Abstract
Background
Specialized hypothalamic systems that increase food intake might also increase ethanol intake. To test this possibility, morphine and receptor-specific opioid agonists were microinjected in the paraventricular nucleus (PVN) of rats that had learned to drink ethanol. To cross-validate the results, naloxone methiodide (m-naloxone), an opioid antagonist, was microinjected with the expectation that it would have the opposite effect of morphine and the specific opioid agonists.
Methods
Sprague-Dawley rats were trained, without sugar, to drink 4% or 7% ethanol and were then implanted with chronic brain cannulas aimed at the PVN. After recovery, those drinking 7% ethanol, with food and water available, were injected with two doses each of morphine or m-naloxone. To test for receptor specificity, two doses each of the μ-receptor agonist [D-Ala2,N-Me-Phe4,Gly5-ol]-Enkephalin (DAMGO), δ-receptor agonist D-Ala-Gly-Phe-Met-NH2 (DALA), or k-receptor agonist U-50,488H were injected. DAMGO was also tested in rats drinking 4% ethanol without food or water available. As an anatomical control for drug reflux, injections were made 2 mm dorsal to the PVN.
Results
A main result was a significant increase in ethanol intake induced by PVN injection of morphine. The opposite effect was produced by m-naloxone. The effects of morphine and m-naloxone were exclusively on intake of ethanol, even though food and water were freely available. In the analysis with specific receptor agonists, PVN injection of the δ-agonist DALA significantly increased 7% ethanol intake without affecting food or water intake. This is in contrast to the k-agonist U-50,488H, which decreased ethanol intake, and the μ-agonist DAMGO, which had no effect on ethanol intake in the presence or absence of food and water. In the anatomical control location 2 mm dorsal to the PVN, no drug caused any significant changes in ethanol, food, or water intake, providing evidence that the active site was close to the cannula tip.
Conclusions
The δ-opioid receptor agonist in the PVN increased ethanol intake in strong preference over food and water, while the k-opioid agonist suppressed ethanol intake. Prior studies show that learning to drink ethanol stimulates PVN expression and production of the peptides enkephalin and dynorphin, which are endogenous agonists for the δ- and k-receptors, respectively. These results suggest that enkephalin via the δ-opioid system can function locally within a positive feedback circuit to cause ethanol intake to escalate and ultimately contribute to the abuse of ethanol. This is in contrast to dynorphin via the k-opioid system, which may act to counter this escalation. Naltrexone therapy for alcoholism may act, in part, by blocking the enkephalin-triggered positive feedback cycle.
Keywords: PVN, hypothalamus, opioids, ethanol, rat
Ethanol is a food containing calories, as well as being a drug of abuse. Hypothalamic peptides are powerful controllers of the urge to eat and to keep eating (Leibowitz and Hoebel, 2004). The expression of these peptides in the hypothalamus is strongly affected by the nutrients that are eaten (Leibowitz and Hoebel, 2004; Leibowitz and Wortley, 2004). This includes ethanol, which can enhance the expression of opioid peptides in the paraventricular nucleus of the hypothalamus (PVN) (Chang et al., 2007a). Ethanol intake may therefore be regulated, in part, by these hypothalamic opioid peptides.
The opioids have long been known to affect food intake through their actions in the hypothalamus. Three major classes of opioid receptors have been characterized: μ, δ, and k (Gianoulakis, 2004), all of which are found in the hypothalamus of the rat (Sharif and Hughes, 1989), including in the PVN (Abbadie et al., 2000; Desjardins et al., 1990; Mansour et al., 1994). Agonists of these opioid receptors in the PVN are found to increase feeding behavior (Gosnell et al., 1986; Leibowitz and Hor, 1982; McLean and Hoebel, 1983; Quinn et al., 2003; Stanley et al., 1988), while antagonists at these receptors cause a suppression of feeding (Gosnell et al., 1986). Peripheral injection of a general opioid agonist or specific k-agonist stimulates cellular activity in the medial PVN (Laorden et al., 2000; Laorden et al., 2003), further identifying this nucleus as an opioid-sensitive site.
Opiates are also known to affect ethanol intake. Morphine given systemically has a bimodal effect, with low doses stimulating and high doses inhibiting intake (Herz, 1997; Hubbell et al., 1986; Ulm et al., 1995). Predictably, systemic naloxone decreases ethanol consumption (Herz, 1997; Hubbell et al., 1986; Oswald and Wand, 2004). The long-acting opioid antagonist naltrexone is used to prevent relapse in alcoholics, particularly in those with the Asp40 allele of the μ-opioid receptor (Anton et al., 2008; O'Brien et al., 1996).
Rats trained sequentially to drink 1%, 2%, 4%, and then 7% ethanol clearly show increases in mRNA and peptide expression of the opioids enkephalin and dynorphin in hypothalamic areas that include the PVN (Chang et al., 2007a; Oliva and Manzanares, 2007). If local injection of opiates in the PVN were found to elicit ethanol intake, this would suggest a positive feedback loop between PVN opioid expression and opiate-induced ethanol intake. This hypothesized positive feedback could ultimately contribute to alcohol abuse. To test this possibility, rats were given injections of morphine, naloxone, and relatively specific agonists of the three major opioid receptors in the PVN. The effects on ethanol, food and water intake were measured.
Materials and Methods
Subjects
Male Sprague-Dawley rats (200-250 g) were obtained from Taconic Farms (Germantown, NY). They were individually housed in hanging wire cages and maintained on a 12:12-hr light-dark cycle with lights off at 6:00 AM and ad libitum access to LabDiet rodent chow (St. Louis, MO) and water, either through a piping system or, when ethanol was present, through graduated cylinders with non-drip sippers (Integrated Laboratory Equipment, Fort Smith, AR) attached to the cages. In total, 39 rats were included in this study.
Ethanol training
Subjects were acclimated to ethanol gradually, using a variant of a two-bottle choice procedure (Martinetti et al., 2000). Unsweetened ethanol was used. Those trained to drink 4% ethanol (n = 6) were given ad libitum access, and those trained to drink 7% (n = 33) were encouraged to drink by presenting the ethanol for 12 h each day, starting 4 h into the dark cycle. The concentration of ethanol was increased every 4 days, from 1%, 2%, 4%, and 7% (v/v). Tests were performed after the subjects had at least 1 week of access to either 4% or 7% ethanol, as specified below. The effects produced by each drug injection were compared to vehicle injections on counterbalanced consecutive days.
Surgery
Subjects were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), supplemented with ketamine as needed. Guide shafts, made of 21-gauge stainless steel, 10 mm in length, were implanted perpendicularly and unilaterally in the PVN (B -1.8, L 0.4, V 3.8), with reference to bregma, the midsaggital sinus, and the level skull surface, with half on the left side and half on the right side. Injectors protruded 4.5 mm beyond the guide shafts. An anatomical control site 2 mm dorsal to this location was also tested. At least one week of recovery was provided after surgery before microinjections began. Between procedures, stainless steel stylets were left in the guide shafts to prevent occlusion.
Microinjection procedures
All solutions were delivered through concentric microinjectors made of 26-gauge stainless steel outside and fused-silica tubing inside that protruded 2.5 mm (74 μm ID, 154 μm OD, Polymicro Technologies, Phoenix AZ). Injector tips reached into the region of interest (V 8.3 for PVN, V 6.3 for anatomical control).
Doses were chosen based on the feeding literature (McLean and Hoebel, 1983; Quinn et al., 2003; Stanley et al., 1988) and on pilot tests designed to determine a dose range that might increase or decrease ethanol intake. The five drugs and doses used for tests of 7% ethanol intake were as follows: 1) morphine sulfate (12.7 nmol, 25.4 nmol), 2) the opioid antagonist naloxone methiodide (m-naloxone; 4.3 nmol, 6.4 nmol), 3) the selective μ-receptor agonist [D-Ala2,N-Me-Phe4,Gly5-ol]-Enkephalin (DAMGO, 2.9 nmol, 5.8 nmol), 4) the δ-receptor agonist D-Ala-Gly-Phe-Met-NH2 (DALA, 7.1 nmol, 14.2 nmol), and 5) the k-receptor agonist (±)-trans-U-50488 methanesulfonate (U-50,488H, 2.2 nmol, 10.7 nmol). Morphine, m-naloxone, DAMGO, and U-50,488H were purchased from Sigma-Aldrich Co. (St. Louis, MO) and DALA from American Peptide Co. Inc. (Sunnyvale, CA). Drugs were dissolved in preservative-free 0.9% NaCl solution (Hospira Inc, Lake Forest, IL) and prepared fresh immediately prior to microinjection. For a test of 4% ethanol intake, DAMGO (2.0 nmol) was dissolved in Ringer solution.
Injections were counterbalanced, so each animal received vehicle or peptide in opposing order on two consecutive days. To minimize stress, animals were handled on an almost daily basis throughout their ethanol training prior to the initiation of microinjections. Injections were given 3.5 h into the dark cycle for all animals, 30 min prior to daily 7% ethanol access. A syringe pump delivered 0.5 μl during 47 sec at a flow rate of 0.6 μl/min, and the microinjector remained in place for another 47 sec to allow diffusion into the injection site. For rats drinking 7% ethanol, the intake of ethanol, food, and water was measured for four hours starting from 30 min after the injections to allow time for the drugs to take effect. For rats drinking 4% ethanol, intake was measured when food and water were not present. Within each group, at least a week elapsed between sets of injections to allow receptor recovery. Each compound was tested in a different group of animals. Tests of each dose included a counterbalanced control injection of saline vehicle. For the anatomical control location, the most effective dose of each drug was tested in a separate group.
Ethanol preference ratios
For the animals consuming 7% ethanol, the ethanol preference ratio was determined (volume of 7% ethanol consumed/total volume of 7% ethanol and water intake consumed).
Blood alcohol assessment
Tail vein blood was sampled for blood alcohol concentration (BAC) 2.5 hours after the start of daily ethanol and at least one week after completing the microinjections (Analox GM7 Fast Enzymatic Metabolic Analyser, Luneburg, MA). At this time, mean BAC for each group was similar, and ranged from 11.2 to 30.1 mg/dl.
Histology and data analysis
Injection sites were verified by injecting 0.25 μl methylene blue dye (Sigma, St. Louis, MO). Brains were kept in formalin for a minimum of 1 week prior to slicing, then cut in 40 μm sections on a freezing microtome and slide-mounted for microscopic examination. Only data from animals with injector tips in the PVN or control area were included in the analysis; data from those with probes 0.5 mm or farther from the target regions, 1 to 2 animals per group, were discarded from the analysis. Data from animals consuming little or no ethanol on measurement days (less than 0.2 g/kg during 4 h) were also excluded.
The measures of ethanol, food, and water intake were statistically analyzed with tests of the simple effects of each dose and time point for the different compounds, using paired, two-tailed t-tests (Keppel and Wickens, 2004). As two doses of each compound were used, a Bonferroni correction was applied, making p < 0.025 the required probability for each of the pairwise comparisons per experiment. As the behavioral effects of opioid injections are known to vary heavily over time (Craft, 2008), and the doses used for each compound were within the same order of magnitude, an overall repeated-measures ANOVA could not be employed. In the figures, data are expressed as mean ± standard error of the difference (SED) between drug and vehicle as recommended for paired t-tests (Keppel and Wickens, 2004). The vehicle intake presented is the average between the two injections, as intake after vehicle did not significantly differ within each group.
Results
Experiment 1: Morphine microinjections in the PVN increase ethanol intake
Injection of morphine in the PVN, compared to vehicle, selectively increased 7% ethanol intake. Tests of the simple effects (Fig. 1) revealed that morphine at the 12.7 nmol dose significantly increased ethanol intake by 44% at 4 h (t = 3.29, df = 6, p < 0.025). The 25.4 nmol dose, while it increased ethanol intake to a greater extent, by 100% at 4 h, did not attain statistical significance (t = 2.49, df = 6, p = 0.047). In contrast, morphine at either dose did not significantly affect food intake or water intake or the ethanol preference ratio in these ethanol-drinking rats (ns). Morphine in the anatomical control site 2 mm above the PVN had no significant effects on intake of ethanol (ns) (Table 1).
Fig. 1.
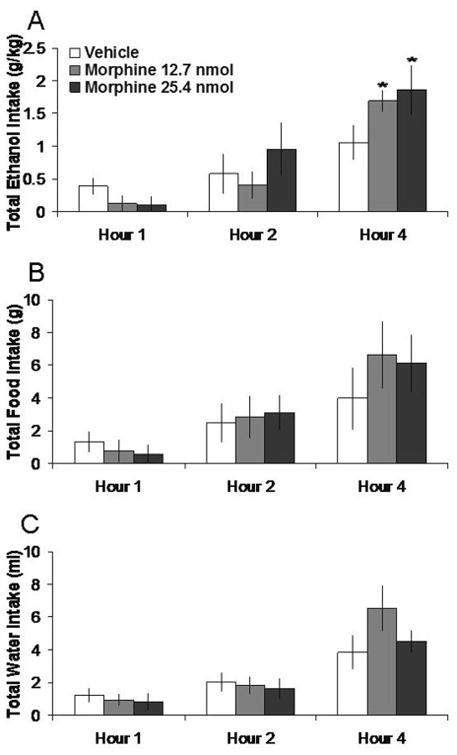
Effects of morphine injection (12.7 nmol, 25.4 nmol, n = 7) in the PVN on ethanol, food, and water consumption in the dark. A. Morphine at the lower dose stimulates 7% ethanol intake. B. Morphine does not significantly affect food intake. C. Morphine does not significantly affect water intake. *p < 0.025. The vehicle shown is averaged from both injections.
Table 1. Anatomical control injection effects on ethanol, food, and water intake.
Injection in the anatomical control location, 2 mm dorsal to the PVN, does not significantly affect ethanol, food, or water intake. Values are mean ± S.E.M.
| Drug | Nutrient | Hour 1 | Hour 2 | Hour 4 |
|---|---|---|---|---|
|
| ||||
| Vehicle | Ethanol (g/kg) | 0.31 ± 0.02 | 0.47 ± 0.08 | 0.92 ± 0.20 |
| Morphine 25.4 nmol | 0.21 ± 0.08 | 0. 54 ± 0.18 | 1.34 ± 0.50 | |
|
| ||||
| Vehicle | Food (g) | 1.68 ± 0.55 | 2.54 ± 0.53 | 5.74 ± 0.71 |
| Morphine 25.4 nmol | 0.84 ± 0.79 | 2.32 ± 1 12 | 5.58 ± 1.94 | |
|
| ||||
| Vehicle | Water (ml) | 1.70 ± 0.54 | 2.30 ± 0.59 | 4.50 ± 0.76 |
| Morphine 25.4 nmol | 0.72 ± 0.24 | 2.16 ± 0.90 | 8.28 ± 3.06 | |
|
| ||||
| Vehicle | Ethanol (g/kg) | 0.31 ± 0.14 | 0.46 ± 0.19 | 0.92 ± 0.33 |
| M-naloxone 6.4 nmol | 0.18 ± 0.09 | 0.34 ± 0.14 | 0.55 ± 0.19 | |
|
| ||||
| Vehicle | Food (g) | 1.60 ± 0.47 | 2.44 ± 0.80 | 6.58 ± 1.15 |
| M-naloxone 6.4 nmol | 0.80 ± 0.53 | 1.34 ± 0.64 | 5.62 ± 1.72 | |
|
| ||||
| Vehicle | Water (ml) | 1.76 ± 0.45 | 3.04 ± 0.62 | 6.10 ± 0.64 |
| M-naloxone 6.4 nmol | 1.36 ± 0.57 | 3.20 ± 1.10 | 8.72 ± 2.43 | |
|
| ||||
| Vehicle | Ethanol (g/kg) | 0.32 ± 0.07 | 0.59 ± 0.12 | 1.08 ± 0.23 |
| DAMGO 5.8 nmol | 0.40 ± 0.13 | 0.77 ± 0.23 | 1.10 ± 0.29 | |
|
| ||||
| Vehicle | Food (g) | 1.62 ± 0.65 | 3.14 ± 0.83 | 6.56 ± 1.14 |
| DAMGO 5.8 nmol | 4.26 ± 1.98 | 5.22 ± 2.01 | 6.48 ± 1.67 | |
|
| ||||
| Vehicle | Water (ml) | 1.92 ± 0.50 | 3.24 ± 0.55 | 7.72 ± 1.55 |
| DAMGO 5.8 nmol | 2.94 ± 1.15 | 6.04 ± 1.76 | 11.56 ± 2.93 | |
|
| ||||
| Vehicle | Ethanol (g/kg) | 0.29 ± 0.12 | 0.52 ± 0.17 | 0.90 ± 0.24 |
| DALA 14.2 nmol | 0.45 ± 0.21 | 0.57 ± 0.22 | 0.90 ± 0.28 | |
|
| ||||
| Vehicle | Food (g) | 1.74 ± 0.37 | 3.00 ± 0.25 | 7.70 ± 1.10 |
| DALA 14.2 nmol | 2.52 ± 0.60 | 3.08 ± 0.61 | 4.80 ± 1.03 | |
|
| ||||
| Vehicle | Water (ml) | 1.62 ± 0.57 | 2.88 ± 0.61 | 6.44 ± 1.38 |
| DALA 14.2 nmol | 1.88 ± 0.77 | 4.46 ± 1.49 | 7.06 ± 1.54 | |
|
| ||||
| Vehicle | Ethanol (g/kg) | 0.32 ± 0.05 | 0.53 ± 0.12 | 0.85 ± 0.22 |
| U-50,488H 2.2 nmol | 0.37 ± 0.15 | 0.54 ± 0.15 | 0.88 ± 0.21 | |
|
| ||||
| Vehicle | Food (g) | 1.60 ± 0.46 | 4.92 ± 0.90 | 8.08 ± 0.76 |
| U-50,488H 2.2 nmol | 1.80 ± 0.70 | 3.50 ± 1.30 | 7.74 ± 1.61 | |
|
| ||||
| Vehicle | Water (ml) | 1.64 ± 0.19 | 3.14 ± 0.42 | 7.44 ± 1.40 |
| U-50,488H 2.2 nmol | 1.86 ± 0.57 | 3.76 ± 0.57 | 9.22 ± 1.58 | |
Experiment 2: M-naloxone microinjections in the PVN decrease ethanol intake
Injection of m-naloxone in the PVN, compared to vehicle, selectively decreased 7% ethanol intake. Tests of the simple effects (Fig. 2) revealed that m-naloxone at 4.3 nmol decreased ethanol intake by 64% at 1 h (t = 3.78, df = 5, p < 0.025). At 6.4 nmol, m-naloxone decreased ethanol intake by 59% at 1h, although this did not reach significance (t = 2.60, df = 5, p = 0.048). In contrast, m-naloxone did not significantly affect food intake or water intake or the ethanol preference ratio (ns). M-naloxone in the anatomical control site 2 mm above the PVN had no significant effects on intake of ethanol, food, or water (ns) (Table 1).
Fig. 2.
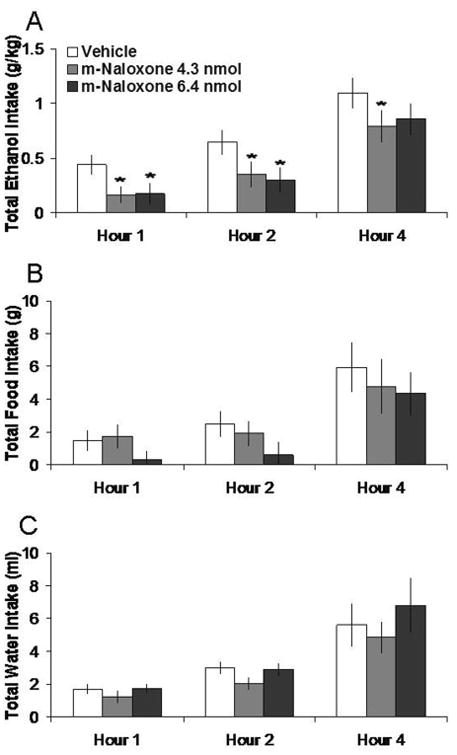
Effects of m-naloxone injection (4.3 nmol, 6.4 nmol, n = 6) in the PVN on ethanol, food, and water consumption. A. M-naloxone at the lower dose suppresses 7% ethanol intake. B. M-naloxone does not significantly affect food intake. C. M-naloxone does not significantly affect water intake. *p < 0.025. The vehicle shown is averaged from both injections.
Experiment 3: DAMGO in the PVN does not significantly affect ethanol intake
As shown in Fig. 3, DAMGO (2.0 nmol), in tests when food and water were not available for consumption, increased 4% ethanol intake by 75% at 1 h, although this effect did not reach significance (t = 2.57, df = 5, p = 0.049). In a different group of rats tested with food and water as well as ethanol available, DAMGO produced no change in intake of 7% ethanol, food or water, as illustrated in Figure 4. In the anatomical control site 2 mm above the PVN, DAMGO also had no significant effects on intake of ethanol, food, or water (ns) (Table 1).
Fig. 3.
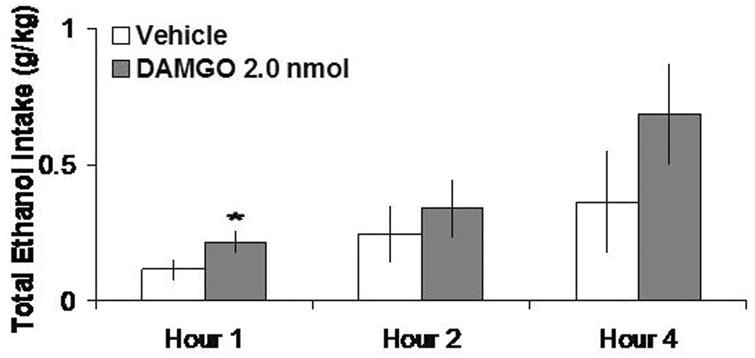
DAMGO (2 nmol, n = 6), with no food or water available, does not significantly stimulate 4% ethanol intake.
Fig. 4.
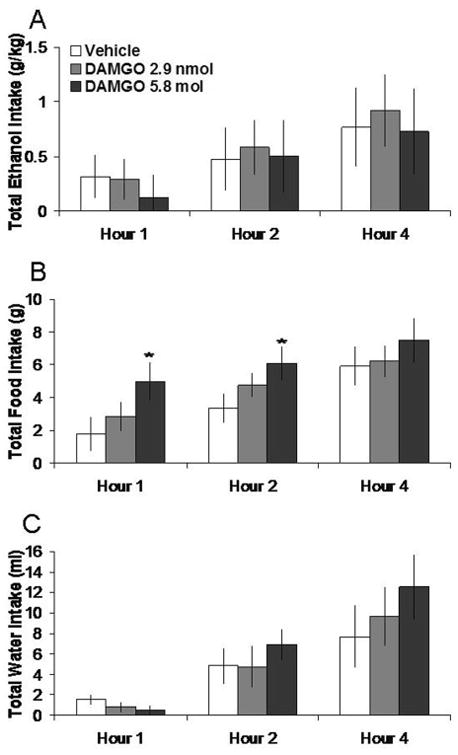
Effects of DAMGO (2.9 nmol, 5.8 nmol, n = 5) in the PVN on ethanol, food, and water consumption. A. DAMGO does not significantly affect 7% ethanol intake. B. DAMGO does not significantly affect food intake. C. DAMGO does not significantly affect water intake. The vehicle shown is averaged from both injections.
Experiment 4: DALA in the PVN increases ethanol intake
Injection of DALA in the PVN, compared to vehicle, selectively increased the consumption of 7% ethanol. Tests of the simple effects revealed that DALA at the 14.2 nmol dose increased ethanol intake by 104% at 1 h (t = 3.85, df = 4, p < 0.025). In contrast, DALA did not significantly affect food intake or water intake (ns). The 14.2 nmol dose of DALA also significantly increased the ethanol preference ratio by 19% at 2 h (0.58 to 0.69, p < 0.025). These effects are illustrated in Figure 5. In the anatomical control site 2 mm above the PVN, DALA had no significant effects on intake of ethanol, food, or water intake (ns) (Table 1).
Fig 5.
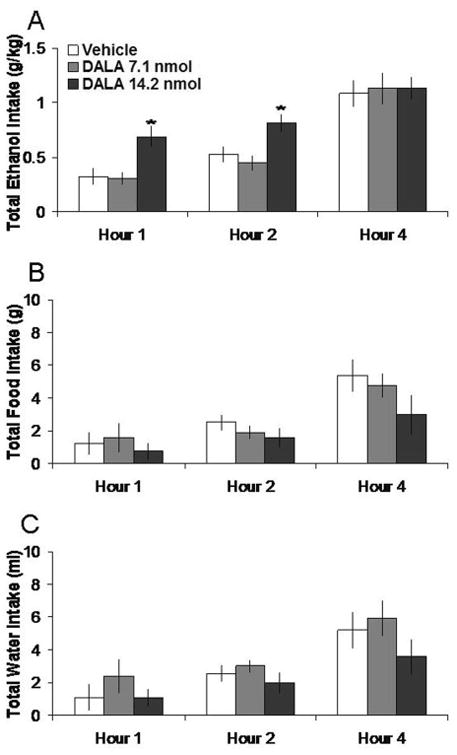
Effects of DALA (7.1 nmol, 14.2 nmol, n = 5) in the PVN on ethanol, food, and water consumption. A. DALA at the higher dose significantly increases 7% ethanol intake. B. DALA does not significantly affect food intake. C. DALA does not significantly affect water intake. *p < 0.025. The vehicle shown is averaged from both injections.
Experiment 5: The kappa agonist in the PVN decreases ethanol intake
Injection of U-50,488H in the PVN, compared to vehicle, selectively decreased 7% ethanol intake. Tests of the simple effects showed that U-50,488H at the 10.7 nmol dose decreased ethanol intake by 40% at 4 h (t = 4.19, df = 4, p < 0.025). In contrast, U-50,488H did not significantly affect food intake or water intake or the ethanol preference ratio (ns). These effects are illustrated in Figure 6. In the anatomical control site 2 mm above the PVN, U-50,488H had no significant effects on intake of ethanol, food, or water intake (ns) (Table 1).
Fig. 6.
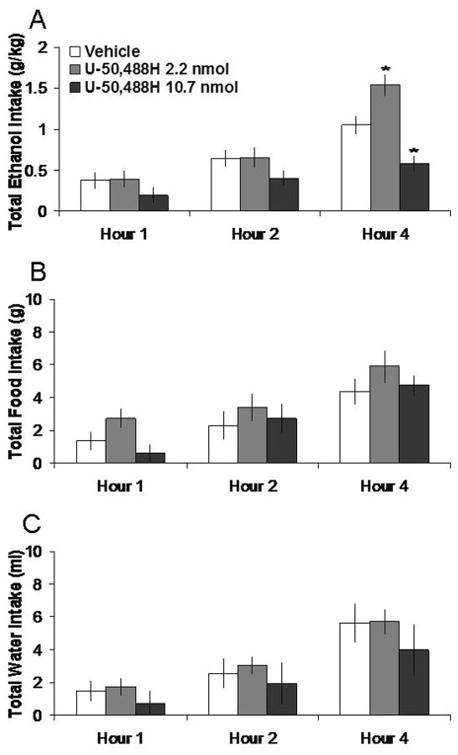
Effects of U-50,488H (2.2 nmol, 10.7 nmol, n = 5) in the PVN on ethanol, food, and water consumption. A. U-50,488H suppresses 7% ethanol intake at the high dose. B. U-50,488H does not significantly affect food intake. C. U-50,488H does not significantly affect water intake. *p < 0.025. The vehicle shown is averaged from both injections.
Histology
Histological verification revealed that PVN injections were made in the ventral, medial parvocellular, and lateral magnocellular parts of the nucleus (Fig. 7A). The anatomical control injections were made primarily in the anteromedial nucleus of the thalamus (Fig. 7B).
Fig 7.
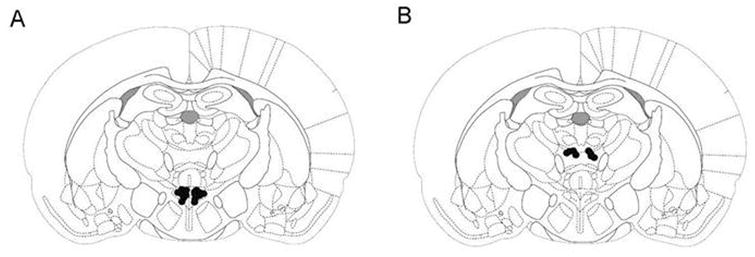
Black dots indicate injector placement for animals included in data analysis. Injections were unilateral and counterbalanced, left and right. Coronal sections are -1.8 mm caudal to Bregma. Adapted from The Rat Brain, compact 3rd edition, G. Paxinos and C. Watson, Copyright 1997, with permission from Elsevier. A. Injection sites for PVN drug injections. B. Injection sites for anatomical control injections.
Discussion
In this study, microinjection of morphine in the PVN increased voluntary intake of unsweetened 7% ethanol even in the presence of food and water. Therefore, opioid receptors in the PVN appear to have an important role in stimulating the selection of ethanol for consumption. The receptor analysis suggests that the δ system is largely responsible for this selective, stimulatory effect on ethanol intake, while the k system may act to counter it. In the present study, the δ-agonists, like morphine, acted in the PVN to increase intake of unsweetened ethanol and enhance ethanol preference. While the μ-receptors could also contribute to ethanol intake in other nuclei, the results with DAMGO in the present study suggest that this effect does not occur in the PVN, a nucleus where δ-receptors are more densely expressed than the μ- or k-receptors (Desjardins et al., 1990).
It is well-known that the μ-opioid receptor system in several brain sites is involved in ethanol intake and the development of alcoholism in humans and laboratory animals (Koob et al., 2003; Oswald and Wand, 2004; Ulm et al., 1995; Zhang and Kelley, 2002). There is evidence, however, that the stimulatory effect on consummatory behavior induced by μ-receptor activation in the PVN may be more an example of the opioids affecting choice behavior based on palatability (Olszewski et al., 2009). Opioids are thought to play a role in food palatability and taste hedonics (Levine, 2006; Zhang and Kelley, 2002), and the opioid antagonist naltrexone causes an increase in aversive reactions along with a decrease in ingestive responses to ethanol (Hill and Kiefer, 1997). Taste reactivity tests demonstrate that rats consider ethanol to contain both sweet and bitter components (Kiefer, 1995). Additionally, in taste preference tests, Sprague-Dawley rats show a preference for ethanol compared to water when it is in concentrations of 1-2% but exhibit a robust avoidance of concentrations above 6% (Morrow et al., 1993; Tordoff et al., 2008). The DAMGO results in the present study are consistent with the view that the μ-receptor system in the PVN may stimulate consummatory behavior as a function of taste (Giraudo et al., 1999; Kelley et al., 2002; Woolley et al., 2007). This μ-opioid was not able to significantly enhance the motivation to drink 7% ethanol when food was available as an alternative or 4% ethanol without food present. The chronic availability of ethanol in the present study may have detracted from the stimulatory effect of DAMGO on food intake that others have seen with ethanol-naïve rats (Leibowitz and Hor, 1982; Quinn et al., 2003).
The finding that a δ-agonist, in contrast to μ-agonist, can preferentially stimulate ethanol intake with food present suggests that this receptor system in the PVN, while able to increase food intake in the absence of ethanol, is more susceptible to being co-opted by voluntary ethanol consumption. With PVN injection of morphine increasing 7% ethanol intake in lieu of food, one can surmise that the δ-receptor system is more likely to be involved in the unhealthy escalation of ethanol intake. Because the opioid receptors may additionally have subtypes and can form dimers that alter agonist drug actions, it is difficult to draw definitive conclusions concerning receptor actions engaged by endogenous agonists (Levac et al., 2002; Pasternak, 2004; Van Rijn et al., 2008). Previous studies from our laboratories and others have shown that, in ethanol-naïve rats, injections of δ-agonists in the PVN produce a significant increase in feeding (McLean and Hoebel, 1983; Stanley et al., 1988). This pattern clearly changes in animals that have learned to drink ethanol, with the δ-agonist stimulating ethanol intake without affecting food intake. This finding is consistent with other studies showing blockade of δ- but not μ-receptors to suppress ethanol intake (Le et al., 1993). Taken together, these results suggest that chronic consumption of ethanol may supplant the feeding-stimulatory effect of endogenous δ-agonists, such as enkephalin, causing ethanol intake to replace the normal opioid-induced feeding response.
In contrast to the δ-agonist, the k-agonist decreased ethanol intake. This is consistent with research showing decreased ethanol intake with peripheral injections of U-50,488H (Lindholm et al., 2001) and enhanced intake with a selective k-antagonist (Mitchell et al., 2005). On the other hand, injection of a low dose of k-agonist has been observed to enhance operant responding for ethanol (Holter et al., 2000). This may be consistent with a “priming effect”(Ulm et al., 1995), where low doses of a drug act as primers for additional stimulation of the same system. Feeding has also been shown to be increased with injection of certain k-agonists in the PVN (Gosnell et al., 1986); however, to our knowledge, effects from U-50,488H have not been investigated. Therefore, it remains unclear if the suppressed ethanol intake observed in the present study is due to a difference in receptor binding properties or in the dose used compared to the k-agonist that led to enhanced food intake.
Studies of endogenous opioids show that ethanol exposure can stimulate the expression of enkephalin and dynorphin mRNA in the PVN (Chang et al., 2007a) and upregulate δ-opioid receptor gene expression in neuronal cell lines (Charness et al., 1993). Together with the present findings showing δ-agonists to increase ethanol intake, this effect on some endogenous opioids suggests a bi-directional, positive relationship between δ-receptor systems and the drinking of ethanol. Other studies have revealed a stimulatory effect of ethanol drinking on the expression of two non-opioid, orexigenic peptides, galanin and orexin, in the hypothalamus (Lawrence et al., 2006; Leibowitz et al., 2003). Similar to δ-agonists, these peptides are known to stimulate food intake in ethanol-naïve rats, and this effect switches to an increase specifically in ethanol intake in rats trained to drink ethanol (Rada et al., 2004; Schneider et al., 2007). It is interesting that all three orexigenic peptides, enkephalin, galanin, and orexin, are also stimulated by chronic or acute consumption of a fat-rich diet, in a manner similar to ethanol (Chang et al., 2007b; Chang et al., 2004). This suggests that some orexigenic peptides in the hypothalamus that stimulate the consumption of nutrients such as ethanol and fat can become over-expressed in response to these nutrients in their diet and thus contribute to their over-consumption.
In contrast, by operating through negative feedback, other orexigenic peptides such as dynorphin, may act to counter this enhanced consumption (Kelley et al., 2005; Koob, 2008). Ethanol intake increases dynorphin mRNA and peptide expression in the PVN (Chang et al., 2007a). In addition, ethanol increases the release of dynorphin peptide in nuclei such as the ventral tegmental area (VTA) (Jarjour et al., 2009), nucleus accumbens (NAc) (Marinelli et al., 2006), and central nucleus of the amygdala (Lam et al., 2008). Interestingly, this release of dynorphin occurs after the release of β-endorphin, which is thought to enhance the rewarding effects of ethanol (Jarjour et al., 2009; Marinelli et al., 2006). This lends support to the idea that dynorphin plays a role in curbing excessive intake.
With any microinjection study, the issue arises concerning anatomical specificity, as injected drugs may diffuse into adjacent brain areas or up along the cannula track. In this study, an injection 2 mm dorsal to the PVN did not produce any observable changes in appetitive behavior, so the effects on ethanol intake with PVN injections were not due to reflux of the peptide up the outside of the cannula. Injection of 0.5 μl of a solution typically spreads laterally only about 0.5 mm (Melzacka et al., 1985; Peciña and Berridge, 2005), and studies suggest that morphine and methylated naloxone remain relatively well at the site of injection (Melzacka et al., 1985; Schroeder et al., 1991). Mapping studies of food intake have shown that opioids act in multiple areas of the brain (Gosnell et al., 1986; Stanley et al., 1988), both hypothalamic and extrahypothalamic. The methods employed in this study do not exclude the possibility that nearby hypothalamic sites participated in the consummatory effects of opioid injections, but they do point to the PVN as an important nucleus in this regard.
Although the circuitry through which PVN opioids increase ethanol intake is not fully understood, one possibility is that the hypothalamic opioids affect consumption, indirectly through their indirect effects on dopamine (DA) release in the NAc. This DA release is believed to be important in appetitive responding for ethanol, as well as in facilitating food intake and drug self-administration (Baldo et al., 2002; Czachowski et al., 2001; Gonzales et al., 2004; Pal and Thombre, 1993; Samson et al., 1999). Projections from the PVN extend rostrally to the NAc, possibly via lateral hypothalamic projections, where they can excite DA nerve terminals (Barone et al., 1981; Brog et al., 1993; Korf, 1984). They also extend caudally from the PVN to the hindbrain, with indirect (Sesack and Pickel, 1992; Wilson et al., 2003) as well as direct (Rodaros et al., 2007) connections to the midbrain VTA where DA cell bodies are concentrated, and project, in part, to the NAc to release DA in the shell and core (Di Chiara, 2002). The evidence that morphine or galanin injection in the PVN increases extracellular DA in the NAc (Barson et al., 2008; Rada et al., 1998) supports a role for this hypothalamic nucleus in controlling the accumbens motivational system. A similar effect on DA is seen with extra-hypothalamic administration of μ- and δ-opioid agonists (Di Chiara and Imperato, 1988; Spanagel et al., 1990). In contrast, DA is decreased in the NAc by k-agonists, at doses that also increase immobility in the forced swim test and elevate thresholds for intracranial self-stimulation (Carlezon et al., 2006; Di Chiara and Imperato, 1988; Spanagel et al., 1990). These divergent effects on DA release may help to explain why the δ-agonist enhanced ethanol intake, while the k-agonist decreased it.
In conclusion, an agonist in the PVN for the δ-opioid receptor can cause rats to respond by drinking ethanol under appropriate conditions. With recent evidence showing ethanol consumption to stimulate the expression and production of enkephalin in the PVN that may further increase its release, these new findings with peptide injections support a role for this PVN opioid in a positive feedback mechanism, which causes ethanol intake to escalate and ultimately play a role in alcohol abuse. This is in contrast with a k-receptor agonist in the PVN, which caused rats to reduce their ethanol consumption. The evidence that ethanol also stimulates the expression and production of dynorphin in the PVN supports a role for this PVN opioid in a negative feedback mechanism that counters further ethanol intake. The additional finding that local injection of m-naloxone has the opposite effect of the general opioid agonist suggests that opioid-antagonist therapy in alcoholic patients may act partly in the PVN ingestive behavior system when it has been diverted to the disservice of ethanol intake.
Acknowledgments
This research was supported by USPHS Grant AA12882 and the E.H. Lane Foundation. We thank Miriam Bocarsly for her technical assistance.
References
- Abbadie C, Gultekin SH, Pasternak GW. Immunohistochemical localization of the carboxy terminus of the novel mu opioid receptor splice variant MOR-1C within the human spinal cord. Neuroreport. 2000;11(9):1953–7. doi: 10.1097/00001756-200006260-00029. [DOI] [PubMed] [Google Scholar]
- Anton R, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65(2):135–44. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137(1-2):165–77. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Barone FC, Wayner MJ, Scharoun SL, Guevara-Aguilar R, Aguilar-Baturoni HU. Afferent connections to the lateral hypothalamus: a horseradish peroxidase study in the rat. Brain Res Bull. 1981;7(1):75–88. doi: 10.1016/0361-9230(81)90101-5. [DOI] [PubMed] [Google Scholar]
- Barson J, Rada P, Leibowitz S, Hoebel B. Neuroscience 2008. Society for Neuroscience; Washington, DC: 2008. Opioids in the hypothalamus raise dopamine and lower acetylcholine release in the nucleus accumbens. [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338(2):255–78. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Carlezon WJ, Béguin C, DiNieri J, Baumann M, Richards M, Todtenkopf M, Rothman R, Ma Z, Lee D, Cohen B. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316(1):440–7. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, Hoebel BG, Leibowitz SF. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007a;31(2):249–59. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Gaysinskaya V, Marwil Z, Leibowitz SF. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. Am J Physiol Endocrinol Metab. 2007b;292(2):E561–70. doi: 10.1152/ajpendo.00087.2006. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145(8):3904–12. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- Charness M, Hu G, Edwards R, Querimit L. Ethanol increases delta-opioid receptor gene expression in neuronal cell lines. Mol Pharmacol. 1993;44(6):1119–27. [PubMed] [Google Scholar]
- Craft RM. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Exp Clin Psychopharmacol. 2008;16(5):376–85. doi: 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alcohol Clin Exp Res. 2001;25(10):1431–40. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Desjardins GC, Brawer JR, Beaudet A. Distribution of mu, delta, and kappa opioid receptors in the hypothalamus of the rat. Brain Res. 1990;536(1-2):114–23. doi: 10.1016/0006-8993(90)90015-4. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137(1-2):75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2004;4(1):39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- Giraudo S, Grace M, Billington C, Levine A. Differential effects of neuropeptide Y and the mu-agonist DAMGO on ‘palatability’ vs. ‘energy’. Brain Res. 1999;834(1-2):160–3. doi: 10.1016/s0006-8993(99)01512-7. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103(2):121–46. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gosnell B, Morley J, Levine A. Opioid-induced feeding: localization of sensitive brain sites. Brain Res. 1986;369(1-2):177–84. doi: 10.1016/0006-8993(86)90526-3. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129(2):99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Hill K, Kiefer S. Naltrexone treatment increases the aversiveness of alcohol for outbred rats. Alcohol Clin Exp Res. 1997;21(4):637–41. [PubMed] [Google Scholar]
- Holter SM, Henniger MS, Lipkowski AW, Spanagel R. Kappa-opioid receptors and relapse-like drinking in long-term ethanol-experienced rats. Psychopharmacology (Berl) 2000;153(1):93–102. doi: 10.1007/s002130000601. [DOI] [PubMed] [Google Scholar]
- Hubbell C, Czirr S, Hunter G, Beaman C, LeCann N, Reid L. Consumption of ethanol solution is potentiated by morphine and attenuated by naloxone persistently across repeated daily administrations. Alcohol. 1986;3(1):39–54. doi: 10.1016/0741-8329(86)90070-4. [DOI] [PubMed] [Google Scholar]
- Jarjour S, Bai L, Gianoulakis C. Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alcoholism-Clinical and Experimental Research. 2009;33(6):1033–1043. doi: 10.1111/j.1530-0277.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- Kelley A, Bakshi V, Haber S, Steininger T, Will M, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76(3):365–77. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493(1):72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens T. Design and Analysis: A Researcher's Handbook. 4th. Prentice Hall; Upper Saddle River, New Jersey: 2004. [Google Scholar]
- Kiefer S. Alcohol, palatability, and taste reactivity. Neurosci Biobehav Rev. 1995;19(1):133–41. doi: 10.1016/0149-7634(94)00027-x. [DOI] [PubMed] [Google Scholar]
- Koob G, Roberts A, Kieffer B, Heyser C, Katner S, Ciccocioppo R, Weiss F. Animal models of motivation for drinking in rodents with a focus on opioid receptor neuropharmacology. Recent Dev Alcohol. 2003;16:263–81. doi: 10.1007/0-306-47939-7_19. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf HW. Neuronal organization of the avian paraventricular nucleus: intrinsic, afferent, and efferent connections. J Exp Zool. 1984;232(3):387–95. doi: 10.1002/jez.1402320303. [DOI] [PubMed] [Google Scholar]
- Lam MP, Marinelli PW, Bai L, Gianoulakis C. Effects of acute ethanol on opioid peptide release in the central amygdala: an in vivo microdialysis study. Psychopharmacology (Berl) 2008;201(2):261–71. doi: 10.1007/s00213-008-1267-8. [DOI] [PubMed] [Google Scholar]
- Laorden ML, Castells MT, Martinez MD, Martinez PJ, Milanes MV. Activation of c-fos expression in hypothalamic nuclei by mu- and kappa-receptor agonists: correlation with catecholaminergic activity in the hypothalamic paraventricular nucleus. Endocrinology. 2000;141(4):1366–76. doi: 10.1210/endo.141.4.7407. [DOI] [PubMed] [Google Scholar]
- Laorden ML, Castells MT, Milanes MV. Effects of U-50488H and U-50488H withdrawal on c-fos expression in the rat paraventricular nucleus. Correlation with c-fos in brainstem catecholaminergic neurons. Br J Pharmacol. 2003;138(8):1544–52. doi: 10.1038/sj.bjp.0705179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A, Cowen M, Yang H, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148(6):752–9. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Quan B, Chow S. The effects of selective blockade of delta and mu opiate receptors on ethanol consumption by C57BL/6 mice in a restricted access paradigm. Brain Res. 1993;630(1-2):330–2. doi: 10.1016/0006-8993(93)90672-a. [DOI] [PubMed] [Google Scholar]
- Leibowitz S, Avena N, Chang G, Karatayev O, Chau D, Hoebel B. Ethanol intake increases galanin mRNA in the hypothalamus and withdrawal decreases it. Physiol Behav. 2003;79(1):103–11. doi: 10.1016/s0031-9384(03)00110-0. [DOI] [PubMed] [Google Scholar]
- Leibowitz S, Hoebel B. Behavioral Neuroscience and Obesity. In: Bray G, Bouchard C, editors. Handbook of Obesity Etiology and Pathophysiology. 2nd. Marcel Dekker, Inc.; New York: 2004. pp. 301–371. [Google Scholar]
- Leibowitz S, Hor L. Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides. 1982;3(3):421–8. doi: 10.1016/0196-9781(82)90102-4. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Wortley KE. Hypothalamic control of energy balance: different peptides, different functions. Peptides. 2004;25(3):473–504. doi: 10.1016/j.peptides.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Levac B, O'Dowd B, George S. Oligomerization of opioid receptors: generation of novel signaling units. Curr Opin Pharmacol. 2002;2(1):76–81. doi: 10.1016/s1471-4892(02)00124-8. [DOI] [PubMed] [Google Scholar]
- Levine A. The animal model in food intake regulation: examples from the opioid literature. Physiol Behav. 2006;89(1):92–6. doi: 10.1016/j.physbeh.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brene S, Franck J. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res. 2001;120(2):137–146. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Meng F, Akil H, Watson SJ. Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA. Mol Cell Neurosci. 1994;5(2):124–44. doi: 10.1006/mcne.1994.1015. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1-8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2006;30(6):982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- Martinetti MP, Andrzejewski ME, Hineline PN, Lewis MJ. Ethanol consumption and the matching law: a choice analysis using a limited-access paradigm. Exp Clin Psychopharmacol. 2000;8(3):395–403. doi: 10.1037//1064-1297.8.3.395. [DOI] [PubMed] [Google Scholar]
- McLean S, Hoebel BG. Feeding induced by opiates injected into the paraventricular hypothalamus. Peptides. 1983;4(3):287–92. doi: 10.1016/0196-9781(83)90134-1. [DOI] [PubMed] [Google Scholar]
- Melzacka M, Nesselhut T, Havemann U, Vetulani J, Kuschinsky K. Pharmacokinetics of morphine in striatum and nucleus accumbens: relationship to pharmacological actions. Pharmacol Biochem Behav. 1985;23(2):295–301. doi: 10.1016/0091-3057(85)90573-8. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182(3):384–92. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Morrow N, Kiefer S, Metzler C. Gustatory and olfactory contributions to alcohol consumption in rats. Alcohol. 1993;10(4):263–7. doi: 10.1016/0741-8329(93)90003-7. [DOI] [PubMed] [Google Scholar]
- O'Brien C, Volpicelli L, Volpicelli J. Naltrexone in the treatment of alcoholism: a clinical review. Alcohol. 1996;13(1):35–9. doi: 10.1016/0741-8329(95)02038-1. [DOI] [PubMed] [Google Scholar]
- Oliva J, Manzanares J. Gene transcription alterations associated with decrease of ethanol intake induced by naltrexone in the brain of Wistar rats. Neuropsychopharmacology. 2007;32(6):1358–69. doi: 10.1038/sj.npp.1301237. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Shaw TJ, Grace MK, Hoglund CE, Fredriksson R, Schioth HB, Levine AS. Complexity of neural mechanisms underlying overconsumption of sugar in scheduled feeding: Involvement of opioids, orexin, oxytocin and NPY. Peptides. 2009;30(2):226–33. doi: 10.1016/j.peptides.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald L, Wand G. Opioids and alcoholism. Physiol Behav. 2004;81(2):339–58. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Pal GK, Thombre DP. Modulation of feeding and drinking by dopamine in caudate and accumbens nuclei in rats. Indian J Exp Biol. 1993;31(9):750–4. [PubMed] [Google Scholar]
- Pasternak G. Multiple opiate receptors: déjà vu all over again. Neuropharmacology. 2004;47(1):312–23. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge K. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25(50):11777–86. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J, O'Hare E, Levine A, Kim E. Evidence for a mu-opioid-opioid connection between the paraventricular nucleus and ventral tegmental area in the rat. Brain Res. 2003;991(1-2):206–11. doi: 10.1016/j.brainres.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena N, Leibowitz S, Hoebel B. Ethanol intake is increased by injection of galanin in the paraventricular nucleus and reduced by a galanin antagonist. Alcohol. 2004;33(2):91–7. doi: 10.1016/j.alcohol.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Rada P, Mark GP, Hoebel BG. Galanin in the hypothalamus raises dopamine and lowers acetylcholine release in the nucleus accumbens: a possible mechanism for hypothalamic initiation of feeding behavior. Brain Res. 1998;798(1-2):1–6. doi: 10.1016/s0006-8993(98)00315-1. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150(1):8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A, Slawecki C, Hodge C. The effects of microinjection of d-amphetamine into the n. accumbens during the late maintenance phase of an ethanol consumption bout. Pharmacol Biochem Behav. 1999;63(1):159–65. doi: 10.1016/s0091-3057(98)00263-9. [DOI] [PubMed] [Google Scholar]
- Schneider E, Rada P, Darby R, Leibowitz S, Hoebel B. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31(11):1858–65. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Schroeder RL, Weinger MB, Vakassian L, Koob GF. Methylnaloxonium diffuses out of the rat brain more slowly than naloxone after direct intracerebral injection. Neurosci Lett. 1991;121(1-2):173–7. doi: 10.1016/0304-3940(91)90678-m. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Dual ultrastructural localization of enkephalin and tyrosine hydroxylase immunoreactivity in the rat ventral tegmental area: multiple substrates for opiate-dopamine interactions. J Neurosci. 1992;12(4):1335–50. doi: 10.1523/JNEUROSCI.12-04-01335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif N, Hughes J. Discrete mapping of brain Mu and delta opioid receptors using selective peptides: quantitative autoradiography, species differences and comparison with kappa receptors. Peptides. 1989;10(3):499–522. doi: 10.1016/0196-9781(89)90135-6. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg T. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990;55(5):1734–40. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Lanthier D, Leibowitz SF. Multiple brain sites sensitive to feeding stimulation by opioid agonists: a cannula-mapping study. Pharmacol Biochem Behav. 1988;31(4):825–32. doi: 10.1016/0091-3057(88)90391-7. [DOI] [PubMed] [Google Scholar]
- Tordoff M, Alarcon L, Lawler M. Preferences of 14 rat strains for 17 taste compounds. Physiol Behav. 2008 doi: 10.1016/j.physbeh.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm RR, Volpicelli JR, Volpicelli LA. Opiates and alcohol self-administration in animals. J Clin Psychiatry. 1995;56(7):5–14. [PubMed] [Google Scholar]
- Van Rijn RM, Bartlett SE, Whistler JL. Society for Neuroscience. Washington, D.C; 2008. Delta opioid receptor subtypes display opposing effects on alcohol intake. vol Program No. 315.3. [Google Scholar]
- Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ. An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1055–65. doi: 10.1152/ajpregu.00108.2003. [DOI] [PubMed] [Google Scholar]
- Woolley J, Lee B, Kim B, Fields H. Opposing effects of intra-nucleus accumbens mu and kappa opioid agonists on sensory specific satiety. Neuroscience. 2007;146(4):1445–52. doi: 10.1016/j.neuroscience.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley A. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159(4):415–23. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]


