Abstract
Introduction
Among other metabolic functions, the apolipoprotein E (APOE) plays a crucial role in neuroinflammation. We aimed at assessing whether APOE ε4 modulates levels of glial cerebrospinal fluid (CSF) biomarkers and their structural cerebral correlates along the continuum of Alzheimer's disease (AD).
Methods
Brain magnetic resonance imaging (MRI) scans were acquired in 110 participants (49 control; 19 preclinical; 27 mild cognitive impairment [MCI] due to AD; 15 mild AD dementia) and CSF concentrations of YKL-40 and sTREM2 were determined. Differences in CSF biomarker concentrations and interactions in their association with gray-matter volume according to APOE ε4 status were sought after.
Results
Preclinical and MCI carriers showed higher YKL-40 levels. There was a significant interaction in the association between YKL-40 levels and gray-matter volume according to ε4 status. No similar effects could be detected for sTREM2 levels.
Discussion
Our findings are indicative of an increased astroglial activation in APOE ε4 carriers while both groups displayed similar levels of CSF AD core biomarkers.
Keywords: Astrogliosis, Microgliosis, TREM2, Glial biomarkers, Inflammation
1. Introduction
Sporadic Alzheimer's disease (AD) is understood as a clinico-biological continuum ranging from normal cognition to dementia [1], [2]. The two major neuropathologic hallmarks of Alzheimer's disease are extracellular fibrillary amyloid-β (Aβ) plaques and intracellular neurofibrillary tau tangles. Amyloid and tau pathology can be monitored in vivo quantifying these proteins in cerebrospinal fluid (CSF) and by positron emission tomography. Before symptomatic stages of AD, this new conceptualization recognizes the presence of an asymptomatic phase, referred to as the preclinical stage of AD, in which cognition is within normal ranges but abnormal amyloid markers are already present [3].
The apolipoprotein E (APOE) is the strongest genetic risk factor for AD. The APOE gene has three major alleles (ε2, ε3, and ε4) which code for different isoforms of the ApoE. ApoE regulates the metabolism of lipids by directing their transport, delivery, and distribution through ApoE receptors and associated proteins [4]. Carriers of at least one ε4 allele (APOE ε4) are 3–4 times more likely to develop AD than noncarriers [5] and show significant atrophy especially in regions susceptible to AD-related atrophy such as the hippocampus [6], [7], [8]. In the central nervous system, the ApoE protein is synthesized and secreted mainly by astrocytes [9], and to a lesser extent by microglia [10]. Microglia and astrocytes are the two major types of glial cells involved in the regulation of the immune response to pathological processes in the brain. Several neuropathological, epidemiological, and genetic studies have shown evidence for the involvement of the innate-immunity in early stages of the pathological cascade leading to Alzheimer's dementia [11], [12]. In addition to its role for lipid metabolism, APOE has an important role in Aβ aggregation and degradation [4], [13] which may be mediated by glial cells: the ApoE has been described to promote the localization and degradation of Aβ deposits by astrocytes [14] and bind to apoptotic neurons to promote phagocytosis by the microglia [15].
Recently, CSF concentrations of YKL-40 and sTREM2 have been linked to the cerebral regulation of astroglial and microglial activation along the AD continuum, respectively. YKL-40 (CHI3L1, HC gp-39) is a homolog to chitotriosidase but lacks chitinolytic activity [16]. YKL-40 secreted into the CSF is mostly produced in reactive astrocytes and has been shown to be increased in Alzheimer's disease and in healthy subjects during late middle age [17], [18], [19]. Previous cross-sectional analyses have not detected differences in CSF YKL-40 levels between APOE ε4 carriers and noncarriers [18], [19], [20], [21]. However, a longitudinal study reported a steeper increase of CSF YKL-40 levels with aging in cognitively healthy middle-aged APOE ε4 carriers relative to noncarriers [19].
The triggering receptor expressed on myeloid cells 2 (TREM2) is an innate immune receptor expressed on the surface of microglia which is involved in regulating phagocytosis, removal of apoptotic neurons and inhibition of proinflammatory response [22], [23], [24], [25]. Homozygous loss-of-function mutations in the TREM2 gene cause Nasu-Hakola disease, a rare and fatal neurodegenerative disorder, and fronto-temporal dementia (FTD)-like syndrome [26]. Heterozygous missense mutations have been recently described to significantly increase the risk of Alzheimer's disease, as well as other neurodegenerative diseases, with an odds ratio similar to that of carrying an APOE ε4 allele [27], [28]. In addition, heterozygous TREM2 mutation carriers display increased density of amyloid plaques and neurofibrillary tangles, to present with upregulated proinflammatory cytokines and with downregulated protective markers [29]. The TREM2 receptor undergoes proteolytic processing, releasing its ectodomain into the extracellular space as a soluble variant (sTREM2) via shedding by a disintegrin and metalloproteinase (ADAM) proteases and can be detected in human plasma and CSF [30], [31]. CSF sTREM2 levels are increased in the early symptomatic stages of AD [32], [33], [34]. The TREM2 receptor has been reported to bind ApoE [15], [35]. In addition, TREM2 expression is differentially modulated by APOE, the ε4 allele causing a proinflammatory environment in brain [36]. However, CSF sTREM2 levels do not seem to differ between APOE ε4 carriers and noncarriers [32], [33].
The association between CSF biomarkers and regional cerebral structural changes contributes to understanding the AD pathological mechanisms. Regarding CSF glial markers in early AD patients, our group has reported positive associations between gray-matter volume versus CSF YKL-40 and sTREM2—independently—in temporal areas, after accounting for p-tau associated atrophy [37], [38]. In a sample of cognitively normal controls and patients with amnestic mild cognitive impairment (MCI), a negative correlation between CSF YKL-40 and cortical thickness in temporal areas was found in subjects with abnormal CSF Aβ levels but not in the amyloid-negative participants [20]. These results illustrate the dynamic nature of the structural correlates of glial CSF biomarkers across the continuum of AD [12].
The aim of this work is to study whether the APOE genotype has an impact on the glial response along the clinico-biological continuum of AD. To this end, we measured the CSF concentrations of two biomarkers associated to astroglial and microglial activation (YKL-40 and sTREM2, respectively) and determined the presence of at least one ε4 allele of the APOE gene in four groups of subjects: healthy controls, a group of preclinical AD subjects, MCI patients due to AD, and a group of patients with mild dementia due to Alzheimer's disease. We then sought for differences between APOE ε4 carriers and noncarriers in the levels of the glial CSF biomarkers, as well as in their respective cerebral volumetric correlates.
2. Materials and methods
2.1. Participants
A sample of 110 participants was recruited at the AD and other cognitive disorders unit, from the Hospital Clinic i Universitari (Barcelona). The study was approved by the local institutional review board (Comitè Ètic de Recerca Clínica de l'Hospital Clínic de Barcelona), and all participants gave written informed consent to participate. Subjects underwent clinical and neuropsychological assessment, lumbar puncture, MRI scanning, and CSF analysis at the local laboratory. Aβ42, tau phosphorylated at position threonine 181 (p-tau), and total tau protein (t-tau) were determined. Genomic DNA was extracted from peripheral blood using the QIAamp DNA blood minikit (Qiagen AG, Basel, Switzerland). APOE genotyping was performed by polymerase chain reaction amplification and HhaI restriction enzyme digestion. Subjects were categorized as APOE ε4 carriers if they had at least one ε4 allele or as noncarriers otherwise.
Clinical and neuropsychological examination was performed by specialized neurologists and neuropsychologists. A clinical committee formed by two neurologists and one neuropsychologist established the diagnoses. All tests were administered in Spanish language; normative neuropsychological data were collected previously from a sample of healthy elders from Spain [39] and cutoff values were derived to be 1.5 standard deviations below the mean, after taking into account age and education. Control subjects (N = 48) were cognitively normal, defined according to Mini–Mental State Examination (MMSE) scores above 24 according to recommended diagnostic cutoffs for the local population [40], objective cognitive performance within the normal range in all tests (test battery below), clinical dementia rating (CDR) scale score of 0, no significant psychiatric symptoms or previous neurological disease, and a CSF biomarker profile inconsistent with AD pathology.
Preclinical AD (Pre-AD) subjects (N = 19) were defined according to well-established research criteria [3]: MMSE scores above the local diagnostic threshold value (24), objective cognitive performance within the normal range in all tests (test battery below), CDR scale score of 0, no significant psychiatric symptoms or previous neurological disease, and decreased CSF Aβ42 below 500 pg/mL. This threshold value was selected according to recommended reference values [41] and corresponded with the 10th percentile in a sample of healthy individuals in that study.
MCI due to AD patients (N = 27) were defined according to the National Institute on Aging -Alzheimer's Association criteria [42]. They had an amnesic MCI clinical picture, MMSE scores equal to or below 24 or abnormal scores in the Free and Cued Selective Reminding Test (FCSRT) according to age and educational level (immediate free recall <15 or immediate total recall <30 or delayed free recall <6 or delayed total recall <11), preserved daily-living activities as measured by the Functional Activities Questionnaire (FAQ, score <6), and CSF Aβ42 levels below 500 pg/mL, plus a marker of neurodegeneration: high t-tau (over 450 pg/mL), p-tau (over 75 pg/mL) [41], or medial temporal atrophy [43].
Mild dementia due to AD (N = 15) was defined according to the NIA-AA criteria [42]. Patients had a clinical picture compatible with probable Alzheimer's disease and verified through a neuropsychological examination. They had abnormal MMSE or FCSRT scores according the previously described cutoff values, plus impaired daily-living activities as measured by the FAQ (≥6) and abnormal CSF biomarkers as defined for the MCI group.
Demographic information, distribution of APOE genotypes, neuropsychology, and CSF biomarker values are detailed in Table 1.
Table 1.
Demographic characteristics of the study participants (mean ± standard deviation)
| Variables | Ctrl |
Pre-AD |
MCI |
AD |
||||
|---|---|---|---|---|---|---|---|---|
| Noncarriers (ε4−) | Carriers (ε4+) | Noncarriers (ε4−) | Carriers (ε4+) | Noncarriers (ε4−) | Carriers (ε4+) | Noncarriers (ε4−) | Carriers (ε4+) | |
| N | 44 | 5 | 11 | 8 | 13 | 14 | 8 | 7∗ |
| Age (years) | 62.18 ± 6.76 | 59.60 ± 7.92 | 68.09 ± 8.42 | 68.38 ± 8.03 | 70.23 ± 8.72 | 70.36 ± 6.16 | 65.88 ± 9.92 | 68.57 ± 10.72† |
| Gender; females, n (%) | 28 (63.64) | 4 (80.00) | 8 (72.73) | 6 (75.00) | 8 (61.54) | 7 (50.00) | 7 (87.50) | 4 (57.14)∗ |
| Aβ42 (mg/mL) | 774.67 ± 186.86 | 670.71 ± 99.86 | 383.66 ± 105.34 | 347.01 ± 52.25 | 330.52 ± 87.10 | 367.24 ± 73.22 | 306.17 ± 60.96 | 302.67 ± 97.87‡ |
| t-tau (mg/mL) | 219.55 ± 69.53 | 225.43 ± 54.42 | 251.40 ± 161.19 | 370.58 ± 133.72 | 691.20 ± 562.45 | 820.83 ± 258.59 | 779.85 ± 494.00 | 945.95 ± 510.99‡ |
| p-tau (mg/mL) | 50.90 ± 12.18 | 46.44 ± 4.36 | 54.73 ± 29.99 | 67.55 ± 19.67 | 98.67 ± 54.97 | 116.19 ± 25.60 | 105.55 ± 47.25 | 124.32 ± 46.64‡ |
| YKL_40 (mg/mL) | 271.36 ± 104.49 | 252.65 ± 32.67 | 283.74 ± 137.05 | 341.16 ± 51.71 | 364.87 ± 123.07 | 490.16 ± 133.03 | 327.79 ± 133.27 | 339.96 ± 107.99‡ |
| sTREM2§ (a.u.) | 0.4257 ± 0.2200 | 0.4042 ± 0.2178 | 0.4793 ± 0.4183 | 0.6050 ± 0.3486 | 0.6772 ± 0.3788 | 0.7400 ± 0.4708 | 0.4359 ± 0.1540 | 0.7588 ± 0.4561‡ |
| MMSE | 28.51± 1.49% | 29.00± 0.71% | 27.91± 1.64% | 26.86± 1.95% | 25.08± 2.23% | 24.79± 3.29% | 23.00± 6.14% | 22.86± 2.91%‡ |
| FCSRT-DTR | 14.65 ± 1.21 | 14.20 ± 1.92 | 14.18 ± 2.27 | 13.29 ± 1.80 | 7.25 ± 5.19 | 5.31 ± 5.44 | 5.83 ± 5.71 | 3.14 ± 3.02‡ |
| FCSRT-DFR | 10.07 ± 2.19 | 11.20 ± 1.48 | 9.45 ± 3.33 | 7.71 ± 2.29 | 3.58 ± 3.78 | 1.69 ± 2.93 | 2.67 ± 3.33 | 0.57 ± 1.13‡ |
| Left hippocampus‖ (cm3) | 5.34 ± 0.38 | 5.07 ± 0.11 | 5.12 ± 0.76 | 5.10 ± 0.43 | 5.31 ± 0.83 | 4.83 ± 0.36 | 5.19 ± 0.80 | 5.32 ± 0.49 |
| Right hippocampus‖ (cm3) | 5.25 ± 0.30 | 5.02 ± 0.18 | 5.19 ± 0.80 | 5.10 ± 0.59 | 5.22 ± 0.77 | 4.89 ± 0.31 | 5.35 ± 0.92 | 5.31 ± 0.79 |
Abbreviations: Pre-AD, preclinical Alzheimer's disease; MCI, mild cognitive impairment; MMSE, Mini–Mental State Examination; FCSRT, Free and Cued Selective Reminding Test; DTR, delayed total recall; DFR, delayed free recall; ANOVA, analysis of variance; CSF, cerebrospinal fluid; TIV, total intracranial volume.
Chi-square test (P < .05).
ANOVA: diagnostic category (P < .05).
ANOVA: diagnostic category after accounting for age and sex (P < .05).
CSF sTREM2 relative to an internal standard (a.u.: arbitrary units).
Hippocampal volumes corrected for TIV (see Methods).
2.2. CSF sampling
CSF was collected by lumbar puncture between 9 and 12 AM. Samples were processed within 1 hour, centrifuged at 4°C for 10 minutes at 2000× g and stored in polypropylene tubes at −80°C. CSF Aβ42, p-tau, and t-tau levels detection was performed by enzyme-linked immunosorbent assay from Innogenetics (Ghent, Belgium). Determination of the CSF YKL-40 followed the procedure described in [37], and determination of the CSF sTREM2 was according the process described in [33].
2.3. Image acquisition and preprocessing
Subjects were examined on a 3T MRI scanner (Magnetom Trio Tim, Siemens Medical Solutions, Germany) with the acquisition protocol described in [37]. The mean time interval between the lumbar puncture and the MRI was 41 days and ranged between 1 and 134 days. No between-group differences were found for the time interval between CSF sampling and MRI scans. MRI data were visually inspected to discard any image artifacts and anatomical anomalies before submission to a voxel-based morphometry (VBM) analysis. Briefly, gray-matter parcellations were normalized, modulated, and smoothed with 8 mm of full width at half maximum (FWHM) Gaussian kernel. See [37] for further details. In addition, hippocampal volumes were automatically calculated for each subject with an in-house implementation for SPM8 of the Individual Atlases using Statistical Parametric Mapping toolbox [44] using the automated anatomical labeling atlas [45]. Hippocampal volumes were corrected for total intracranial volume (TIV), calculated as the sum of gray-matter, white-matter, and CSF volumes. Corrected values were calculated as the unstandardized residuals of the regression between hippocampal values and TIV, plus the mean value of the uncorrected hippocampal volumes.
2.4. Statistical analysis
The probability distribution of both CSF glial biomarkers did not meet normality criteria according to the Shapiro-Wilk normality test (both, P < .001). Therefore, values were log-transformed (after transformation: YKL-40, P = .227; sTREM2, P = .699). In the remainder of the text, CSF YKL-40 and sTREM2 will refer to the log-transformed variables. Finally, a regression analysis was performed to assess whether CSF YKL-40 and sTREM2 were associated between them in the whole sample and broken down by diagnostic group.
Age-corrected core AD biomarkers were compared between carriers and noncarriers for each diagnostic group. For exploring the independent relationship between each CSF YKL-40 and sTREM2 and relevant factors, two analysis of covariance (ANCOVA) models were set up. The first one (model 1) included age and sex as covariates and diagnostic group and APOE ε4 status as categorical factors (diagnostic model). In the second one (model 2), diagnostic group was substituted by CSF Aβ and p-tau levels (biomarker model). In both models, the dependent variable was the concentration of glial CSF biomarkers. Model 1 allowed us to assess the impact of APOE ε4 genotype on these concentrations by modeling AD progression with diagnostic categories, whereas in model 2, disease progression is modeled using core CSF AD biomarkers as continuous covariates. Initially, all two-way interactions were included in the model but were removed if they did not reach a statistical significance level of P < .1. In addition, two-sample t tests were computed to compare age-corrected glial biomarker levels between APOE ε4 carriers and noncarriers for each diagnostic group. For all tests, statistical significance was considered if P < .05.
After the first set of statistical analyses, the design matrix for neuroimaging data was created. VBM analyses were performed by fitting a voxelwise general linear model as implemented in SPM12. The design involved gender (F/M), age (interacting with APOE ε4 status), p-tau, diagnostic category (Ctrl/PreAD/MCI/AD), APOE ε4 status (carrier/noncarrier), and the CSF glial biomarkers interacting with APOE ε4 status. The interaction between age and APOE ε4 was considered after previous reports of carriers showing a steeper age-related decline of cortical thickness not only in brain regions associated with aging but also with Alzheimer's disease and Aβ accumulation [46]. As data were modulated, total intracranial volume was used as a global regressor. Therefore, voxelwise gray-matter volumes were modeled as follows:
| (1) |
where CSF_biomark was substituted by CSF YKL-40 and sTREM2, accordingly. For both CSF glial biomarkers, differences between correlation slopes between carriers and noncarriers were sought after with t-contrasts for each diagnostic group and for the whole sample. Another model was essayed including CSF Aβ as regressor that was finally discarded as this variable had no impact on the results. The statistical significance threshold was set to P < .001 uncorrected. Only clusters that survived an extent threshold of k = 100 voxels were considered.
3. Results
3.1. Demographics
Table 1 displays the demographic characteristics and CSF biomarker concentrations for the different diagnostic groups broken down by APOE ε4 status. The distribution between carriers and noncarriers was generally balanced between diagnostic groups, except for controls in which the number of ε4+ subjects (N = 5) significantly (P < .05) differed from that of ε4− (N = 44). Only four subjects in our sample were APOE ε4 homozygotes (2 MCI and 2 AD) which prevented us from performing any comparisons against heterozygotes. Levels of core Alzheimer's disease CSF biomarkers were slightly more altered in APOE ε4 carriers versus noncarriers. Specifically, APOE ε4 carriers displayed lower CSF Aβ through the studied sample as well higher concentrations of CSF p-tau and t-tau. In addition, carriers also displayed slightly lower TIV-corrected hippocampal volumes (Table 1). However, none of these differences reached statistical significance for any biomarker or diagnostic group. CSF YKL-40 and TREM values were significantly associated in the whole sample (P < .001; R2 = 0.252). When broken down by diagnostic group, CSF YKL-40 and sTREM were significantly associated in Ctrl (P = .009; R2 = 0.371) and Pre-AD (P = .018; R2 = 0.537) and showed a tendency to signification in MCI (P < .109; R2 = 0.315) and AD (P < .056; R2 = 0.503).
3.2. Statistical associations of CSF YKL-40
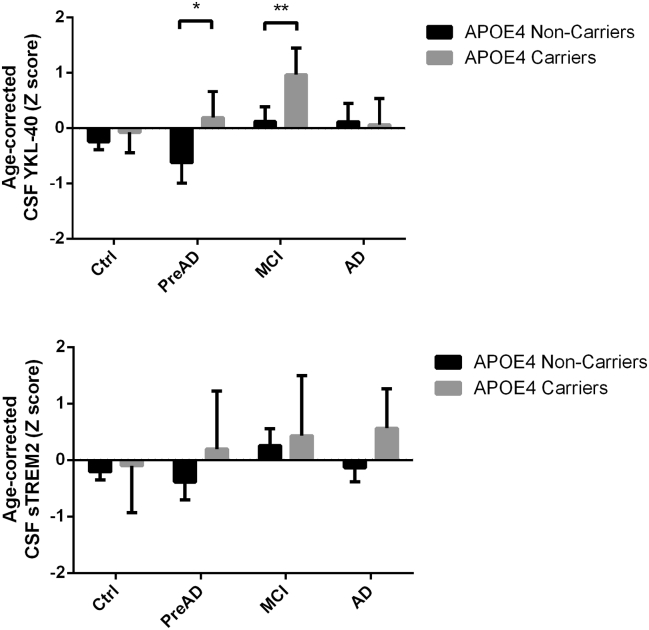
All two-way interactions did not reach statistical significance and were removed from the two statistical models. Therefore, model 1 (diagnostic model) included demographic factors (age and sex), APOE ε4 status, and diagnostic category, whereas in model 2 (biomarker model), the diagnostic factor was substituted by core AD CSF biomarkers as covariates. In both statistical models, CSF YKL-40 levels were increased in APOE ε4 carriers (P = .016 and P = .015 for models 1 and 2, respectively) and positively associated with age (P < .001), but not sex (see Table 2 for further details). In the diagnostic model, diagnostic category significantly predicted CSF YKL-40 (P = .015): Age-corrected CSF YKL-40 levels were significantly higher for MCI APOE ε4 carriers than in noncarriers (P = .023), and this pattern showed a tendency to significance on Pre-AD subjects (P = .074; Fig. 1). In the biomarker model, CSF YKL-40 was positively associated with p-tau (P < .001) and showed a tendency to significance to be inversely associated with CSF Aβ.
Table 2.
Results (F-statistic; P-value) of the univariate analyses of variance (ANOVAs) to test for associations between levels of glial CSF biomarkers and APOE ε4 status, demographic factors, and disease stage (model 1) or core CSF AD biomarkers (model 2)
| CSF YKL-40 |
CSF sTREM2 |
|||
|---|---|---|---|---|
| Model 1 (diagnostic) | Model 2 (biomarker) | Model 1 (diagnostic) | Model 2 (biomarker) | |
| Age | F(1,103) = 16.969; P < .001 | F(1,104) = 19.770; P < .001 | F(1,103) = 4.285; P = .041 | F(1,104) = 4.329; P = .040 |
| Sex | F(1,103) = 0.452; P = .503 | F(1,104) = 0.379; P = .539 | F(1,103) = 0.626; P = .431 | F(1,104) = 0.413; P = .522 |
| APOE ε4 | F(1,103) = 6.024; P = .016 | F(1,104) = 6.107; P = .015 | F(1,103) = 2.664; P = .106 | F(1,104) = 2.070; P = .153 |
| Diag | F(3,103) = 3.679; P = .015 | - | F(3,103) = 1.450; P = .233 | - |
| Aβ | - | F(1,104) = 3.292; P = .073 | - | F(1,104) = 2.203; P = .141 |
| p-tau | - | F(1,104) = 20.777; P < .001 | - | F(1,104) = 20.735; P < .001 |
Abbreviations: CSF, cerebrospinal fluid; AD, Alzheimer's disease.
NOTE: Statistically significant factors (P < .05) in bold.
Fig. 1.
Age-corrected levels of CSF YKL-40 (top) and sTREM2 (bottom) for all diagnostic groups broken down by APOE4 genotype. *P < .1; **P < .05. Abbreviations: CSF, cerebrospinal fluid; MCI, mild cognitive impairment; Pre-AD, preclinical Alzheimer's disease.
3.3. Statistical associations of CSF sTREM2
All two-way interactions did not reach statistical significance and were removed from the models. CSF sTREM2 showed a significant direct association with age in both statistical models (P = .041 and P = .040 for models 1 and 2, respectively) but not APOE ε4 genotype (P = .106 and P = .153 for models 1 and 2, respectively) nor sex (see Table 2 for additional details). In the diagnostic model, diagnostic category did not have a significant impact on CSF sTREM2 levels (P = .233). In the biomarker model, CSF sTREM2 concentrations were positively associated with CSF p-tau (P < .001) but not with Aβ (P = .141). Age-corrected CSF sTREM2 levels were not significantly different between APOE ε4 carriers and noncarriers for any diagnostic group.
3.4. Brain volumetric correlates of CSF YKL-40 by APOE ε4 genotype in the AD continuum
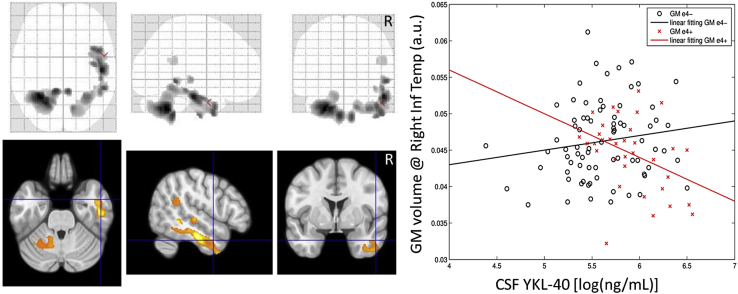
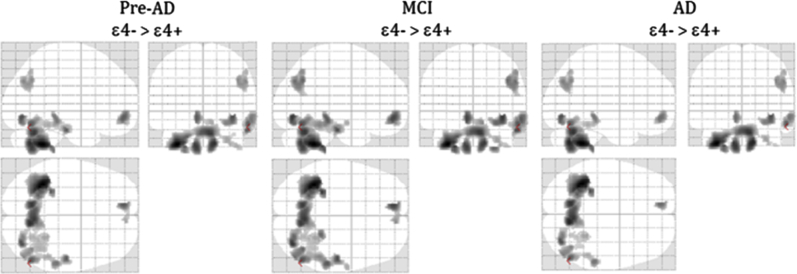
In the correlation analysis in the whole sample, which included p-tau and the diagnostic group as covariates, APOE ε4 carriers showed an inverse (negative) association between CSF YKL-40 and gray-matter volumes, whereas this association was direct (positive) in noncarriers. The regression slopes of carriers and noncarriers were significantly different (P < .001) as determined by the interaction analysis. Brain areas displaying such behavior included the cerebellum, fusiform, angular, and temporal regions (Table 3). Fig. 2 shows this pattern, which included areas that were previously described to negatively correlate with CSF YKL-40 in amyloid-positive cognitively normal controls and patients with amnestic MCI [20]. Similar areas showed an inverted u-shape association with CSF YKL-40 in early Alzheimer's disease patients [37]. On the other hand, the inverse contrast did not show any significant cluster neither at the group nor whole sample levels. Fig. 3 shows equivalent maps broken down by diagnostic category, except for controls given the low number of APOE ε4 carriers in this group (N = 4). The pattern of APOE ε4 modulation of the association between CSF YKL-40 and gray-matter volume is remarkably consistent along the clinico-biological continuum of AD.
Table 3.
Location of the clusters found in the maps from the correlation of the GM volume in ε4− w.r.t. ε4+ associated with the CSF YKL-40 in the AD continuum
| Group (contrast) | Distribution | Brain regions | MNI coordinates |
Cluster size | Z-score | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| AD continuum (ε4− > ε+) | Cluster 1 | Cerebellum Crus L | −34 | −50 | −42 | 2473 | 4.50 |
| Cerebellum L & R | |||||||
| Vermis | |||||||
| Cluster 2 | Fusiform R | 36 | −76 | −8 | 226 | 3.87 | |
| Occipital Inf R | |||||||
| Lingual R | |||||||
| Cluster 3 | Temporal Inf R | 56 | −66 | −18 | 511 | 3.81 | |
| Temporal Mid R | |||||||
| Cluster 4 | Frontal Med Orb L | −10 | 52 | −10 | 206 | 3.81 | |
| Rectus R | |||||||
| Frontal Med Orb R | |||||||
| Cluster 5 | Angular R | 42 | −64 | 36 | 309 | 3.60 | |
| Occipital Mid R | |||||||
Abbreviations: GM, gray matter; CSF, cerebrospinal fluid; AD, Alzheimer's disease.
Fig. 2.
Interaction in the volumetric correlates of CSF YKL-40 between APOE ε4 carriers and noncarriers throughout all diagnostic groups. (Left) Brain areas showing a statistically significant interaction. (Right) Scatterplot and linear fits of gray-matter volume in the right inferior temporal cortex versus CSF YKL-40 for APOE ε4 carriers (red) and noncarriers (black). Abbreviations: a.u., arbitrary units; CSF, cerebrospinal fluid; GM, gray matter.
Fig. 3.
Gray-matter volume interactions between CSF YKL-40 concentrations and APOE4 genotype at each diagnostic group. Glass brains show areas in which the regression slope was significantly higher (P < .001, uncorrected) in noncarriers (ε4−) versus carriers (ε4+). The control group was excluded from the analysis due to the small number of APOE4 carriers (N = 4). Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; GM, gray matter; MCI, mild cognitive impairment.
3.5. Brain volumetric correlates of CSF sTREM2 by APOE ε4 genotype in the AD continuum
No statistically significant results were found in any of the analyses comparing this biomarker in accordance to APOE ε4 status. In the VBM analysis, no voxels survived the significance thresholds in any of the t-contrasts comparing CSF sTREM2 regression slopes between carriers and noncarriers.
4. Discussion
In this work, we have explored how the APOE genotype modulates CSF YKL-40 and sTREM2 concentrations and their brain structural correlates across cognitively healthy individuals free of (Ctrls) and with (Pre-AD) amyloid pathology, patients of MCI due to AD, and AD patients. To this regard, it is important to take disease stage into account when considering CSF YKL-40 and sTREM2 as AD biomarkers. Our main finding is that APOE ε4 has a significant impact on CSF YKL-40 concentrations and its cerebral structural correlates. APOE ε4 carriers present with increased CSF YKL-40 levels, particularly at the MCI and Pre-AD stages (Fig. 1). In addition, we found that APOE ε4 status modulated the association between CSF YKL-40 levels and gray-matter volume in brain areas previously described to be associated with this biomarker in preclinical, prodromal, and early AD [20], [37] (Fig. 2). In these areas and throughout the full AD continuum, APOE ε4 carriers showed an inverse association between gray-matter volume and CSF YKL-40 levels, whereas noncarriers displayed a positive one (Fig. 3). These two regression slopes were significantly different as we specifically sought for their interactions depending on the APOE ε4 genotype. Please note that CSF p-tau levels have been taken into account in all of the analyses here reported, and therefore, our findings should be regarded to be independent of tau-related cerebral atrophy. In addition, in our analysis, we also took into account the distinct impact of APOE ε4 status on age-related decline of gray-matter volume in accordance with previous reports [46].
On the other hand, we did not detect any significant differences between APOE ε4 carriers and noncarriers with respect CSF sTREM2, in agreement with [32], [33]. The TREM2 receptor has been described to recognize ApoE not only as a free soluble protein but also as a constituent of amyloid plaques. APOE ε4 shows a similar binding affinity to the TREM2 receptor as compared to the other APOE isoforms [15]. This fact might account for the similar CSF sTREM2 levels here observed in APOE ε4 carriers and noncarriers.
Our finding of increased CSF YKL-40 levels in APOE ε4 carriers contrasts with previous literature [18], [19], [20], including one article published with the same sample here studied [21]. Unlike these, we here sought for differences between carriers and noncarriers after accounting for demographic and diagnostic factors. By taking them into account, we improved the statistical power to detect differences associated to the APOE genotype. It could be hypothesized that increased amyloid pathology in APOE ε4 carriers could explain this difference. However, although APOE ε4 carriers in our sample tended to present with slightly more abnormal AD progression biomarkers within each diagnostic group—including lower CSF Aβ—these differences did not reach statistical significance. On the other hand, we had previously described an inverted u-shape relationship between CSF YKL-40 and gray-matter volume in the same brain areas [37]: At lower concentrations, CSF YKL-40 was positively associated with gray-matter volume but for levels over 400 ng/mL, approximately, this association changed sign and became negative. Therefore, APOE ε4 carriers showing an increased astroglial activation in response to similar levels of brain pathology could simultaneously explain both the higher YKL-40 levels in CSF and the inverse association with gray-matter volume observed in this group as compared with the positive one in noncarriers. However, the evidence presented in this cross-sectional article does not allow us to confirm this hypothesis and further longitudinal studies are needed.
The main limitations of our work are the reduced sample size and the lack of longitudinal data to track the described dynamic changes in CSF YKL-40 and sTREM2 concentrations and their associated cerebral correlates. Therefore, our findings require confirmation in larger longitudinal studies. Another relevant limitation of analyzing a reduced sample size is that our VBM results were not corrected for multiple comparisons. In this regard, our results can be useful for performing statistical power calculations to estimate sample size requirements for future studies. In addition, it remains to be determined whether elevations in glial CSF biomarkers actually correspond with increased cerebral astroglial and/or microglial activation. To this end, longitudinal in vivo studies of astroglial and microglial activation and its structural correlates along the AD continuum are most needed.
In conclusion, we detected increased CSF YKL-40 in APOE ε4 carriers with abnormal AD core CSF biomarkers. APOE ε4 carriers showed significantly lower regression slopes with gray-matter volume across the full continuum of AD. These findings are indicative of an increased astroglial activation in APOE ε4 carriers at similar levels of alterations in AD core biomarkers. On the other hand, we did not detect any differences between carriers and noncarriers with respect CSF levels of sTREM2 or its cerebral structural correlates.
Research in context.
-
1.
Systematic review: Literature was reviewed using traditional sources, and studies investigating the relationship between APOE ε4, cerebrospinal fluid (CSF) glial markers YKL-40, and sTREM-2 with regional brain volumes across the spectrum of Alzheimer's disease (AD) are cited throughout the article.
-
2.
Interpretation: We identify that APOE ε4 carriers display greater CSF levels of YKL-40 but not of sTREM2. In addition, carriers showed a distinct association between CSF YKL-40 and regional brain volumes compatible with a greater astrocytic response. These results support for a role of APOE ε4 in the regulation of astroglial response in the AD continuum.
-
3.
Future directions: (1) To track the fate of the swollen brain regions in longitudinal studies. (2) To ascertain whether elevated CSF glial markers actually correlate with neuroinflammation along the AD continuum.
Acknowledgments
This publication is part of the AETIONOMY project (Organising Mechanistic Knowledge about Neurodegenerative Diseases for the Improvement of Drug Development and Therapy) of the EU/EFPIA Innovative Medicines Initiative Joint Undertaking AETIONOMY grant number 115568. This work was also partially financed by grant to Dr. Albert Lladó (PI14/00282) from the Spanish Ministry of Economy and Competitiveness ISCIII and cofunded by the European Regional Development Fund (ERDF). Juan D. Gispert holds a “Ramón y Cajal” fellowship (RYC-2013-13054) and Lorena Rami is part of the “Programa de investigadores del sistema nacional Miguel Servet II” (CPII14/00023; IP: Lorena Rami). This work was supported by the Deutsche Forschungsgemeinschaft (German Research Foundation) within the framework of the Munich Cluster for Systems Neurology (EXC 1010 SyNergy), Cure Alzheimer's Fund, and MetLife Foundation Award (to Christian Haass).
Contributor Information
Juan Domingo Gispert, Email: jdgispert@fpmaragall.org.
José Luis Molinuevo, Email: jlmolinuevo@fpmaragall.org.
References
- 1.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 3.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verghese P.B., Castellano J.M., Holtzman D.M. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gispert J.D., Rami L., Sanchez-Benavides G., Falcon C., Tucholka A., Rojas S. Nonlinear cerebral atrophy patterns across the Alzheimer's disease continuum: impact of APOE4 genotype. Neurobiol Aging. 2015;36:2687–2701. doi: 10.1016/j.neurobiolaging.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y., Yu J.T., Wang H.F., Han P.R., Tan C.C., Wang C. APOE genotype and neuroimaging markers of Alzheimer's disease: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86:127–134. doi: 10.1136/jnnp-2014-307719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolk D.A., Dickerson B.C., Alzheimer's Disease Neuroimaging Initiative Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Q., Bernardo A., Walker D., Kanegawa T., Mahley R.W., Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J., Basak J.M., Holtzman D.M. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eikelenboom P., Veerhuis R., van Exel E., Hoozemans J.J., Rozemuller A.J., van Gool W.A. The early involvement of the innate immunity in the pathogenesis of late-onset Alzheimer's disease: neuropathological, epidemiological and genetic evidence. Curr Alzheimer Res. 2011;8:142–150. doi: 10.2174/156720511795256080. [DOI] [PubMed] [Google Scholar]
- 12.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyss-Coray T., Loike J.D., Brionne T.C., Lu E., Anankov R., Yan F. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 14.Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 15.Atagi Y., Liu C.C., Painter M.M., Chen X.F., Verbeeck C., Zheng H. Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2) J Biol Chem. 2015;290:26043–26050. doi: 10.1074/jbc.M115.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattsson N., Tabatabaei S., Johansson P., Hansson O., Andreasson U., Mansson J.E. Cerebrospinal fluid microglial markers in Alzheimer's disease: elevated chitotriosidase activity but lack of diagnostic utility. Neuromolecular Med. 2011;13:151–159. doi: 10.1007/s12017-011-8147-9. [DOI] [PubMed] [Google Scholar]
- 17.Bonneh-Barkay D., Bissel S.J., Kofler J., Starkey A., Wang G., Wiley C.A. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol. 2012;22:530–546. doi: 10.1111/j.1750-3639.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig-Schapiro R., Perrin R.J., Roe C.M., Xiong C., Carter D., Cairns N.J. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutphen C.L., Jasielec M.S., Shah A.R., Macy E.M., Xiong C., Vlassenko A.G. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol. 2015;72:1029–1042. doi: 10.1001/jamaneurol.2015.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcolea D., Vilaplana E., Pegueroles J., Montal V., Sanchez-Juan P., Gonzalez-Suarez A. Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer's disease. Neurobiol Aging. 2015;36:2018–2023. doi: 10.1016/j.neurobiolaging.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Antonell A., Mansilla A., Rami L., Llado A., Iranzo A., Olives J. Cerebrospinal fluid level of YKL-40 protein in preclinical and prodromal Alzheimer's disease. J Alzheimers Dis. 2014;42:901–908. doi: 10.3233/JAD-140624. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh C.L., Koike M., Spusta S.C., Niemi E.C., Yenari M., Nakamura M.C. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009;109:1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klesney-Tait J., Turnbull I.R., Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K., Rochford C.D., Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., Cella M., Mallinson K., Ulrich J.D., Young K.L., Robinette M.L. TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borroni B., Ferrari F., Galimberti D., Nacmias B., Barone C., Bagnoli S. Heterozygous TREM2 mutations in frontotemporal dementia. Neurobiol Aging. 2014;35:934.e7–934.e10. doi: 10.1016/j.neurobiolaging.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P.V., Snaedal J. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roussos P., Katsel P., Fam P., Tan W., Purohit D.P., Haroutunian V. The triggering receptor expressed on myeloid cells 2 (TREM2) is associated with enhanced inflammation, neuropathological lesions and increased risk for Alzheimer's dementia. Alzheimers Dement. 2015;11:1163–1170. doi: 10.1016/j.jalz.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinberger G., Yamanishi Y., Suarez-Calvet M., Czirr E., Lohmann E., Cuyvers E. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014;6:243ra86. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- 31.Piccio L., Buonsanti C., Cella M., Tassi I., Schmidt R.E., Fenoglio C. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain. 2008;131:3081–3091. doi: 10.1093/brain/awn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heslegrave A., Heywood W., Paterson R., Magdalinou N., Svensson J., Johansson P. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer's disease. Mol Neurodegener. 2016;11:3. doi: 10.1186/s13024-016-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suarez-Calvet M., Kleinberger G., Araque Caballero M.A., Brendel M., Rominger A., Alcolea D. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer's disease and associate with neuronal injury markers. EMBO Mol Med. 2016;8:466–476. doi: 10.15252/emmm.201506123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piccio L., Deming Y., Del-Aguila J.L., Ghezzi L., Holtzman D.M., Fagan A.M. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 2016;131:925–933. doi: 10.1007/s00401-016-1533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey C.C., DeVaux L.B., Farzan M. The triggering receptor expressed on myeloid cells 2 binds Apolipoprotein E. J Biol Chem. 2015;290:26033–26042. doi: 10.1074/jbc.M115.677286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X., Montine K.S., Keene C.D., Montine T.J. Different mechanisms of apolipoprotein E isoform-dependent modulation of prostaglandin E2 production and triggering receptor expressed on myeloid cells 2 (TREM2) expression after innate immune activation of microglia. FASEB J. 2015;29:1754–1762. doi: 10.1096/fj.14-262683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gispert J.D., Monte G.C., Falcon C., Tucholka A., Rojas S., Sanchez-Valle R. CSF YKL-40 and pTau181 are related to different cerebral morphometric patterns in early AD. Neurobiol Aging. 2016;38:47–55. doi: 10.1016/j.neurobiolaging.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 38.Gispert J.D., Suarez-Calvet M., Monté G.C., Tucholka A., Falcon C., Rojas S. Cerebrospinal fluid sTREM2 levels are associated with gray matter volume increases and reduced diffusivity in early Alzheimer's Disease. Alzheimers Dement. 2016;12:1259–1272. doi: 10.1016/j.jalz.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Pena-Casanova J., Aguilar M., Santacruz P., Bertran-Serra I., Hernandez G., Sol J.M. Adaptation and normalization of the Alzheimer's disease Assessment Scale for Spain (NORMACODEM) (II)Neurologia. 1997;12:69–77. [PubMed] [Google Scholar]
- 40.Blesa R., Pujol M., Aguilar M., Santacruz P., Bertran-Serra I., Hernandez G. Clinical validity of the ‘mini-mental state’ for Spanish speaking communities. Neuropsychologia. 2001;39:1150–1157. doi: 10.1016/s0028-3932(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 41.Sjogren M., Vanderstichele H., Agren H., Zachrisson O., Edsbagge M., Wikkelso C. Tau and Abeta42 in cerebrospinal fluid from healthy adults 21-93 years of age: establishment of reference values. Clin Chem. 2001;47:1776–1781. [PubMed] [Google Scholar]
- 42.Jack C.R., Jr., Albert M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molinuevo J.L., Gispert J.D., Dubois B., Heneka M.T., Lleo A., Engelborghs S. The AD-CSF-index discriminates Alzheimer's disease patients from healthy controls: a validation study. J Alzheimers Dis. 2013;36:67–77. doi: 10.3233/JAD-130203. [DOI] [PubMed] [Google Scholar]
- 44.Alemán-Gómez Y, Melie-García L, Valdés-Hernandez P. IBASPM: toolbox for automatic parcellation of brain structures. 12th Annual Meeting of the Organization for Human Brain Mapping. Florence, Italy 2006.
- 45.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 46.Espeseth T., Westlye L.T., Fjell A.M., Walhovd K.B., Rootwelt H., Reinvang I. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiol Aging. 2008;29:329–340. doi: 10.1016/j.neurobiolaging.2006.10.030. [DOI] [PubMed] [Google Scholar]