 0.6 y | 2.75 ± 0.27 ×10−3 | 2.77 ± 0.27 ×10−3 | 2.52 ± 0.30 ×10−3 | .030* | 0.73 (0.58–0.88) |
| Hippocampal fraction at age 72.8 ± 0.5 y | 2.64 ± 0.31 ×10−3 | 2.67 ± 0.29 ×10−3 | 2.17 ± 0.29 ×10−3 | .001** | 0.89 (0.81–0.98) |
| Hippocampal fraction/Δt, per y | −2.77 ± 3.41 ×10−5 | −2.35 ± 2.95 ×10−5 | −8.55 ± 4.17 ×10−5 | .001** | 0.91 (0.79–1.00) |
Abbreviations: AD, Alzheimer's dementia; SD, standard deviation; ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval.
3.4. Logit regression analysis—The value of adding a rate of change
Table 2 shows the value of adding a rate of change in the prediction of AD according to the following standard logit regression equation where the probability of AD is probAD
Table 2.
Predicting AD outcome from a combination of baseline size and subsequent rate of change
| Intercept | βbaseline | P value for βbaseline | βrate | P value for βrate | ROC AUC (95% CI) | |
|---|---|---|---|---|---|---|
| Scheltens' scale | −3.368 | 3.631 | .003** | 8.768 | .004** | 0.80 (0.64–0.96) |
| Rounding ratio | −2.527 | −0.175 | .961 | −6.553 | .715 | 0.60 (0.40–0.80) |
| Volume mm3 | 16.8 | −0.00567 | <.001*** | −0.0350 | <.001*** | 0.96 (0.90–1.00) |
| Hippocampal fraction | 7.292 | −4365 | .005** | −35770 | <.001*** | 0.95 (0.91–0.99) |
Abbreviations: AD, Alzheimer's dementia; ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval.
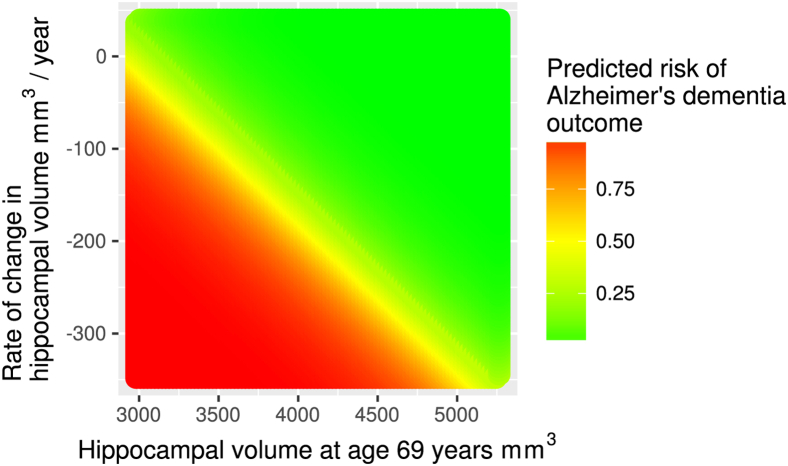
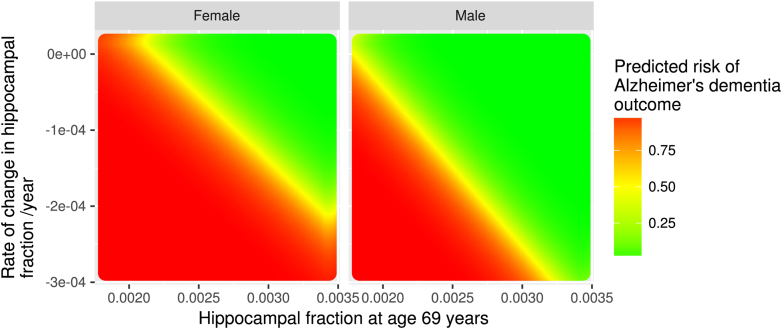
Combining a baseline measurement with a rate of change improves the AUC for Scheltens' scale from 0.64 to 0.78, for volume from 0.82 to 0.95, and for hippocampal fraction from 0.61 to 0.94. The significant P-values for βrate indicate that this is a statistically significant predictor in the logit regression model. The risk of AD as predicted by baseline volume and a rate of change is illustrated in Fig. 3.
Fig. 3.
Risk of AD according to hippocampal volume at age 69 years and subsequent rate of change. The values of hippocampal size are the average of the left and the right hippocampi.
3.5. Logit regression analysis—The value of adding sex
Because hippocampal volume and hippocampal fraction at baseline were significantly different between men and women, we considered the effect of an interaction term βbaseline×sex
The β coefficient for the male-female difference is statistically significant, indicated by the P value of .07. The improvement in AUC from 0.94 to 0.95 is minimal (Table 3).
Table 3.
Effect of adding the male-female difference in hippocampal size at age 69 years
| Intercept | βbaseline | P value for βbaseline | βrate | P value for βrate | βbaseline×sex | P value for βbaseline×sex | ROC AUC (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Volume mm3 | 17.0 | −0.00568 | <.001*** | −0.0391 | <.001*** | −0.000370 | .248 | 0.95 (0.86–1.00) |
| Hippocampal fraction | 19.8 | −8795 | .001** | −51059 | <.001*** | −1896 | .007** | 0.95 (0.88–1.00) |
Abbreviations: ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval.
NOTE. In this table, the βbaseline thi is the value for changing from female to male.
The risk of AD modeled with the aforementioned parameters is illustrated graphically in the Supplementary Fig. 2.
4. Discussion
At age 69 years, automated hippocampal volumetry is superior to manual measures for identifying people who are developing AD. This is shown by its ROC AUC of 0.84 (good) compared with 0.67 (poor) for Scheltens' scale and 0.52 (fail) for RR.
The ability of a hippocampal size measurement to predict AD is improved by combining a baseline measurement with a rate of change. This is significant for Scheltens' scale and for automated volumetry. Therefore, in cases of diagnostic uncertainty, the waiting time and expense of a second MRI scan may add value for diagnosis.
Average hippocampal volume in mm3 combined with a rate of change in mm3/y gives the highest ROC AUC of 0.96 (excellent). The predicted AD risk derived from these two factors is shown graphically in Fig. 3.
RR performs poorly for predicting AD development. This is in contrast to Scheltens' scores which were performed by the same observer, and which correlated strongly with automated volumetry. Early disease, a limited coronal resolution of 1.6 mm, measurement variability, and a limited number of AD outcomes in a normally aging cohort are possible explanations for RR's poor performance.
Prior studies have shown that the annual hippocampal atrophy rate is greater in patients with known AD compared with healthy controls, for example, 2.7% versus 0.8% in Hua et al [21], 5.59% versus 0.66% in Morra et al 2009 [22], 1.5% versus 0.6% in Sluimer et al [23], and 4.66% versus 1.41% in Barnes et al [24] meta-analysis of prior studies. Our rates of hippocampal volume loss were 3.42% in participants who are developing AD and 0.85% in participants who did not develop AD.
In 2007, Ridha et al [4] compared different manual measures of hippocampal atrophy in 47 patients with clinically probable AD and in 26 controls aged approximately 65 years. Participants were scanned twice, at an interval of 481 ± 302 days (personal communication). Using manual tracing, volume atrophy rates were higher at 4.49% per year in AD compared with 0.37% per year in controls (P ≤ .001). Scheltens' scores in the study by Ridha et al deteriorated by 0.15 units/y in AD and, opposite to expected, deteriorated even more quickly in controls at 0.20 units/y although this was not significant. In our study, Scheltens' scores deteriorated faster in AD at 0.09 compared with 0.01 units/y.
Ridha et al [4] have also performed ROC analysis. The AUC for volume atrophy rate was better than for Scheltens' scale and for a novel measure of regional signal intensity. The AUC numerical values were not reported, in contrast to our study.
Longitudinal follow-up studies of participants without clinical dementia are limited.
Jack et al [25] assessed 91 normal elderly subjects at baseline and 1.4 years later, of whom two developed AD. In contrast to our study, hippocampal annual percentage change was not significantly associated with conversion in the normal participants.
Henneman et al [26] studied the added value of atrophy rates to baseline volume in identifying AD. In a group comprising 44 participants with MCI, 26 patients with subjective complaints, and 8 healthy volunteers, those with low hippocampal volume at baseline and a high hippocampal atrophy rate had a hazard ratio of 61.1 for developing AD. Our study confirms that adding atrophy rate to baseline volume improves prediction, as shown by improved ROC values, in a larger group without dementia at baseline.
Our study remains novel in that we worked from a large, well-characterized initially normal cohort, had longitudinal imaging over 4 years, had clinical follow-up 11 years after the first MRI, and directly compared manual to automated methods of image analysis. We have used ROC to assess predictive ability and reported AUCs. We have combined the predictive power of baseline and follow-up hippocampal volumes and produced graphical plots to easily visualize the risk of a person developing AD.
Our study has limitations. It may not be generalizable outside our selected population, and therefore, the model would benefit from validation in other cohorts. We have used clinical diagnoses of AD rather than postmortem neuropathologic diagnoses, and there is known to be a mismatch rate between these [27]. This is a limitation of all clinical studies that do not have postmortem pathology available. The correlation between hippocampal size and the neuropathologic severity of AD is imperfect [28], [29], especially if outlier points are included [30].
We chose the subset of participants who returned for a second MRI scan at age 73 years, excluding those for whom a follow-up MRI was unavailable. Many individuals were diagnosed with AD between the first and second MRI scans. In the clinical scenario, it would be advantageous to know the rate of change in hippocampal size before 4 years have elapsed.
Our axial plane of volumetric image acquisition would have maximized measurement uncertainty in the vertical direction. More modern scanners have better spatial resolution for volumetric images, and this would improve the quality of multiplanar reconstructions (e.g., the ADNI criteria, which were published after our data collection, require <1.5 mm slice thickness compared with our 1.6 mm [31], and direct angled coronal acquisitions as used by Adachi et al [6] are optimal for manual measurements). In our study, the comparison of different measurement methods against each other remains valid, and the comparison of rates of change over time using consistent image quality is valid. It remains clear that Scheltens' scale is superior to RR and that volumetric analysis is superior to manual measurements for the detection of AD. Because volumetric series are acquired routinely in clinical settings, it is feasible to incorporate automated volumetry into routine clinical practice. We envisage that with continuing increases in everyday computing power, automated volumetry will become a useful adjunct in the clinical setting.
5. Conclusion
A combination of baseline hippocampal volume and subsequent rate of change in hippocampal volume is a good predictor of older people who are developing AD.
Research in context.
-
1.
Systematic review: MEDLINE searches with the terms “Alzheimer's disease” (AD) and “hippocampal atrophy rate” and a book chapter by Chong et al [32] were used as start points. References of articles were screened for other potentially relevant articles. The majority of studies examine hippocampal volume and incidence of dementia in samples of patients who already have cognitive impairment. The added value of hippocampal atrophy rate for predicting AD in normal individuals is reported.
-
2.
Interpretation: If one wishes to screen for people at risk of AD using a quick, manual hippocampal measurement at age 69 years, then Scheltens' scale is useful, whereas rounding ratio is not. If taking a single measurement at age 69 years, then absolute hippocampal volume is best. For all measures, adding a rate of change to the baseline measurement improves the accuracy for detecting AD. Automated volumetry plus a rate of change is an excellent predictor of AD, and this is illustrated graphically.
-
3.
Future directions: Validation of the model in other large cohorts is required. The cost-effectiveness of measuring hippocampal atrophy compared with other biomarkers is needed for judging clinical value.
Acknowledgments
The authors would like to thank all radiographers and doctors involved in gathering the data. A special thanks is given to the participants from the Aberdeen 1936 Birth Cohort studies for their voluntary contribution to these projects.
R.T.S. receives funding from TauRx. A.D.M. provides brain imaging advice to TauRx but receives no remuneration for this. She has previously received honoraria from GE Healthcare for educational lectures on brain imaging in dementia and parkinsonian disorders. The other authors report no disclosures.
Data collection was funded by grants from the Alzheimer's Research Trust (now Alzheimer's Research UK, grant reference ART/SPG2003B), Alzheimer's Research UK (grant reference ARUK-SB2012B-2), and the University of Aberdeen Development Trust. DG002 RGB3109. L.J.W., R.T.S., and A.D.M. acquired funding for the study and are steering committee members and guarantors of the imaging data.
L.J.W. recruited participants. A.D.M. supervised all imaging acquisitions.
Conflicts of interest: None declared
Statistical analyses completed by Dr Arnab Rana, Aberdeen Biomedical Imaging Center
Industry sponsorship: None.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2016.11.007.
Supplementary data
Supplementary Figure 1.
Supplementary Figure 2.
References
- 1.Scheltens P., Leys D., Barkhof F., Huglo D., Weinstein H.C., Vermersch P. Atrophy of medial temporal lobes on MRI in ”probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen Q., Loewenstein D.A., Potter E., Zhao W., Appel J., Greig M.T. Volumetric and visual rating of magnetic resonance imaging scans in the diagnosis of amnestic mild cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2011;7:e101–e108. doi: 10.1016/j.jalz.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi M., Kawakatsu S., Sato T., Ohshima F. Correlation between volume and morphological changes in the hippocampal formation in Alzheimer's disease: rounding of the outline of the hippocampal body on coronal MR images. Neuroradiology. 2012;54:1079–1087. doi: 10.1007/s00234-012-1019-7. [DOI] [PubMed] [Google Scholar]
- 4.Ridha B.H., Barnes J., van de Pol L.A., Schott J.M., Boyes R.G., Siddique M.M. Application of automated medial temporal lobe atrophy scale to Alzheimer disease. Arch Neurol. 2007;64:849–854. doi: 10.1001/archneur.64.6.849. [DOI] [PubMed] [Google Scholar]
- 5.Scheltens P., van de Pol L. Impact commentaries. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 2012;83:1038–1040. doi: 10.1136/jnnp-2012-302562. [DOI] [PubMed] [Google Scholar]
- 6.Adachi M., Kawanami T., Kawakatsu S., Shibata A., Ohshima F. Rounding of the hippocampus in Alzheimer's disease: a study by routine coronal magnetic resonance imaging. Radiat Med. 2007;25:224–228. doi: 10.1007/s11604-007-0130-x. [DOI] [PubMed] [Google Scholar]
- 7.FreeSurfer. Available at: http://surfer.nmr.mgh.harvard.edu/. Accessed January 12, 2017.
- 8.Sedgwick P. Receiver operating characteristic curves. BMJ. 2013;346 doi: 10.1136/bmj.h2464. [DOI] [PubMed] [Google Scholar]
- 9.Whalley L.J., Murray A.D., Staff R.T., Starr J.M., Deary I.D., Fox H.C. How the 1932 and 1947 mental surveys of Aberdeen schoolchildren provide a framework to explore the childhood origins of late onset disease and disability. Maturitas. 2011;69:365–372. doi: 10.1016/j.maturitas.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Scheltens P., Launer L.J., Barkhof F., Weinstein H.C., van Gool W.A. Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurol. 1995;242:557–560. doi: 10.1007/BF00868807. [DOI] [PubMed] [Google Scholar]
- 11.Segonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Dale A., Fischl B., Sereno M.I. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 13.Talairach J., Tournoux P. Georg Thieme Verlag; Stuttgart: 1988. Co-planar stereotaxic atlas of the human brain: three-dimensional proportional system. [Google Scholar]
- 14.Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 15.Klauschen F., Goldman A., Barra V., Meyer-Lindenberg A., Lundervold A. Evaluation of automated brain MR image segmentation and volumetry methods. Hum Brain Mapp. 2009;30:1310–1327. doi: 10.1002/hbm.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-Subject Template Estimation for Unbiased Longitudinal Image Analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 18.Becker JP, Knowles JE. Convenience functions for education data, 2015.
- 19.Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Jean-Charles, Sanchez J.-C. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 21.Hua X., Hibar D.P., Ching C.R., Boyle C.P., Rajagopalan P., Gutman B.A. Unbiased tensor-based morphometry: improved robustness and sample size estimates for Alzheimer's disease clinical trials. Neuroimage. 2013;66:648–661. doi: 10.1016/j.neuroimage.2012.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morra J.H., Tu Z., Apostolova L.G., Green A.E., Avedissian C., Madsen S.K. Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. Neuroimage. 2009;45:S3–S15. doi: 10.1016/j.neuroimage.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sluimer J.D., van der Flier W.M., Karas G.B., van Schijndel R., Barnes J., Boyes R.G. Accelerating regional atrophy rates in the progression from normal aging to Alzheimer's disease. Eur Radiol. 2009;19:2826–2833. doi: 10.1007/s00330-009-1512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes J., Bartlett J.W., van de Pol L.A., Loy C.T., Scahill R.I., Frost C. A meta-analysis of hippocampal atrophy rates in Alzheimer's disease. Neurobiol Aging. 2009;30:1711–1723. doi: 10.1016/j.neurobiolaging.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jack C.R., Shiung M.M., Weigand S.D., O'Brien P.C., Gunter J.L., Boeve B.F. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henneman W.J.P., Sluimer J.D., Barnes J., van der Flier W.M., Sluimer I.C., Fox N.C. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72:999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beach T.G., Monsell S.E., Phillips L.E., Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71:266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack C., Dickson D.W., Parisi J.E., Xu Y.C., Cha R.H., O'Brien P.C. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton E.J., Mukaetova-Ladinska E.B., Perry R.H., Jaros E., Barber R., O'Brien J.T. Quantitative neurodegenerative pathology does not explain the degree of hippocampal atrophy on MRI in degenerative dementia. Int J Geriatr Psychiatry. 2012;27:1267–1274. doi: 10.1002/gps.3774. [DOI] [PubMed] [Google Scholar]
- 30.Apostolova L.G., Zarow C., Biado K., Hurtz S., Boccardi M., Somme J. Relationship between hippocampal atrophy and neuropathology markers: A 7T MRI validation study of the EADC-ADNI Harmonized Hippocampal Segmentation Protocol. Alzheimers Dement. 2015;11:139–150. doi: 10.1016/j.jalz.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jack C.R., Jr., Bernstein M.A., Fox N.C., Thompson P., Alexander G., Harvey D. The Alzheimers Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chong MS, Lee TS. Understanding Alzheimer's Disease, InTech, chap. 15. Predicting Cognitive Decline in Alzheimer's Disease (AD): The Role of Clinical, Cognitive Characteristics and Biomarkers. 2013:375–408.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.