Human Molecular Genetics 2014 23:7; pp. 1869–1878; doi: 10.1093/hmg/ddt579
Unfortunately, this article included errors involving the antibody directed against HMOX1 supplied by Aviva Systems Biology, San Diego, CA, USA (Catalogue #ARP-45222_P050) that were not discovered until follow-up studies using HMOX1 transgenic mouse models. Further western blotting with this antibody on mouse muscles including a Hmox1-null mouse (1) and a transgenic mouse expressing human HMOX1 (2) clearly showed that the commercial antibody detected the human HMOX1 transgene and a cross-reactive protein slightly larger than the one expressed by the transgenic animal and not missing from the Hmox1-null mouse (Corrigendum Fig. 4C). This Hmox1 Aviva antibody was used in Fig. 4C and indicated that HMOX1 was increased in expression in mdx5cv mice treated with the PDE5 inhibitor sildenafil. Follow-up experiments with a second HMOX1 antibody (Enzo Life Sciences Catalogue #ADI-SPA-895) showed that while the antibody detected the proper-sized protein missing from the Hmox1-null mouse it failed to detect any indication of induction of HMOX1 following sildenafil treatment both in the original samples used in the manuscript nor in additional mouse muscles in sildenafil-treated mdx5cv mice (see Corrigendum Fig. 4C). The same Hmox1 Aviva antibody was used in Fig. 2C where it detected an increase of HMOX1 in dystrophin-deficient zebrafish treated with aminophylline as well as other compounds. Given the new results in mice with this second Enzo Life Sciences antibody and the fact that zebrafish hmox1 protein sequence in the region of the epitope to which the Aviva antibody was directed was substantially less conserved than the mouse sequence we decided to redo the zebrafish experiments with both antibodies on new preparations of zebrafish muscle following aminophylline treatment. Using both commercial Hmox1 antibodies on new westerns of zebrafish muscle treated with aminophylline (or vehicle control) alongside lanes of muscle from the transgenic mice expressing human HMOX1 and mouse muscle from Hmox1-null mice, we found that both antibodies detected a band on western blots which migrated at the same size as that produced by the transgenic animal and was missing from the muscle of the Hmox1-null mouse. Neither antibody detected an increase of hmox1 protein in these fish muscles following aminophylline treatment (Corrigendum Fig. 2C). We regret this error but remain confident that the compounds presented in this manuscript improves muscle function in zebrafish, albeit not through directly increasing hmox1 as originally proposed. The original authors of this manuscript wish to acknowledge Jamie L. Marshall who uncovered these issues related to the HMOX1 antibody and performed the experiments presented here in this Corrigendum notice.
1. J. Clin. Invest., 1999 Apr;103(8):R23–9.
2. Stem Cells. Jan 2011; 29(1): 99–107.
Figure Legend for Corrigendum
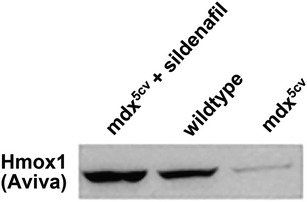
Original Figure 4C: Western blot for Hmox1 protein (Aviva antibody; 1:500 dilution) in the TA (tibialis anterior) muscles of mdx5cv mice treated with sildenafil (400 mg/l) for 7–8 weeks, wild-type and untreated mdx5cv mice.
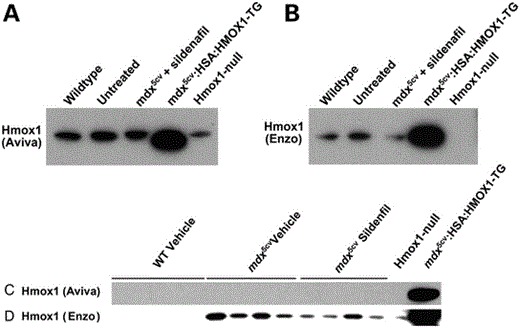
Corrigendum Figure 4C: (A and B). Western blot for Hmox1 protein in wild-type (WT) untreated (mdx5cv mice), mdx5cv + sildenafil (400 mg/l concentration in drinking water for 7–8 weeks), mdx5cv:HSA:HMOX1-TG mice (mdx5cv mice that overexpress a muscle-specific human HMOX1 transgene in human skeletal actin/HSA myofiber promoter; positive control), and Hmox1 knockout (KO) mice (negative control). (A) The Hmox1 (Aviva antibody; diluted at 1:500). (B) The other Hmox1 antibody (Enzo antibody; diluted at 1:500). Note the cross-reactive band in (A) that appears in the Hmox1-null mouse muscle protein lysate. (C and D) Western blot for Hmox1 protein in WT untreated (mdx5cv mice), mdx5cv + sildenafil (400 mg/l concentration in drinking water for 7–8 weeks), mdx5cv:HSA:HMOX1-TG mice (mdx5cv mice that overexpress a muscle-specific human HMOX1 transgene in human skeletal actin/HSA myofiber promoter; positive control), and Hmox1 knockout (KO) mice (negative control). (C) The Hmox1 (Aviva antibody; diluted at 1:5000). (D) The other Hmox1 antibody (Enzo antibody; diluted at 1:5000). Note the loss of the cross-reacting band in c. as the antibody has been diluted by ten-fold.
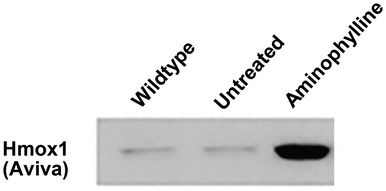
Original Figure 2C: Western blot for Hmox1 protein in Wildtype (AB strain), Untreated and Aminophylline sapje (dystrophin mutant) at 20 days post fertilization (dpf) of whole-body zebrafish protein lysates. Zebrafish were treated with Aminophylline (2.5 µg/ml) added to their water starting at 5 dpf and ending at 20 dpf.
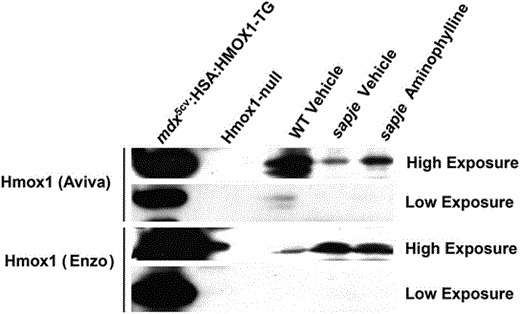
Corrigendum Figure 2C: Western blot for Hmox1 protein in the whole body of WT, sapje vehicle and sapje Aminophylline (2.5 µg/ml), along with positive (mdx5cv:HSA:HMOX1-TG mouse TA muscle lysate) and negative (Hmox1 KO mouse TA muscle lysate) controls. Both the Hmox1 Aviva and Hmox1 Enzo antibodies were used a dilutions of 1:5000, with low and high exposures on X-ray film shown.


