Abstract
Background and Objectives:
Transanal endoscopic operation (TEO) is a minimally invasive technique used for local excision of benign and selected malignant rectal lesions. The purpose of this study was to investigate the feasibility, safety, and oncological outcomes of the procedure and to report the experience in 3 centers.
Methods:
Retrospective review of a prospectively collected database was performed of all patients with benign lesions or ≤cT1N0 rectal cancer who underwent TEO with curative intent at 3 Belgian centers (2012 through 2014).
Results:
Eighty-three patients underwent 84 TEOs for 89 rectal lesions (37 adenomas, 43 adenocarcinomas, 1 gastrointestinal stromal tumor, 1 lipoma, 2 neuroendocrine tumors, and 5 scar tissues). Operative time was associated with lesion size (P < .001). Postoperative complications occurred in 13 patients: 7 hemorrhages, 1 urinary tract infection, 1 urinary retention, 2 abscesses, 1 anastomotic stenosis, and 1 entrance into the peritoneal cavity. Median hospital stay was 3 days (range, 1–8). During a median follow-up of 13 months (range, 2–27), there was 1 recurrence.
Conclusion:
Although longer follow-up is still necessary, TEO appears to be an effective method of excising benign tumors and low-risk T1 carcinomas of the rectum. However, TEO should be considered as part of the diagnostic work-up. Furthermore, the resected specimen of a TEO procedure allows adequate local staging in contrast to an endoscopic piecemeal excision. Nevertheless, definitive histology must be appreciated, and in case of unfavorable histology, radical salvage resection still has to be performed.
Keywords: Benign rectal lesion, Early rectal cancer, Transanal endoscopic operation
INTRODUCTION
Colorectal cancer is one of the leading causes of cancer-related deaths in the Western world. Total mesorectal excision (TME) remains the gold standard treatment for any stage of rectal cancer, especially in more advanced disease, as it most effectively treats the mesorectal lymph nodes and reduces recurrence.1 However, TME is accompanied by significant morbidity and mortality.2 Therefore, in early rectal cancer, the role of organ-preserving techniques is growing, not only in patients unfit for radical resection.
Transanal endoscopic microsurgery (TEM), which was developed and defined by Prof. G. F. Buess, has been generally accepted for the treatment of early rectal cancers and benign rectal lesions not amendable for colonoscopic excision.3–5 TEM is a 3-dimensional viewing system with a rectoscope and the creation of a pneumorectum, which allows access to the entire rectum.6 Because of its high cost, the learning curve, and the complexity of the equipment, TEM is not commonly used.7
Transanal endoscopic operation (TEO) has evolved from TEM as a new technique. A recently published prospective randomized trial comparing TEM and TEO showed no technical or clinical differences between the 2 systems, except a significantly lower cost.8 Moreover, Nieuwenhuis et al9 showed improved ergonomics with TEO.
The purpose of this study was to investigate the feasibility, safety and oncological outcome of the procedure and to report the experience in 3 Belgian medical centers.
PATIENTS AND METHODS
Patients
A retrospective review of a prospectively collected database of all patients with benign rectal lesions or ≤cT1N0 rectal cancer, who underwent TEO with curative intent, was performed at 3 Belgian centers from January 2012 through December 2014. In case of preoperatively confirmed malignancy, endorectal ultrasonography (endo-US) and magnetic resonance imaging (MRI) of the pelvis were performed to determine tumor depth and nodal stage.
For all patients, the following variables were collected: demographics, medical comorbidities, preoperative diagnosis, location of the lesion (distance from anal verge/direction), details of operative procedure, length of hospital stay, intra- and postoperative complications, final pathology, and oncologic outcome.
Surgical Technique
Before surgery, all patients received a phosphate-based enema. They were given standard antibiotic prophylaxis for colorectal surgery to treat Gram-negative and anaerobic bacteria. In all patients, a urinary catheter was placed. Preoperative rectoscopy was performed to assess the exact location of the tumor and to explore for resectability. Subsequently, the patient was positioned in a position with the tumor downward (lithotomy, lateral position or prone position). Standard laparoscopic instruments were used, such as a grasper, aspirator, dissector, and monopolar cautery hook. Rectal insufflation was set at 20 mm Hg. At the beginning of the operation, the margin of clearance was tattooed with dots using coagulation circumferentially all around the lesion. In large lesions, this procedure was sometimes difficult. The margin then has to be completed further during the resection. Consequently a 5-mm margin in benign lesions—10 mm in cases where a carcinoma was suspected—was attained. Once a good view was obtained, a standard laparoscopic dissector and the monopolar cautery hook were used for tumor excision. For all lesions (benign and malignant) full-thickness resection of the bowel wall was performed down to the level of the perirectal fat. Occasionally a LigaSure device (Covidien, Norwalk, Connecticut, USA) was used for optimal hemostasis. The wound was irrigated with iodine solution. Final hemostasis was performed, and the partial defect was closed with a V-Loc suture (Covidien). In large defects, reduction of the rectal insufflation pressure can help in approximating the borders of the wound. The orientation of the resected specimen was marked for final pathologic examination.
Postoperative Care
The urinary catheter was removed when the patient was awake enough (within 24 hours). An oral diet was initiated the evening of the operation and increased progressively. Postoperative analgesia was given on demand, in the form of paracetamol. Patients were discharged on the condition that they had passed stool. The first outpatient clinic appointment was planned 3 weeks after discharge.
Data Collection and Statistical Analysis
SAS version 9.1.3 (Cary, North Carolina, USA) was used for all statistical analyses. Fisher's exact test was used for statistical analysis of discrete variables and Student's t test was used for continuous variables. Values are expressed as medians. Statistical significance was set at an α = 0.05 for all analyses.
RESULTS
We enrolled 83 patients in the study: 58 men and 25 women (median age, 66 years; range, 39–88). Table 1 shows the patient demographics. Eighty-three patients underwent 84 TEO procedures (1 patient underwent a revision) for a total of 89 rectal lesions (1 patient had 6 adenomas). Median distance from the anal verge was 6 cm (range, 2–15). The median lesion diameter was 35 mm (range, 5–110).
Table 1.
Patient and Lesion Characteristics
| Patient characteristics (n = 83) | ||
|---|---|---|
| Sex (F/M) | 25/58 | |
| Age (years) | Median 67 | 39–88 |
| ASA score | ||
| 1 | 23 | |
| 2 | 49 | |
| 3 | 11 | |
| Lesion characteristics (n = 89) | ||
| Height | ||
| High rectal (>12 cm) | 10 | |
| Mid rectal (12–7 cm) | 29 | |
| Low rectal (≤6 cm) | 50 | |
| Location | ||
| Dorsal | 35 | |
| Ventral | 32 | |
| Lateral left | 14 | |
| Lateral right | 7 | |
| Circular | 1 | |
Data are the number of cases of each characteristic, unless otherwise noted.
Median operative time was 60 minutes (range, 40–180). The operative time was associated with lesion size (P < .0001). All lesions except 1 (an adenoma with high-grade dysplasia) were radically excised.
No perioperative complications occurred. Postoperative complications occurred in 13 patients: 7 hemorrhages, of which 4 were treated conservatively and 3 required reintervention. None of the patients needed a blood transfusion. One patient had a urinary tract infection and 1 had urinary retention. Two patients developed an abscess for which antibiotic therapy was started. One patient who underwent surgery for a circular lipoma developed a stenosis of the anastomosis, which was treated with balloon dilation. The median hospital stay was 3 days (range, 1–8).
In 2 patients, a frozen section was obtained during the procedure because of uncertainty about the T-stage after endorectal-US and MRI (uT1N0, possibly uT2N0). Frozen section confirmed a T2 adenocarcinoma; therefore, in both cases, an additional laparoscopic total mesorectal excision (TME) was performed immediately.
Final pathology revealed 37 tubulovillous adenomas (25 cases of low/mild-grade dysplasia and 12 cases of high-grade dysplasia), 43 adenocarcinomas, 1 gastrointestinal stromal tumor, 2 neuroendocrine tumors, 1 lipoma, and 5 cases with only residual scar tissue after incomplete polypectomy. One patient had 6 tubulovillous adenomas, of which the largest lesion of 35 mm turned out to be a pT1sm2 lesion on final pathology. Details of the pathologic results of cases with clinical and/or pathologic evidence of adenocarcinoma are shown in Table 2.
Table 2.
Pathologic Characteristics of Adenocarcinoma
| Case | Age | Sex | cTstage | Size (cm) | pTstage | Invasion Depth | LVI | PNI | TME |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | M | TA | 1.5 | Tis | Mucosa | 0 | 0 | |
| 2 | 81 | M | TA | 1.8 | Tis | Mucosa | 0 | 0 | |
| 3 | 74 | F | TA | 2.0 | Tis | Mucosa | 0 | 0 | |
| 4 | 80 | F | TA | 2.6 | Tis | Mucosa | 0 | 0 | |
| 5 | 54 | F | TA | 3.0 | Tis | Mucosa | 0 | 0 | |
| 6 | 56 | M | TA | 3.0 | Tis | Mucosa | 0 | 0 | |
| 7 | 72 | F | TA | 3.5 | Tis | Mucosa | 0 | 0 | |
| 8 | 41 | M | TA | 4.0 | Tis | Mucosa | 0 | 0 | |
| 9 | 72 | M | TA | 4.0 | Tis | Mucosa | 0 | 0 | |
| 10 | 39 | F | TA | 8.0 | Tis | Mucosa | 0 | 0 | |
| 11 | 74 | F | TA | 9.0 | Tis | Mucosa | 0 | 0 | |
| 12 | 50 | F | TA | 3.5 | T1 | Sm1 | 1 | 0 | |
| 13* | 70 | M | TA | 2.5 | T1 | Sm2 | 0 | 0 | Refused |
| 14† | 55 | M | TA | 3.8 | T1 | Sm2 | 0 | 0 | T1N1(1/14) |
| 15‡ | 81 | F | TA | 4.0 | T3 | Subserosa | 0 | 0 | Unfit |
| 16 | 65 | M | Tis | 1.0 | TA | TA | 0 | 0 | |
| 17 | 66 | M | Tis | 1.5 | TA | TA | 0 | 0 | |
| 18 | 54 | F | Tis | 2.5 | TA | TA | 0 | 0 | |
| 19§ | 64 | M | Tis | 0 | Scar tissue | - | 0 | 0 | |
| 20§ | 68 | M | Tis | 1.5 | Scar tissue | - | 0 | 0 | |
| 21 | 75 | M | Tis | 1.3 | Tis | Mucosa | 0 | 0 | |
| 22 | 72 | M | Tis | 3.5 | Tis | Mucosa | 0 | 0 | |
| 23 | 41 | F | Tis | 3.5 | Tis | Mucosa | 0 | 0 | |
| 24 | 85 | M | Tis | 4.5 | Tis | Mucosa | 0 | 0 | |
| 25 | 46 | M | Tis | 5.5 | Tis | Mucosa | 0 | 0 | |
| 26 | 68 | F | Tis | 5.5 | Tis | Mucosa | 0 | 0 | |
| 27 | 71 | M | Tis | 6.5 | Tis | Mucosa | 0 | 0 | |
| 28 | 68 | M | Tis | 8.0 | Tis | Mucosa | 0 | 0 | |
| 29 | 49 | M | Tis | 9.0 | Tis | Mucosa | 0 | 0 | |
| 30‖ | 82 | M | Tis | 0.8 | T1 | Sm1 | 0 | 0 | |
| 31† | 61 | M | Tis | 4.0 | T1 | Sm3 | 0 | 0 | T1N0 |
| 32‡ | 65 | F | Tis | 6.0 | T1 | Sm3 | 0 | 0 | Refused |
| 33† | 67 | M | Tis | 6.0 | T1 | Sm3 | 0 | 0 | T1N0 |
| 34†,¶ | 68 | M | Tis/T1? | 8.5 | T2 | Muscularis | 0 | 0 | T2N0 |
| 35§ | 65 | M | T1 | 0.5 | Scar tissue | - | 0 | 0 | |
| 36§ | 65 | M | T1 | 0 | Scar tissue | - | 0 | 0 | |
| 37§ | 52 | F | T1 | 0 | Scar tissue | - | 0 | 0 | |
| 38 | 76 | F | T1 | 6.0 | TA | TA | 0 | 0 | |
| 39‖ | 82 | M | T1 | 0.6 | T1 | Sm1 | 0 | 0 | |
| 40 | 62 | F | T1 | 1.0 | T1 | Sm1 | 0 | 0 | |
| 41 | 53 | F | T1 | 2.2 | T1 | Sm1 | 0 | 0 | |
| 42# | 64 | M | T1? | 3.0 | T1 | Sm1 | 0 | 0 | |
| 43 | 55 | M | T1 | 4.5 | T1 | Sm1 | 0 | 0 | |
| 44‡ | 70 | M | T1 | 0.5 | T1 | Sm2 | 0 | 0 | Refused |
| 45‡ | 64 | M | T1 | 2.5 | T1 | Sm2 | 0 | 0 | Refused |
| 46‡ | 82 | M | T1 | 2.8 | T1 | Sm2 | 0 | 0 | Unfit |
| 47† | 69 | M | T1/T2? | 2.5 | T1 | Sm3 | 0 | 0 | T1N1(1/12) |
| 48† | 64 | M | T1 | 4.0 | T1 | Sm3 | 0 | 0 | T1N0 |
| 49‡ | 88 | M | T1 | 4.0 | T1 | Sm3 | 1 | 0 | Unfit |
| 50† | 76 | M | T1 | 2.5 | T2 | Muscularis | 0 | 0 | T2N1(1/14) |
| 51†,¶ | 72 | F | T1? | 5.0 | T2 | Muscularis | 0 | 0 | T2N2(4/13) |
| 52‡ | 69 | M | T2 | 2.5 | T1 | Sm2 | 0 | 0 | Unfit |
Included are 9 cases with overstaging and 11 cases with understaging. TA, tubulovillous adenoma; Tis, carcinoma in situ.
No Australian Prudential Regulation Authority risk assessment was performed.
Patient underwent salvage TME.
Patient was advised to undergo TME but refused TME or was medically unfit to undergo TME.
Residual scar tissue after endomucosal resection.
Local recurrence after endoscopic polypectomy.
On pre-operative imaging Endo-US/MRI there was uncertainty about T stage T1/T2. Frozen section during the TEO procedure showed T2, therefore a completion laparoscopic TME was performed immediately.
No preoperative biopsy.
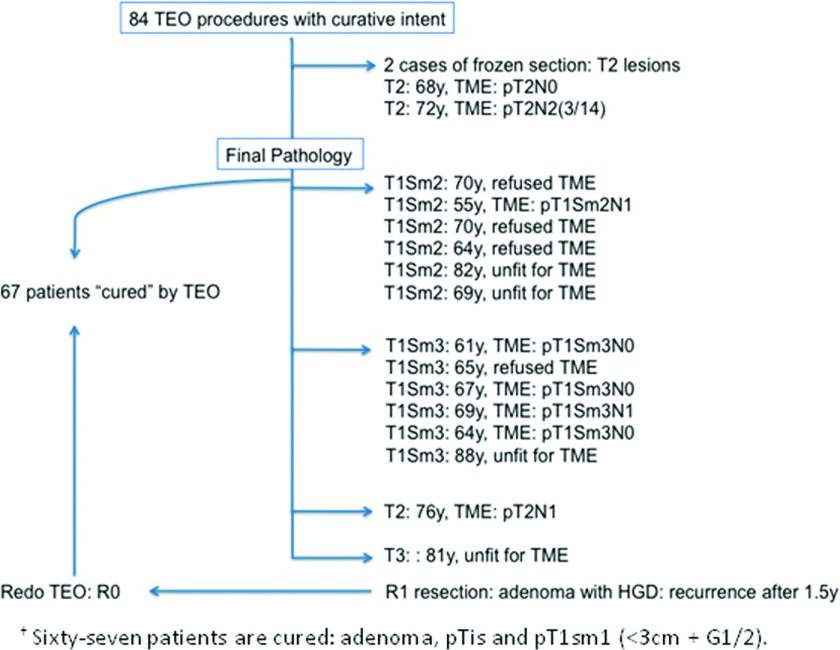
Six patients underwent further radical surgery (laparoscopic TME) 8 wk (range, 7–11) after TEO because of unfavorable final pathology (≥T1sm2 or T2 lesions). Four other patients refused an additional TME and 4 others were unfit for further radical surgery (Figure 1).
Figure 1.
Flowchart showing an overview of the 84 TEO procedures performed with curative intent (83 patients, of which 1 had a second TEO procedure 1.5 years later because of a local recurrence of an adenoma with high-grade dysplasia after a primary R1-resection).
The median follow-up was 13 months (range, 2–27). One patient had recurrence of an adenoma 18 months after the initial TEO procedure; this was the case with the initial R1 resection. The adenoma was excised in a revision TEO procedure.
Sixty-seven patients out of the 83 were cured by TEO. Of all 43 adenocarcinomas that were evaluated before surgery by endo-US and MRI, 20 carcinomas in situ, 19 T1 lesions, 3 T2 lesions, and 1 T3 lesion were diagnosed. Of those, 11 carcinomas in situ, 7 T1 lesions, and 3 T2 lesions were understaged. On the other hand, lesions in 9 patients (4 cases of an adenoma in final pathology and 5 cases of residual scar tissue) were overstaged.
In the first 11 cases, where the carcinoma in situ was initially thought to be an adenoma, there were no therapeutic consequences, and the patients were treated curatively with the TEO procedure. This also applied to the carcinomas in situ and the cT1 lesions that were restaged to pT1sm1. TEO therefore prevents overtreatment and reduces the cost of cure.
Only 4 patients with a T2–3 lesion and 6 patients with a pT1sm3 lesion required further radical surgery. The possibility of a second operation should be discussed with the patients in advance.
Six other patients with a pT1sm2 lesion remained in the gray zone, and additional treatment was proposed. Four patients refused further radical surgery, of whom 1 patient should have undergone an abdominoperineal resection. Four patients were declared unfit for surgery.
DISCUSSION
Almost 3 decades after its introduction by Buess, TEM is having a revival. It has gained a role in the resection of benign and malignant neoplasms of the rectum, and its role in the management of early rectal cancer is increasing. Initially indicated for curative resection of benign tumors, TEM is now also recommended for carcinoid tumors and selective T1 adenocarcinomas.10
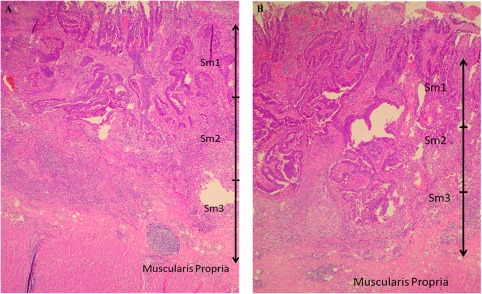
TEM currently is indicated as a curative treatment of malignant neoplasms that are histologically confirmed as pT1sm1 carcinomas.11 T1 sm2–3 (Figure 2A, B) and T2 lesions should at present be included in prospective trials. The management of the T1sm2 tumors without any unfavorable criteria remains a gray zone due to lymph node positivity. In a T1sm1 lesion, 1–3% of the nodes are positive, whereas in T1sm2, 8 to 10% of nodes and in T1sm3 in up to 25% are positive. The findings should therefore be discussed in a patient-by-patient evaluation.12 Furthermore, TEM plays a role in the histopathologic staging in indeterminate cases, and finally in palliative resection in patients medically unfit for radical resection, in symptomatic patients (hemorrhage, tenesmus, or mucous-producing lesion), or in patients who refuse radical treatment.10
Figure 2.
A, Histopathological image of a T1sm2 lesion. Associated lymph node positivity is present in 8–10% of the cases. B, Histopathological image of a T1sm3 lesion. Associated lymph node positivity is present in up to 25% of the cases.
It has been shown that TEO has a lower cost with the use of standard laparoscopic equipment,8 better ergonomics (owing to the camera),9 and a shorter learning curve. Still, more than 90% of the studies report on TEM. There is limited experience with TEO and as a consequence, there also are a limited number of studies.
Consequently, the TEO procedure is being performed in the 3 Belgian centers.
The operation time (median 60 min, range 40–180) is in accordance with the time reported in the literature (median 55 minutes, range 25–165).9 Thirteen postoperative complications occurred, with 3 reinterventions caused by hemorrhage, a well known complication of TEO.13
Six patients underwent a TME because of unfavorable final pathology (Figure 1). Completion of surgery has been demonstrated to be safe and returns oncological outcome to that of primary radical TME surgery. This effect is also seen in series where immediate reoperation is performed.14,15
All lesions except 1 were radically excised. The patient concerned had a recurrence of an adenoma 18 months after the initial TEO procedure. Several factors have been shown to predict recurrence after TEM for adenomas. Positive margin is a strong predictor of recurrence, and large adenomas have a higher rate of recurrence.16 The adenoma was excised by an additional TEO procedure. Repeat TEM is highly successful and safe in treating recurrence.17,18
In 2016, the indication for local excision combined with neoadjuvant therapies is growing. The cost of cure with TME is high, taking into account a longer hospital stay, infectious complications, urinary dysfunction, sexual dysfunction, defecatory dysfunction (low anterior resection syndrome), and sometimes the need of a temporary or even permanent stoma.19–21 An abdominoperineal excision confers a 3.5% longer life expectancy, but this advantage is lost when the quality of life reduction reported by patients with stoma is taken into account. Only a minority of patients would not sacrifice a percentage of life expectancy to avoid a permanent stoma.22
The rate of local recurrence and 5-year survival after local resection of pT1 lesions using TEM is not significantly different from the rates reported for conventional surgery.23 TEM after long-course neoadjuvant radiotherapy may be a valuable equivalent to laparoscopic TME in selected patients.24 It has been established in recent literature that developments in neo-adjuvant chemoradiotherapy techniques decrease rates of local recurrence following surgery for even more advanced rectal cancers.25 Prospective randomized research will dictate how we identify higher risk tumors before surgery and who will benefit from neoadjuvant therapy.
In conclusion, although longer follow-up is still necessary, TEO appears to be an effective method of excising benign tumors and low-risk T1 carcinomas of the rectum. TME remains the gold standard, especially in lesions ≥pT1sm2. However, TEO should be considered part of the diagnostic work-up. Furthermore, the resected specimen of a TEO procedure allows adequate local staging in contrast to an endoscopic piecemeal excision. Nevertheless, definitive histology needs to be appreciated and in cases of unfavorable histology radical salvage resection still has to be performed.
Contributor Information
Mathieu D'Hondt, Department of General and Digestive Surgery, Groeninge Hospital, Kortrijk, Belgium..
Emi Yoshihara, Department of General and Digestive Surgery, Groeninge Hospital, Kortrijk, Belgium..
Lieven Dedrye, Department of General and Digestive Surgery, Jan Yperman Hospital, Ieper, Belgium..
Koen Vindevoghel, Department of General and Digestive Surgery, OLV van Lourdes Hospital, Waregem, Belgium..
Frederiek Nuytens, Department of General and Digestive Surgery, Groeninge Hospital, Kortrijk, Belgium..
Hans Pottel, Interdisciplinary Research Center, Catholic University Leuven, Kortrijk, Belgium..
References:
- 1. MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–460. [DOI] [PubMed] [Google Scholar]
- 2. Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol. 2012;13:e403–e408. [DOI] [PubMed] [Google Scholar]
- 3. Casadeus D. Surgical resection of rectal adenoma: a rapid review. World J Gastroenterol. 2009;15:3844–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suppiah A, Maskelar S, Alabi A, Hartley JE, Monson JR. Transanal endoscopic microsurgery in early rectal cancer: time for a trial? Colorectal Dis. 2008;10:314–327. [DOI] [PubMed] [Google Scholar]
- 5. Bach SP, Hill J, Simson JN, Lane L, Merrie A, Warren B, Mortensen NJ. Association of Coloproctology of Great Britain and Ireland Transanal Endoscopic Microsurgery (TEM) Collaboration. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg. 2009;96:280–290. [DOI] [PubMed] [Google Scholar]
- 6. Bretagnol F, Merrie A, George B, Warren BF, Mortensen NJ. Local excision of rectal tumours by transanal endoscopic microsurgery. Br J Surg. 2007;94:627–633. [DOI] [PubMed] [Google Scholar]
- 7. Maslekar S, Pillinger SH, Sharma A, Taylor A, Monson JR. Cost analysis of transanal endoscopic microsurgery for rectal tumours. Colorectal Dis. 2007;9:229–234. [DOI] [PubMed] [Google Scholar]
- 8. Serra-Aracil X, Mora-Lopez L, Alcantara-Moral M, Caro-Tarrago A, Navarro-Soto S. Transanal endoscopic microsurgery with 3-D (TEM) or high-definition 2-D transanal endoscopic operation (TEO) for rectal tumors: a prospective, randomized clinical trial. Int J Colorectal Dis. 2014;29:605–610. [DOI] [PubMed] [Google Scholar]
- 9. Nieuwenhuis DH, Draaisma WA, Verberne GH, van Overbeeke AJ, Consten EC. Transanal endoscopic operation for rectal lesions using two-dimensional visualization and standard endoscopic instruments: a prospective cohort study and comparison with the literature. Surg Endosc. 2009;23:80–86. [DOI] [PubMed] [Google Scholar]
- 10. Tsai BM, Finne CO, Nordenstam JF, Christoforidis D, Madoff RD, Mellgren A. Transanal endoscopic microsurgery resection of rectal tumors: outcomes and recommendations. Cis Colon Rectum. 2010;21:1870–1874. [DOI] [PubMed] [Google Scholar]
- 11. Morino M, Arezzo A, Allaix ME. Transanal endoscopic microsurgery. Tech Coloprotol. 2013;1:S55–861. [DOI] [PubMed] [Google Scholar]
- 12. Lartigau C, Lebreton G, Alves A. Local resection for small rectal cancer. J Visc Surg. 2013;150:325–331. [DOI] [PubMed] [Google Scholar]
- 13. Arezzo A, Passera R, Saito Y, et al. Systematic review and meta-analysis of endoscopic submucosal dissection versus transanal endoscopic microsurgery for large noninvasive rectal lesions. Surg Endosc. 2014;28:427–438. [DOI] [PubMed] [Google Scholar]
- 14. Borschitz T, Heintz A, Junginger T. The influence of histopathologic criteria on the long-term prognosis of locally excised pT1 rectal carcinomas: results of local excision (transanal endoscopic microsurgery) and immediate reoperation. Dis Colon Rectum. 2006;49:1492–1506. [DOI] [PubMed] [Google Scholar]
- 15. Wu ZY, Zhao G, Chen Z, Du JL, Wan J, Lin F, Peng L. Oncological outcomes of transanal local excision for high risk T(1) rectal cancers. World J Gastrointest Oncol. 2012;4:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allaix ME, Arezzo A, Cassoni P, Famiglietti F, Morino M. Recurrence after transanal endoscopic microsurgery for large rectal adenomas. Surg Endosc. 2012;26:2594–2600. [DOI] [PubMed] [Google Scholar]
- 17. Khoury W, Gilshtein H, Nordkin D, Kluger Y, Duek SD. Repeated transanal endoscopic microsurgery is feasible and safe. J Laparoendosc Adv Surg Tech. 2013;23:216–219. [DOI] [PubMed] [Google Scholar]
- 18. Smart CJ, Cunningham C, Bach SP. Transanal endoscopic microsurgery. Best Pract Res Clin Gastroenterl. 2014;28:143–157. [DOI] [PubMed] [Google Scholar]
- 19. Hendren SK, O'Connor BI, Liu M, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg. 2005;242:212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Temple LK, Bacik J, Savatta SG, et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum. 2005;48:1353–1365. [DOI] [PubMed] [Google Scholar]
- 21. Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255:922–928. [DOI] [PubMed] [Google Scholar]
- 22. Johnston CF, Tomlinson G, Temple LK, Baxter NN. The management of patients with T1 adenocarcinoma of the low rectum: a decision analysis. Dis Colon Rectum. 2013;56:400–407. [DOI] [PubMed] [Google Scholar]
- 23. Winde G, Nottberg H, Keller R, Schmid KW, Bünte H. Surgical cure for early rectal carcinomas (T1): transanal endoscopic microsurgery vs. anterior resection. Dis Colon Rectum. 1996;39:969–976. [DOI] [PubMed] [Google Scholar]
- 24. Lezoche E, Baldarelli M, Lezoche G, Paganini AM, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg. 2012;99:1211–1218. [DOI] [PubMed] [Google Scholar]
- 25. Guerrieri M, Gesuita R, Ghiselli R, Lezoche G, Budassi A, Baldarelli M. Treatment of rectal cancer by transanal endoscopic microsurgery: experience with 425 patients. World J Gastroenterol. 2012;28:20:9556–9563. [DOI] [PMC free article] [PubMed] [Google Scholar]