Abstract
Early life experiences, particularly the experience with parents, are crucial to phenotypic outcomes in both humans and animals. Although the effects of maternal deprivation on offspring well-being have been studied, paternal deprivation (PD) has received little attention despite documented associations between father absence and children health problems in humans. In the present study, we utilized the socially monogamous prairie vole (Microtus ochrogaster), which displays male-female pair bonding and bi-parental care, to examine the effects of PD on adult behaviors and neurochemical expression in the hippocampus. Male and female subjects were randomly assigned into one of two experimental groups that grew up with both the mother and father (MF) or with the mother-only (MO, to generate PD experience). Our data show that MO subjects received less parental licking/grooming and carrying and were left alone in the nest more frequently than MF subjects. At adulthood (~75 days of age), MO subjects displayed increased social affiliation towards a conspecific compared to MF subjects, but the two groups did not differ in social recognition and anxiety-like behavior. Interestingly, MO subjects showed consistent increases in both gene and protein expression of the brain-derived neurotrophic factor (BDNF) and tropomyosin receptor kinase B (TrkB) as well as the levels of total histone 3 and histone 3 acetylation in the hippocampus compared to MF subjects. Further, PD experience increased glucocorticoid receptor beta (GRβ) protein expression in the hippocampus of females as well as increased corticotrophin receptor 2 (CRHR2) protein expression in the hippocampus of males, but decreased CRHR2 mRNA in both sexes. Together, our data suggest that PD has a longlasting, behavior-specific effect on social affiliation and alters hippocampal neurochemical systems in the vole brain. The functional role of such altered neurochemical systems in social behaviors and the potential involvement of epigenetic events should be further studied.
Keywords: parental behavior, social affiliation, hippocampus, BDNF, oxytocin, CRH, epigenetics
Introduction
Variations in parental environment during development can affect adult phenotype in profound ways (Heim et al., 2002; Repetti et al., 2002; O’Donnell et al., 2014; Samek et al., 2015). In humans, for example, childhood family environments characterized by high conflict and low quality attachments are associated with vulnerability to social and emotional processing deficits (Repetti et al., 2002) as well as an increase in the risk for developing psychopathology in adulthood (Bifulco et al., 1991; Brown and Anderson, 1991; Felitti et al., 1998; Heim et al., 2002; Yam et al., 2015). It has been shown that father absence is a risk factor for a host of negative outcomes such as the development of depressive symptoms and externalizing behaviors (Culpin et al., 2013; McLanahan et al., 2013). In contrast, increased maternal responsiveness is associated with an increase in infant development including social, emotional, and cognitive competence (Landry et al., 2006). Therefore, early parental environment predicts susceptibility and resiliency to adult psychopathology (Smith and Prior, 1995; O’Donnell et al., 2014). Studies using functional magnetic resonance imaging (fMRI) have elucidated some of the brain regions, including the hippocampus as well as the cingulate and prefrontal cortices, that are affected by early childhood adversity (Brooks et al., 2014; Elton et al., 2014; Sripada et al., 2014; Jensen et al., 2015). However, the neurochemical systems that are affected by early life perturbations are less known due to the inherent difficulty of human studies.
Animal models demonstrating the effects of early life experience on adult outcome have shown high predictive and face validity, and have provided insights into neural mechanisms. Studies in rats and mice have shown that prenatal stress or maternal separation results in increased anxiety- and depressive-like behaviors in the adult offspring, as well as an increased neuroendocrine response to stress with a host of neurochemicals acting in brain regions involved in stress responses (Plotsky and Meaney, 1993; Pryce and Feldon, 2003; Daniels et al., 2004; Binder et al., 2011; Barbosa Neto et al., 2012). Notably, alterations in neurochemicals involved in plasticity and gene expression have been found in the hippocampus (Suri et al., 2013; Nishi et al., 2014; Sousa et al., 2014; Shin et al., 2016) – a brain region important for learning and memory as well as for the regulation of stress responses via interactions with the hypothalamic pituitary adrenal (HPA) axis (Kim et al., 2015). For example, maternal separation is associated with alterations in the expression of neurotrophic factors in the hippocampus of offspring, including brain derived neurotrophic factor (BDNF) and its receptor tropomyosin receptor kinase B (TrkB), as well as altered performance in hippocampal dependent cognitive tasks (Marco et al., 2013; Suri et al., 2013; Hill et al., 2014). Furthermore, adult offspring of rat dams that received more maternal licking/grooming during development displayed higher levels of maternal licking/grooming towards their own offspring but lower levels of anxiety-like behaviors, stress-induced circulating corticosterone, and glucocorticoid receptor (GR) expression in the hippocampus, compared to ones that received less maternal licking/grooming during development (Liu et al., 1997; Francis et al., 1999). Interestingly, epigenetic events have been implicated in mediating the effects of maternal environment on hippocampal neurochemical expression and associated behaviors in the adult offspring (Fish et al., 2004; Champagne et al., 2006; Pan et al., 2014; Wang et al., 2014).
While data from animal studies have revealed new knowledge regarding the effects of maternal environment on adult outcome and the underlying neurochemical mechanisms, the effects of paternal deprivation (PD) are less studied. In humans, paternal care is related to adult well-being and PD has been found to be associated with a variety of negative outcomes (McLanahan et al., 2013). Additionally, according to reports from the U.S Census Bureau and Center for Disease Control, the number of children living in father absent homes is prevalent and increasing in the United States and this corresponds to less paternal-offspring contact (U. S. Census Bureau, 2012; Jones and Mosher, 2013). Considering that PD is prevalent and increasing in our society, it is imperative to understand the neural mechanisms underlying the negative association between early father absence and adult well-being for the prevention and treatment of related psychopathologies. While common laboratory rats and mice cannot serve as appropriate models as they do not naturally display paternal behavior, the emergence of the socially monogamous prairie vole (Microtus ochrogaster) model has provided an opportunity to study male parental behavior and its effects on offspring cognitive and behavioral functions as well as the underlying neurochemical mechanisms.
The prairie vole is a rodent species that displays behavioral features of social monogamy including long-term bonding between opposite-sex mates (pair bonding) (Williams et al., 1992; Getz and Carter, 1996; Lim et al., 2004), and thus has been established as an animal model to study the neurochemical regulation of social attachment (Carter et al., 1995; Young et al., 1998). Prairie vole fathers contribute to the care of their young, displaying the full range of maternal behaviors with the exception of nursing (Wang and Novak, 1992; Wang and Insel, 1996). Furthermore, PD affects the behavior of prairie vole offspring: reducing the display of alloparental behavior by juveniles towards their younger siblings (Wang and Novak, 1994) and impairing their ability to form a pair bond in adulthood (Ahern and Young, 2009; Ahern et al., 2011). In the present study, we aimed to characterize the behavioral and neurobiological consequences of paternal deprivation in prairie voles. We compared adult male and female voles that grew up with or without a father, and examined their social and anxiety-like behaviors as well as the gene expression of several neurochemicals in the hippocampus that have been implicated in regulating stress resiliency and memory deficits in response to stressful life experience. We hypothesized that in the absence of early paternal experience, voles would show altered social and anxiety-like behaviors associated with alterations in hippocampal neurochemical systems.
Experimental procedures
Experimental animals
All animals used in our experiments were prairie voles (M. ochrogaster) produced by a breeding colony housed at Florida State University. Animals had ad libitum access to food and water, and cages were maintained on a 14:10 light:dark cycle with lights on at 0700. Subjects were the offspring of adult male and female prairie voles that were paired at the beginning of the experiment and were sexually naïve upon pairing. Paired animals were housed in large polycarbonate cages (45 × 22 × 20 cm) lined with cedar chip bedding and two cotton nestlets were provided for nest identification. Fifteen pairs gave birth to 56 offspring subjects over a period of five days. Upon litter birth (postnatal day 1: PND1), fathers remained with the female partners in the home cages (n=8 pairs) or were permanently removed from the home cages (n=7 pairs). Thus, subjects were either housed with both a mother and father (MF; n=31) or mother only to create paternal deprivation experience (MO; n=25) from PND1 until PND21. Notably, during litter births we removed the father from every other litter so that the treatment groups were created in a counterbalanced manner. At weaning (PND21), subjects were weighed and housed until adulthood with a same sex conspecific that went through the same experimental treatment. Subjects were assigned identification numbers and half of the subjects were ear-punched to distinguish them from their cage mates. All experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Florida State University.
Spot Checks
Spot checks were conducted in the morning (0830) and evening (1900) daily between PND2 and PND21, based on previous published protocols (Hammock et al., 2005; Ahern and Young, 2009; Ahern et al., 2011). Each spot check consisted of five behavioral checks at five minute intervals. During spot checks, a researcher would observe the behaviors of parents in a consecutive order and record tally marks corresponding to observed behaviors on a sheet of paper which was later transferred to an SPSS document. Cage order was counterbalanced between checks. On PND1, a patch of fur was shaven off the backs of fathers in the MF group in order to differentiate them from the mothers. Behavioral measures scored included nursing, licking/grooming, huddling and carrying pups, as well as nest building, self-grooming and the instances of each parent being either in the nest or outside of the nest. Huddling was scored when the father was hovering over its pups with an arched back. Since huddling is always accompanied by pups’ located ventral to the parent, huddling by the mother made it implausible to distinguish with certainty if any pups were attached to the nipple. Therefore, huddling by the mother was scored as nursing/huddling. Pup carrying was defined when a parent carried a pup via their mouth.
Body Weights
All subjects were weighed at weaning (PND21) and as adults. Two MF males were found dead in their home cage soon after weaning and, therefore, their data were excluded from data analyses. All adult weights were taken on the same day, which was at least two days before the first behavioral test for each subject began, during which subject’s age ranged from 68–73 days old.
Behavioral Tests
Subjects were split into two cohorts, each consisting of an equal representation of subjects across litters. Subjects in Cohort 1 (M/MF; n = 6, F/MF; n = 11, M/MO; n = 6, F/MO; n = 7) went through the parental behavior (PB), social affiliation (SOA), social recognition (SOR), and elevated plus maze (EPM) tests, respectively, with one test per day over a four day period. Due to the number of subjects and experimental groups, several behavioral tests, and a narrow window for each behavioral test, we managed to begin the first day of behavioral testing for each subject when they reached PND74-76. Each behavioral test began around the same time every day and all behavioral tests were conducted between the hours of 0900 and 1500 in a behavioral testing room controlled for light illumination (about 300 lx), as described previously (Sun et al., 2014). Behaviors were video recorded and scored at a later time via JWatcher software (NIH, Bethesda, MD) by a researcher unknowing to the experimental condition of the subjects. The day after the last behavioral test, subjects from cohort 1 were sacrificed. To avoid potential effects of behavioral testing on the neurochemical expression in the brain, subjects in Cohort 2 (n=24; 6 subjects per each of the 4 experimental groups) did not go through the behavioral tests but remained in home cages without disturbance until PND79 during which subjects were euthanized for brain collection.
Parental Behavior Test
Subject’s parental behavior was tested towards a conspecific pup for 10 mins using an established protocol (Lonstein and De Vries, 1999). The testing apparatus consisted of a polycarbonate cage (45 × 22 × 20 (H) cm) lined with cedar chip bedding. The subject was placed into the center of the cage for 10-mins habituation. Thereafter, a novel 2-day old stimulus pup was placed at the furthermost corner of the testing cage from the subject. The stimulus pup was the offspring of colony breeders and was cleaned with distilled water and a cotton swab immediately before the test. Parental behaviors were scored as pup licking/grooming, huddling, and carrying. Other behaviors such as nest building, self-grooming, locomotion, and resting were also scored. In the instances when a subject displayed aggressive behavior towards the pup, the researcher promptly intervened to remove the pup and the behavioral test was stopped.
Social Affiliation Test
Subject’s social affiliation (SOA) towards a same sex conspecific was tested also using an established method (Pan et al., 2009). Briefly, the testing apparatus consisted of two polycarbonate chambers (13 × 18 × 29 (H) cm) connected by a hollow tube (7.5 × 16 cm). A stimulus animal of the same sex and at a similar age as the subject was tethered in one chamber, and the subject was placed into the empty chamber and had access to the entire testing apparatus during the 1-hr test. Customized light sensors were attached to the hollow tube and connected to a computer to record the subject’s entry into each chamber. The subject’s time spent in each cage and cage crossing as well as the duration and frequency of side-by-side contact with the stimulus animal were recorded.
Social Recognition Test
Subject’s social recognition (SOR) was tested using an established method (Lieberwirth et al., 2012). The test consisted of four trials, each five minutes long and separated by a five minute interval. Subjects were allowed to habituate to the testing apparatus (a 25 × 45 × 20 (H) cm polycarbonate cage) for 10 mins. At the beginning of each trial, a juvenile stimulus animal was placed into the testing cage with the subject. All stimulus animals were the same sex as the subject and approximately 30 days old. At the end of each trial, the stimulus animal was removed from the testing cage and placed into a separate cage while the subject remained in the testing cage. During trials 1 to 3, the same stimulus animal was placed into the experimental cage. During trial 4, a different stimulus animal was used. The duration and frequency of subject’s sniffing (including anogenital and head sniffing) were recorded.
Elevated plus maze test
Subject’s anxiety-like behaviors were tested in an EPM test (Liu et al., 2014). Briefly, the testing apparatus (Columbus Instruments, Columbus, OH, USA) consisted of two open arms and two closed arms (35 × 6.5 × 15 (H) cm) that cross in the middle and are elevated 45 cm off the floor. At the beginning of the 5-min test, the subject is placed into the center of the platform facing a closed arm. Time that subjects spent in each arm and in center stage, entries into each arm, and latency to enter the open arm were recorded.
Tissue processing
Subjects were rapidly decapitated. Brains were extracted, immediately frozen on dry ice and subsequently stored at −80°C. Coronal sections (200µm thick) were cut on a cryostat and thaw-mounted on slides. Bilateral tissue punches 1mm in diameter of the posterior medial hippocampus were taken at −40 °C and then stored at −80 °C until further processing. Protein and RNA were isolated from 14 tissue punches of the posterior medial hippocampus using Tri-Reagent according to the manufacturer instructions (Molecular Research Center, Cincinnati, OH). Proteins were stored in Laemmle buffer in −80°C. RNA was dissolved in DEPC water and also stored in −80°C until further processing.
Western Blotting
Protein samples, 20 µg each, were loaded into 12% sodium dodecyl sulfate (SDS) polyacrylamide gels (Bio-Rad) for electrophoresis. Proteins were run on gels at 80 volts (V) for approximately 20 mins and thereafter at 120 V for 1 hr. Proteins were then transferred to nitrocellulose membranes at 100 V for 1 hr at 4 °C and subsequently blocked in either 5% milk or Superblock (Bio-Rad). Membranes were incubated at 4 °C for 1–2 days with the following primary antibodies: rabbit anti-tropomyosin receptor kinase B (TrkB) (1:200, Santa Cruz), rabbit anti-brain derived neurotrophic factor (BDNF) (1:200, Santa Cruz), rabbit anti-glucocorticoid receptor (GR) (1:1000, Santa Cruz), goat anti-oxytocin receptor (OXTR) (1:500, Santa Cruz), rabbit anti-corticotrophin releasing hormone receptor 1 (CRHR1) (1:300, Novus Biologicals), and 2 (CRFR2) (1:500, Novus Biologicals), goat anti-vasopressin receptor (V1aR) (1:300, Santa Cruz), rabbit anti-corticotrophin releasing hormone (CRH) (1:300, Proteintech), rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1000, Santa Cruz), mouse anti-total histone 3 (H3total) (Millipore, 1:1000), rabbit anti-histone 3 acetylation (H3ace) (1:1000, Millipore), or rabbit antihistone 3 acetylation at lysine 9 (H3K9ace) (1:1000, Millipore). Thereafter, membranes were washed in phosphate buffered saline (PBS) and incubated for 1 hr in the following secondary antibodies: horseradish peroxidase (HRP) conjugated anti-rabbit (1:20,000, Santa Cruz), HRP anti-goat (Santa Cruz, 1:20,000), DYLight 550 anti-rabbit (1:20,000, Thermo Fisher Scientific), DYLight 650 anti-mouse (1:20,000, Thermo Fisher Scientific) or IRDye 680 anti-rabbit (1:20,000 Li-Cor Biosciences). Membranes were then washed again and membranes that were incubated in a HRP conjugated secondary antibody were further incubated in a chemiluminescence HRP substrate (SuperSignal West Dura Extended Duration Substrate, Thermo Fisher Scientific) for 10 minutes. All proteins were visualized on the ChemiDoc MP System (Bio-Rad). The specificities of the primary antibodies were validated in previous studies in voles as well as in rats and mice for the CRH system and H3k9ace, which share high homology with prairie voles (Huang et al., 2009; Greenberg et al., 2012; Gupta-Agarwal et al., 2012; Wang et al., 2013; Wu et al., 2014; Galinato et al., 2015; Duclot et al., 2016; Yadawa and Chaturvedi, 2016) . It should be mentioned that the OTR antibody was shown to be able to detect OTR protein in the vole brain tissue via antibody neutralization with an OTR blocking peptide but the signal reduction by overnight incubation with the blocking peptide was not complete (Duclot et al., 2016). Therefore, caution needs to be taken for data interpretation. Western bands were quantified via ImageJ (NIH, Bethesda Maryland). All data were normalized within the same membrane to GAPDH while H3ace and H3K9ace was further normalized to H3total. All data are expressed as percent change from the mean of the MF groups.
Semi-quantitative real-time polymerase chain reaction (RT-PCR)
400 ng of total RNA was processed on the MyCycler Thermal Cycler (Bio Rad) for complementary DNA synthesis with the Cloned AMV First-Strand cDNA Synthesis Kit (Invitrogen, Thermo Fisher Scientific) according to manufacturer instructions. All primers and cDNA samples were diluted 1/10, loaded in triplicates with SYBR Green Supermix (Bio Rad) to a 384-well PCR plate and ran on the CFX 384 Thermal Cycler (Bio Rad). Results were normalized to the reference gene nicotinamide adenine dinucleotide dehydrogenase (NADH). Primer sequences were summarized in Table 1. Primer specificity was verified by melting curve analysis. Data are presented as the percent change from controls (MF groups).
Table 1.
Primer Sequences (5' - 3')
| Target | Primer Sequence | |
|---|---|---|
| GRα | forward | GGTTGGTGCTTCTACCCTGA |
| reverse | GTACACTGGGCCGCCTTTCGGTA | |
| GRβ | forward | ACAGACTTTCGGCTTCTGGA |
| reverse | AAAGGTGCTTTGGTCTGTGG | |
| CRHR1 | forward | GTCAGCTTCCACAGCATCAA |
| reverse | AGCCCAGTGCTGTGAGCTAT | |
| CRHR2 | forward | CAGCTTCCACAGCATCAAGC |
| reverse | CACCCAAGGGTCAGTGTAGC | |
| BDNF | forward | CCATAAGGACGCGGACTTGTAC |
| reverse | TTGGAGATGTGGTGGAGAGG | |
| TrkBFL | forward | TATGCTGTGGTGGTGATTGC |
| reverse | TTGGAGATGTGGTGGAGAGG | |
| TrkBt | forward | GGCTGCTCCTAACCTCACTG |
| reverse | TTCACGGAATCCTGGTCTTC | |
| NADH | forward | CTATTAATCCCCGCCTGACC |
| reverse | GGAGCTCGATTTGTTTCTGC | |
List of primer sequences for all targets. OXTR, oxytocin receptor; GRα, glucocorticoid receptor alpha; GRβ, glucocorticoid receptor beta; CRHR1, corticotrophin releasing hormone receptor one; CRHR2, corticotrophin releasing hormone receptor two; BDNF, brain derived neurotrophic factor; TrkBFL, tropomyosin receptor kinase receptor B full length; TrkBt; tropomyosin receptor kinase receptor B truncated; NADH, nicotinamide adenine dinucleotide dehydrogenase.
Statistical analysis
All statistical analyses were performed using SPSS Statistics software (IBM) and p < 0.05 was deemed statistically significant. All data were checked for homogeneity of variance and were either normalized when necessary by square root transformations, unless otherwise noted, or analyzed with non-parametric tests. Independent sample T-tests were conducted to compare spot checking behaviors between MF and MO groups, mothers and fathers in the MF group, and mothers from MO and MF groups, respectively. Tally marks totaling morning and evening spot checks per behavior were used for data analysis. All other data were analyzed by two-way ANOVA (group X sex) except for the social recognition data which was analyzed by two-way ANOVA with repeated measures (trials). Significant interactions were followed up with Fisher’s Least Significant Difference (LSD) post hoc tests. Data are depicted as mean+SEM.
Results
Spot checks
Under the MF condition (Figure 1a), sex differences were found - mothers licked/groomed their pups (t(14) = 4.426, p < 0.001) and adopted an overhanging posture over pups (nurse/huddle) (t(14) = 8.216, p < 0.001) more than fathers. No differences were found in other behaviors including pup carrying, nest building, and self-grooming. Mothers in the MF and MO conditions did not differ in any behaviors measured (Figure 1b). Interestingly, pups in the MF condition were licked/groomed (t(13) = 2.365, p < 0.05) and carried (H(2) = 4.801, p < 0.05) more by parents and were left alone in the nest less frequently (t(13) = 3.429, p < 0.01), compared to pups in the MO condition (Figure 1c). At weaning (PND 21), MF males weighed less compared to MF females as well as to MO males and females (F(1, 49) = 6.884, p < 0.05) (Figure 1d). At adulthood, however, males weighed more than females, regardless of rearing condition (F(1, 49) = 6.596, p < 0.05).
Figure 1.
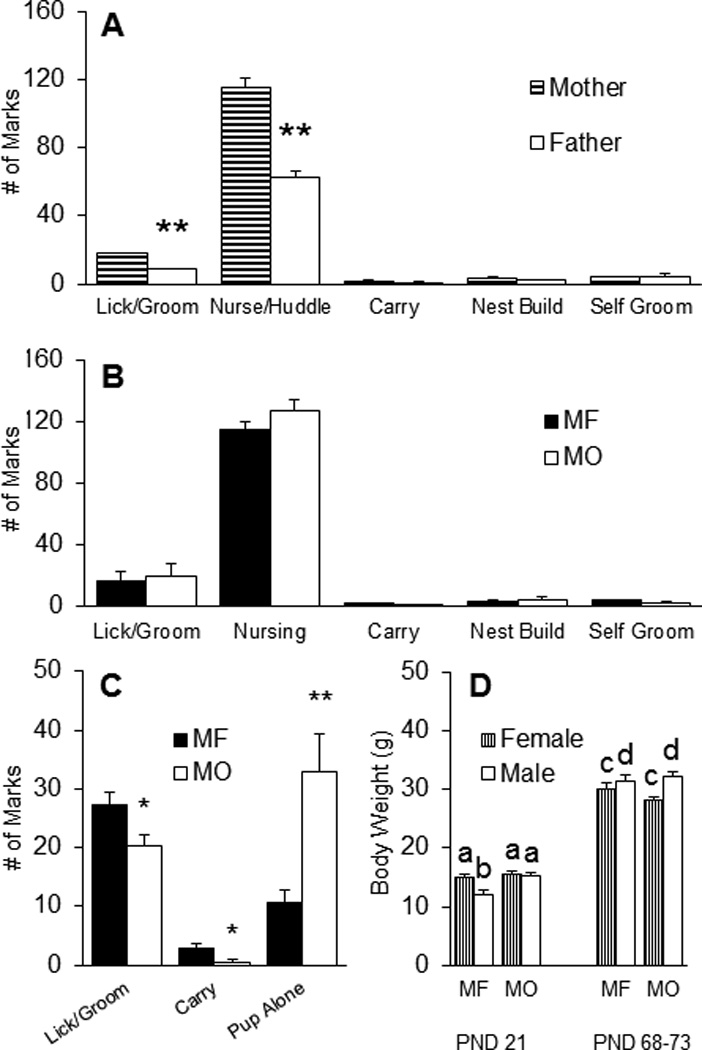
Paternal deprivation impacts offspring care. (A) When both the mother and father are present (MF), mothers display more pup licking/grooming and adopt an overarching position (Nurse/Huddle) over pups compared to fathers, but they do not differ on levels of pup carrying, nest building, or self-grooming behaviors. (B) There were no differences in pup licking and grooming, nursing, pup carrying, nest building, and self-grooming between MF mothers and mothers only (MO), without the father. (C) There were lower levels of pup directed licking and grooming and pup carrying in MO nests compared to MF nests. Additionally, there were higher instances of offspring left alone on the nest in the MO condition. (D) Finally, MF males weighed less at weaning (PND21) while MO males weighed the same as females. In adulthood, male subjects weigh more than female subjects, regardless of rearing condition. Data are shown as mean + SEM. *p < 0.05, **p < 0.01. Alphabetic letters indicate the results from a post-hoc test following an ANOVA. Bars labeled with different letters differ significantly from each other.
Behavioral tests
The number of subjects per treatment group that went through the following behavioral tests included 6 M/MF, 6 M/MO, 11 F/MF, and 7 F/MO. All subjects in cohort 1 went through all behavioral tests and all of their data is reported, unless otherwise noted.
Parental behavior
During the parental behavioral test, 33.7% (4/11) of females and 50% (3/6) of males in the MF group as well as 28.6% (2/7) of females and 33.4% (2/6) of males in the MO group displayed spontaneous parental behavior. No group and sex differences were found in the percentages of the subjects displaying parental behavior. Due to the limited numbers of the subjects displaying parental behavior, we were not able to conduct statistical analyses on the specific behaviors.
Social affiliation and recognition
In the SOA test, two subjects from the M/MO group were excluded from analysis due to poor video recording quality. Significant group and sex differences were found in the duration of side by side contact with the stimulus animal (Figure 2a). MO subjects spent more time in side by side contact with the stimulus animal than MF subjects (F(1, 24) =4.511, p < 0.05). In addition, males had more contact with the stimulus animal compared to females (F(1, 24) =5.756, p < 0.05). No significant group by sex interaction was found in the contact duration. No differences were found in the frequency of the social contact (Figure 2b). In the SOR test, no group and sex differences were found in the duration and frequency of social sniffing (Figure 2c & d). All subjects displayed decreased duration in olfactory investigation of a stimulus animal repeatedly presented over trials 1 to 3 and then increased olfactory investigation upon presentation of a novel stimulus animal during trial 4 (F1, 75 = 19.985, p <.001, Figure 2c). This similar pattern was also found in the frequency of olfactory investigation (Figure 2d).
Figure 2.
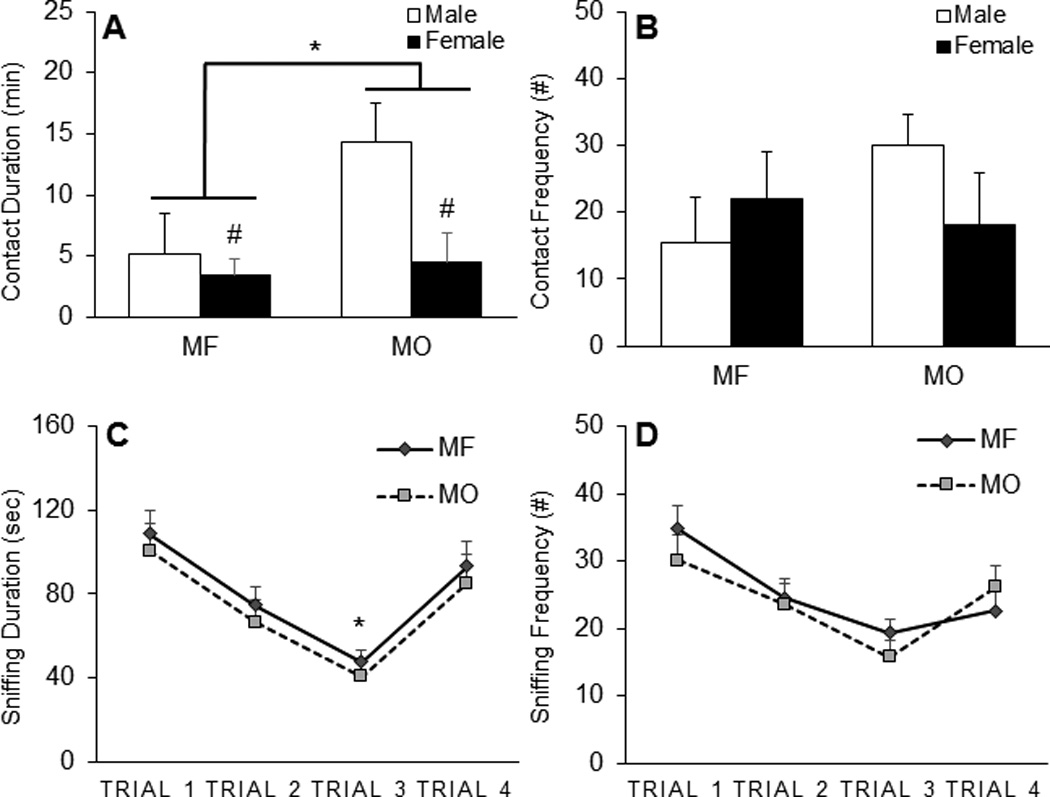
Paternal deprivation affects adult affiliation, but not recognition. (A) Paternally deprived subjects reared with a mother only (MO) spent more time in side by side contact with a conspecific during the social affiliation test (SA) than subjects that were reared by a mother and father (MF). No differences were found in side by side contact frequency (B). There were no group differences in the social recognition test (SR) including in sniffing duration (C) and frequency (D). Data are shown as mean + SEM. *p < 0.05; #: sex difference (p < 0.05).
Anxiety-like behavior
In the EPM test, one subject from the F/MF group and one subject from the F/MO group were excluded from analysis due to poor video quality and one subject falling off the EPM for more than half of the test. During the EPM test, there were no group or sex differences on any indicators of anxiety-like behavior including open arm latency, percentages of open arm entries or durations (Table 2). Treatment effect was found in total arm entries (F(1, 24) = 7.367, p < 0.01) with MF subjects displaying more arm entries than MO subjects. A two-way ANCOVA was then conducted to determine if there were group or sex differences in the behavioral measurements while controlling for locomotion (total arm frequency). When controlling for total arm crosses, a significant group effect was detected for open arm latency (F(1, 23) = 0.028, p < 0.05): MO subjects ( 14 ± 8 seconds) had decreased open arm latencies compared to MF subjects (40 ± 7 seconds).
Table 2.
Elevated Plus Maze Test
| Group | MF | MO | p | ||||
|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Female | Group | Sex | G × S |
| Open arm latency (sec) | 35.6 ± 14.4 | 28.9 ± 8.0 | 16.1 ± 6.9 | 28.4 ± 14.7 | 0.23 | 0.92 | 0.53 |
| Open arm duration (%) | 34.8 ± 9.1 | 29.7 ± 6.8 | 36.7 ± 7.9 | 22.8 ± 8.2 | 0.76 | 0.26 | 0.60 |
| Open (%) arm frequency | 28.2 ± 5.9 | 28.5 4.9 ± | 27.7 8.8 ± | 24.7 ± 7.0 | 0.76 | 0.85 | 0.82 |
| Total Arm crosses (#) | 27.7 ± 4.0 | 21.7 ± 3.0 | 17.0 ± 2.3 | 15.7 ± 1.4 | 0.01 | 0.26 | 0.46 |
Paternal deprivation affects locomotion, but not anxiety like behaviors in adulthood. There were no behavioral differences in indicators of anxiety between subjects that grew up with a mother and father (MF) or mother only (MO) including latency to enter the open arm, percent time spent on the open arm, and percent crosses into the open arm. However, MO subjects had fewer total arm crosses than MF subjects in the EPM test indicating decreased locomotion. Data are shown as mean ± SEM. Significance values are rounded up to the nearest hundredths.
Neurochemical protein expression in the hippocampus
Oxytocin, vasopressin, and glucocorticoid receptors
A significant group by sex interaction was found for OXTR protein expression in the hippocampus (F1, 20 =8.795, p < 0.05; Table 3). MF males had a higher level of OXTR expression than MF females and MO males. A group by sex interaction was also found for GRβ protein expression in the hippocampus (F1, 20 =8.641, p < 0.05). MO females had a higher level of GRβ expression than MO males and MF females. No significant differences were found in V1aR and GRα protein expression in the hippocampus.
Table 3.
Hippocampal protein levels
| MF | MO | p | |||||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Group | Sex | G × S | |
| OXTR | 146.3 ± 35.9 | 53.7 ± 13.9 | 68.6 ± 17.6 | 109.4 ± 21.6 | 0.80 | 0.35 | 0.01 |
| V1aR | 101.7 ± 9.3 | 98.3 ± 7.3 | 115.3 ± 16.4 | 111.8 ± 10.4 | 0.38 | 0.03 | 0.39 |
| GRα | 106.4 ± 34.2 | 92.3 ± 22.7 | 96.5 ± 35.7 | 208.6 ± 53.0 | 0.19 | 0.23 | 0.13 |
| GRβ | 116.4 ± 16.2 | 83.6 ± 11.7 | 79.1 ± 9.9 | 135.3 ± 20.4 | 0.64 | 0.45 | 0.01 |
Paternal deprivation alters protein levels in the hippocampus. Males that grew up with a mother only (M/MO) had decreased OXTR protein expression in the hippocampus compared to males that grew up with both a mother and father (M/MF). There were no differences in protein levels of vasopressin receptor type 1a (V1aR), nor glucocorticoid receptor alpha (GRα) in the hippocampus. Compared to MF females (F/MF), MO females (F/MO) had higher glucocorticoid beta receptor (GRβ) protein levels in the hippocampus. Data are shown as mean ± SEM. Significance values are rounded up to the nearest hundredths.
Corticotrophin releasing hormone and its receptors
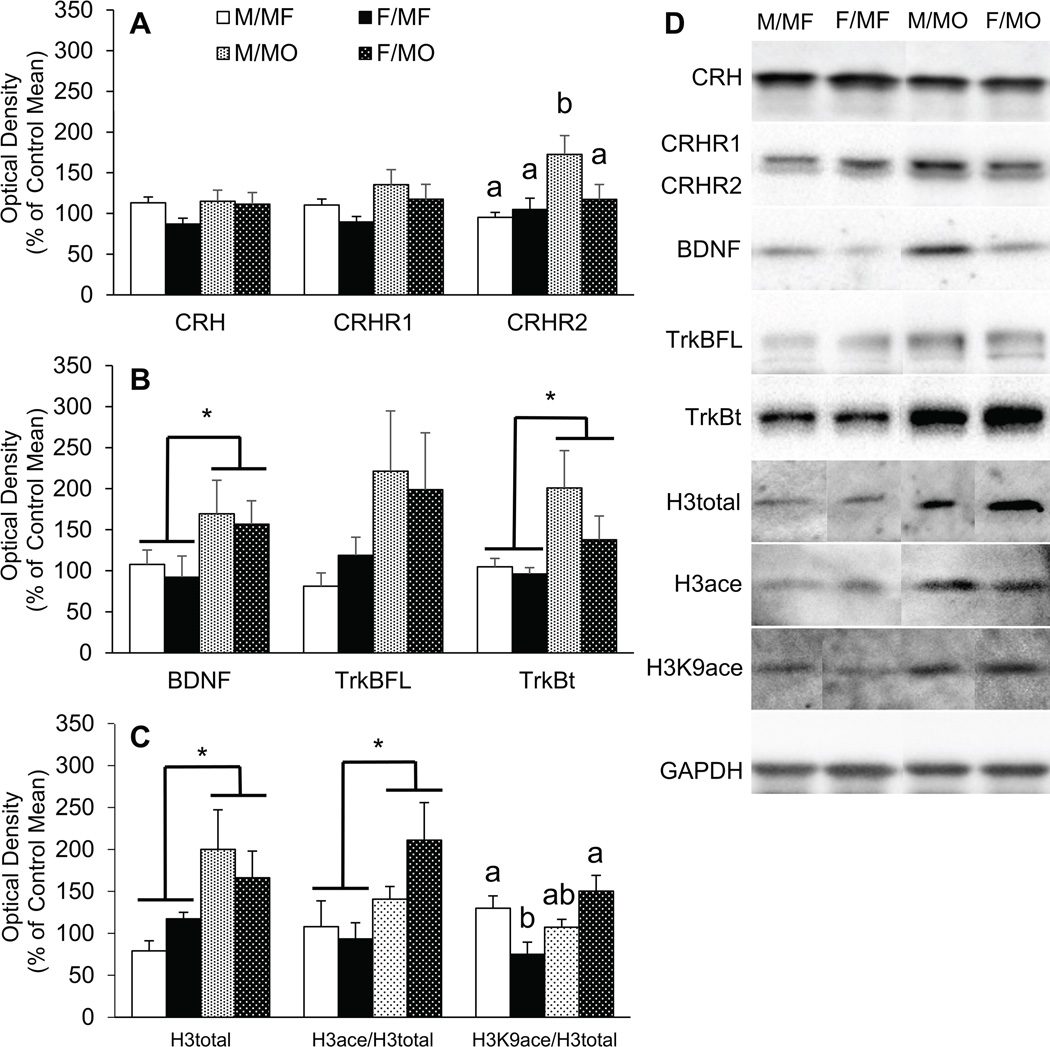
No differences were found in the levels of CRH and CRHR1 in the hippocampus (Figure 3a). A significant group by sex difference was found for CRHR2 expression in the hippocampus (F1, 20 = 8.635, p < 0.05). MO males had a higher level of CRHR2 protein expression than MO females as well as MF males and females (Figure 3a). Interestingly, CRHR2 protein levels were correlated with CRHR1 (r(24) = 0.704, p < 0.05), CRH (r(24) = 0.553, p < 0.05), TrkBt (r(23) = 0.803, p < 0.05), TrkBFL (r(22) = 0.495, p < 0.05), and BDNF (r(24) = 0.502, p < 0.05) protein levels.
Figure 3.
Paternal deprivation alters adult hippocampal protein expression and histone acetylation. (A) Males that grew up with a mother only (M/MO) had increased corticotrophin releasing hormone receptor type 2 (CRHR2) protein expression in the hippocampus compared to males that grew up with both a mother and father (M/MF). (B) Compared to males and females reared by a mother and father (M/MF, F/MF), males and females reared without a father (M/MO, F/MO) had higher protein levels of brain derived neurotrophic factor (BDNF) as well as its receptor, tropomyosin receptor kinase B in the truncated form (TrkBt) and trending in the full length form (TrkBFL) in the hippocampus. (C) MO subjects also had higher protein levels of total histone 3 (H3total) in the hippocampus as well as higher levels of histone acetylation (H3ace). Finally, compared to MF females, MO females had a higher ratio of histone 3 acetylation at lysine 9 (H3K9ace) to H3total. Data are shown as mean + SEM. *p <.05. Alphabetic letters indicate the results from a post-hoc test following an ANOVA. Bars labeled with different letters differ significantly from each other.
Brain derived neurotrophic factor and tropomyosin receptor kinase B
Significant group effects were found in the levels of BDNF (F1, 20 = 4.598, p < 0.05) and TrkBt (95kd) (F1, 14.043 = 5.078, p < 0.05) in the hippocampus (Figure 3b). MO subjects had higher levels of BDNF and TrkBt in the hippocampus than MF subjects. A similar trend was also found in the level of TrkBFL (145kd) protein expression in the hippocampus (F1, 17 = 4.055, p =.060). Interestingly, BDNF protein levels in the hippocampus were correlated with TrkBt (r(21) = 0.545, p < 0.05), CRH (r(22) = 0.423, p < 0.05), CRHR1 (r(22) = 0.555, p < 0.05), and CRHR2 (r(22) = 0.502, p < 0.05) protein levels, and trending with TrkBFL (r(22) = 0.411, p = 0.057). Additionally, BDNF levels were negatively correlated with the licking and grooming frequency per nest, (r(22) = −0.503, p < 0.05).
Histone 3
Significant treatment effects were found in the levels of H3total (F1, 19 = 7.680, p < 0.05) and the ratio of H3ace to H3total (F1, 18 = 5.885, p < 0.05). MO subjects had higher levels of H3total and H3ace/H3total compared to MF subjects (Figure 3c). A treatment by sex interaction was found for H3K9ace/H3total in the hippocampus (F1, 18 = 10.270, p < 0.05). MF females had a lower level of H3K9ace/H3total compared to MF males and MO females (Figure 3c).
Neurochemical mRNA expression in the hippocampus
This was focused only on the neurochemical markers where protein levels indicated potential group differences in gene expression. Significant treatment effects were found in the levels of CRHR2 (F1, 19 = 10.078, p < 0.01), BDNF (F1, 17 = 4.553, p < 0.05), and TrkBFL (F1, 20 = 7.620, p < 0.05) mRNA expression in the hippocampus (Figure 4). MO subjects had a lower level of CRHR2 but higher levels of BDNF and TrkBFL, compared to MF subjects. Interestingly and similar to the protein data, BDNF mRNA was negatively correlated with total levels of licking and grooming (r(19) = −0.533, p < 0.05). BDNF levels were also strongly correlated with both the full length (r(19) = 0.616, p < 0.01) and truncated TrkB levels (r(21) = 0.627, p < 0.01). No sex or group by sex interactions were found. In addition, no differences were found in the GRα, and GRβ mRNA expression in the hippocampus.
Figure 4.
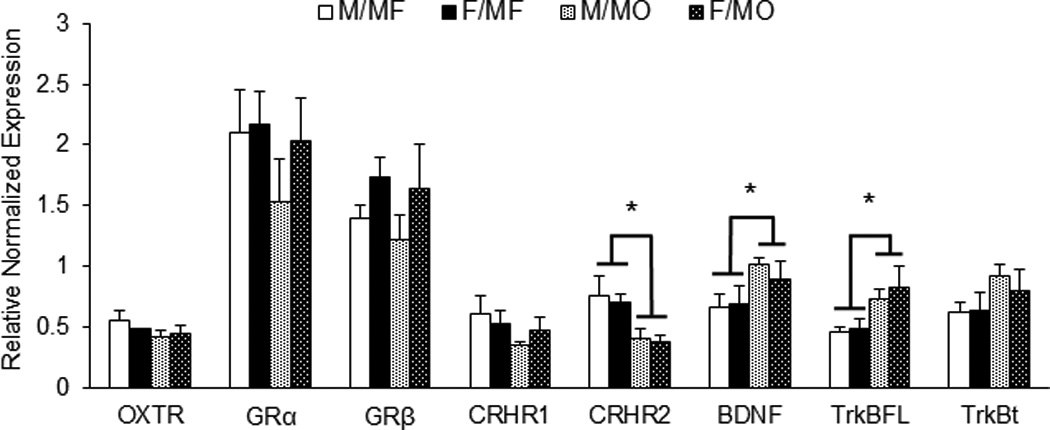
Paternal deprivation alters hippocampal mRNA in adulthood. Quantitative real time polymerase chain reaction revealed that subjects that were reared with a mother only (MO) had decreased levels of mRNA for corticotrophin releasing hormone receptor 2 (CRHR2) in the hippocampus compared to subjects that were reared with both a mother and father (MF). Additionally, MO subjects had higher mRNA levels for brain derived neurotrophic factor (BDNF) and its full length receptor, tropomyosin receptor kinase B (TrkBFL). Data are shown as mean + SEM. *p <.05.
Discussion
It has been well documented that early life experience, including experience with parents, can shape adult cognitive and behavioral functions (Felitti et al., 1998; Repetti et al., 2002; Nishi et al., 2014; Sripada et al., 2014; Jensen et al., 2015; Samek et al., 2015; Yam et al., 2015). In humans, father presence during early development is associated with decreased risk for various psychopathologies and increased well-being of the children (Sarkadi et al., 2008; McLanahan et al., 2013; Tikotzky et al., 2015), whereas father absence can lead to negative outcomes including the development of depressive symptoms and externalizing behaviors (Phares and Compas, 1992; Culpin et al., 2013; McLanahan et al., 2013). In the present study, using the socially monogamous prairie voles which naturally display both maternal and paternal behaviors, we found that paternal deprivation (PD) during early development has long-lasting effects on social affiliation of the offspring when they became adults, and this effect is behavior-specific. In addition, our data indicate that such PD experience also alters the expression of several neurochemicals and epigenetic markers in the hippocampus, indicating their potential roles in mediating altered social behavior.
PD affected parental environment
As expected, prairie vole fathers contributed to the care of offspring and displayed pup licking/grooming, huddling and carrying as well as nest building like mothers did with the exception of nursing (Wang and Novak, 1992; Ahern and Young, 2009; Ahern et al., 2011). Maternal behavior did not differ between the MF and MO groups, suggesting that mothers do not compensate for the absence of the father, which is in agreement with previous studies in prairie voles (Wang and Novak, 1992; Ahern and Young, 2009; Ahern et al., 2011) and in other bi-parental species such as octodon degus (Helmeke et al., 2009). However, PD resulted in reduced parental care received by the offspring, indicated by a decrease in the levels of pup licking/grooming and carrying as well as an increase in pups being left alone in the nest. This altered parental care was associated with an increase in the body weight of males at weaning but did not lead to a significant effect on body weight in adulthood in our study. It should be noted that in a previous study, PD experience was associated with lower pup body weight at weaning in prairie voles although this might have been confounded by weekly handling and in-utero effects upon removal of the father about 6–10 days before litter birth (Ahern and Young, 2009).
PD experience facilitated social affiliation
An interesting finding in the present study is that PD prairie voles displayed a significant increase in contact with a conspecific during a social affiliation test in adulthood, indicating that PD experience during early development has a long-lasting effect on social affiliation. Importantly, this effect was not due to altered social recognition, and thus the effect was behavior-specific. It is interesting to mention that a similar effect was found in adult female prairie voles that experienced 6 weeks of social isolation (Lieberwirth et al., 2012) and in male prairie voles that experienced partner loss (Sun et al., 2014). The presence of a conspecific may be anxiolytic, decreasing biobehavioral anxiety responses in animals (Smith and Wang, 2014). Because our PD voles did not show increases in their anxiety-like behavior, the enhanced social affiliation may likely be driven by increased social seeking rather than stress coping. Mild stressors, like perhaps that induced by a behavioral test, have also been shown to enhance affiliative behavior in rats and prairie voles in a sex-dependent manner (DeVries et al., 1996; Beery and Kaufer, 2015; Muroy et al., 2016). It is worth mentioning that in our study male PD voles seemed to display a higher level of social affiliation than females, and this was likely the driving force for treatment differences. In male mandarin voles, PD experience also led to increased body contact with a conspecific, probably due to decreased adult aggression (Frazier et al., 2006; Wang et al., 2012). Indeed in male prairie voles, increased social affiliation induced by partner loss was associated with a decrease in aggression (Sun et al., 2014).
Stress during early development can increase either anxiety-like behavior (Brunton, 2013; Maccari et al., 2014) or resilience to stressful stimuli (Faure et al., 2007; Zhang et al., 2014; Beery and Kaufer, 2015; Rana et al., 2015) in adulthood. Studies using maternal deprivation as an early life stressor have found both increased (Ishikawa et al., 2015) and decreased anxiety in adulthood in rats (Zhang et al., 2014). In monogamous rodent species, PD has been shown to decrease anxiety-like behavior in prairie voles (Ahern and Young, 2009) but increase the same behavior in mandarin voles (Jia et al., 2009). Our data indicate that PD experience had no significant effects on anxiety-like behavior at the time when our animals were tested. Our PD paradigm either was not effective to have long-lasting effects on anxiety-like behavior or effectively enhanced the resilience to stressful stimuli associated with the EPM test. The decreased locomotor activity in PD voles should be noted as it is unclear whether it might have secondary effects on anxiety-like behavior. Indeed, when locomotion was controlled for, subjects reared without a father entered the open arms of the EPM sooner than subjects reared with both parents. Parental deprivation during early development has been shown to affect locomotor activity in other bi-parental species such as marmosets (Dettling et al., 2002) and mandarin voles (Jia et al., 2009; Cao et al., 2014). In mandarin voles, PD experience also decreased locomotor activity (Jia et al., 2009).
PD experience altered neurochemical expression in the hippocampus
Several neurochemicals and their receptors in the hippocampus were examined because of their demonstrated roles in learning & memory, stress responses, and social behaviors (Carter et al., 1995; Kim et al., 2015; Lieberwirth et al., 2016). Our data indicate that PD experience affected some of these markers in a neurochemical-specific manner. The most striking and consistent group difference was found on the BDNF system in the hippocampus. MO voles showed enhanced RNA and protein expression of BDNF and its TrkB receptors in the hippocampus compared to control voles raised by both parents (MF). This effect seemed to be similar in both males and females. Extensive data have indicated that stressful stimuli during early development can induce changes in the BDNF system in a brain region- and stress-specific manner. For example, maternal separation can increase BDNF in the hippocampus of rats post-weaning and this is associated with alterations in anxiety-like behaviors and HPA axis activity (Faure et al., 2007; Daniels et al., 2009; Suri et al., 2013). Interestingly, hippocampal BDNF and TrkB levels are most consistently found to be increased post-weaning and decreased in later adulthood in response to early maternal separation in rodents (Lee et al., 2012; Suri et al., 2013; Daskalakis et al., 2015). In adult rats, early maternal separation decreases hippocampal BDNF, resulting in impaired recognition memory in females and impaired working memory in males (Marco et al., 2013; Suri et al., 2013; Hill et al., 2014). The effects of maternal separation on the stress response have been found to be further mediated by the age of experience and duration of maternal separation (Levine et al., 1991; Levine, 2005). It has been suggested that enhanced BDNF activity in the brain in response to early life stress may act as a mechanism to protect against future stress exposure (Daniels et al., 2009; Suri et al., 2013; Daskalakis et al., 2015). For example, early maternal separation associated with increased hippocampal BDNF expression in rats post-weaning is associated with decreased anxiety-like behaviors and increased performances in cognitive behavioral tasks (Daniels et al., 2009; Suri et al., 2013). Interestingly, our data indicate that PD experience increased not only BDNF but also both the truncated and full length TrkB receptor isoforms in prairie voles. These were further demonstrated by positive correlations between BDNF and TrkB receptor mRNA levels as well as between levels of BDNF and TrkBt proteins (trending with TrkBFL protein). Truncated and full-length TrkB receptors have been implicated in regulating dendritic growth in distinct ways (Yacoubian and Lo, 2000; Hartmann et al., 2004). Thus, PD experience may induce adaptive plasticity changes by the BDNF system in response to emotional challenges associated with paternal deprivation and inoculate voles against future life stressors, much like the effects of environmental enrichment and early handling in other rodent species (Meaney et al., 1993).
It is also worth mentioning that our data indicated positive correlations in protein levels between the BDNF and CRH systems in the hippocampus. Interactions between the BDNF and CRH systems have been implicated in regulating neural plasticity. For example, CRH has been shown to increase or decrease BDNF levels, depending on the brain region, and alter spine density accordingly (Bayatti et al., 2005; de la Tremblaye et al., 2016). As BDNF acts on TrkB receptors to regulate dendritic spine growth (McAllister et al., 1995; An et al., 2008) and the CRH system can regulate this process (Bayatti et al., 2005; de la Tremblaye et al., 2016), these correlations between BDNF and CRH markers may further support the notion that increased BDNF activity associated with PD may alter hippocampal spine density.
PD experience altered hippocampal epigenetic marker expression
Recent data have indicated that early life experience may affect adult behavior via epigenetic-mediated mechanisms (Babenko et al., 2015). For example, in a rat model of early life variation in maternal care received, offspring of high licking/grooming dams, compared to ones from the low licking/grooming dams, are more resilient to stress due to increased GR expression in the hippocampus which is regulated by increased H3 acetylation, specifically at lysine 9, and decreased methylation at the GR gene promotor region (Fish et al., 2004; Zhang et al., 2013). In mice, maternal separation during development resulted in increased H3 acetylation in the hippocampus which was paralleled by increased spine density and complexity in the CA3 region (Xie et al., 2013). Further, social stress, crowding, and novel environment during the peripubertal-juvenile period increased hippocampal H3 acetylation and mossy fibers while decreasing depressive-like behaviors and stressinduced plasma CORT (Oztan et al., 2011). An interesting finding in our study is that the PD experience was associated with increased H3total and H3ace in the vole hippocampus. Further, PD experience significantly elevated H3K9ace in the hippocampus in female, but not male, voles. These data indicate that early PD experience may affect histone acetylation in the hippocampus possibly in a sex-dependent manner, although we still do not know the specific gene(s) on which histone acetylation occurred and the functional significance of such epigenetic events on the brain and behavior. It is worth mentioning that epigenetic events have been implicated in the regulation of social bonding behavior in both male and female prairie voles (Wang et al., 2013; Duclot et al., 2016).
Several issues need to be mentioned. First, our data show that PD experience also affected other neurochemical markers in the vole hippocampus. For example, PD experience elevated GRβ in females but decreased OXTR and increased CRHR2 protein levels in males, indicating a potential sex-specific effect of PD on neurochemical receptor expression. Interestingly, GRα and GRβ RNA levels were positively correlated with TrkB RNA levels indicating interaction between both systems, an interaction previously implicated in regulating synaptic plasticity (Jeanneteau and Chao, 2013). Although the underlying mechanisms and functional significance of such effects are still unknown, these interesting data should not be ignored especially given the roles of GR and CRHR in stress responses and stress coping (McEwen, 2007; Bosch et al., 2009, 2016; Farrell and O’Keane, 2016; Vyas et al., 2016). Second, in our present study only certain percentages of sexually naïve male and female prairie voles displayed spontaneous parental behavior towards conspecific pups, which is consistent with previous studies (Lonstein and De Vries, 2001; Ahern and Young, 2009). Unfortunately, the limited numbers of subjects prevented us from examining the PD effects on the specific patterns of parental behavior. We believe that this interesting question should still be pursued in further studies, as it has been shown that individual characteristics of parental behavior can be transgenerational (Francis et al., 1999; Champagne and Meaney, 2007) and regulated by epigenetic mechanisms (Champagne et al., 2006). Third, the convincing treatment effect observed on the BDNF system and H3 acetylation in the vole hippocampus indicate potential epigenetic regulation of BNDF and TrkB and its subsequent behavior – an interesting question which should be followed. In a previous study, epigenetic regulation of BDNF in the hippocampus was involved in mediating depression-like behavior in mice (Tsankova et al., 2006). Finally, a caveat in the present study should be mentioned. Our experiments were initially designed to focus on assessing the effects of PD on anxiety-like and social behaviors, including social recognition which reflects social memory processes. In future studies, it will be interesting to examine the effects of PD on learning and memory tasks using the behavioral tests such as the Morris water maze, Barnes maze, and Radial arm maze (Lieberwirth et al., 2016).
Conclusion
Data from our present study indicate that PD experience during early development affected social affiliation of the offspring when they became adults, in a behavior-specific manner. In addition, such PD experience was associated with enhanced gene and protein expression of BDNF and its tropomyosin receptor kinase B as well as the levels of total histone 3 and histone 3 acetylation in the hippocampus. Together, these data indicate that paternal experience during development may alter gene transcription and synaptic plasticity in the adult hippocampus of the offspring via epigenetic mechanisms. This, in turn, may serve to alter social affiliative behaviors in adulthood. Indeed, PD was found to affect synaptic plasticity in a neuron type- and brain region-specific manner in another biparental rodent species, Octodon degus (Pinkernelle et al., 2009). In our prairie voles, the levels of licking and grooming received by the offspring were negatively correlated with BDNF mRNA and protein expression in the hippocampus. Alternations in the hippocampal BDNF system (and its mediated changes in synaptic plasticity) in PD voles may reflect a compensatory mechanisms for deficits in neurochemical and synaptic plasticity due to reduced parental contact during early development. In addition, it may reflect a developmental adaption serving to inoculate subjects against future life stressors (Helmeke et al., 2001).
Finally, considering the negative association between early father absence and adult well-being largely reported in the human literature (McLanahan et al., 2013), it is surprising that we were unable to find clear deficits in the social behaviors of PD prairie voles in the present study. This may be due to multiple factors including suboptimal times of experimental manipulation (e.g., father removing) and/or behavioral testing (e.g., adolescence vs adulthood), as hinted by human studies (McLanahan et al., 2013). Furthermore, father absence during development in humans may be associated with a societal stigma that can compound the effects of father absence on adult outcome, and this factor would not apply in the context of our study. Nevertheless, further studies are needed for our better understanding of the consequences as well as the underlying neuronal and neurochemical mechanisms of father deprivation during early development.
Pups raised by both parents (MF) received more parental care than ones raised by mothers only (MO)
Paternal deprivation (PD) during early development led to an increase in social affiliation
PD experience was associated with enhanced BDNF, TrkB, and H3ace protein expression in the hippocampus
PD experience also affected hippocampal GRβ, OTR, and CRHR2 protein expression in a sex-specific manner
Altered neurochemical expression in the hippocampus may mediate social affiliation
Acknowledgments
We would like to acknowledge and thank Randy Altshuler for his assistance with spot checks and Dr. Hui Wang for his technical assistance with western blots and qPCR. This work was supported by the National Institutes of Health grants R01 MH058616 and R01 MH089852 to ZXW. MT was supported by the NIH program training grant (T32 MH093311, P.K. Keel and L.A. Eckel).
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CRH
corticotrophin releasing hormone
- CRHR1
corticotrophin releasing hormone receptor 1
- CRHR2
corticotrophin releasing hormone receptor 2
- EPM
elevated plus maze
- fMRI
functional magnetic resonance imaging
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GR
glucocorticoid receptor
- GRα
glucocorticoid receptor alpha
- GRβ
glucocorticoid receptor beta
- HPA
hypothalamic pituitary adrenal
- H3ace
histone 3 acetylation
- H3K9ace
histone 3 acetylation at lysine 9
- H3total
total histone 3
- MF
mother and father
- MO
mother only
- NADH
nicotinamide adenine dinucleotide dehydrogenase
- OXTR
oxytocin receptor
- PB
parental behavior
- PD
paternal deprivation
- PND
postnatal day
- SOA
social affiliation
- SOR
social recognition
- TrkB
tropomyosin receptor kinase B
- TrkBt
tropomyosin receptor kinase B truncated
- TrkBFL
tropomyosin receptor kinase B full length
- V1aR
vasopressin 1a receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing financial interests.
References
- Ahern TH, Hammock EAD, Young LJ. Parental division of labor, coordination, and the effects of family structure on parenting in monogamous prairie voles (Microtus ochrogaster) Dev Psychobiol. 2011;53:118–131. doi: 10.1002/dev.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JJ, Gharami K, Liao G-Y, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3’ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko O, Kovalchuk I, Metz GAS. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev. 2015;48:70–91. doi: 10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Barbosa Neto JB, Tiba PA, Faturi CB, de Castro-Neto EF, da Graça Naffah-Mazacoratti M, de Jesus Mari J, de Mello MF, Suchecki D. Stress during development alters anxiety-like behavior and hippocampal neurotransmission in male and female rats. Neuropharmacology. 2012;62:518–526. doi: 10.1016/j.neuropharm.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Hermann H, Lutz B, Behl C. Corticotropin-releasing hormone-mediated induction of intracellular signaling pathways and brain-derived neurotrophic factor expression is inhibited by the activation of the endocannabinoid system. Endocrinology. 2005;146:1205–1213. doi: 10.1210/en.2004-1154. [DOI] [PubMed] [Google Scholar]
- Beery AK, Kaufer D. Stress, social behavior, and resilience: insights from rodents. Neurobiol Stress. 2015;1:116–127. doi: 10.1016/j.ynstr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, Adler Z. Early sexual abuse and clinical depression in adult life. Br J Psychiatry J Ment Sci. 1991;159:115–122. doi: 10.1192/bjp.159.1.115. [DOI] [PubMed] [Google Scholar]
- Binder E, Malki K, Paya-Cano JL, Fernandes C, Aitchison KJ, Mathé AA, Sluyter F, Schalkwyk LC. Antidepressants and the resilience to early-life stress in inbred mouse strains. Pharmacogenet Genomics. 2011;21:779–789. doi: 10.1097/FPC.0b013e32834b3f35. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, Neumann ID, Young LJ. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology. 2016;64:66–78. doi: 10.1016/j.psyneuen.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Dalvie S, Cuzen NL, Cardenas V, Fein G, Stein DJ. Childhood adversity is linked to differential brain volumes in adolescents with alcohol use disorder: a voxel-based morphometry study. Metab Brain Dis. 2014;29:311–321. doi: 10.1007/s11011-014-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GR, Anderson B. Psychiatric morbidity in adult inpatients with childhood histories of sexual and physical abuse. Am J Psychiatry. 1991;148:55–61. doi: 10.1176/ajp.148.1.55. [DOI] [PubMed] [Google Scholar]
- Brunton PJ. Effects of maternal exposure to social stress during pregnancy: consequences for mother and offspring. Reprod Camb Engl. 2013;146:R175–R189. doi: 10.1530/REP-13-0258. [DOI] [PubMed] [Google Scholar]
- Cao Y, Wu R, Tai F, Zhang X, Yu P, An X, Qiao X, Hao P. Neonatal paternal deprivation impairs social recognition and alters levels of oxytocin and estrogen receptor α mRNA expression in the MeA and NAcc, and serum oxytocin in mandarin voles. Horm Behav. 2014;65:57–65. doi: 10.1016/j.yhbeh.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121:1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver ICG, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Culpin I, Heron J, Araya R, Melotti R, Joinson C. Father absence and depressive symptoms in adolescence: findings from a UK cohort. Psychol Med. 2013;43:2615–2626. doi: 10.1017/S0033291713000603. [DOI] [PubMed] [Google Scholar]
- Daniels WMU, Fairbairn LR, van Tilburg G, McEvoy CRE, Zigmond MJ, Russell VA, Stein DJ. Maternal separation alters nerve growth factor and corticosterone levels but not the DNA methylation status of the exon 1(7) glucocorticoid receptor promoter region. Metab Brain Dis. 2009;24:615–627. doi: 10.1007/s11011-009-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels WMU, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, De Kloet ER, Yehuda R, Malaspina D, Kranz TM. Early Life Stress Effects on Glucocorticoid-BDNF Interplay in the Hippocampus. Front Mol Neurosci. 2015;8:68. doi: 10.3389/fnmol.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Tremblaye PB, Linares NN, Schock S, Plamondon H. Activation of CRHR1 receptors regulates social and depressive-like behaviors and expression of BDNF and TrkB in mesocorticolimbic regions following global cerebral ischemia. Exp Neurol. 2016;284:84–97. doi: 10.1016/j.expneurol.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacol Biochem Behav. 2002;73:259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc Natl Acad Sci U S A. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclot F, Wang H, Youssef C, Liu Y, Wang Z, Kabbaj M. Trichostatin A (TSA) facilitates formation of partner preference in male prairie voles (Microtus ochrogaster) Horm Behav. 2016;81:68–73. doi: 10.1016/j.yhbeh.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Tripathi SP, Mletzko T, Young J, Cisler JM, James GA, Kilts CD. Childhood maltreatment is associated with a sex-dependent functional reorganization of a brain inhibitory control network. Hum Brain Mapp. 2014;35:1654–1667. doi: 10.1002/hbm.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell C, O’Keane V. Epigenetics and the glucocorticoid receptor: A review of the implications in depression. Psychiatry Res. 2016;242:349–356. doi: 10.1016/j.psychres.2016.06.022. [DOI] [PubMed] [Google Scholar]
- Faure J, Uys JDK, Marais L, Stein DJ, Daniels WMU. Early maternal separation alters the response to traumatization: resulting in increased levels of hippocampal neurotrophic factors. Metab Brain Dis. 2007;22:183–195. doi: 10.1007/s11011-007-9048-3. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Frazier CRM, Trainor BC, Cravens CJ, Whitney TK, Marler CA. Paternal behavior influences development of aggression and vasopressin expression in male California mouse offspring. Horm Behav. 2006;50:699–707. doi: 10.1016/j.yhbeh.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Galinato MH, Orio L, Mandyam CD. Methamphetamine differentially affects BDNF and cell death factors in anatomically defined regions of the hippocampus. Neuroscience. 2015;286:97–108. doi: 10.1016/j.neuroscience.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz LL, Carter CS. Prairie-Vole Partnerships. Am Sci. 1996;84:56–62. [Google Scholar]
- Greenberg GD, van Westerhuyzen JA, Bales KL, Trainor BC. Is it all in the family? The effects of early social structure on neural–behavioral systems of prairie voles (Microtus ochrogaster) Neuroscience. 2012;216:46–56. doi: 10.1016/j.neuroscience.2012.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta-Agarwal S, Franklin AV, DeRamus T, Wheelock M, Davis RL, McMahon LL, Lubin FD. G9a/GLP Histone Lysine Dimethyltransferase Complex Activity in the Hippocampus and the Entorhinal Cortex is Required for Gene Activation and Silencing during Memory Consolidation. J Neurosci. 2012;32:5440–5453. doi: 10.1523/JNEUROSCI.0147-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EaD, Lim MM, Nair HP, Young LJ. Association of vasopressin 1a receptor levels with a regulatory microsatellite and behavior. Genes Brain Behav. 2005;4:289–301. doi: 10.1111/j.1601-183X.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Brigadski T, Erdmann KS, Holtmann B, Sendtner M, Narz F, Lessmann V. Truncated TrkB receptor-induced outgrowth of dendritic filopodia involves the p75 neurotrophin receptor. J Cell Sci. 2004;117:5803–5814. doi: 10.1242/jcs.01511. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Poeggel G, Braun K. Differential emotional experience induces elevated spine densities on basal dendrites of pyramidal neurons in the anterior cingulate cortex of Octodon degus. Neuroscience. 2001;104:927–931. doi: 10.1016/s0306-4522(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Seidel K, Poeggel G, Bredy TW, Abraham A, Braun K. Paternal deprivation during infancy results in dendrite- and time-specific changes of dendritic development and spine formation in the orbitofrontal cortex of the biparental rodent Octodon degus. Neuroscience. 2009;163:790–798. doi: 10.1016/j.neuroscience.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Hill RA, Klug M, Kiss Von Soly S, Binder MD, Hannan AJ, van den Buuse M. Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling. Hippocampus. 2014;24:1197–1211. doi: 10.1002/hipo.22302. [DOI] [PubMed] [Google Scholar]
- Huang M, Kempuraj D, Papadopoulou N, Kourelis T, Donelan J, Manola A, Theoharides TC. Urocortin induces interleukin-6 release from rat cardiomyocytes through p38 MAP kinase, ERK and NF-kappaB activation. J Mol Endocrinol. 2009;42:397–405. doi: 10.1677/JME-08-0120. [DOI] [PubMed] [Google Scholar]
- Ishikawa J, Nishimura R, Ishikawa A. Early-life stress induces anxiety-like behaviors and activity imbalances in the medial prefrontal cortex and amygdala in adult rats. Eur J Neurosci. 2015;41:442–453. doi: 10.1111/ejn.12825. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Chao MV. Are BDNF and glucocorticoid activities calibrated? Neuroscience. 2013;239:173–195. doi: 10.1016/j.neuroscience.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SKG, Dickie EW, Schwartz DH, Evans CJ, Dumontheil I, Paus T, Barker ED. Effect of Early Adversity and Childhood Internalizing Symptoms on Brain Structure in Young Men. JAMA Pediatr. 2015;169:938–946. doi: 10.1001/jamapediatrics.2015.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia R, Tai F, An S, Zhang X, Broders H. Effects of neonatal paternal deprivation or early deprivation on anxiety and social behaviors of the adults in mandarin voles. Behav Processes. 2009;82:271–278. doi: 10.1016/j.beproc.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Jones J, Mosher W. Fathers’ involvement with their children: United States, 2006–2010. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- Kim EJ, Pellman B, Kim JJ. Stress effects on the hippocampus: a critical review. Learn Mem Cold Spring Harb N. 2015;22:411–416. doi: 10.1101/lm.037291.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Swank PR. Responsive parenting: establishing early foundations for social, communication, and independent problem-solving skills. Dev Psychol. 2006;42:627–642. doi: 10.1037/0012-1649.42.4.627. [DOI] [PubMed] [Google Scholar]
- Lee K-Y, Miki T, Yokoyama T, Ueki M, Warita K, Suzuki S, Ohta K-I, Wang Z-Y, Jamal M, Yakura T, Liu J-Q, Hosomi N, Takeuchi Y. Neonatal repetitive maternal separation causes long-lasting alterations in various neurotrophic factor expression in the cerebral cortex of rats. Life Sci. 2012;90:578–584. doi: 10.1016/j.lfs.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Levine S, Huchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev Psychobiol. 1991;24:547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Horm Behav. 2012;62:357–366. doi: 10.1016/j.yhbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Pan Y, Liu Y, Zhang Z, Wang Z. Hippocampal adult neurogenesis: Its regulation and potential role in spatial learning and memory. Brain Res. 2016;1644:127–140. doi: 10.1016/j.brainres.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazábal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal Care, Hippocampal Glucocorticoid Receptors, and Hypothalamic-Pituitary-Adrenal Responses to Stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lieberwirth C, Jia X, Curtis J, Meredith M, Wang Z. Chemosensory cues affect amygdaloid neurogenesis and alter behaviors in the socially monogamous prairie vole. Eur J Neurosci. 2014;39:1632–1641. doi: 10.1111/ejn.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Sex differences in the parental behaviour of adult virgin prairie voles: independence from gonadal hormones and vasopressin. J Neuroendocrinol. 1999;11:441–449. doi: 10.1046/j.1365-2826.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Social influences on parental and nonparental responses toward pups in virgin female prairie voles (Microtus ochrogaster) J Comp Psychol Wash DC. 2001;1983(115):53–61. doi: 10.1037/0735-7036.115.1.53. [DOI] [PubMed] [Google Scholar]
- Maccari S, Krugers HJ, Morley-Fletcher S, Szyf M, Brunton PJ. The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. J Neuroendocrinol. 2014;26:707–723. doi: 10.1111/jne.12175. [DOI] [PubMed] [Google Scholar]
- Marco EM, Valero M, de la Serna O, Aisa B, Borcel E, Ramirez MJ, Viveros M-P. Maternal deprivation effects on brain plasticity and recognition memory in adolescent male and female rats. Neuropharmacology. 2013;68:223–231. doi: 10.1016/j.neuropharm.2012.08.014. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McLanahan S, Tach L, Schneider D. The Causal Effects of Father Absence. Annu Rev Sociol. 2013;399:399–427. doi: 10.1146/annurev-soc-071312-145704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Bhatnagar S, Diorio J, Larocque S, Francis D, O’Donnell D, Shanks N, Sharma S, Smythe J, Viau V. Molecular basis for the development of individual differences in the hypothalamic-pituitary-adrenal stress response. Cell Mol Neurobiol. 1993;13:321–347. doi: 10.1007/BF00711576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroy SE, Long KLP, Kaufer D, Kirby ED. Moderate Stress-Induced Social Bonding and Oxytocin Signaling are Disrupted by Predator Odor in Male Rats. Neuropsychopharmacology. 2016;41:2160–2170. doi: 10.1038/npp.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Horii-Hayashi N, Sasagawa T. Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents. Front Neurosci. 2014;8:166. doi: 10.3389/fnins.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KA, Gaudreau H, Colalillo S, Steiner M, Atkinson L, Moss E, Goldberg S, Karama S, Matthews SG, Lydon JE, Silveira PP, Wazana AD, Levitan RD, Sokolowski MB, Kennedy JL, Fleming A, Meaney MJ. The Maternal Adversity, Vulnerability and Neurodevelopment Project: Theory and Methodology. Can J Psychiatry Rev Can Psychiatr. 2014;59:497–508. doi: 10.1177/070674371405900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztan O, Aydin C, Isgor C. Stressful environmental and social stimulation in adolescence causes antidepressant-like effects associated with epigenetic induction of the hippocampal BDNF and mossy fibre sprouting in the novelty-seeking phenotype. Neurosci Lett. 2011;501:107–111. doi: 10.1016/j.neulet.2011.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Fleming AS, Lawson D, Jenkins JM, McGowan PO. Within- and between-litter maternal care alter behavior and gene regulation in female offspring. Behav Neurosci. 2014;128:736–748. doi: 10.1037/bne0000014. [DOI] [PubMed] [Google Scholar]
- Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci Lett. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares V, Compas BE. The role of fathers in child and adolescent psychopathology: make room for daddy. Psychol Bull. 1992;111:387–412. doi: 10.1037/0033-2909.111.3.387. [DOI] [PubMed] [Google Scholar]
- Pinkernelle J, Abraham A, Seidel K, Braun K. Paternal deprivation induces dendritic and synaptic changes and hemispheric asymmetry of pyramidal neurons in the somatosensory cortex. Dev Neurobiol. 2009;69:663–673. doi: 10.1002/dneu.20726. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Rana S, Pugh PC, Jackson N, Clinton SM, Kerman IA. Inborn stress reactivity shapes adult behavioral consequences of early-life maternal separation stress. Neurosci Lett. 2015;584:146–150. doi: 10.1016/j.neulet.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- Samek DR, Rueter MA, Keyes MA, McGue M, Iacono WG. Parent involvement, sibling companionship, and adolescent substance use: A longitudinal, genetically informed design. J Fam Psychol JFP J Div Fam Psychol Am Psychol Assoc Div. 2015;43(29):614–623. doi: 10.1037/fam0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi A, Kristiansson R, Oberklaid F, Bremberg S. Fathers’ involvement and children’s developmental outcomes: a systematic review of longitudinal studies. Acta Paediatr Oslo Nor. 2008;1992(97):153–158. doi: 10.1111/j.1651-2227.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Shin SY, Han SH, Woo R-S, Jang SH, Min SS. Adolescent mice show anxiety- and aggressive-like behavior and the reduction of long-term potentiation in mossy fiber-CA3 synapses after neonatal maternal separation. Neuroscience. 2016;316:221–231. doi: 10.1016/j.neuroscience.2015.12.041. [DOI] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Prior M. Temperament and stress resilience in school-age children: a within-families study. J Am Acad Child Adolesc Psychiatry. 1995;34:168–179. doi: 10.1097/00004583-199502000-00012. [DOI] [PubMed] [Google Scholar]
- Sousa VC, Vital J, Costenla AR, Batalha VL, Sebastião AM, Ribeiro JA, Lopes LV. Maternal separation impairs long term-potentiation in CA1-CA3 synapses and hippocampal-dependent memory in old rats. Neurobiol Aging. 2014;35:1680–1685. doi: 10.1016/j.neurobiolaging.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Swain JE, Evans GW, Welsh RC, Liberzon I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2014;39:2244–2251. doi: 10.1038/npp.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, Wang Z. Breaking bonds in male prairie vole: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav Brain Res. 2014;265:22–31. doi: 10.1016/j.bbr.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri D, Veenit V, Sarkar A, Thiagarajan D, Kumar A, Nestler EJ, Galande S, Vaidya VA. Early stress evokes age-dependent biphasic changes in hippocampal neurogenesis, BDNF expression, and cognition. Biol Psychiatry. 2013;73:658–666. doi: 10.1016/j.biopsych.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikotzky L, Sadeh A, Volkovich E, Manber R, Meiri G, Shahar G. Infant sleep development from 3 to 6 months postpartum: links with maternal sleep and paternal involvement. Monogr Soc Res Child Dev. 2015;80:107–124. doi: 10.1111/mono.12147. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau DIS. Children Under 18 Years Living With Mother Only, by Marital Status. Washington, DC: U.S. Census Bureau; 2012. CH-5. [Google Scholar]
- Vyas S, Rodrigues AJ, Silva JM, Tronche F, Almeida OFX, Sousa N, Sotiropoulos I. Chronic Stress and Glucocorticoids: From Neuronal Plasticity to Neurodegeneration. Neural Plast. 2016;2016:6391686. doi: 10.1155/2016/6391686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Nie W, Li H, Hou Y, Yu Z, Fan Q, Sun R. Epigenetic upregulation of corticotrophin-releasing hormone mediates postnatal maternal separation-induced memory deficiency. PloS One. 2014;9:e94394. doi: 10.1371/journal.pone.0094394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Duclot F, Liu Y, Wang Z, Kabbaj M. Histone deacetylase inhibitors facilitate partner preference formation in female prairie voles. Nat Neurosci. 2013;16:919–924. doi: 10.1038/nn.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tai F, Yan X, Yu P. Paternal deprivation alters play-fighting, serum corticosterone and the expression of hypothalamic vasopressin and oxytocin in juvenile male mandarin voles. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198:787–796. doi: 10.1007/s00359-012-0748-8. [DOI] [PubMed] [Google Scholar]
- Wang Z, Insel T. Parental Care: Evolution, Mechanisms, And Adaptive Significance. Academic Press; 1996. [Google Scholar]
- Wang Z, Novak MA. Influence of the social environment on parental behavior and pup development of meadow voles (Microtus pennsylvanicus) and prairie voles (M. Ochrogaster) J Comp Psychol. 1992;106:163–171. [Google Scholar]
- Wang Z, Novak MA. Alloparental care and the influence of father presence on juvenile prairie voles, Microtus ochrogaster. Anim Behav. 1994;47:281–288. [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Wu R, Song Z, Wang S, Shui L, Tai F, Qiao X, He F. Early Paternal Deprivation Alters Levels of Hippocampal Brain-Derived Neurotrophic Factor and Glucocorticoid Receptor and Serum Corticosterone and Adrenocorticotropin in a Sex-Specific Way in Socially Monogamous Mandarin Voles. Neuroendocrinology. 2014;100:119–128. doi: 10.1159/000366441. [DOI] [PubMed] [Google Scholar]
- Xie L, Korkmaz KS, Braun K, Bock J. Early life stress-induced histone acetylations correlate with activation of the synaptic plasticity genes Arc and Egr1 in the mouse hippocampus. J Neurochem. 2013;125:457–464. doi: 10.1111/jnc.12210. [DOI] [PubMed] [Google Scholar]
- Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- Yadawa AK, Chaturvedi CM. Expression of stress hormones AVP and CRH in the hypothalamus of Mus musculus following water and food deprivation. Gen Comp Endocrinol. 2016 doi: 10.1016/j.ygcen.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Yam K-Y, Naninck EFG, Schmidt MV, Lucassen PJ, Korosi A. Early-life adversity programs emotional functions and the neuroendocrine stress system: the contribution of nutrition, metabolic hormones and epigenetic mechanisms. Stress Amst Neth. 2015;18:328–342. doi: 10.3109/10253890.2015.1064890. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Insel TR. Neuroendocrine bases of monogamy. Trends Neurosci. 1998;21:71–75. doi: 10.1016/s0166-2236(97)01167-3. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Labonté B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2013;38:111–123. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang B, Jin J, An S, Zeng Q, Duan Y, Yang L, Ma J, Cao X. Early deprivation reduced anxiety and enhanced memory in adult male rats. Brain Res Bull. 2014;108:44–50. doi: 10.1016/j.brainresbull.2014.08.005. [DOI] [PubMed] [Google Scholar]