Abstract
Background
Recent reports support the involvement of hypothalamic orexigenic peptides in stimulating ethanol intake. Our previous studies have examined the effects of ethanol on hypothalamic peptide systems of the paraventricular nucleus of the hypothalamus (PVN) and identified a positive feedback loop in which PVN peptides, such as enkephalin and galanin, stimulate ethanol intake and ethanol, in turn, stimulates the expression of these peptides. Recently, orexin (OX), a peptide produced mainly by cells in the perifornical lateral hypothalamus (PFLH), has been shown to play an important role in mediating the rewarding aspects of ethanol intake. However, there is little evidence showing the effects that ethanol itself may have on the OX peptide system. In order to understand the feedback relationship between ethanol and the OX system, the current investigation was designed to measure OX gene expression in the PFLH following acute as well as chronic ethanol intake.
Methods
In the first experiment, Sprague-Dawley rats were trained to voluntarily consume a 2% or 9% concentration of ethanol, and the expression of OX mRNA in the PFLH was measured using quantitative real-time polymerase chain reaction (qRT-PCR). The second set of experiments tested the impact of acute oral gavage of 0.75 and 2.5 g/kg ethanol solution on OX expression in the PFLH using qRT-PCR, as well as radiolabeled in situ hybridization. Further tests using digoxigenin-labeled in situ hybridization and immunofluorescence histochemistry allowed us to more clearly distinguish the effects of acute ethanol on OX cells in the lateral hypothalamic (LH) vs perifornical (PF) regions.
Results
The results showed chronic consumption of ethanol vs water to dose-dependently reduce OX mRNA in the PFLH, with a larger effect observed in rats consuming 2.5 g/kg/day (-70%) or 1.0 g/kg/day (-50%) compared to animals consuming 0.75 g/kg/day (-40%). In contrast to chronic intake, acute oral ethanol compared to water significantly enhanced OX expression in the PFLH, and this effect occurred at the lower (0.75 g/kg) but not higher (2.5 g/kg) dose of ethanol. Additional analyses of the OX cells in the LH vs PF regions identified the former as the primary site of ethanol's stimulatory effect on the OX system. In the LH but not the PF, acute ethanol increased the density of OX-expressing and OX-immunoreactive neurons. The increase in gene expression was detected only at the lower dose of ethanol (0.75 g/kg), whereas the increase in OX peptide was seen only at the higher dose of ethanol (2.5 g/kg).
Conclusion
These results lead us to propose that OX neurons, while responsive to negative feedback signals from chronic ethanol consumption, are stimulated by acute ethanol administration, most potently in the LH where OX may trigger central reward mechanisms that promote further ethanol consumption.
Keywords: Orexin, Lateral hypothalamus, Perifornical hypothalamus, Ethanol, Sprague-Dawley Rat
Introduction
Worldwide, alcohol use accounts for 4% of global disease burden and is related to approximately 1.8 million deaths each year (WHO, 2009). Alcoholism, also known as alcohol dependence, is a chronic debilitating disorder characterized by excessive ingestion of alcohol, the development of tolerance and withdrawal, and impairment in social and occupational functioning (APA, 2000; Becker, 2008). Although physiological systems involved in and affected by alcoholism have been extensively studied, the neurobiological mechanisms that contribute to ethanol intake and abuse are not well understood.
The lateral hypothalamus has been suggested to play an important role in reward-related behaviors for over fifty years now. Early studies of intracranial self-stimulation (ICSS) determined that animals would repeatedly press a lever associated with an electrical stimulation of the lateral hypothalamus, suggesting that this region is involved in reinforcing behavior (Olds, 1958; Olds and Milner, 1954). In addition, systemic administration of a low to moderate dose of ethanol, similar to cocaine, reduces the rate of responding for ICSS of the lateral hypothalamus, suggesting that the rewarding effects of ethanol and cocaine may be mediated directly by this brain region (Fish et al., 2010; Schaefer and Michael, 1987). Although we now understand that the lateral hypothalamus is intimately involved in reward, a precise understanding of the local systems mediating such positive reinforcement has only surfaced in the last decade. In 1989, two independent research groups isolated and cloned the peptide, hypocretin, also known as orexin (OX), which is produced mainly by neurons located in the perifornical lateral hypothalamus (PFLH) (de Lecea et al., 1998; Sakurai et al., 1998a). Originally, OX was shown to play a role in feeding behavior; however, subsequent studies have revealed an important role for this peptide in waking, arousal, and later in drug addiction (Chemelli et al., 1999; Georgescu et al., 2003; Hara et al., 2001; Lin et al., 1999; Sakurai et al., 1998b). Investigations examining the involvement of OX pathways in reward-seeking behavior have distinguished OX neurons in the perifornical (PF) and lateral hypothalamic (LH) regions and found the former to be involved in sleep and arousal and the latter to be more sensitive to rewarding substances, such as morphine, cocaine and food (DiLeone et al., 2003; Estabrooke et al., 2001; Georgescu et al., 2003; Harris et al., 2005).
Recent studies suggest a role for the OX peptide system in ethanol-seeking and drinking behaviors. For example, central injection of OX in the LH stimulates ethanol consumption (Schneider et al., 2007), and ethanol-paired stimuli increase the number of Fos-positive, OX neurons in the PFLH region (Dayas et al., 2008). OX projections from the PFLH directly innervate and stimulate the dopamine (DA) reward pathway, depolarizing DA neurons in the ventral tegmental area (VTA) and increasing DA release in terminal regions (Li and van den Pol, 2005; Narita et al., 2006; Vittoz and Berridge, 2006). Moreover, systemic administration of an OX-R1 antagonist alleviates reinstatement and operant responding for ethanol in ethanol-preferring rats (Harris et al., 1978; Lawrence et al., 2006), and injection of OX peptide directly into the VTA reinstates drug-seeking behavior (Wang et al., 2009). These and other studies suggest that the OX system, originating in the PFLH, is actively involved in the rewarding aspect of ethanol, as well as other drugs of abuse (Borgland et al., 2006; Boutrel et al., 2005).
In order to understand the role of OX neurons in controlling ethanol intake, it is important to examine the impact that ethanol itself has on the expression and production of this peptide in the PFLH, where OX neurons originate. Whereas little is known about the relationship of ethanol to endogenous OX, recent studies of other orexigenic peptides in the hypothalamus have shown ethanol to have a strong, stimulatory effect on their expression. These peptides include galanin (GAL) and the opioid, enkephalin (ENK), which similar to OX increase ethanol intake when injected into the paraventricular nucleus (PVN) of the hypothalamus (Lewis et al., 2004; Rada et al., 2004; Schneider et al., 2007). Studies measuring these endogenous peptides in the hypothalamus have shown both acute and chronic ethanol intake to increase their peptide expression and levels specifically in the PVN (Chang et al., 2007; Leibowitz et al., 2003). Together, these reports of injections and measurements of GAL and ENK have led to the proposal that these peptides function within a positive feedback circuit to stimulate the consumption of ethanol that, in turn, activates the endogenous peptide systems to promote further ethanol intake (Leibowitz, 2007). The question to be addressed in the present study is whether OX is similar to GAL and ENK peptides in terms of its response to acute as well as chronic manipulations with ethanol. The first experiment examined the impact of chronic ethanol consumption on OX expression in the PFLH, while the next two experiments explored the effect of acute oral administration of ethanol. The final experiment performed an anatomically more precise analysis of ethanol's effects on OX-expressing neurons in the LH vs PF regions of the posterior hypothalamus. The results obtained from these experiments revealed clear differences between the effects of acute vs chronic ethanol on OX neurons in the PFLH and also distinguished the LH and PF areas in terms of their responsiveness to ethanol.
Materials and Methods
Subjects
Adult, male Sprague-Dawley rats (Charles River Breeding Labs, Kingston, NY) were housed individually, on a 12-h reversed light/dark cycle in a fully accredited American Association for the Accreditation of Laboratory Animal Care facility, according to institutionally approved protocols as specified in the NIH Guide to the Use and Care of Animals and also with the approval of the Rockefeller University Animal Care Committee. The rats in each set of water- and ethanol-drinking groups were approximately matched for body weight, with an overall range of 300-350 g at the start of the experiment and 400-475 g at the end (for Experiment 1). All animals were allowed 1 week to acclimate to their individual housing conditions, during which time they were given ad libitum access to standard rodent chow (LabDiet Rodent Chow 5001, St. Louis, MO; 12% fat, 60% carbohydrate, and 28% protein) and water.
Test Procedures
In Experiment 1, rats were given ad libitum access to lab chow, water and in some cases ethanol over a 28-day period. The water-drinking control rats (n=5-10/experiment) were maintained on chow and water, and the ethanol-drinking experimental groups (n=30/experiment) were additionally given access to ethanol (95% ethanol, David Sherman Corp., St. Louis, MO) diluted with water. This was presented in the home cage in a 100-mL graduated glass cylinder, which was fitted with a sipper tube containing a steel ball as a tip valve to prevent spillage. In order to increase the amount of daily ethanol consumed, access to the ethanol-containing cylinder over the 28 days was provided for 12 h rather than 24 h each day, as described in our previous publications (Chang et al., 2007; Leibowitz et al., 2003). In one subset of animals (n=15), the concentration of ethanol was increased stepwise, every 4 days, from 1% to 2%, 4%, 7% and then 9% v/v, with the rats maintained for an additional 8 days on 9% ethanol. In a second subset of animals (n=15), ethanol was provided as a 1% solution for the first four days and then kept at 2% for the remainder of the experiment. Animals consuming the 2% and 9% ethanol solution were further subdivided based on low and high daily ethanol consumption, with the 2% low drinkers consuming an average of 0.25 g/kg/day and the high drinkers consuming 0.75 g/kg/day and the 9% low drinkers consuming 1.0 g/kg/day and the high drinkers consuming 2.5 g/kg/day. Body weight and daily food intake were measured every 4 days and showed no significant differences between groups. Following the 28 days of ethanol exposure, animals showed no physical or affective disturbances, no motor abnormalities, convulsions or autonomic disturbances, in the absence of ethanol, indicating that they were not dependent. On the final day of ethanol drinking, chow was provided for 4 h after dark onset, to allow the animals to be satiated. The food was then removed to minimize its effect on ethanol consumption or absorption, and the rats were given water alone (control group) or ethanol plus water and allowed to drink ad libitum for the next 2 h. With this intermittent access schedule, the rats consume a large percentage (25-30%) of their daily ethanol intake during the first few hours of exposure. While this leads to peak BEC values within the 2 h consumption period, these values do not rise to the level that might occur with 12 h of consumption. After being allowed to drink for 2 h, the water- and ethanol-drinking rats were sacrificed by rapid decapitation, their brains were removed and examined for peptide gene expression using quantitative real-time polymerase chain reaction (qRT-PCR), and trunk blood was collected for BEC measurements.
In Experiment 2, three additional groups of rats (n=5/group) were used to determine the effects of acute ethanol administration on OX expression in the PFLH. Oral gavage was used to administer ethanol in order to minimize the effects of stress on peptide expression. Rats were separated into three groups of equal body weights the day before the experiment and were given two days of mock gavages prior to the test day. Animals received a single gavage of water (n=5), 0.75 g/kg ethanol (n=5), or 2.5 g/kg ethanol (n=5) of a 30% (v/v) ethanol solution. Tail vein blood was collected 15 minutes following oral gavage in order to measure blood ethanol concentration (BEC). Animals were sacrificed 2 h after gavage procedure by rapid decapitation. Brains were processed according to experimental procedures described below for qRT-PCR and trunk blood collected for further analysis.
In Experiment 3, a new set of animals was used to confirm the qRT-PCR results obtained in Experiment 2. Briefly, animals received an acute dose of ethanol or water, as described in more detail in Experiment 2. They were sacrificed 2 h later, and their brains were processed for analysis using radiolabeled in situ hybridization (ISH), as described below.
In Experiment 4, three additional groups of animals were examined for a more precise analysis of the OX-expressing cells in the PF and LH. The three groups received a single gavage of water (n=5), 0.75 g/kg ethanol (n=5), or 2.5 g/kg ethanol (n=5) of a 30% (v/v) ethanol solution, and they were sacrificed 2 h later. Their brains were examined using ISH with a digoxigenin-labeled probe, to measure the density of OX-expressing cells in the PF and LH, while immunoflourescence histochemistry in adjacent sections was used to measure OX peptide levels in these regions.
Blood Ethanol Concentration
In Experiment 1, trunk blood at the time of sacrifice was used for BEC measurements. In Experiment 2, BEC values were measured from tail vein blood collected 15 minutes following ethanol gavage and trunk blood collected during the sacrifice procedure 2 h after gavage. BEC measurements were made using the Analox GM7 Fast Enzymatic Metabolic Analyser (Lunenburg, MA) and reported as mg/dl.
Brain Dissections
Immediately after sacrifice, the brains for Experiment 1 and 2 were removed for peptide measurements using qRT-PCR. Brains were placed, with the ventral surface facing up in a matrix and three 1.0 mm coronal sections were made, with the middle optic chiasm as the anterior boundary (Paxinos and Watson, 1986). For microdissection, the sections were placed on a glass slide and the PFLH (Bregma - 2.8 to - 3.6 mm) was removed under a microscope, using the fornix and third ventricle as landmarks. The PFLH was taken from the area surrounding the fornix, within a range of 0.2 mm medial and ventral to the fornix, 0.3 mm dorsal and 0.4 mm lateral. These dissections were stored in RNA later (Sigma-Aldrich Co., St. Louis, MO) until processed.
Quantitative Real-time PCR Analysis
In Experiments 1 and 2, qRT-PCR was used to measure OX mRNA levels in the PFLH. As previously described (Chang et al., 2004), total RNA from pooled microdissected hypothalamic samples (n=10) was extracted with Trizol reagent and treated with RNase-free DNase 1. The brain regions were pooled in order to maximize the amount and purity of extracted RNA from brain regions of interest. The cDNA and minus RT were synthesized using an oligo-dT primer with or without SuperScript II reverse transcriptase. The qRT-PCR experiments were conducted with Applied Biosystems (ABI) system. With Applied Biosystems Primer Express V1.5a software, primers were designed to have a melting temperature of 58-60°C and to produce an amplicon of 50-160 base pairs. The last five bases on the 3′ end contained no more than 2 G and/or C bases, to reduce the possibility of nonspecific product formation.
The SYBR Green PCR core reagents kit (ABI, CA) was used with cyclophilin (cyc) as an endogenous control. PCR was performed in MicroAmp Optic 96-well Reaction Plates (ABI) on an ABI PRISM 7900 Sequence Detection system, with the condition of 2 min at 50°C, 10 min at 95°C, then 40 cycles of 15 sec at 95°C and 1 min at 60°C. Each study consisted of 4 independent runs of PCR in triplicate, and each run included a standard curve, non-template control, and negative RT control. The levels of target gene expression were quantified relative to the level of cyc by standard curve method, based on threshold with Ct value of 18-25 for the different genes. For our initial OX expression experiments, we used cyc, β- actin and GAPDH as controls. Since cyc gave the most reliable data with no region or treatment specific changes in quantity, we continued to use cyc to normalize our data for OX expression. The primers, designed with ABI Primer Express V.1.5a software based on published sequences, were: 1) cyc: 5′- GTGTTCTTCGACATCACGGCT -3′ (forward) and 5′- CTGTCTTTGGAACTTTGTCTGCA -3′ (reverse); and 2) OX: 5′-AGATACCATCTCTCCGGATTGC -3′ (forward) and 5′-CCAGGGAACCTT TGT AGAAGGA-3′ (reverse). The concentrations of primers were 100 to 200 nM, and all reagents, unless indicated, were from Invitrogen (Carlsbad, CA). The specificities of RT-PCR products were confirmed by both a single dissociation curve of the product and a single band with a corresponding molecular weight revealed by an agarose gel electrophoresis. In addition to the non-template control and a negative RT control, the specificity of the quantitative PCR was verified with an anatomical negative control by using the corpus callossum in the same brain. No signals above threshold of all 7 targeted genes were detected by qRT-PCR in all of the controls.
Radiolabeled In Situ Hybridization Histochemistry
In Experiment 3, the mRNA levels of OX were also measured by radiolabeled-ISH histochemistry in animals treated with water or an acute gavage of 0.75 g/kg ethanol or 2.5 g/kg ethanol (n=8/group). The animals were sacrificed by rapid decapitation, and the brains were immediately removed and fixed in 4% paraformaldehyde PB (0.1M pH 7.2) for 48-72 h, cryoprotected in 25% sucrose for 48-72 h, and then frozen and stored at -80°C. The antisense and sense RNA probes were donated by Dr. Luis de Lecea and labeled with 35S-UTP (Perkin Elmer, Waltham, MA) as described (Wortley et al., 2003). Free-floating 30 μm coronal sections were processed as follows: 10 min in 0.001% proteinase K, 5 min in 4% paraformaldehyde, and 10 min each in 0.2 N HCl and acetylation solution, with 10 min wash in PB between each step. After washing, the sections were hybridized with 35S-labeled probe (103 cpm/ ml) at 55°C for 18 h. Following hybridization, the sections were washed in 4 × SSC, and nonspecifically bound probe was removed by RNase (Sigma, St. Louis, MO) treatment for 30 min at 37°C. Then, sections were run through a series of stringency washes with 0.1 M dithiothreicitol (Sigma, St. Louis, MO) in 2 × SSC and 1 × SSC and 0.1 × SSC at 55°C. Finally, sections were mounted, air-dried and exposed to Kodak BioMax MR film for 8-18 h at -80°C, developed and microscopically analyzed. The sense probe control was performed in the same tissue, and no signal was found.
Gene expression level was determined with a computer-assisted microdensitometry of autoradiographic images on the MCID image analysis system (Image Research, Inc., St. Catherines, Canada) as described (Lucas et al., 1998; Reagan et al., 2004). Microscale 14C standards (Amersham Biosciences, Piscataway, NJ) were exposed on the same Kodak film with the sections and digitized. Gray level/optical density calibrations were performed by using a calibrated film strip ladder (Imaging Research, St. Catherines, ON, Canada) for optical density. Optical density was plotted as a function of microscale calibration values. It was determined that all subsequent optical density values of digitized autoradiographic images fell within the linear range of the function. The values obtained represent the average of measurements taken from 10-12 sections per animal. In each section, the optical density for the PFLH was recorded, from which the background optical density from a same size area in the thalamus was subtracted. The mean value of the 0.75 g/kg ethanol and 2.5 g/kg ethanol groups in each experiment was reported as percentage of the water group.
Digoxigenin-labeled In Situ Hybridization Histochemistry
As previously described (Chang et al., 2008), digoxigenin-labeled antisense RNA probes and 30-lm free-floating cryostat sections were used for ISH histochemistry. AP-conjugated sheep anti-digoxigenin Fab fragments (1:1000, Roche, Nutley, NJ) and NBT / BCIP (Roche, Nutley, NJ) were used to visualize the signal. Gene expression level was measured by semi-quantification with Image-Pro Plus software, version 4.5 (Media Cybernetics, Inc., Silver Spring, MD, USA) and was expressed as cells /mm2, reflecting density of mRNA containing cells. Densitometry was performed adjacent, anatomically matched sections. In all analyses, the cell number was counted only on one plane in each section, and only those cells containing a nucleus in the plane (> 10 lm2) were counted, thereby excluding fractions of cells. All OX cells lateral to the fornix were considered to be in the LH, and all OX neurons located dorsal and 0.4 mm medial to the fornix were considered to be in the PF. The average cell density in each region for the different groups was compared and statistically analyzed, with the analyses being performed by an observer who was blind to the identity of the rats.
Immunofluorescence Histochemistry
Adjacent sections from Experiment 4 were used to measure OX peptide immunoreactivity via immunofluorescence histochemistry, specifically the more lateral LH area and more medial PF area, as previously described (Chang et al., 2008). Briefly, 30 μm free-floating sections were used for immunofluorescence histochemistry. First, sections were blocked in 1% normal donkey serum containing 0.02% triton X-100 PBS for 1 h, then incubated in primary antiserum overnight (anti-OX IgG 1:200, Santa Cruz, CA). The primary antibody used, orexin-A (C-19), is an affinity purified goat polyclonal antibody raised against a peptide mapping at the C-terminus of orexin-A of human origin. This antibody is recommended for detection of orexin-A processed active peptide of rat by immunoflourescence. After a 30-min rinse in PBS, the sections were incubated in FITC-conjugated Donkey anti-goat IgG (1:100, Jackson ImmunoRes PA) for 2 h. After a 10-min rinse in PBS, the sections were mounted and coverslipped with vectashield mounting medium (Vector, CA). Immunofluorescence image was captured with a Zeiss fluorescence microscope with Met Vue software. Density of immunofluorescence objects was quantified with ImagePro software as described (Chang et al., 2008) and reported as density (objects/ μm2). The LH and PF regions were defined by parameters described in the previous section.
Data Analysis
The values in the figures are expressed as mean ± SEM. Statistical analyses of these data were performed using a one-way analysis of variance (ANOVA) followed by post-hoc tests (Bonferroni or Fisher for the peptides and Holm-Sidak for blood ethanol) for multiple comparisons between groups, or using an unpaired t-test where appropriate. Two-way repeated measure ANOVA (RM-ANOVA) followed by post-hoc Holm-Sidak test was used to compare acute blood ethanol measurements taken at two different time points. The different within-group measures were related using a Pearson's product-moment correlation. The probability values given in the text or legends to the figures and tables reflect the results of these tests.
Results
Experiment 1: Effect of Chronic Ethanol Intake on OX Expression in the PFLH Using qRT-PCR
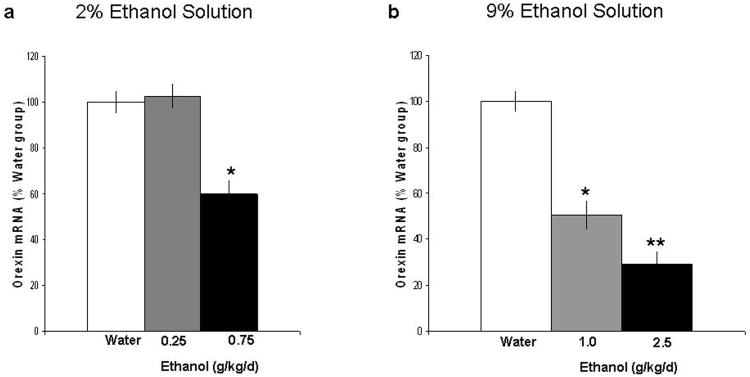
This experiment compared rats given only water to those on 2% ethanol, which consumed an average of 0.25 g/kg/day or 0.75 g/kg/day over 28 days, or those on 9% ethanol, which consumed an average of 1 g/kg/day or 2.5 g/kg/day. As depicted in Fig. 1a, chronic consumption of 2% ethanol produced an unexpected result, a strong reduction in OX expression in the PFLH [F(2,15) = 30.8, p<0.001]. Whereas the group consuming 0.25 g/kg/day showed no difference compared to the water control group, the rats drinking the higher daily amount of ethanol (0.75 g/kg/day) exhibited significantly lower (-40%) levels of OX mRNA expression. As shown in Fig. 1b, chronic consumption of 9% ethanol solution caused a similar reduction in OX mRNA expression in the PFLH [F(2,15) = 41.5, p<0.001]. This effect was dose related; the 2.5 g/kg/day concentration produced a significantly greater reduction (-70%) than that seen with the 1.0 g/kg/day concentration (-50%) (p<0.001), which was slightly greater than the effect seen in the 0.75 g/kg/day rats on 2% ethanol. Measurements of circulating ethanol levels at the time of sacrifice (Table 1) indicated a significant rise in BEC values in animals consuming 0.75 g/kg/day on the 2% ethanol solution [F(2,15) = 25.7, p<0.001] but not those consuming 0.25 g/kg/day. In the animals consuming the 9% ethanol solution, BEC values were dose-dependently increased [F(2,15) = 39.6, p<0.001], with the rats consuming 1.0 g/kg/day or 2.5 g/kg/day ethanol showing significantly greater BEC values compared to the water-drinking rats (p<0.001). These results demonstrate that chronic, voluntary ethanol consumption in Sprague-Dawley rats leads to a dose-dependant reduction in OX mRNA expression in the PFLH. This change occurs only when BEC values are elevated, and it is unlikely to be due to any difference in total calories consumed, as the ethanol-drinking rats showed no difference in body weight compared to the water-drinking rats, and the ethanol they consumed accounted for only a small percentage (<8%) of their daily intake.
Figure 1.
Effects of chronic ethanol intake on the expression of OX in the PFLH, as measured by qRT-PCR (Experiment 1). In the 2% ethanol drinking group (a) three groups of rats (n=5/group), water, 0.25 g/kg/day and 0.75 g/kg/day ethanol drinking animals were examined. The data (mean ± SEM) revealed a significant reduction in expression of OX in the 0.75 g/kg/day ethanol group [F(2,15) =30.8, p<0.001]. In the 9% ethanol drinking group (b) three groups of rats (n=5/group), water, 1.0 g/kg/day and 0.75 g/kg/day ethanol drinking animals were examined. The data (mean ± SEM) revealed a significant reduction in expression of OX in the 1.0 g/kg/day and 2.5 g/kg/day ethanol groups [F(2,15) = 41.5, p<0.001]. This effect was statistically significant in the 0.75 g/kg, 1.0 g/kg and 2.5 g/kg ethanol drinking groups compared to their respective water group (*p<0.001), and the suppression in the 2.5 g/kg/day ethanol drinkers was significantly greater than that in the 1.0 g/kg ethanol drinkers (** p<0.001).
Table 1. Blood ethanol concentration (BEC) following ethanol intake.
| BEC (mg/dl) | ||
|---|---|---|
|
| ||
| Chronic | 120 min | |
|
|
||
| 2% Ethanol Solution | ||
| Water | 9.52 ± 1.23 | |
| 0.25 g/kg/d ethanol | 13.02 ± 2.45 | |
| 0.75 g/kg/d ethanol | 25.05 ±2.12* | |
| 9% Ethanol Solution | ||
| Water | 8.73 ± 1.57 | |
| 1.0 g/kg/d ethanol | 29.32 ± 2.37* | |
| 2.5 g/kg/d ethanol | 38.45 ± 3.48* | |
| BEC (mg/dl) | ||
|
| ||
| Acute | 15 min | 120 min |
|
|
||
| Water | 8.50 ± 2.29 | 9.30 ± 1.37 |
| 0.75 g/kg ethanol | 32.90 ± 3.34* | 11.60 ± 1.20 |
| 2.5 g/kg ethanol | 150.03 ±12.67* | 90.00 ±5.91** |
Compared to water drinking animals, rats chronically consuming 0.75 g/kg/d, 1.0 g/kg/d and 2.5 g/kg/d average daily ethanol had elevated BEC values (*p<0.001). In animals given an acute dose of ethanol versus water, we measured a significant increase in BEC values 15 minutes following gavage of 0.75 and 2.5 g/kg ethanol (*p<0.001) and a significant increase in BEC values 120 minutes following gavage of 2.5 g/kg ethanol (**p<0.001).
Experiment 2: Effect of Acute Ethanol on OX Expression in the PFLH Using qRT-PCR
This experiment tested the effects of acute ethanol administration at different doses on OX expression in the PFLH. The rats received a single gavage of either water or ethanol, at 0.75 g/kg or 2.5 g/kg, and they were examined 2 h later. In contrast to chronic ethanol intake, acute oral administration of ethanol was found to stimulate OX expression compared to the water control group [F(2,17) = 11.6, p<0.001] (Fig. 2). This effect was detected at the lower 0.75 g/kg dose, which produced a 60% increase in OX mRNA in the PFLH (p<0.001), but not at the higher 2.5 g/kg dose, which produced no change in OX mRNA. This dose relationship may be attributed to differences detected in the measurements of BEC at different times after the gavage (Table 1). A significant main effect in BEC values [F(1,18) = 45.31, p<0.001] reflected a moderate and transient rise (4-fold) in the 0.75 g/kg group that was evident only at the 15-min time point, in contrast to a much larger and sustained rise in the 2.5 g/kg group apparent at both the 15-min (20-fold) and 120-min (10-fold) measurement periods. These results suggest that the stimulatory effect of acute ethanol on OX expression in the PFLH is evident at lower doses of ethanol (0.75 g/kg), when BEC values range from 25-35 mg/dl, but not when ethanol intake and BEC values rise to higher levels.
Figure 2.
Effects of acute ethanol gavage on the expression of OX in the PFLH, as measured by qRT-PCR (Experiment 2). Three groups of rats (n=5/group), water, 0.75 g/kg and 2.5 g/kg ethanol, were tested 2 h after gavage. The data (mean ± SEM) showed a significant increase [F(2,17) =11.6, p<0.001] in OX expression after low-dose (0.75 g/kg) of ethanol vs water gavage (*p<0.001).
Experiment 3: Effect of Acute Ethanol on OX Expression in the PFLH Using Radiolabeled ISH
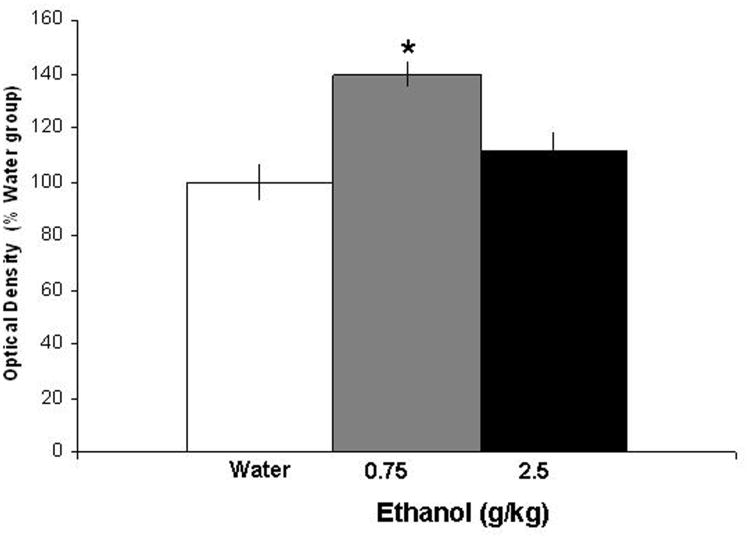
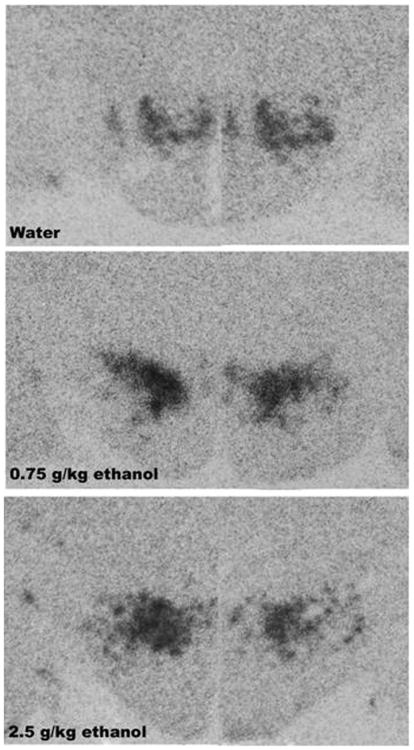
This experiment with oral administration of ethanol used radiolabeled ISH to provide a further test of the stimulatory effect of acute ethanol on OX in the PFLH. Examination of the brains showed that the distribution of the radioactive probe was contained within the medial and lateral hypothalamus and that the dose-related increase in OX expression observed using qRT-PCR in Experiment 2 was confirmed. As shown in Fig. 3 and illustrated in the photomicrographs of Fig. 4, oral administration of 0.75 g/kg ethanol compared to water produced a significant, 40% increase in mRNA expression in the PFLH [F(2,15) = 4.56, p<0.05]. As with qRT-PCR, OX mRNA was unaffected at the higher 2.5 g/kg dose. These results with acute manipulations confirm the responsiveness of OX neurons to the lower dose of ethanol and the lack of this response at the higher dose.
Figure 3.
Effects of acute ethanol gavage on the expression of OX in the PFLH, as measured by radiolabeled ISH (Experiment 3). The data (mean ± SEM) for the 3 groups (n=5/group), water, 0.75 g/kg ethanol and 2.5 g/kg ethanol are presented as % of water control. They showed a significant increase in expression of OX [F(2,15) =4.56, p<0.05] with the low dose of ethanol (0.75 g/kg) as compared with the water group (*p<0.05).
Figure 4.
Photomicrographs illustrating the stimulatory effect of 0.75 g/kg ethanol on OX mRNA expression in the PFLH, as measured using radiolabeled ISH and graphed in Fig. 3 (Experiment 3).
Experiment 4: Effect of Acute Ethanol on OX Expression and Peptide Levels in the PF vs LH Regions
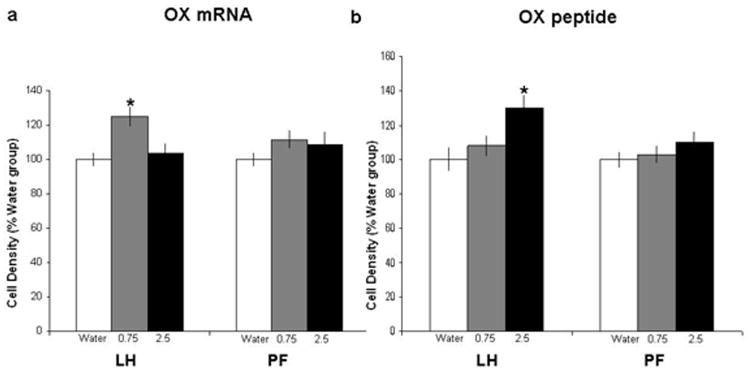
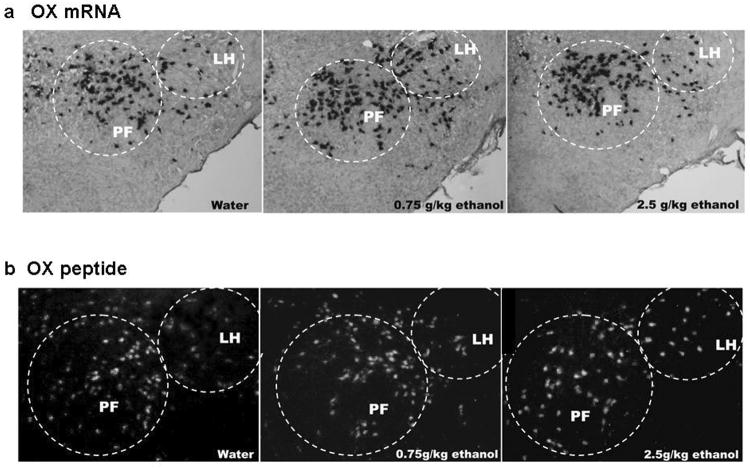
In order to better visualize and anatomically differentiate OX cells in the lateral LH vs more medial PF areas, digoxigenin-labeled ISH and immunofluorescence histochemistry were performed in adjacent brain sections in an additional set of animals given acute gavage of ethanol, as performed in Experiments 2 and 3. When compared to the water group, the 0.75 g/kg oral dose of ethanol produced a significant increase in the density of OX-expressing cells specifically in the LH [F(2,15) = 3.87, p<0.05] but not the PF [F(2,15) = 0.34, n.s.] (Fig. 5a), as illustrated in the photomicrographs (Fig. 6a). Consistent with results of Experiments 2 and 3, this effect of acute ethanol on gene expression was not observed at the higher, 2.5 g/kg dose of ethanol, in either the LH or PF. Measurements of peptide immunoreactivity also showed a stimulatory effect of acute ethanol that was anatomically specific but revealed a different dose relationship. The density of OX-immunoreactive cells was significantly increased in the LH [F(2,15) = 4.21 p<0.05] but not the PF [F(2,15) = 0.46, n.s] (Figs. 5b and 6b). In contrast to the change in mRNA, this effect on peptide was strongest at the 2.5 g/kg dose of ethanol and not statistically significant at the 0.75 g/kg dose. Thus, in addition to showing a change in peptide as well as mRNA in the LH, these findings suggest that the increase in OX peptide requires a higher dose of ethanol than the increase in OX mRNA.
Figure 5.
Effects of acute ethanol gavage on OX mRNA and peptide expression in the LH vs PF regions, as measured via (a) digoxigenin-labeled ISH and (b) immunofluorescence histochemistry (Experiment 4). The data (mean ±SEM) for the 3 groups (n=5/group), water, 0.75 g/kg ethanol and 2.5 g/kg ethanol showed a significant increase [F(2,15) = 3.87, p<0.05] in OX mRNA expression in the LH at the lower dose (0.75 g/kg) of ethanol compared to the water or 2.5 g/kg group (*p<0.05). The immunoflourescence data demonstrated a significant increase in peptide levels of OX in the LH [F(2,15) = 4.21, p<0.05] at the higher dose (2.5 g/kg) compared to the lower dose (0.75 g/kg) or water group (* p<0.05).
Figure 6.
Photomicrographs illustrating the stimulatory effect of 0.75 g/kg ethanol on OX (a) mRNA expression and 2.5 g/kg ethanol on (b) peptide levels in the LH, as measured using digoxigenin-labeled ISH and immunofluorescence histochemistry as graphed in Fig. 5 (Experiment 4).
Discussion
The present study is the first to examine the consequences of acute and chronic ethanol on OX expression and peptide levels. Whereas chronic consumption of ethanol profoundly suppresses OX expression, the results with acute oral administration of ethanol reveal a stimulatory effect. This effect, which is only observed in the LH and absent in the PF region, is reflected by an increase in gene expression at the lower dose of ethanol and increased peptide levels at the higher ethanol dose.
Effects of Chronic Ethanol on OX Expression
With chronic, voluntary consumption of ethanol in Sprague-Dawley rats, the present study demonstrates a suppression of OX expression in the PFLH, which becomes stronger as the amount of ethanol consumed rises from 0.25 g/kg/day to 2.5 g/kg/day, and BEC values increase, respectively, from 13 to 38mg/dl. Although a study of chronic ethanol intake in selectively-bred, ethanol-preferring rats revealed little change in the density of OX-expressing neurons with higher daily ethanol consumption (5g/kg/day) (Lawrence et al., 2006), the suppression of OX mRNA seen here with chronic ethanol intake in Sprague-Dawley rats is similar to that produced by chronic treatment with morphine or cocaine in this same rat strain (Zhou et al., 2006; Zhou et al., 2008). Together, these studies suggest that repeated intake of rewarding substances leads to an overall reduction in OX gene expression in the PFLH, which may be influenced by a negative feedback circuit. This finding clearly distinguishes OX in the PFLH from other peptides, GAL and ENK, in the PVN, which are known to increase ethanol intake (Leibowitz, 2007; Lewis et al., 2004; Rada et al., 2004). In contrast to OX, these peptides are stimulated by chronic consumption of ethanol (Chang et al., 2007; Leibowitz et al., 2003), which has led to the hypothesis that they function within a positive feedback loop to promote excess ethanol intake (Leibowitz, 2007).
There are several possible mechanisms that may underlie this negative feedback of chronic ethanol on the OX system. One may involve the direct pharmacological action of ethanol on the inhibitory amino acid, γ-aminobutyric acid (GABA), in the PFLH (Kaneyuki et al., 1995). Acting via ionotropic GABA-A receptor sites, ethanol is known to reduce neuronal activity (Deitrich et al., 1989), and these receptors are found to be located on OX neurons in the PFLH, where chronic ethanol may produce a long-term suppression of this peptide (Backberg et al., 2004; Moragues et al., 2003). Another explanation may involve negative feedback by monoaminergic projections to OX neurons. Ethanol is known to activate serotonin (5-HT) and dopamine (DA) neurons of the dorsal raphe and VTA, respectively (Brodie et al., 1990; Gessa et al., 1985; Yamane et al., 2003), which send projections to the PFLH. The inhibitory feedback from these projections is likely to occur through activation of the 5-HT1A and DA-D2 receptor sites, which are found to exist on and inhibit OX neurons of the PFLH (Alberto et al., 2006; Bubser et al., 2005; Muraki et al., 2004; Xie et al., 2006). Given the stimulatory effects of ethanol on each of these neurochemicals that inhibit OX neurons (Brodie et al., 1990; Gessa et al., 1985; Khatib et al., 1988; Yamane et al., 2003; Yoshimoto et al., 1992), the observed suppression of OX expression with chronic ethanol consumption may be a consequence of these negative feedback systems.
Effects of Acute Ethanol on OX Expression
With evidence that the suppressive effect of chronic ethanol intake on OX mRNA occurs with relatively low BEC values, we were encouraged to test acute doses of ethanol, which yield higher BEC values and may stimulate rather than inhibit the OX system, similar to that shown for GAL and ENK peptides in the PVN (Chang et al., 2007). The results revealed such an effect, showing OX mRNA in the PFLH to be stimulated by acute, oral administration of ethanol at the lower dose of 0.75 g/kg but not at the higher, 2.5 g/kg. This dose-related effect was demonstrated in two separate experiments using qRT-PCR and radiolabeled ISH. The bimodal pattern, a stimulation at low doses and no effect or suppression at moderately high doses, has been seen in other studies examining the effects of ethanol on such measures as beta-endorphin or DA release, inducible nitric oxide synthase activity and locomotor activity (Davis and de Fiebre, 2006; Gingras and Cools, 1996; Jarjour et al., 2009; Mocsary and Bradberry, 1996). The measurements of BEC suggest that this effect is closely related to circulating ethanol levels. The stimulatory effect on OX expression is evident with a transient and small rise in BEC to approximately 30mg/dl, but it is lost with a greater and longer-lasting increase in BEC to >35 mg/dl. We propose that this larger, sustained rise in BEC, acting through inhibitory neurotransmitter systems as described above (Alberto et al., 2006; Backberg et al., 2004; Bubser et al., 2005; Muraki et al., 2004; Xie et al., 2006), may provide negative feedback to OX neurons as ethanol remains in the blood for a prolonged period of time.
Differential Effects of Ethanol on OX Expression in the LH and PF
With digoxigenin-labeled ISH and immunofluorescence histochemistry, the stimulatory effect of acute ethanol on OX neurons was found to occur predominantly in the LH rather than the PF regions. The available evidence suggests that this sub-population of OX-expressing neurons in the LH is more closely linked to reward-related behaviors than the sub-population in the PF (Harris and Aston-Jones, 2006). Whereas PF OX neurons are involved in arousal, the LH OX neurons have a more prominent role in reward-driven behaviors, specifically those activated by morphine, cocaine and food in a conditioned place preference paradigm (Estabrooke et al., 2001; Harris et al., 2005). Although Lawrence and colleagues found no change in the density of OX neurons in response to ethanol intake, they described an increase in the area of OX neurons in the LH but not the PF (Lawrence et al., 2006). Anatomical differences between the LH- and PF-OX neurons may account for these differential functions. Whereas the majority of OX neurons originating in the LH project directly to the VTA and activate key reward DA pathways (Fadel and Deutch, 2002; Korotkova et al., 2003; Narita et al., 2006), most PF efferent projections extend to brain regions related to arousal (Espana et al., 2005; Peyron et al., 1998). Although ethanol-associated stimuli may activate OX neurons in both the LH and PF, this effect in the PF is more evident under conditions of greater arousal (Dayas et al., 2008). In the present study, ethanol was acutely administered at low-to-moderate doses via a relatively, un-stressful oral gavage and thus very likely activated predominantly reward mechanisms mediated by LH-OX neurons, while sparing arousal-related pathways involving PF-OX neurons.
Our results with acute ethanol further demonstrated a stimulation of OX mRNA expression in the LH with the lower dose of ethanol (0.75 g/kg) but an increase in peptide immunoreactivity at the higher dose (2.5 g/kg). These findings may be explained by the differential time course of mRNA vs protein expression, as well as the difference in dose. For example, a moderate dose of ethanol increases c-fos mRNA, a marker of neuronal activation, in the hypothalamus within 45 minutes (Hansson et al., 2008), while stimulating Fos peptide levels only at 2 h after injection (Ogilvie et al., 1998; Ryabinin et al., 1997; Thiele et al., 1997). An increased expression of c-fos in the hypothalamus is even seen within 10 minutes of oral administration of a moderately high dose of ethanol, underscoring the rapid nature of this phenomenon as dose increases (Ogilvie et al., 1998). Further, changes in Fos protein levels are found to be dose dependent. Whereas a low ethanol dose of 0.75 g/kg has minimal effect, a higher dose of 3g/kg produces a marked increase in Fos protein levels at 2 h after administration (Chang et al., 1995). Thus, these studies with moderate-to-high doses of ethanol reveal rapid changes in gene expression followed by an increase in Fos protein, and with lower doses, they suggest a slower stimulation of gene expression followed by peptide changes at a later time point. Although we measured and describe mRNA and peptide changes 120 minutes following ethanol administration as BEC levels are declining, it is important to note that the gene expression changes observed with the low dose of ethanol most likely occurred at an earlier time point. In studies by Ogilvie and colleagues (Ogilvie et al., 1997; Ogilvie et al., 1998), BEC levels were found to peak within the first 15-30 minutes after oral administration of ethanol (1-3 g/kg), while mRNA changes occurred within the first 30-60 minutes of this treatment and began to decline at 180 minutes post-treatment (Ogilvie et al., 1997; Ogilvie et al., 1998). In all, the results of the present study with measurements at 2 h are consistent with this evidence, showing the lower, 0.75 g/kg dose of ethanol to stimulate gene expression while having no effect on OX peptide levels and the higher, 2.5 g/kg dose of ethanol to produce no change in OX mRNA while producing an increase in peptide levels.
In summary, the results presented herein demonstrate that acute ethanol stimulates and chronic ethanol reduces OX mRNA expression in the PFLH, a pattern related to the amount of ethanol consumed and corresponding BEC values achieved. These differential effects of acute and chronic ethanol on mRNA expression are consistent with previous results obtained with injection studies. Whereas acute injection of OX into the LH has a stimulatory effect on ethanol intake (Schneider et al., 2007) and food intake (Haynes et al., 1999; Sakurai, 1999; Sweet et al., 1999), the effect of chronic injection of OX on feeding diminishes or is reversed to a suppression (Novak and Levine, 2009; Rossi et al., 1997; Yamanaka et al., 1999). Together, these studies suggest that OX may function within a negative feedback circuit, in contrast to the proposed positive feedback system regulating GAL and ENK in the PVN (Chang et al., 2007; Leibowitz et al., 2003).
Acknowledgments
This research was supported by USPHS Grant AA12882. We would like to thank Ambrose Carr, Yu-Wei Chen and Siyi Chang for their technical assistance. We would also like to thank Dr. de Lecea for his generosity in providing the RNA probe used for this study.
Support: USPHS Grant AA12882
References
- Alberto CO, Trask RB, Quinlan ME, Hirasawa M. Bidirectional dopaminergic modulation of excitatory synaptic transmission in orexin neurons. J Neurosci. 2006;26(39):10043–10050. doi: 10.1523/JNEUROSCI.1819-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association; Washington: 2000. [Google Scholar]
- Backberg M, Ultenius C, Fritschy JM, Meister B. Cellular localization of GABA receptor alpha subunit immunoreactivity in the rat hypothalamus: relationship with neurones containing orexigenic or anorexigenic peptides. J Neuroendocrinol. 2004;16(7):589–604. doi: 10.1111/j.1365-2826.2004.01207.x. [DOI] [PubMed] [Google Scholar]
- Becker C. Alcohol dependence, withdrawal, and relapse. Alcohol Research & Health. 2008;31(4):348–351. [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102(52):19168–73. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508(1):65–9. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Bubser M, Fadel J, Jackson L, Meador-Woodruff J, Jing D, Deutch A. Dopaminergic regulation of orexin neurons. Eur J Neurosci. 2005;21(11):2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28(46):12107–19. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, Hoebel BG, Leibowitz SF. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007;31(2):249–59. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145(8):3904–12. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679(1):89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol's actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29(3):179–85. [PMC free article] [PubMed] [Google Scholar]
- Dayas C, McGranahan T, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63(2):152–7. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff T, Peyron C, Gao X, Foye P, Danielson P, Fukuhara C, Battenberg E, Gautvik V, Bartlett Fn, Frankel W, van den Pol A, Bloom F, Gautvik K, Sutcliffe J. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitrich RA, Dunwiddie TV, Harris RA, Erwin VG. Mechanism of action of ethanol: initial central nervous system actions. Pharmacol Rev. 1989;41(4):489–537. [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73(6):759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Espana RA, Reis KM, Valentino RJ, Berridge CW. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J Comp Neurol. 2005;481(2):160–78. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21(5):1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–87. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fish EW, Riday TT, McGuigan MM, Faccidomo S, Hodge CW, Malanga CJ. Alcohol, Cocaine, and Brain Stimulation-Reward in C57Bl6/J and DBA2/J Mice. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2009.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23(8):3106–11. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348(1):201–3. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gingras MA, Cools AR. Analysis of the biphasic locomotor response to ethanol in high and low responders to novelty: a study in Nijmegen Wistar rats. Psychopharmacology (Berl) 1996;125(3):258–64. doi: 10.1007/BF02247337. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Rimondini R, Neznanova O, Sommer WH, Heilig M. Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur J Neurosci. 2008;27(8):1912–22. doi: 10.1111/j.1460-9568.2008.06159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Harris AR, Fang SL, Azizi F, Lipworth L, Vagenakis AG, Barverman LE. Effect of starvation on hypothalamic-pituitary-thyroid function in the rat. Metabolism: Clinical & Experimental. 1978;27(9):1074–1083. doi: 10.1016/0026-0495(78)90153-1. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29(10):571–7. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, Arch JR. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides. 1999;20(9):1099–1105. doi: 10.1016/s0196-9781(99)00105-9. [DOI] [PubMed] [Google Scholar]
- Jarjour S, Bai L, Gianoulakis C. Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alcoholism-Clinical and Experimental Research. 2009;33(6):1033–1043. doi: 10.1111/j.1530-0277.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- Kaneyuki T, Morimasa T, Shohmori T. Neurotransmitter interactions in the striatum and hypothalamus of mice after single and repeated ethanol treatment. Acta Med Okayama. 1995;49(1):13–7. doi: 10.18926/AMO/30415. [DOI] [PubMed] [Google Scholar]
- Khatib SA, Murphy JM, McBride WJ. Biochemical evidence for activation of specific monoamine pathways by ethanol. Alcohol. 1988;5(4):295–9. doi: 10.1016/0741-8329(88)90068-7. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23(1):7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148(6):752–9. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF. Overconsumption of dietary fat and alcohol: mechanisms involving lipids and hypothalamic peptides. Physiol Behav. 2007;91(5):513–21. doi: 10.1016/j.physbeh.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF, Avena NM, Chang GQ, Karatayev O, Chau DT, Hoebel BG. Ethanol intake increases galanin mRNA in the hypothalamus and withdrawal decreases it. Physiol Behav. 2003;79(1):103–111. doi: 10.1016/s0031-9384(03)00110-0. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Johnson DF, Waldman D, Leibowitz SF, Hoebel BG. Galanin microinjection in the third ventricle increases voluntary ethanol intake. Alcohol Clin Exp Res. 2004;28(12):1822–1828. doi: 10.1097/01.alc.0000148099.12344.c8. [DOI] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Direct and indirect inhibition by catecholamines of hypocretin/orexin neurons. J Neurosci. 2005;25(1):173–83. doi: 10.1523/JNEUROSCI.4015-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98(3):365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Pompei P, Ono J, McEwen BS. Effects of adrenal steroids on basal ganglia neuropeptide mRNA and tyrosine hydroxylase radioimmunoreactive levels in the adrenalectomized rat. J Neurochem. 1998;71(2):833–843. doi: 10.1046/j.1471-4159.1998.71020833.x. [DOI] [PubMed] [Google Scholar]
- Mocsary Z, Bradberry CW. Effect of ethanol on extracellular dopamine in nucleus accumbens: comparison between Lewis and Fischer 344 rat strains. Brain Res. 1996;706(2):194–8. doi: 10.1016/0006-8993(95)01200-1. [DOI] [PubMed] [Google Scholar]
- Moragues N, Ciofi P, Lafon P, Tramu G, Garret M. GABAA receptor epsilon subunit expression in identified peptidergic neurons of the rat hypothalamus. Brain Res. 2003;967(1-2):285–9. doi: 10.1016/s0006-8993(02)04270-1. [DOI] [PubMed] [Google Scholar]
- Muraki Y, Yamanaka A, Tsujino N, Kilduff T, Goto K, Sakurai T. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J Neurosci. 2004;24(32):7159–66. doi: 10.1523/JNEUROSCI.1027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26(2):398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Levine JA. Daily Intraparaventricular Orexin-A Treatment Induces Weight Loss in Rats. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Rivier C. Effect of three different modes of alcohol administration on the activity of the rat hypothalamic-pituitary-adrenal axis. Alcohol Clin Exp Res. 1997;21(3):467–76. doi: 10.1111/j.1530-0277.1997.tb03792.x. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Lee S, Rivier C. Divergence in the expression of molecular markers of neuronal activation in the parvocellular paraventricular nucleus of the hypothalamus evoked by alcohol administration via different routes. J Neurosci. 1998;18(11):4344–52. doi: 10.1523/JNEUROSCI.18-11-04344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958;127(3294):315–24. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of Comparative and Physiological Psychology. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Sydney: 1986. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, Van Den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Avena NM, Leibowitz SF, Hoebel BG. Ethanol Intake is Increased by PVN Galanin Injection and Reduced by a GAL Antagonist. Alcohol. 2004;33(2):91–97. doi: 10.1016/j.alcohol.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Rosell DR, Wood GE, Spedding M, Munoz C, Rothstein J, McEwen BS. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc Natl Acad Sci U S A. 2004;101(7):2179–2184. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Choi SJ, O'Shea D, Miyoshi T, Ghatei MA, Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138(1):351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2(1):32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- Sakurai T. Orexins and orexin receptors: implication in feeding behavior. Regul Pept. 1999;85(1):25–30. doi: 10.1016/s0167-0115(99)00076-2. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli R, Tanaka H, Williams S, Richarson J, Kozlowski G, Wilson S, Arch J, Buckingham R, Haynes A, Carr S, Annan R, McNulty D, Liu W, Terrett J, Elshourbagy N, Bergsma D, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998a;92(5) doi: 10.1016/s0092-8674(02)09256-5. 1 page following 696. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998b;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schaefer GJ, Michael RP. Ethanol and current thresholds for brain self-stimulation in the lateral hypothalamus of the rat. Alcohol. 1987;4(3):209–13. doi: 10.1016/0741-8329(87)90045-0. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: Differential effects of orexin, galanin, and ghrelin. Alcoholism-Clinical and Experimental Research. 2007;31(11):1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Sweet DC, Levine AS, Billington CJ, Kotz CM. Feeding response to central orexins. Brain Res. 1999;821(2):535–538. doi: 10.1016/s0006-8993(99)01136-1. [DOI] [PubMed] [Google Scholar]
- Thiele TE, van Dijk G, Bernstein IL. Ethanol-induced c-Fos expression in rat lines selected for low and high alcohol consumption. Brain Res. 1997;756(1-2):278–82. doi: 10.1016/s0006-8993(97)00228-x. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31(2):384–95. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65(10):857–62. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Management of Substance Abuse: Alcohol. Vol. 2009. World Health Organization; 2009. [Google Scholar]
- Wortley K, Chang G, Davydova Z, Leibowitz S. Peptides that regulate food intake: orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1454–65. doi: 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- Xie XM, Crowder TL, Yamanaka A, Morairty SR, LeWinter RD, Sakurai T, Kilduff TS. GABA(B) receptor-mediated modulation of hypocretin/orexin neurones in mouse hypothalamus. J Physiol-London. 2006;574(2):399–414. doi: 10.1113/jphysiol.2006.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999;849(1-2):248–252. doi: 10.1016/s0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- Yamane F, Tohyama Y, Diksic M. Continuous ethanol administration influences rat brain 5-hyroxytrytamine synthesis non-umiformly: alpha-[14C]methyl-L-trytophan autoradiographic measurements. Alcohol Alcohol. 2003;38(2):115–20. doi: 10.1093/alcalc/agg053. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9(1):17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191(1):137–45. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience. 2008;153(4):1225–34. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]