Abstract
Purpose
In women with early stage, estrogen-receptor (HR)-positive breast cancer, the 21-gene recurrence score (RS) assay quantifies recurrence risk and predicts chemotherapy responsiveness. Recent data suggest that not all women with early-stage, HR+ disease receive this testing. We examined socio-demographic, clinical, and attitudinal factors associated with RS testing receipt and the RS testing impact on chemotherapy use in Black and White patients.
Patients and Methods
Women with newly diagnosed invasive, non-metastatic breast cancer were recruited and interviewed to collect socio-cultural and healthcare process data; clinical data were collected from charts. Of the sample (n=359), 270 had HR-positive disease. Primary analysis focused on those with HR-positive node negative disease (n=143); secondary analyses included node positive women. Logistic regression models evaluated factors associated with receipt of RS testing and chemotherapy.
Results
Among women eligible for the 21-gene assay, 43% received RS testing. In multivariable analysis, higher age (OR=1.04 per one year increase; 95% CI: 1.01–1.08) was associated with RS testing adjusting for covariates. Chemotherapy use was 23%. In multivariable analysis, positive attitudes about chemotherapy and higher risk of recurrence were associated with chemotherapy use (p<.05).
Conclusion
Patterns of genomic testing may vary by age. Efforts to understand factors associated with low testing will be important.
Introduction
Breast cancer remains the most common cancer among US women, with more than 230,000 new diagnoses and 40,000 deaths each year, 1 along with decrements in quality of life.2–4 Half of newly-diagnosed patients present with estrogen-receptor positive and early-stage disease.5 Increased clinical genomic profiling of breast tumors, in combination with traditional factors such as age, tumor size, and grade, determine recurrence estimates and guide adjuvant treatment decisions.6 The 21-gene recurrence score (RS) assay (Oncotype DX; Genomic Health Inc., Redwood City, CA) is a validated test that quantifies risk of recurrence and predicts benefit from chemotherapy in patients with early stage, estrogen-receptor and progesterone-receptor positive (HR+) node negative breast cancer treated with Tamoxifen.7 The use of RS testing has been integrated into three sets of clinical guidelines.6,8,9
Several studies demonstrate that the RS impacts treatment utilization.10–14 Specifically, RS alters oncologists’ chemotherapy treatment recommendations in 25%–44% of cases,11,13,15 usually from combined chemo-hormonal therapy to hormone therapy alone.10,12,16–18 Beyond decreasing chemotherapy, testing also results in more specific reclassification of women. With an accurate understanding of the severity, treatment can be tailored to a patient’s specific tumor.19 Ultimately, the RS can become one of the first commonplace genomic tests that shift patterns of care in routine practice.19
Recent studies suggest that not all women with early-stage, hormone-positive disease receive RS testing. Studies utilizing large cohorts have demonstrated that Black women are less likely to be tested than Whites.19,20 Lund and colleagues (2012)20 found that testing bias may attenuate racial differences in RS, and disparate outcomes may be explained in part by differences in RS, although compliance and pharmacogenomics also may play a role. Given persistent disparities in breast cancer survival,21,22 even among HR positive women,23 it may be critical to understand potential racial disparities in RS testing. Because socio-cultural factors as religiosity and medical mistrust have been associated with receipt of genetic risk assessment in minority women, we examine these factors in our study.24,25 Such factors are regarded to influence both patient-provider interactions and decision-making in minority women.26
Healthcare practice setting also appears to play a role in the receipt of testing 27. Other healthcare related factors such as patient-provider communication may also impact receipt of RS testing. Patient-provider communication has been associated with receipt of cancer screening tests,28 cancer treatment,26,29 and other genomic tests.30 Patients with better communication with their providers may be more likely to request the test, have a better understanding of the purpose and/or rationale of the test and thus be more likely to make decisions to have the test.31,32 In a small sample of mostly White patients, Tzeng and colleagues32 found that receiving information about RS testing from an oncologist was associated with women’s willingness to receiving testing. We will expand knowledge in this area by including attitudinal and socio-cultural variables that are hypothesized to impact cancer behaviors particularly in Black women but have not been empirically examined within the RS testing context.
We hypothesized that Black women and those of lower socioeconomic status would be less likely to receive RS but that this would be attenuated after controlling for tumor characteristics. We also expected that women without RS would be more likely to have chemotherapy compared to those with RS, but that RS would be correlated with chemotherapy use among those with RS.
Methods
Setting and Population
This report is a part of a larger study that has been detailed elsewhere.33 Briefly, after Institutional Review Board approval from all institutions, a convenience sample was recruited via hospitals in Washington D.C. (n=3), in Detroit (n=1), and self-referrals from outreach activities (e.g. fliers, posters, mailings) between July 2006 and April 2011. Since recommendations for Oncotype DX testing were added to NCCN and ASCO guidelines in 2007 we focused only on women who were diagnosed when the guidelines had integrated RS testing. Thus, participants diagnosed in 2006 were excluded from the analysis.6,34
Eligible women were ≥ 21 years old and diagnosed with invasive non-metastatic disease for whom systemic adjuvant therapy would be considered with curative intent. Black women were oversampled to facilitate race comparisons and to investigate within race-group differences. Women with ductal and lobular carcinoma in-situ, distant metastasis, recurrent disease, second primaries, who were not English speakers, who did not self-identify as Black or White, or unable to provide informed consent were excluded.
A total of 678 potentially eligible patients were screened for the study, 477 were eligible and of that number 82.8% were interviewed and 350 (90.9%) had complete medical records. Women with HR negative cancers and those with Stage III breast cancer were excluded from analyses. There were 270 women with HR positive disease and 146 who were both HR positive and node negative; the latter group is the primary focus of this manuscript. 34 We also explored RS patterns in the overall group of women recruited that included both node negative and node positive disease (n=270). The reason for including patients with node-positive tumors was to explore use of RS among women for whom testing was not standard practice to assess if some women may have requested or sought testing regardless of NCCN guidelines and/or recommendations.35
Data Collection
Clinical research assistants confirmed eligibility and obtained consent for interviews and chart reviews. Interviews were conducted by trained staff using a standardized computer-assisted telephone survey that lasted about 50 minutes. The survey included information about socio-demographic factors, attitudes about therapy, socio-cultural factors, and interactions with providers. On average interviews were conducted within 14 weeks post definitive surgery. Treatment and clinical variables (e.g., 21 gene assay, etc.) were abstracted from medical records 12–18 months after interviews. Participants received a $25 incentive.
Measures
Outcomes were obtained from medical records and included RS (yes or no), hormonal use (yes vs. no), and chemotherapy use of any regimen (yes or no).26 Among women with RS, we abstracted patients’ recurrence risk score and 10-year recurrence risk from medical records.
Socio-demographic variables were self-reported race, age, education, marital status, employment, education, and insurance type (private vs. public) status. Clinical factors abstracted from medical records were estrogen or progesterone receptor (HR) status (positive vs. negative), surgery type (lumpectomy or mastectomy), nodal status (positive or negative), pathological tumor size, and breast cancer stage categorized from I to III, which was categorized similar to other reports as positive, negative, or unknown.36 Comorbidity was measured using the Charlson comorbidity index score.37 Body mass index was calculated from data in the medical charts and categorized as either normal (kg/m2 < 25) or overweight/obese (kg/m2 ≥25).38
Communication about chemotherapy was assessed using the Makoul Communication Scale (7-items) (Cronbach’s alpha: overall= .83).39 The scale includes key dimensions of communication such as information-giving (e.g. “the doctor fully explained the risks of chemotherapy”) and physicians’ solicitation behaviors (e.g. “the doctor asked your opinion about taking chemotherapy”); scores above the median (=28) reflect self-reported perceptions of greater communication.
Chemotherapy attitudes items captured women’s perceptions about the efficacy and benefits (vs. side effects) of therapy (“chemotherapy does not help you live longer”) (Cronbach’s alpha overall= .60); scores above the median (=20) reflected positive attitudes.40
The suspicion subscale (alpha=.84) of the Group-Based Medical Mistrust Scale was used to measure medical mistrust; higher scores indicated higher mistrust.41 Self-efficacy in understanding (alpha=.77) and participating in healthcare with providers (alpha=.76) were measured by the Communication Attitudinal Self-Efficacy Scale (CASE).42 Religiosity was analyzed based on the Lukwago Religiosity scale (alpha=.99) that measured women’s beliefs, values, and religious preferences; higher scores indicating higher religiosity.43
Statistical Analysis
Study sample constitutes HR positive node negative breast cancer patients. Demographic, clinical, behavioral, and attitudinal characteristics of the participant women were described. Chi-square tests were conducted to examine bivariate associations with receipt of RS testing and chemotherapy initiation (yes or no). Independent sample t-tests were used for continuous variables. Multivariable logistic regression models were used to examine receipt of RS testing and also chemotherapy initiation. Variables significant (p<.05) in the bivariate analysis were included into the models. All models included race and models were adjusted for type of surgery and hormonal use. We used logistic regression to explore RS use in node positive women.
Result
Sample characteristics of the 143 women with HR positive and node negative cancer are detailed in Table 1. Participants’ ages ranged from 28 to 89 (m=55.9; SD=11.0) and 54% were Black. All patients were insured and most had private insurance. There were a few differences in socio-demographic factors by race. Compared to White women, a higher proportion of Black women had public insurance (76.25% vs. 23.75%, respectively), were in single/unpartnered households (73.68% vs. 26.31%) and had less than a college education (82.59% vs. 17.31%) (data not shown).
Table 1.
Sample Characteristics, Recurrence Score (RS) Test Status and Adjusted ORs for Likelihood of 21-Gene Assay Tests in HR positive and Node Negative Breast Cancer Women, N=143
| Characteristic | Total N(%) | Yes RS N(%) | No RS N(%) | P value | Adjusted ORs 95% CI |
|---|---|---|---|---|---|
| Age Mean (SD) | 55.9(11.0) | 58.7(10.1) | 53.8(11.3) | .011 | 1.04 (1.01, 1.08)† |
| Race | |||||
| Black | 78(54.5) | 33(42.3) | 45(57.7) | .647 | 0.71 (.35, 1.45) |
| White | 65 (45.7) | 30(46.2) | 35(53.8) | ref | |
| Married/Partnered | |||||
| Yes | 71(49.3) | 32(45.1) | 39(54.9) | .809 | |
| No | 74(50.3) | 31(43.1) | 41(56.9) | ||
| Education | |||||
| ≤HS | 26(18.2) | 11(42.3) | 15(57.7) | .843 | |
| >HS | 117(81.8) | 52(44.4) | 65(55.6) | ||
| Highest Grade Completed | |||||
| ≤HS | 26(18.2) | 11(42.3) | 15(57.7) | .248 | |
| Any college | 41(28.7) | 14(34.1) | 27(65.9) | ||
| ≥College degree | 76(53.1) | 38(50) | 38(50) | ||
| Employed full-time | |||||
| Yes | 47(35.6) | 22(46.8) | 25(53.2) | .978 | |
| No | 85(64.4) | 40(47.1) | 45(52.9) | ||
| Insurance | |||||
| Private | 84(66.1) | 36(42.9) | 48(57.1) | .259 | |
| Public | 43(33.9) | 23(53.5) | 20(46.5) | ||
| Tumor Size | |||||
| <2cm | 92(66.2) | 41(44.6) | 51(55.4) | .989 | |
| ≥2 cm | 47(33.8) | 21(44.7) | 26(55.3) | ||
| Stage | |||||
| I | 86(67.2) | 42(48.8) | 44(51.2) | .902 | |
| II | 42(32.8) | 21(50) | 21(50) | ||
| Surgery | |||||
| Lumpectomy | 97(67.8) | 41(42.3) | 56(57.7) | .536 | |
| Mastectomy | 46(32.2) | 22(47.8) | 24(52.2) | ||
| Initiated Chemotherapy | |||||
| Yes | 31(21.7) | 13(41.9) | 18(58.1) | .788 | |
| No | 112(78.3) | 50(44.6) | 62(55.4) | ||
| Hormonal Therapy | |||||
| Yes | 115 (80.4) | 57 (49.6) | 58(50.4) | .005 | 3.82 (1.38, 10.57)† |
| No | 28 (19.6) | 6 (21.4) | 22(78.6) | ref | |
| Initiated radiation therapy | |||||
| Yes | 86(60.1) | 40(46.5) | 46(53.5) | .468 | |
| No | 57(39.9) | 23(40.4) | 34(59.6) | ||
| Comorbidity | |||||
| No Comorbidity | 42(29.4) | 18(42.9) | 24(57.1) | .853 | |
| One or more | 101(70.6) | 45(44.6) | 56(55.4) | ||
| BMI | |||||
| <25 Normal | 46(34.8) | 22(47.8) | 24(52.2) | .886 | |
| ≥25 Overweight/obese | 86(65.2) | 40(46.5) | 46(53.5) | ||
| Chemo-Communication | |||||
| Low | 77(53.8) | 36(46.8) | 41(53.2) | .4843 | |
| High | 66(46.2) | 27(40.9) | 39(59.1) | ||
| Chemotherapy Attitudes | |||||
| Low | 76(53.5) | 39(51.3) | 37(48.7) | .0728 | |
| High | 66(46.5) | 24(36.4) | 42(63.6) | ||
| Understanding and participation in care | |||||
| Low | 61(46.6) | 30(49.2) | 31(50.8) | .694 | |
| High | 70(53.4) | 32(45.7) | 38(54.3) | ||
| Maintaining a positive attitude | |||||
| Low | 60(46.5) | 26(43.3) | 34(56.7) | .502 | |
| High | 69(53.5) | 34(49.3) | 35(50.7) | ||
| Suspicion of mistrust | |||||
| Low | 66 (40.9) | 22(39.3) | 34(60.7) | .377 | |
| High | 81(59.1) | 38(46.9) | 43(53.1) | ||
| Religiosity | |||||
| Low | 66(50.0) | 32(48.5) | 34(51.5) | .729 | |
| High | 66(50.0) | 30(45.5) | 36(54.5) | ||
| Race-based discrimination | |||||
| Yes | 47(32.9) | 17(36.2) | 30(63.8) | .181 | |
| No | 96(67.1) | 46(47.9) | 50(52.1) | ||
P<.05
Factors Associated with Receipt of RS 21 Gene Assay
Forty-three percent of the HR positive node negative sample had RS testing. There were no differences in receipt of RS testing by race (p >.05). Age was the only socio-demographic factor associated with RS testing. There was also no association of RS testing according to tumor characteristics, patient-provider communication or socio-cultural factors. There was a trend towards an association between chemotherapy attitudes (p=.07). In multivariate analysis, per 1 year increased in age was associated with a greater likelihood (OR: 1.04; 95%CI:1.01–1.08) of RS testing.
Exploratory Analysis
In the secondary analysis of both node positive and node negative women (n=270; 13% node positive, 87% node negative), 16% of those with node positive disease received RS testing (data not shown). In contrast to the primary analysis (node negative disease only), lower stage and smaller tumor size were associated with higher likelihood of having RS testing. Positive attitudes about chemotherapy were also associated with higher likelihood of RS testing. In multivariable analysis, women with private insurance (OR: 2.8; 95% CI: 1.05–7.27) were more likely to receive RS (data not shown).
Risk Profile of Study Participants
Among women with RS testing, risk of having recurrence in 10 years ranged from 0 to 58 (m=20.3, SD=11.6). Black and White women had similar mean RS scores (20.2±10.5 for Black vs. 20.5±12.9 for White; p=.897) (data not shown). Overall, 46% had scores that fell within the low risk category. Fifteen percent of Black women compared to 10% of White had high recurrence risk, while low to intermediate risk of recurrence was 85% among Blacks and 90% among Whites.
Chemotherapy Use and RS Testing in HR Positive Node Negative Women
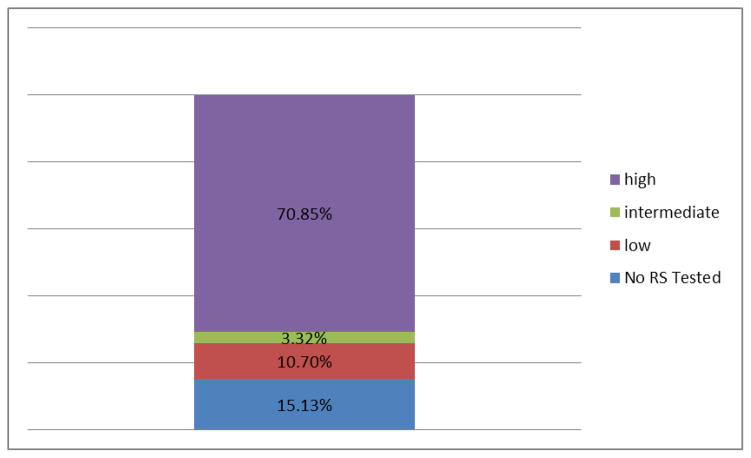
Twenty-three percent of women with hormone positive and node negative disease initiated chemotherapy. Younger women were more likely to have chemotherapy compared to older women (p=.020). Women with high risk of recurrence were most likely to have chemotherapy (70.8%; p=.03) compared to the low risk group (3.3%) (Figure 1). More aggressive disease characteristics (i.e., higher stage, tumor size >=2cm) were associated with higher odds of having chemotherapy in bivariate analysis (see Table 1 in Appendix). Women with more positive attitudes about chemotherapy were more likely to initiate therapy than those with less positive attitudes (p<.001). In multivariable analysis, only attitudes about chemotherapy and risk of recurrence were significant controlling for other factors (p<.05).
Figure 1.
Overall Recurrence by Categories.
Appendix Table 1.
Chemotherapy Initiation Rates and Adjusted ORs in Women with HR Positive and Node Negative Breast Cancer, N=143
| Characteristic | Total N(%) | Chemotherapy Initiated | P value | Adjusted ORs and 95% CI | |
|---|---|---|---|---|---|
|
| |||||
| Yes N(%) | No N(%) | ||||
| Age: Mean (SD) | 55.9(11.4) | 51.9(11.81) | 57.1(11.07) | .020 | 0.96 (.88,1.05) |
| Race | |||||
| Black | 78(54.5) | 19 (24.4) | 59 (75.6) | .392 | 1.29 (.24, 6.98) |
| White | 65(45.5) | 12 (18.5) | 54 (81.5) | ref. | |
| Married/Partnered | |||||
| Yes | 71 (49.7) | 13 (18.3) | 58 (81.7) | .333 | |
| No | 72 (50.3) | 18 (25) | 54 (75) | ||
| Education | |||||
| ≤HS | 26 (18.2) | 8 (30.8) | 18 (69.2) | .265 | |
| >HS | 117 (81.8) | 23 (19.7) | 94 (80.3) | ||
| Highest Grade Completed | |||||
| ≤HS | 26 (18.2) | 8 (30.8) | 18 (69.2) | .481 | |
| Any college | 41 (28.7) | 9 (22.0) | 32 (78.0) | ||
| ≥College degree | 76 (53.1) | 14 (18.4) | 62 (81.6) | ||
| Employed full-time | |||||
| Yes | 47 (34.8) | 10 (21.3) | 37 (78.7) | .886 | |
| No | 85 (65.2) | 19 (22.4) | 66 (77.6) | ||
| Insurance | |||||
| Private | 84(66.1) | 16(19.1) | 68(80.9) | .413 | 2.12 (.25, 18.31) |
| Public | 43(33.9) | 11(25.6) | 32(74.4) | ref | |
| Tumor Size | |||||
| <2cm | 92 (65.5) | 13 (14.1) | 79 (85.9) | .0068 | 2.39 (.38 14.97) |
| ≥2 cm | 47 (34.5) | 17 (36.2) | 30 (63.8) | ref | |
| Stage | .0128 | ||||
| I | 86 (65.6) | 14 (16.3) | 72 (83.7) | 1.01(.08, 12.32) | |
| II | 42 (34.4) | 16 (38.1) | 26 (61.9) | ref | |
| Surgery | |||||
| Lumpectomy | 97 (67.8) | 20 (20.6) | 77 (79.4) | .663 | |
| Mastectomy | 46 (32.2) | 11 (23.9) | 35 (76.1) | ||
| Hormonal Therapy | |||||
| Yes | 115 (80.4) | 29 (25.2) | 86 (74.8) | .008 | |
| No | 28 (19.6) | 2 (7.1) | 26 (92.9) | ||
| Radiation therapy | |||||
| Yes | 86 (60.1) | 20 (23.3) | 66 (76.7) | .571 | |
| No | 57 (39.9) | 11 (19.3) | 46 (80.7) | ||
| Comorbidity | |||||
| No Comorbidity | 42 (29.4) | 9 (21.4) | 33 (78.6) | .963 | |
| One or more | 101 (70.6) | 22 (21.8) | 79 (78.2) | ||
| BMI | |||||
| <25 Normal | 46 (34.8) | 8 (17.4) | 38 (82.6) | .337 | |
| ≥25 Overweight/obese | 86 (65.2) | 21 (24.4) | 65 (75.6) | ||
| Chemo-Communication | |||||
| Low | 77 (53.8) | 15 (19.5) | 62 (80.5) | .495 | |
| High | 66 (46.2) | 16(24.2) | 50 (75.8) | ||
| Chemotherapy Attitudes | <0.0001 | ||||
| Low Efficacy | 76 (53.5) | 5 (6.6) | 71 (93.4) | ref. | |
| High Efficacy | 66 (46.5) | 26 (39.4) | 40 (60.6) | 9.74 (1.39, 67.91)‡ | |
| Understanding Care participation in care | |||||
| Low | 61 (46.6) | 15 (24.6) | 46 (75.4) | .532 | |
| High | 70 (53.4) | 14 (20.0) | 56 (80.0) | ||
| Positive attitude | |||||
| Low | 60 (46.5) | 14(23.3) | 46 (76.7) | .679 | |
| High | 69 (53.5) | 14(20.3) | 55 (79.7) | ||
| Suspicion of mistrust | |||||
| Low | 56 (40.9) | 11 (19.6) | 45 (79.0) | .848 | |
| High | 81 (59.1) | 17 (80.4) | 64 (21.0) | ||
| Religiosity | |||||
| Low | 66 (50) | 13 (20.6) | 53 (79.4) | .530 | |
| High | 66 (50) | 16 (25.4) | 50 (74.6) | ||
| Discrimination | |||||
| Yes | 47 (32.9) | 12 (25.5) | 35 (74.5) | .450 | |
| No | 96 (67.1) | 19 (19.8) | 77 (80.2) | ||
| Risk of recurrence | |||||
| RS not tested | 80(55.9) | 18(22.5) | 62(77.5) | .037 | 1.50 (.51, 4.43) |
| Low/Intermediate | 55(37.7) | 8(14.5) | 47(85.5) | ref. | |
| High | 8(5.5) | 5(62.5) | 3(37.5) | 14.23 (1.35, 149.92) | |
P<.01
Table 2 shows the descriptive results for the women who underwent chemotherapy only. Among the women who had chemotherapy, 58.8% had a tumor size ≥2 cm, the majority (76.5%) were diagnosed with stage II, and had lumpectomy (70.6%).
Table 2.
Demographic and Clinical Characteristics of Women with Chemotherapy and Without RS Testing, N=17
| Variable | N | % |
|---|---|---|
| Race | ||
| African American | 15 | 88.2 |
| White | 2 | 11.8 |
|
| ||
| Marriage Status | ||
| Married | 4 | 23.5 |
| Currently Single | 13 | 76.5 |
|
| ||
| Education | ||
| Less than High School | 10 | 58.8 |
| More than high school | 7 | 41.2 |
|
| ||
| Highest grade completed | ||
| <= high school | 10 | 58.8 |
| Any college | 4 | 23.5 |
| Bachelors and above | 3 | 17.6 |
|
| ||
| Employment status | ||
| Full time employed | 2 | 14.3 |
| Not full time employed | 12 | 85.7 |
|
| ||
| Tumor Size | ||
| >=2 cm | 10 | 58.8 |
| <2 cm | 7 | 41.2 |
|
| ||
| Stage | ||
| I | 4 | 23.5 |
| II | 13 | 76.5 |
|
| ||
| Surgery Type | ||
| Lumpectomy | 12 | 70.6 |
| Mastectomy | 5 | 29.4 |
|
| ||
| Radiation Therapy | ||
| Yes | 15 | 88.2 |
| NO | 2 | 11.8 |
Discussion
In this observational study, less than half of breast cancer patients diagnosed between 2006 and 2011 with node negative, hormone receptor positive disease received RS testing. The rate of RS testing of node negative women fell within ranges found in other studies.44,45 In concert with Nguyen and colleagues (2012) our results show that RS testing increased over the study period suggesting greater dissemination and/or adoption of testing.45 Most of the initial studies of RS testing were in samples of mostly Whites (e.g., De Frank et al, 2013).46 By including a proportional representative sample of Blacks our data adds to the growing body of literature that includes diverse breast cancer patients. Contrary to our hypothesis, we did not find differences by race or other sociodemographic factors among the group of women for whom tested was recommended (i.e., node negative).
Our null findings regarding the impact of race on RS testing are in contrast to Hassett and colleagues who found that Black patients were less likely to have RS.19 Lund and colleagues also found that Black women were half as likely as White women to receive RS in their sample but data were lacking on insurance and education.20 This study did not have information about psychosocial attitudes. One explanation as to why we did not find racial disparities in RS testing may be that women in our study had higher SES backgrounds as noted by the level of education (only 18% had high school or less) and private insurance status among the Black and the Whites. Among women who were tested we did find that more Black women were in the highest risk category. This is concert with other analyses.20,47
In general, few demographic factors have been consistently associated with RS.44 Age was the only significant demographic variable in our study. The relationship between age and receipt of RS may relate to patient and/or provider practices. For example, patients and their physicians may be inclined to seek additional information about risk of recurrence in older women to avoid unnecessary chemotherapy given concerns about competing comorbidities or toxicities.48 Studies that have examined the association between age and RS testing have produced mixed results.49 For instance, DeFrank and colleagues found slightly higher RS testing rates in younger women.46 In contrast, Hassett and colleagues found patients aged 50–59 had higher testing in comparison to patients below 50 or those over 70 years.19 Given equivocal findings to date this will be an important area for future research.
RS testing allows for a more accurate understanding regarding the tumor’s nature. Study findings supported our second hypothesis that women without RS would be more likely to have chemotherapy compared to those with RS (58.1% vs. 41.9%). Also, as expected women in the highest risk category who received RS, had the highest use of chemotherapy (55%) suggesting that RS results may have influenced treatment decisions. These results further support the studies that demonstrate that the RS impacts treatment utilization.10–14 For example, RS information has been found to change oncologists’ chemotherapy treatment recommendations in 25%–44% of cases,11,13,15 usually from combined chemo-hormonal therapy to hormone therapy alone.10,11,16 Beyond this overall shift to less chemotherapy, testing also results in reclassification of some women with clinically low-risk disease to higher risk disease that requires chemotherapy and other women with clinically high-risk disease to a low risk categorization thus enabling them to avoid chemotherapy.19 Ultimately, the RS may represent one of the first genomic tests in common medical use that shifts patterns of care in routine practice.19
While chemotherapy utilization is largely influenced by clinical characteristics (e.g., tumor size, etc.), patients’ attitudes about therapy also appear to be important. Our data add to the limited literature about the influence of women’s attitudes on chemotherapy use as few studies have examined attitudes within the context of recurrence risk. We found that those with higher risk of recurrence had more favorable attitudes about the efficacy of chemotherapy. Due to the cross-sectional nature of the study, we cannot infer causation however, this finding may imply that women who received RS testing understood that chemotherapy had greatest benefit in women with tumors with high risk and, conversely, no demonstrated benefit if low risk of recurrence. Additionally, these women may have more discussions with their providers about chemotherapy. Richman and colleagues examined knowledge about RS testing in patients and found that while most women understood discussion about test results, about one third showed limited understanding, suggesting the need to improve risk communication.32,50
It was interesting to note in the secondary analysis that 16% of women with node positive disease had RS testing, despite the fact that testing guidelines over the time of this study only recommended testing for those with node negative disease. Utility of testing among node-positive patients is currently the primary aim of an ongoing clinical trial (RxPONDER).51 Among this group, those with private insurance had access to testing although it was not clinical practice; data did not emerge until 2013 that would support this practice. Minority and lower SES groups had slower adoption of genetic testing for BRCA1/2 mutations.52,53 Evidence of rapid or early uptake of new evidence-based treatment and diagnostic procedures/tests may lead to disparities.54
Limitations of this study include our inability to attribute lack of RS testing based on physician’s orders. Physicians or patients were not queried about their decisions or communication about RS results. This will be an important area for future research. Also, some estimates had wide confidence intervals due to the modest sample size. We did not utilize a population-based sample so generalizability of results may be limited. Additionally, the cross-sectional design of the study prevents us from establishing causal relationships. Furthermore, the sample includes women who were insured so those without insurance may have different experiences with receipt of testing since some data suggests lower testing among uninsured women or those seen in other settings.55 Because most large studies of RS testing lack information collected from patients, there are several strengths of our approach. These include oversampling of Black patients and inclusion of patient reported factors relating to communication and attitudes about chemotherapy. Although some socio-cultural and psychosocial factors were not associated with RS testing, it is important to assess the impact of these understudied factors. On balance this study extends knowledge about patient and clinical factors associated with receipt of RS testing and the relationship between receipt of RS testing and chemotherapy. In insured higher educated samples, racial disparities in RS may not be as prominent.
MicroAbstract.
Studies suggest that not all women with early-stage, hormone-positive (HR) disease receive RS testing. This paper examined the influence of socio-demographic, clinical, and attitudinal factors associated with receipt of RS testing and the impact of RS testing on chemotherapy use in Black and White patients. The study sample consisted of 270 HR positive women. Among those who were node negative, 43% received RS testing. No differences were found in RS testing by race but testing varied by age. The results of the study enhance the body of knowledge regarding RS testing in a diverse clinical sample by including self-reported psychosocial and attitudinal factors in patient's interactions with their providers.
Clinical Practice Points.
What is already known about this subject?
The 21-gene recurrence score (RS) assay testing is a validated test integrated into clinical guidelines
RS impacts treatment utilization in patients with early stage, hormone positive (HR+), node negative breast cancer
RS is underused in some populations. Psychosocial factors associated with RS have been understudied
What are the new findings?
Examination of psychosocial factors
There were no race differences in RS use
Age was the only significant demographic factor associated with RS use
Women with higher risk of recurrence had more favorable attitudes about the efficacy of chemotherapy
How might it impact on clinical practice in the foreseeable future?
Results of the study will enhance the body of knowledge regarding RS testing in a diverse clinical sample
Acknowledgments
This work was funded in part by grants from the American Cancer Society (Sheppard: PI MRSGT-06-132 CPPB; O’Neil: PI MRSG-10-110-01-CPPB), Komen for the Cure, Inc. (PI: Sheppard POP0503398), and the National Cancer Institute (Pl: Sheppard R01CA154848). It was also supported by the Biostatistics and Bioinformatics Shared Resource and the Nontherapeutic Subject Registry (NTSR) Shared Resource (Isaacs) at Lombardi Comprehensive Cancer Center under NCI Grant #P30CA51008.
Footnotes
Conflicts of interest: None
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). She is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from vls3@georgetown.edu.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Lundh MH, Lampic C, Nordin K, et al. Changes in health-related quality of life by occupational status among women diagnosed with breast cancer-a population-based cohort study. Psychooncology. 2013 doi: 10.1002/pon.3285. [DOI] [PubMed] [Google Scholar]
- 3.Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29(9):1101–1109. doi: 10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epplein M, Zheng Y, Zheng W, et al. Quality of life after breast cancer diagnosis and survival. J Clin Oncol. 2011;29(4):406–412. doi: 10.1200/JCO.2010.30.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeSantis C, Howlader N, Cronin KA, Jemal A. Breast cancer incidence rates in U.S. women are no longer declining. Cancer Epidemiol Biomarkers Prev. 2011;20(5):733–739. doi: 10.1158/1055-9965.EPI-11-0061. [DOI] [PubMed] [Google Scholar]
- 6.Harris L, Fritsche H, Mennel R, et al. American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 7.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. 1533-4406; 0028-4793. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. breast cancer. version 1.2012. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 9.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: Highlights of the st gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albanell J, Gonzalez A, Ruiz-Borrego M, et al. Prospective transGEICAM study of the impact of the 21-gene recurrence score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Ann Oncol. 2012;23(3):625–631. doi: 10.1093/annonc/mdr278. [DOI] [PubMed] [Google Scholar]
- 11.Asad J, Jacobson AF, Estabrook A, et al. Does oncotype DX recurrence score affect the management of patients with early-stage breast cancer? Am J Surg. 2008;196(4):527–529. doi: 10.1016/j.amjsurg.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Geffen DB, Abu-Ghanem S, Sion-Vardy N, et al. The impact of the 21-gene recurrence score assay on decision making about adjuvant chemotherapy in early-stage estrogen-receptor-positive breast cancer in an oncology practice with a unified treatment policy. Ann Oncol. 2011;22(11):2381–2386. doi: 10.1093/annonc/mdq769. [DOI] [PubMed] [Google Scholar]
- 13.Henry LR, Stojadinovic A, Swain SM, Prindiville S, Cordes R, Soballe PW. The influence of a gene expression profile on breast cancer decisions. J Surg Oncol. 2009;99(6):319–323. doi: 10.1002/jso.21244. [DOI] [PubMed] [Google Scholar]
- 14.Partin JF, Mamounas EP. Impact of the 21-gene recurrence score assay compared with standard clinicopathologic guidelines in adjuvant therapy selection for node-negative, estrogen receptor-positive breast cancer. Ann Surg Oncol. 2011;18(12):3399–3406. doi: 10.1245/s10434-011-1698-z. [DOI] [PubMed] [Google Scholar]
- 15.Oratz R, Paul D, Cohn AL, Sedlacek SM. Impact of a commercial reference laboratory test recurrence score on decision making in early-stage breast cancer. J Oncol Pract. 2007;3(4):182–186. doi: 10.1200/JOP.0742001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rayhanabad JA, Difronzo LA, Haigh PI, Romero L. Changing paradigms in breast cancer management: Introducing molecular genetics into the treatment algorithm. Am Surg. 2008;74(10):887–890. [PubMed] [Google Scholar]
- 17.Davidson JA, Cromwell I, Ellard SL, et al. A prospective clinical utility and pharmacoeconomic study of the impact of the 21-gene recurrence score(R) assay in oestrogen receptor positive node negative breast cancer. Eur J Cancer. 2013;49(11):2469–2475. doi: 10.1016/j.ejca.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Jain S, Gradishar WJ. The application of oncotype DX in early-stage lymph-node-positive disease. Curr Oncol Rep. 2014;16(1) doi: 10.1007/s11912-013-0360-2. 360-013-0360-2. [DOI] [PubMed] [Google Scholar]
- 19.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30(18):2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lund MJ, Mosunjac M, Davis KM, et al. 21-gene recurrence scores: Racial differences in testing, scores, treatment, and outcome. Cancer. 2012;118(3):788–796. doi: 10.1002/cncr.26180. [DOI] [PubMed] [Google Scholar]
- 21.Olopade OI, Grushko TA, Nanda R, Huo D. Advances in breast cancer: Pathways to personalized medicine. Clin Cancer Res. 2008;14(24):7988–7999. doi: 10.1158/1078-0432.CCR-08-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: Biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18(9):986–993. doi: 10.1634/theoncologist.2013-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham JE, Montero AJ, Garrett-Mayer E, Berkel HJ, Ely B. Racial differences in the incidence of breast cancer subtypes defined by combined histologic grade and hormone receptor status. Cancer Causes Control. 2009:1573–7225. doi: 10.1007/s10552-009-9472-2. [DOI] [PubMed] [Google Scholar]
- 24.Sheppard VB, Mays D, LaVeist T, Tercyak KP. Medical mistrust influences black women’s level of engagement in BRCA 1/2 genetic counseling and testing. J Natl Med Assoc. 2013;105(1):17–22. doi: 10.1016/s0027-9684(15)30081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagos VI, Perez MA, Ricker CN, et al. Social-cognitive aspects of underserved latinas preparing to undergo genetic cancer risk assessment for hereditary breast and ovarian cancer. [Accessed 20080813];Psychooncology. 2008 17(8):774–782. doi: 10.1002/pon.1358. [DOI] [PubMed] [Google Scholar]
- 26.Sheppard VB, Adams IF, Lamdan R, Taylor KL. The role of patient-provider communication for black women making decisions about breast cancer treatment. Psychooncology. 2011;20(12):1309–1316. doi: 10.1002/pon.1852. [DOI] [PubMed] [Google Scholar]
- 27.Guth AA, Fineberg S, Fei K, Franco R, Bickell NA. Utilization of oncotype DX in an inner city population: Race or place? Int J Breast Cancer. 2013;2013:653805. doi: 10.1155/2013/653805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Underhill ML, Kiviniemi MT. The association of perceived provider-patient communication and relationship quality with colorectal cancer screening. Health Educ Behav. 2012;39(5):555–563. doi: 10.1177/1090198111421800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandelblatt JS, Sheppard VB, Hurria A, et al. Breast cancer adjuvant chemotherapy decisions in older women: The role of patient preference and interactions with physicians. J Clin Oncol. 2010;28(19):3146–3153. doi: 10.1200/JCO.2009.24.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graves KD, JLC, TMH, Peshkin BN, Isaacs C, VBS Providers’ perceptions and practices regarding/BRCA1/2/genetic counseling and testing in African American women. Journal of Genetic Counseling. 2011 doi: 10.1007/s10897-011-9396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipkus IM, Vadaparampil ST, Jacobsen PB, Miree CA. Knowledge about genomic recurrence risk testing among breast cancer survivors. J Cancer Educ. 2011;26(4):664–669. doi: 10.1007/s13187-011-0248-5. [DOI] [PubMed] [Google Scholar]
- 32.Tzeng JP, Mayer D, Richman AR, et al. Women’s experiences with genomic testing for breast cancer recurrence risk. Cancer. 2010;116(8):1992–2000. doi: 10.1002/cncr.24990. [DOI] [PubMed] [Google Scholar]
- 33.Sheppard VB, Isaacs C, Luta G, et al. Narrowing racial gaps in breast cancer chemotherapy initiation: The role of the patient-provider relationship. Breast Cancer Res Treat. 2013;139(1):207–216. doi: 10.1007/s10549-013-2520-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Comprehensive Cancer Network. Breast cancer treatment guidelines for patients NCCN v. VIII. 2006. [Google Scholar]
- 35.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizzo M, Lund MJ, Mosunjac M, et al. Characteristics and treatment modalities for African American women diagnosed with stage III breast cancer. Cancer. 2009;115(13):3009–3015. doi: 10.1002/cncr.24334. 0008-543; 0008-543. [DOI] [PubMed] [Google Scholar]
- 37.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. 0021-9681; 0021-9681. [DOI] [PubMed] [Google Scholar]
- 38.Bird ST, Bogart LM. Perceived race-based and socioeconomic status(SES)-based discrimination in interactions with health care providers. Ethn Dis. 2001;11(3):554–563. 1049-510. [PubMed] [Google Scholar]
- 39.Makoul G, Arntson P, Schofield T. Health promotion in primary care: Physician-patient communication and decision making about prescription medications. Soc Sci Med. 1995;41(9):1241–1254. doi: 10.1016/0277-9536(95)00061-b. 0277-9536. [DOI] [PubMed] [Google Scholar]
- 40.Sheppard VB, Isaacs C, Luta G, et al. Narrowing racial gaps in breast cancer chemotherapy initiation: The role of the patient-provider relationship. Breast Cancer Res Treat. 2013;139(1):207–216. doi: 10.1007/s10549-013-2520-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson EA, Montgomery A, Douglas D, Reilly D. A pilot, randomized, double-blinded, placebo-controlled trial of individualized homeopathy for symptoms of estrogen withdrawal in breast-cancer survivors. J Altern Complement Med. 2005;11(1):13–20. doi: 10.1089/acm.2005.11.13. 1075-5535. [DOI] [PubMed] [Google Scholar]
- 42.Wolf MS, Chang CH, Davis T, Makoul G. Development and validation of the communication and attitudinal self-efficacy scale for cancer (CASE-cancer) Patient Educ Couns. 2005;57(3):333–341. doi: 10.1016/j.pec.2004.09.005. 0738-3991. [DOI] [PubMed] [Google Scholar]
- 43.Lukwago SN, Kreuter MW, Bucholtz DC, Holt CL, Clark EM. Development and validation of brief scales to measure collectivism, religiosity, racial pride, and time orientation in urban African American women. Fam Community Health. 2001;24(3):63–71. doi: 10.1097/00003727-200110000-00008. 0160-6379. [DOI] [PubMed] [Google Scholar]
- 44.Chen C, Dhanda R, Tseng WY, Forsyth M, Patt DA. Evaluating use characteristics for the oncotype dx 21-gene recurrence score and concordance with chemotherapy use in early-stage breast cancer. J Oncol Pract. 2013;9(4):182–187. doi: 10.1200/JOP.2012.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen MT, Stessin A, Nagar H, et al. Impact of oncotype DX recurrence score in the management of breast cancer cases. Clin Breast Cancer. 2013 doi: 10.1016/j.clbc.2013.12.002. S1526-8209(13)00307-8 [pii] [DOI] [PubMed] [Google Scholar]
- 46.DeFrank JT, Salz T, Reeder-Hayes K, Brewer NT. Who gets genomic testing for breast cancer recurrence risk? Public Health Genomics. 2013;16(5):215–222. doi: 10.1159/000353518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the southwest oncology group. J Natl Cancer Inst. 2009;101(14):984–992. doi: 10.1093/jnci/djp175. 1460-2105; 0027-8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mandelblatt JS, Sheppard VB, Hurria A, et al. Breast cancer adjuvant chemotherapy decisions in older women: The role of patient preference and interactions with physicians. J Clin Oncol. 2010;1(28):3146–3156. doi: 10.1200/JCO.2009.24.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siegelmann-Danieli N, Silverman B, Zick A, Beit-Or A, Katzir I, Porath A. The impact of the oncotype DX recurrence score on treatment decisions and clinical outcomes in patients with early breast cancer: The Maccabi healthcare services experience with a unified testing policy. Ecancermedical science. 2013;7:380. doi: 10.3332/ecancer.2013.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richman AR, Tzeng JP, Carey LA, Retel VP, Brewer NT. Knowledge of genomic testing among early-stage breast cancer patients. Psychooncology. 2011;20(1):28–35. doi: 10.1002/pon.1699. [DOI] [PubMed] [Google Scholar]
- 51.Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemp Clin Trials. 2013;34(1):1–9. doi: 10.1016/j.cct.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.KA, Micco E, Carney A, JS, Putt M. Racial differences in the use of BRCA1/2 testing among women with family history of breast or ovarian cancer. JAMA. 2005;293(14):1729–1736. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- 53.Susswein LR, Skrzynia C, Lange LA, Booker JK, Graham ML, 3rd, Evans JP. Increased uptake of BRCA1/2 genetic testing among African American women with a recent diagnosis of breast cancer. J Clin Oncol. 2008;26(1):32–36. doi: 10.1200/JCO.2007.10.6377. [DOI] [PubMed] [Google Scholar]
- 54.Silva A, Rauscher GH, Hoskins K, Rao R, Ferrans CE. Assessing racial/ethnic disparities in chemotherapy treatment among breast cancer patients in context of changing treatment guidelines. Breast Cancer Res Treat. 2013;142(3):667–672. doi: 10.1007/s10549-013-2759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vataire AL, Laas E, Aballea S, Gligorov J, Rouzier R, Chereau E. Cost-effectiveness of a chemotherapy predictive test. Bull Cancer. 2012;99(10):907–914. doi: 10.1684/bdc.2012.1652. [DOI] [PubMed] [Google Scholar]