Abstract
ATP11C is a homolog of ATP8B1, both of which catalyze the transport of phospholipids in biological membranes. Mutations in ATP8B1 cause progressive familial intrahepatic cholestasis type1 in humans, which is characterized by a canalicular cholestasis. Mice deficient in ATP11C are characterized by a conjugated hyperbilirubinemia and an unconjugated hypercholanemia. Here, we have studied the hypothesis that ATP11C deficiency interferes with basolateral uptake of unconjugated bile salts, a process mediated by organic anion-transporting polypeptide (OATP) 1B2. ATP11C localized to the basolateral membrane of central hepatocytes in the liver lobule of control mice. In ATP11C-deficient mice, plasma total bilirubin levels were 6-fold increased, compared to control, of which ~65% was conjugated and ~35% unconjugated. Plasma total bile salts were 10-fold increased and were mostly present as unconjugated species. Functional studies in ATP11C-deficient mice indicated that hepatic uptake of unconjugated bile salts was strongly impaired whereas uptake of conjugated bile salts was unaffected. Western blotting and immunofluorescence analysis demonstrated near absence of basolateral bile salt uptake transporters OATP1B2, OATP1A1, OATP1A4, and Na+-taurocholate-cotransporting polypeptide only in central hepatocytes of ATP11C-deficient liver. In vivo application of the proteasome inhibitor, bortezomib, partially restored expression of these proteins, but not their localization. Furthermore, we observed post-translational down-regulation of ATP11C protein in livers from cholestatic mice, which coincided with reduced OATP1B2 levels.
Conclusions
ATP11C is essential for basolateral membrane localization of multiple bile salt transport proteins in central hepatocytes and may act as a gatekeeper to prevent hepatic bile salt overload. Conjugated hyperbilirubinemia and unconjugated hypercholanemia and loss of OATP expression in ATP11C-deficient liver strongly resemble the characteristics of Rotor syndrome, suggesting that mutations in ATP11C can predispose to Rotor syndrome.
ATP11C belongs to the P4 type family of P-type ATPases (P4 ATPase), members of which catalyze the intramembranous transport of phospholipids and are termed phospholipid flippases.(1) Phospholipid flippases are important for creation of phospholipid asymmetry in biological membranes and for biogenesis of intracellular transport vesicles.(2) Like most P4 ATPases, ATP11C functions as a heterodimer with CDC50A protein, in which ATP11C is the α-subunit and CDC50A the β-subunit.(3) Assembly of α- and β-subunit is essential for endoplasmic reticulum (ER) exit and activity of the heterodimer.(4–6) Recently, two laboratories independently generated multiple ATP11C-deficient mice denoted emptyhive, spelling, and ambrosius that all displayed similar phenotypes, that is, a B-cell lymphopenia and a conjugated hyperbilirubinemia.(7–9) Atp11c hemizygous males and homozygous females displayed a chronic conjugated hyperbilirubinemia with relatively high levels in juvenile animals that decline gradually overtime, but do not normalize to littermate control values.(8) In a follow-up study, Siggs et al.(7) showed that ATP11C-deficient mice also accumulated bile salts. Both hypercholanemia and hyperbilirubinemia were independent of B-cell lymphopenia, identifying ATP11C as a P4 ATPase essential for normal liver function.
ATP11C belongs to the same family of proteins as ATP8B1, the deficiency of which results in progressive familial intrahepatic cholestasis type 1 (PFIC1) and benign recurrent intrahepatic cholestasis type 1.(10) Using ATP8B1-deficient mice, we have shown previously that ATP8B1 plays a crucial role in maintaining lipid asymmetry of the canalicular membrane of hepatocytes, thereby providing protection to high bile salt concentrations present in the canalicular lumen.(1) ATP8B1-deficient mice displayed no hyperbilirubinemia; however, plasma bile salts were significantly elevated at baseline to ~15–75 μM and were predominantly conjugated.(11) Despite elevated plasma bile salt levels, no overt liver damage was observed. When fed a cholate-supplemented diet, ATP8B1-deficient mice develop severe cholestasis.(12)
In ATP11C-deficient mice, plasma bile salts at baseline were elevated to ~90 μM with cholate as the main species.(7) Only mild liver pathology was observed, that is, extracellular matrix deposition and portal inflammation. From these data, the researchers hypothesized that, analogous to ATP8B1, ATP11C may have an important role in regulation of canalicular membrane asymmetry, the regulation of canalicular membrane protein trafficking or intrahepatic bile salt transport.(7) Thus far, however, the molecular mechanisms underlying the conjugated hyperbilirubinemia/hypercholanemia attributed to ATP11C deficiency have not been elucidated.
We have now studied how hepatic ATP11C deficiency causes conjugated hyperbilirubinemia/hypercholanemia. Our data indicate that ATP11C, together with its β-subunit, CDC50A, is essential for basolateral localization of multiple bile salt uptake transporters in central hepatocytes and may act as a novel gatekeeper of bile salt overload.
Materials and Methods
Details about western blotting, quantitative polymerase chain reaction analysis of gene expression and immunofluorescent stainings are provided in the Supporting Information.
MATERIALS AND ANTIBODIES
Cholyl-lysyl-fluorescein (CLF) was a generous gift from Norgine International Ltd. (Amsterdam, The Netherlands). Acetonitrile (high-performance liquid chromatography [HPLC] grade) was from Biosolve (Lexington, MA). [3H]taurocholate ([3H]TC) and [3H]cholate ([3H]CA) were a generous gift of Alan Hofmann. Muricholate bile salts were obtained from Steraloids (Newport, RI) and bortezomib from Selleckchem.com. All other chemicals were from Sigma-Aldrich (St. Louis, MO). Rabbit polyclonal antibodies used were: anti-ATP11C (AP5446c; Abgent, San Diego, CA); anti-CDC50A,(13) anti-organic anion-transporting polypeptide (OATP) 1A1, Na+-taurocholate-cotransporting polypeptide (NTCP), multidrug resistance-associated protein 2 (MRP2), and bile salt export pump (BSEP; kindly provided by Prof. B. Stieger); anti-OATP1B2 (kindly provided by Dr. Richard Kim(14)); anti-ATP1A1 (kindly provided by Dr. J. Koenderink)(15); anti-ATP8B1 (kindly provided by Dr. Leo Klomp)(11); mouse monoclonals to glutamine synthetase (BD Transduction Laboratories, San Jose, CA); and goat monoclonal to OATP1A4 (sc-18436; Santa Cruz Biotechnology, Santa Cruz, CA).
ANIMALS
ATP11C-deficient mice have a one-nucleotide mutation in the X-linked Atp11c gene, introducing a stop codon at amino acid 655 (also called Atp11cemp for emptyhive) and were generated by chemical mutagenesis as described (kindly provided by Drs. B. Beutler and O. Siggs(8)). Experiments were performed with hemizygous and littermate control male mice. ATP8B1-deficient mice and feeding regimens were described.(16) All animals were on a C57BL/6J background. For adeno-associated virus serotype 8 (AAV8)-mediated knockdown experiments, FVB mice were obtained from Harlan Laboratories (Indianapolis, IN). Animal experiments were approved by the Institutional Animal Care and Use Committee of the Academic Medical Center (AMC; Amsterdam, The Netherlands).
PLASMA BIOCHEMISTRY AND BILE SALT SPECIES DETERMINATION
Alanine aminotransferase (ALT), alkaline phosphatase (ALP), and aspartate aminotransferase (AST) were determined by the clinical chemistry lab of the AMC. Feces were treated with 10 volumes of 50% tertiary butanol at 4°C. Homogenates were sonicated and centrifuged (10 minutes, 20,000g). The supernatant was dried at 60°C, and the residue was dissolved in 25% methanol. Bile salt species were quantified by reverse-phase HPLC using a Nano Quantity Analyte Detector QT-500 (Quant Technologies, Minneapolis, MN) as described.(17) Bilirubin (conjugates) were measured with HPLC as described.(18)
HEPATIC BILE SALT UPTAKE AND BILIARY SECRETION IN MICE
Mice were anesthetized with a mix of Hypnorm (11.8 mg/kg of fluanisone and 0.37 mg/kg of fentanyl-citrate; VetaPharma Ltd., Sherburn in Elmet, UK) and diazepam (5.9 mg/kg of valium; Centrafarm B.V., Etten-Leur, The Netherlands). Body temperature was maintained at 36 ± 1°C on thermostatted heating pads. Gallbladders were cannulated with PE10 polyethylene tubing and CLF (100 μL 1 mM), [3H]CA (100 μL 0.5 mM), or [3H]TC (100 μL 2 mM) were injected in the jugular vein. Bile was collected in 10-minute fractions; blood was drawn at the end of the experiment. CLF-containing samples were diluted with 0.1% Triton X-100 and quantitated by fluorimetric measurement at λexc 485 nm and λem 520 nm using a NovoStar analyzer (BMG LABTECH, Ortenberg, Germany).(19) Radioactivity was counted in a scintillation counter.
KNOCKDOWN CDC50A IN HEPATOCYTES OF WILD-TYPE MICE
Wild-type (WT) FVB mice were injected with AAV8 through the tail vein, delivering a short hairpin RNA (shRNA) to Cdc50a or a short hairpin control in a single dose of 1 × 10e13 gc/kg diluted in physiological salt solution. Seven days postinjection, mice were subjected to CLF perfusion experiments as described above.
STATISTICAL ANALYSIS
Statistical differences were determined by an unpaired Student t test. All data were expressed as means ± standard deviation.
Results
PLASMA CONCENTRATIONS OF BILIRUBIN AND BILE SALT SPECIES IN ATP11C-DEFICIENT MICE
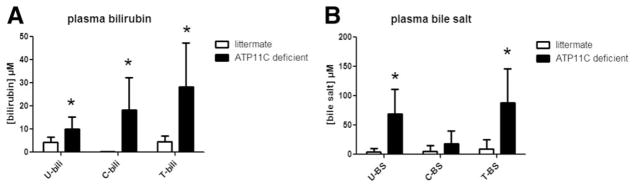
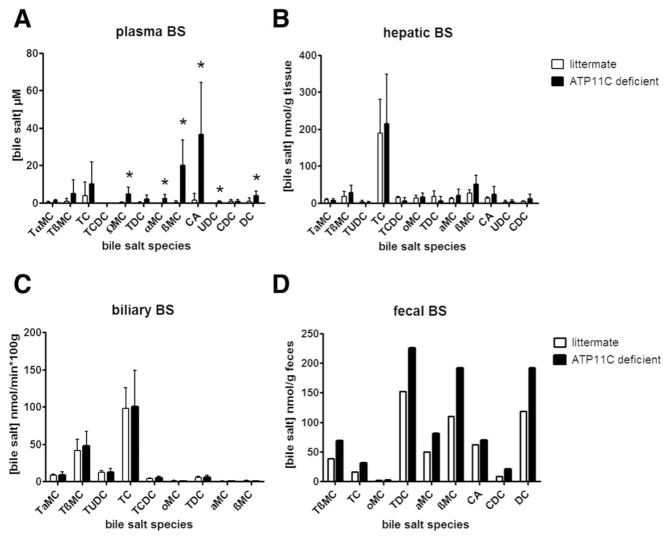
ATP11C-deficient mice did not display elevated plasma AST, ALP, and ALT levels (Supporting Fig. 1) and had elevated plasma total bilirubin (~6-fold) and total bile salt (~10-fold) levels compared to litter-mate controls (Fig. 1).(7) Plasma bilirubin was present as conjugated bilirubin (64 ± 49% of total and almost exclusively bilirubin monoglucuronides [BMGs; Supporting Fig. 2]) and a significant fraction of unconjugated bilirubin (UCB; 36 ± 18% of total; Fig. 1A). Plasma bile salts were mostly present as unconjugated bile salts (78% ± 48% of total; Fig. 1B). Bile salt species determination showed that concentrations of all unconjugated bile salts, but not (taurine-)conjugated bile salts, were increased, (Fig. 2A). No changes in distribution of hepatic and biliary bile salt species were observed (Fig. 2B,C). Fecal bile salt output was slightly elevated in ATP11C-deficient mice (Fig. 2D).
FIG. 1.
Plasma bilirubin (A) and bile salt (B) levels. Data show means ± standard deviation for male ATP11C-deficient (n = 9) and littermate control mice (n =5). U =unconjugated, C = conjugated, T = total, bili = bilirubin, and BS = bile salts. Statistical significance was tested by a Student t test: *P < 0.05.
FIG. 2.
Analysis of bile salt species in plasma (A), liver (B), bile (C), and feces (D). Data show means ± standard deviation for ATP11C-deficient (n = 9) and littermate controls (n = 5). T = tauro, MC = muricholate, C = cholate, CDC = chenodeoxycholate, DC = deoxycholate, and UDC = ursodeoxycholate. Plasma levels are expressed in μM, hepatic levels in nmol/g liver, biliary and fecal output in nmol/min*100 g and nmol/g feces, respectively. Standard deviation is not included in the fecal determinations because feces were collected from cages housing 3 ATP11C-deficient or 3 littermate controls. Statistical significance was tested by a Student t test: *P < 0.05.
HEPATIC UPTAKE OF BILE SALTS IN ATP11C-DEFICIENT MICE
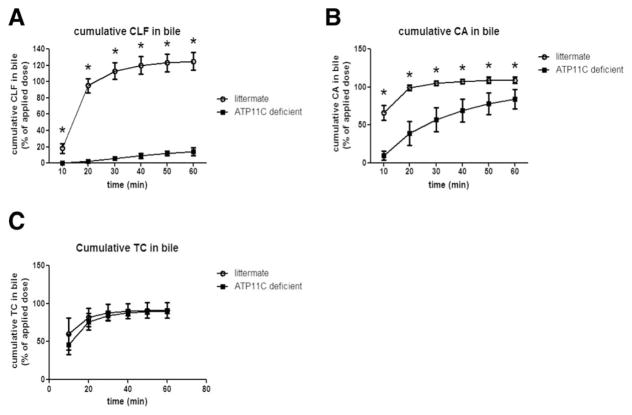
Because the Atp11c phenotype strongly resembled that of Oatp1a/1b−/− mice,(18) which have a defect in the basolateral uptake of unconjugated bile salts and BMG, we studied hepatic uptake of the fluorescently labeled bile salt analog, CLF, CA, and TC, by measuring appearance in bile after bolus application. CLF is taken up by OATP-mediated transport and is excreted into bile by MRP2.(19) Figure 3A shows that already 30 minutes after CLF administration, 113 ± 10% of the administrated dose was recovered in bile of litter-mate controls, whereas this was approximately 6 ± 2% in ATP11C-deficient mice. The amount of CLF recovered in plasma at the end of the experiment (60 minutes) was 91 ± 35% and 0.3 ± 0.1% of the applied dose in ATP11C-deficient mice and littermate controls, respectively. Similarly, administration of CA led to almost 100% recovery in bile of littermates 20 minutes after administration as opposed to 39 ± 16% in ATP11C-deficient mice (Fig. 3B). At the end of the experiment, 3.89 ± 2.03% and 0.029 ± 0.003% of the applied dose was recovered in plasma of ATP11C-deficient mice and littermate controls, respectively. In contrast, no differences were observed between littermates and ATP11C-deficient mice in the hepatic handling of TC (Fig. 3C). Furthermore, bile formation was not affected, as demonstrated by intravenous infusion of increasing concentrations of TC (Supporting Fig. 3). These data indicate that ATP11C deficiency impairs hepatic OATP-mediated uptake of unconjugated bile salts, but does not affect canalicular excretion.
FIG. 3.
Biliary excretion of CLF and CA, but not of TC, is impaired in ATP11C-deficient mice. Mean cumulative appearance ± standard deviation of CLF (A), CA (B), and TC (C) in bile of ATP11C-deficient mice (n = 4) and littermate controls (n = 4) is shown. Data are expressed as percent of the applied dose. Statistical significance was tested by a Student t test: *P < 0.05.
PROTEIN LEVELS OF BASOLATERAL MEMBRANE TRANSPORTERS ARE REDUCED IN ATP11C-DEFICIENT LIVER
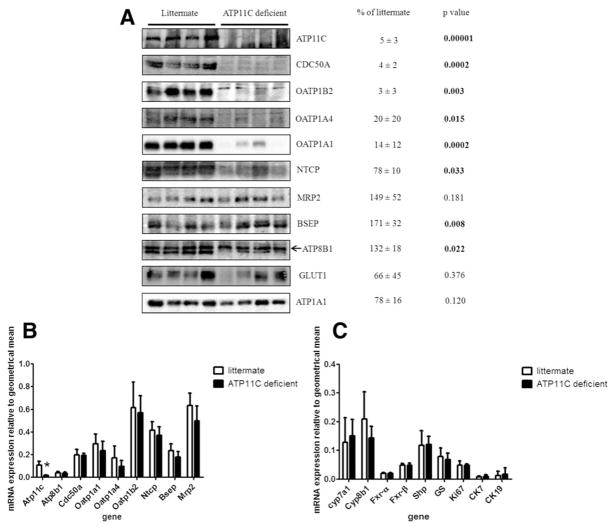
Protein levels of membrane proteins involved in bile salt transport were studied by western blotting analyses of liver membrane preparations (Fig. 4A). Interestingly, protein levels of the basolateral-localized proteins, OATP1B2, OATP1A1, and OATP1A4, were dramatically (>90%) reduced, whereas levels of NTCP were reduced to 78 ± 10% of litter controls. The β-subunit for ATP11C, CDC50A, was virtually absent from ATP11C-deficient liver. Protein levels of several basolateral membrane proteins not involved in bile salt transport, including glucose transporter 1 (GLUT1) and ATP1A1, were not affected. Similarly, protein levels of the canalicular transport proteins, MRP2, BSEP, and ATP8B1, were unaffected or increased. The protein level of a protein that cross-reacts with the ATP8B1 antibody (and which may be a close homolog of ATP8B1 of yet unknown identity [Supporting Fig. 4]) was reduced in ATP11C-deficient liver (20 ± 19% of litter controls; P = 0.002).
FIG. 4.
Protein levels and mRNA expression of proteins and genes involved in bile salt transport and homeostasis. (A) Western blotting analysis of mouse liver plasma membranes isolated from ATP11C-deficient and littermate control liver. Arrow indicates protein band corresponding to ATP8B1 (see Supporting Fig. 4; the lower band does not represent ATP8B1 because it is present in the ATP8B1-deficient liver, but may represent a related P4 ATPase the identity of which we have not yet characterized). Protein levels were quantified by densitometric analysis using ImageJ software (National Institutes of Health, Bethesda, MD), normalized to ATP1A1 and expressed as mean percentage ± standard deviation of protein present in ATP11C-deficient versus littermate control liver (n = 4). (B,C) Mean relative mRNA expression levels ± standard deviation of indicated genes in liver of ATP11C-deficient (n = 4) mice and littermate controls (n = 4). Statistical significance was tested by a Student t test: *P < 0.05. Abbreviations: CK, cytokeratin; Fxr, farnesoid X receptor; Shp, small heterodimer partner.
MESSENGER RNA EXPRESSION OF GENES INVOLVED IN BILE SALT METABOLISM
To analyze whether reduced basolateral protein expression in ATP11C-deficient liver was attributed to transcriptional down-regulation, we determined their messenger RNA (mRNA) levels. Figure 4B,C shows that mRNA levels of Oatp1b2, Oatp1a1, Oatp1a4, and Ntcp were unaffected in ATP11C-deficient liver. mRNA levels of canalicular transporters Mrp2 and Bsep, nuclear receptors Shp and Fxr, and bile salt synthesis enzymes Cyp7a1 and Cyp8b1 were also not affected. Atp11c expression was strongly reduced, probably caused by increased degradation often observed for mRNAs with a nonsense mutation causing a premature stop codon (nonsense-mediated mRNA decay). mRNA expression levels of the proliferation marker, Ki67, and of cytokeratins 7 and 19, markers for hepatic progenitor cells, cholangiocytes, and hepatocellular carcinoma, were not affected, confirming the absence of significant hepatocyte damage. These data indicate that reduced presence of basolateral bile salt transport proteins in ATP11C-deficient liver is not attributed to reduced gene expression, but is caused by a post-transcriptional mechanism.
LOCALIZATION OF TRANSPORT PROTEINS IN ATP11C-DEFICIENT LIVER
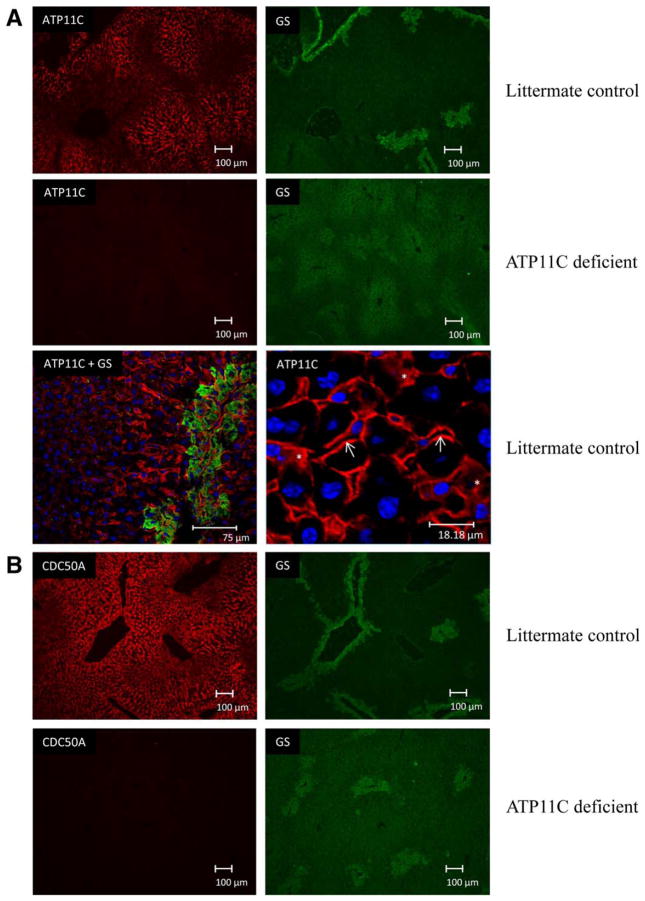
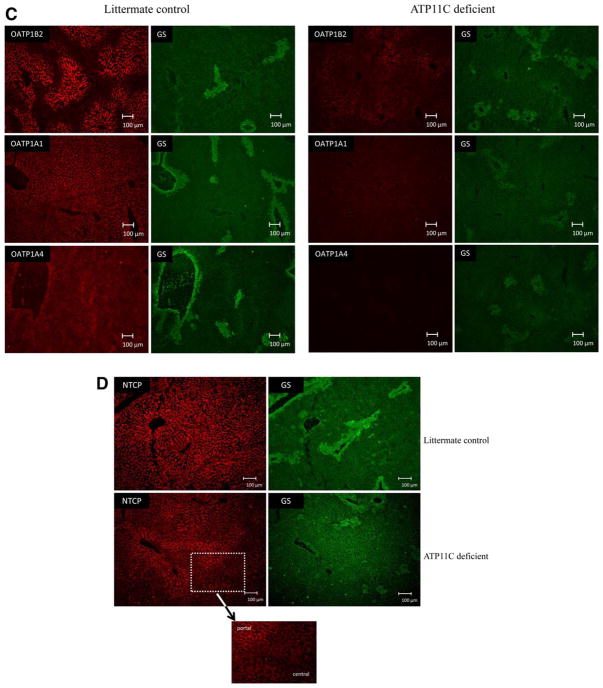
Next, we analyzed the localization of hepatic transporters in ATP11C-deficient liver. ATP11C immunostaining was detected in membranes of cells lining the hepatic sinusoids and was restricted to the pericentral area of the liver lobule, as evidenced by costaining with the centrally expressed enzyme glutamine synthetase (GS; Fig. 5A). Confocal imaging and higher magnification also indicated intracellular ATP11C immunostaining. A similar distribution pattern was observed for CDC50A, the β-subunit for ATP11C (Fig. 5B). ATP11C and CDC50A immunostaining were completely absent from ATP11C-deficient liver. OATP1B2 immunostaining was restricted to the basolateral membrane of central hepatocytes in control liver. In ATP11C-deficient liver, this staining was virtually absent (Fig. 5C). A similar immunostaining pattern was observed for OATP1A1 and OATP1A4, both of which were below detection in ATP11C-deficient liver. Strikingly, basolateral NTCP immunostaining was homogenously distributed throughout the liver lobule of control liver, however, was almost completely absent only from central hepatocytes in ATP11C-deficient liver (Fig. 5D). Immunostaining of basolateral non-bile-salt transporters GLUT1 and ATP1A1 and of canalicular proteins, including BSEP, MRP2, and ATP8B1, were not different between mutant and control liver (Supporting Fig. 5). These results indicate that ATP11C is essential for correct localization of multiple basolateral bile salt transport proteins, but only in central hepatocytes.
FIG. 5.
Immunofluorescent imaging of ATP11C and various basolateral bile salt transporters. (A) ATP11C immunostaining is concentrated in the pericentral area of the liver (indicated by GS immunostaining), localizes to basolateral membrane in littermate controls and is absent from ATP11C-deficient livers. Confocal images highlight ATP11C membrane (indicated by a white arrow) and intra-cellular staining (indicated by an asterisk). (B) CDC50A localizes to the basolateral membrane of central hepatocytes and is below detection limits in ATP11C-deficient liver. (C) OATP1B2, OATP1A1, and OATP1A4 immunostaining is detected in the pericentral area of control liver and is hardly detectable in ATP11C-deficient liver. (D) NTCP immunostaining is homogenously detected in control liver and is absent from the pericentral area of the liver lobule in ATP11C-deficient liver (see insert). GS stains are included to indicate the central hepatocytes. Blue: nuclear 4′,6-diamidino-2-phenylindole staining. Bar represents depicted length in μm.
ATP11C AND CDC50A FORM A FUNCTIONAL HETERODIMER
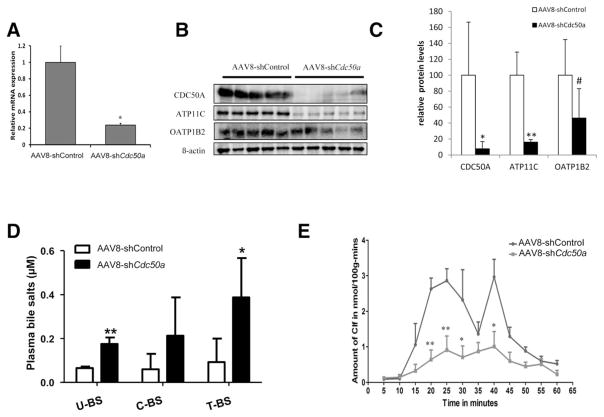
The near-absence of CDC50A protein in ATP11C-deficient liver and the overlapping localization of ATP11C and CDC50A suggest that they function as a heterodimer. To study this, we depleted CDC50A in hepatocytes of WT mice by AAV8-mediated delivery of shRNAs to Cdc50a and analyzed ATP11C and OATP1B2 expression, plasma bile salts, and hepatic CLF handling (Fig. 6). No liver damage or toxicity of treatment was observed in mice. Cdc50a mRNA and protein levels were reduced by ~80% and ~90%, respectively, whereas ATP11C protein was reduced by ~80 %. ATP8B1 protein levels were not affected (Supporting Fig. 6). OATP1B2 expression was reduced by ~50%, which coincided with a significant increase in plasma unconjugated bile salt concentrations. Similar to ATP11C-deficient mice, biliary recovery of bolus-injected CLF was significantly delayed in shCdc50a-treated mice. These data indicate that basolateral localization of OATP1B2 depends both on ATP11C and CDC50A, and strongly suggest that ATP11C and CDC50A function as a heterodimer in central hepatocytes.
FIG. 6.
Hepatic Cdc50a knockdown mice phenocopy ATP11C-deficient mice. WT FVB mice were injected with AAV8-shControl (n = 5) or AAV8-shCdc50a (n = 5) and livers were analyzed for RNA, protein, plasma bile salts, and CLF clearance. All data are expressed as mean ± standard deviation. (A) Cdc50a mRNA expression. *P < 0.005. (B) Western blotting analysis of CDC50A, ATP11C, and OATP1B2 protein in liver of Cdc50a knockdown mice. (C) Quantification of protein levels (normalized to β-actin) in (B) by densitometric analysis. Statistical significance was tested by a Student t test: *P = 0.01; **P = 0.0002; #P = 0.07. (D) Plasma bile salt levels are expressed as means of 5 different mice and are significantly elevated in Cdc50a knockdown mice: *P < 0.05; **P < 0.01. U = unconjugated, C = conjugated, T = total, and BS = bile salts. (E) Recovery of biliary CLF, after jugular vein injection of 1 mmol/L of CLF, is delayed in Cdc50a knockdown mice. Statistical significance was tested by a Student t test: *P < 0.05; **P < 0.01.
PROTEASOMAL DEGRADATION OF BASOLATERAL TARGETED PROTEINS
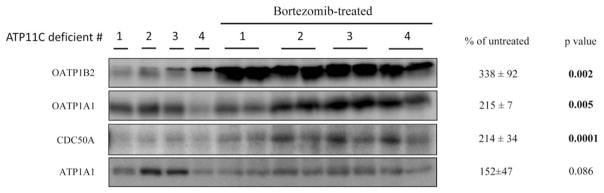
We hypothesized that ATP11C mediates targeting of bile salt uptake transporters to the basolateral membrane and that, in ATP11C deficient hepatocytes, basolateral proteins are retained in the ER or trans-Golgi network (TGN) and, consequently, are targeted for proteasomal degradation. Proteasome inhibition studies in isolated primary hepatocytes failed because of the very poor quality and survival (i.e., less than 10%) of ATP11C-deficient hepatocytes (not shown). Therefore, the effect of proteasome inhibition was studied in vivo. ATP11C-deficient mice were treated with the proteasome inhibitor, bortezomib, for 6 hours, and livers were analyzed for OATP1B2, OATP1A1, and CDC50A protein levels by western analysis. Figure 7 shows that bortezomib treatment resulted in a partial recovery of mature, fully glycosylated protein levels. However, no restored plasma membrane localization of the proteins was observed (Supporting Fig. 7). OATP1A4 protein levels remained below detection after bortezomib treatment. These data suggest that in ATP11C-deficient liver, basolateral transporters are targeted for proteasomal degradation.
FIG. 7.
In vivo inhibition of the proteasome by bortezomib partially restores OATP1B2, OATP1A1, and CDC50A protein expression in ATP11C-deficient mice. ATP11C-deficient mice were treated with vehicle (n = 4) or with 0.5 mg/kg of bortezomib (n = 4) and liver proteins were extracted. From bortezomib-treated livers, protein was extracted from two different lobules. Protein levels were quantified by densitometric analysis, normalized to ATP1A1, and expressed as mean percentage ± standard deviation of protein levels in bortezomib-treated Atp11c mutants versus vehicle-treated ATP11C-deficient mice. Statistical significance was determined by a Student t test.
ATP11C EXPRESSION IS DOWN-REGULATED IN LIVERS OF CHOLESTATIC ATP8B1-DEFICIENT MICE
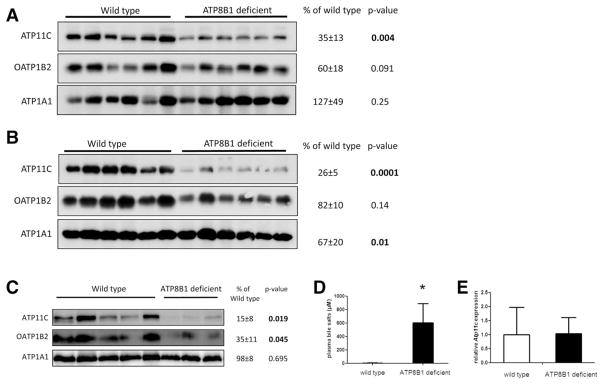
Given that ATP11C deficiency impaired expression of all basolateral bile salt uptake transporters, we hypothesized that ATP11C could have a role in protection of the liver to bile salt overload by regulating OATP protein levels. It has been shown recently that caspase activation (during apoptosis) leads to cleavage and inactivation of ATP11C.(21) We reasoned that ATP11C inactivation and consequent impairment of OATP trafficking might represent a protective mechanism against bile salt overload. We thus analyzed ATP11C and OATP1B2 protein levels in livers from WT and ATP8B1-deficient mice fed a control- or a 0.5% CA-supplemented diet for 6 days. Even though serum bile salt levels are minimally increased in control-fed ATP8B1-deficient mice,(22) hepatic ATP11C and OATP1B2 protein levels were ~60% and ~40% reduced, respectively, in ATP8B1-deficient mice compared to WTs (Fig. 8A). Feeding ATP8B1-deficient mice a 0.5% CA-supplemented diet for 6 days resulted in a further decrease of these proteins (~75% and ~20% for ATP11C and OATP1B2, respectively; Fig. 8B); this treatment was accompanied by a ~30-fold increase in plasma bile salt levels.(22) In an independent set of liver samples from WT and ATP8B1-deficient mice fed a 0.1% CA-supplemented diet for a longer period (12 days), ATP11C and OATP1B2 protein levels were ~85% and ~65% reduced, respectively, in ATP8B1-deficient mice (Fig. 8C), which coincided with a ~150-fold increase in plasma bile salt levels (Fig. 8D). Most important, even under this condition, Atp11c mRNA expression was unaffected (Fig. 8E). These data indicate that ATP11C levels in cholestatic mice are regulated at the post-translational level, and suggest that ATP11C is important for protecting the liver to bile salt overload by regulating protein levels of bile salt uptake transporters.
FIG. 8.
ATP11C is post-translationally down-regulated in cholestatic liver. (A) ATP11C and OATP1B2 levels in liver of WT (n = 6) and ATP8B1-deficient mice (n = 6) fed a control diet for 6 days. (B) ATP11C and OATP1B2 levels in liver of WT and ATP8B1-deficient mice fed a 0.5% CA-supplemented diet for 6 days. (C) ATP11C and OATP1B2 levels in liver of WT (n = 5) and ATP8B1-deficient mice (n = 3) fed a 0.1% CA-supplemented diet for 12 days. ATP1A1 is included as a loading control. Protein levels in all blots were quantified and expressed as mean percentage ± standard deviation of protein present in ATP8B1-deficient versus control liver. (D) Mean total bile salt levels ± standard deviation in plasma of control and ATP8B1-deficient mice fed a 0.1% CA-supplemented diet for 12 days. (E) Mean relative Atp11c mRNA expression levels ± standard deviation in liver of control (n = 6) and ATP8B1-deficient mice (n = 6) fed a 0.1% CA-supplemented diet for 12 days. Statistical significance was determined by a Student t test. *P < 0.004.
Discussion
Here, we show that the P4 ATPase, ATP11C, is expressed in central hepatocytes and is essential for basolateral localization of multiple bile salt uptake transporter proteins in these hepatocytes, including OATP1B2, OATP1A1, OATP1A4, and NTCP. In the absence of ATP11C, the level of these transporters is strongly reduced in pericentral hepatocytes, and, because the OATPs are predominantly expressed in zone 3, this reduction causes conjugated hyperbilirubinemia and unconjugated hypercholanemia. Our findings are in line with the data very recently published by Matsuzaka et al., who reported reduced OATP and NTCP protein levels in liver of ATP11C-deficient mice.(23)
ATP11C-deficient mice share several phenotypic characteristics with Oatp1b2−/− and Oatp1a/1b−/− mice, the latter of which are deficient not only in OATP1B2, but also in OATP1A1 and OATP1A4.(18,24) First, ATP11C-deficient, Oatp1a/1b−/− and Oatp1b2−/− mice display 14-, 20-, and 13-fold increased plasma levels of all unconjugated bile salts (except for chenodeoxycholate), compared to their controls. Given that OATP1B2 is the major uptake transporter for unconjugated bile salts, its reduced expression in these models seems to explain the hypercholanemia.(25,26) Second, ATP11C-deficient and Oatp1a/1b−/− mice have strongly increased (6- and 40-fold, respectively) plasma bilirubin levels, most of which is attributed to conjugated bilirubin (~67% and ~95% of total bilirubin in ATP11C-deficient and Oatp1a/1b−/− mice, respectively). The accumulation of monoglucuronides in plasma of ATP11C-deficient, Oatp1a/1b−/− and Oatp1b2−/− mice seems, as previously proposed by Van de Steeg et al., to be caused by impaired bilirubin monoglucuronide reuptake attributed to absence of these OATPs.(18) Third, ATP11C-deficient mice accumulated unconjugated bilirubin, as was also observed in Oatp1a/1b−/− mice.(18) This may be attributed to reduced uptake of unconjugated bilirubin by the hepatocyte because of the absence of one or more transporters. The observation by Van de Steeg et al. that the increase in unconjugated bilirubin levels in Oatp1a/1b−/− mice could be reversed by transgenic expression of the human orthologs of OATP1B2, OATP1B1, or OATP1B3 does suggest a potential role of these transporters in the uptake of this metabolite by the liver.(27) This is also supported by in vitro studies revealing a role of OATP1B1 in the selective uptake of UCB.(28) However, contradictory results have also been published, and given that UCB is very hydrophobic, passive diffusion over the hepatocyte membrane cannot be ruled out. Indeed, an alternative explanation for the mild accumulation of UCB could be the hemolytic anemia displayed by these ATP11C-deficient mice.(29)
Rotor syndrome is a rare and benign autosomal-recessive disorder characterized by a nonhemolytic, predominantly conjugated hyperbilrubinemia.(30) Recently, Van de Steeg et al. showed that Rotor syndrome is caused by combined deficiency of OATP1B1 and OATP1B3.(27) The phenotypic similarities between ATP11C-deficient mice and Oatp1a/1b−/− mice suggest that mutations in ATP11C in humans cause a Rotor syndrome-like phenotype. Given that Rotor syndrome is an asymptomatic disorder, patients with ATP11C mutations may also be difficult to identify. Furthermore, ATP11C deficiency in mice does not impair life expectancy despite the multiple phenotypes.(8,9,29) In contrast, homozygous ATP11C-deficient female mice displayed a high incidence (>50%) of dystocia, a condition leading to stillbirth.(7) It thus cannot be excluded that ATP11C deficiency-associated phenotypes are incompatible with life in humans. Searches of the Ensembl and PheGenI integrator databases do not report any association between ATP11C variants and human disease. Thus, ATP11C deficiency is either lethal or very rare or it does not present with clear symptoms.
In contrast to the transporter for unconjugated bile salts OATP1B2, the transporter for conjugated bile salts NTCP is homogenously distributed throughout the liver lobule with a faint portal-to-central gradient in control liver. Apparently, the uptake capacity for conjugated bile salts is spread over the entire lobule whereas for unconjugated bile salts this is restricted to central hepatocytes. As with OATP1B2, NTCP was absent from central hepatocytes of ATP11C-deficient livers; demonstrating its localization in these cells relies on the function of ATP11C. In portal hepatocytes that do not express ATP11C, however, NTCP expression was unaffected, suggesting a role for other proteins for its targeting in these cells. Lack of NTCP expression in central hepatocytes did not impair clearance capacity of conjugated bile salts, evidenced by normal biliary excretion and plasma clearance upon bolus application of TC.
CDC50A heterodimerizes with 11 of 14 mammalian P4 ATPases,(3,31) of which nine are expressed in the liver (J. Naik, unpublished data), and which is critical for ER exit and catalytic activity of the P4 ATPase. Hepatic ATP11C most likely is quantitatively the most important P4 ATPase to heterodimerize with CDC50A, and ATP11C deficiency obviously leads to strongly reduced CDC50A protein levels. ATP8B1, which deficiency causes PFIC1, also heterodimerizes with CDC50A.(31–33) Still, expression level and localization of ATP8B1 as well as canalicular bile formation were not affected in ATP11C-deficient livers. Apparently, in ATP11C-deficient hepatocytes, there still is sufficient CDC50A protein produced to interact with ATP8B1 (and other P4 ATPases). Alternatively, hepatic ATP8B1 heterodimerizes with CDC50B, a close homolog of CDC50A, which also can interact with ATP8B1 in vitro.(31,32) The near absence of CDC50A in ATP11C-deficient liver may thus be caused by ER retention and consequent proteasomal degradation of this protein attributed to absence of its major binding partner in hepatocytes, ATP11C. The partial restoration of CDC50A protein levels in ATP11C-deficient mice upon proteasome inhibition by bortezomib supports such a mechanism.
We propose a role for ATP11C-CDC50A heterodimer in basolateral targeting of membrane proteins from the TGN to the plasma membrane in central hepatocytes. By flipping phosphatidylserine to the cytosolic leaflet of the TGN membrane, ATP11C initiates a membrane curvature that provides a docking platform for proteins of the vesicle-generating machinery. ATP11C deficiency thus blocks this process, and, as a consequence, cargo proteins, including OATPs and NTCP, are targeted for proteasomal degradation. We could show that proteasome inhibition by bortezomib led to partial restoration of mature CDC50A and OATP expression, suggesting a defect at the TGN rather than in the ER. Indeed, multiple P4 ATPases have shown to fulfil critical roles in biogenesis and transport of intracellular transport vesicles in the biosynthetic and endocytic pathways.(1,2) For instance, ATP11B, a close homolog of ATP11C, has been implicated in the biogenesis and transport of cisplatin-containing vesicles at the TGN.(34) Furthermore, we have recently shown that ATP8B1 mediates the apical targeting of the apical sodium-dependent bile acid transporter SLC10A2/ASBT, either directly from the TGN or by recycling from a subapical vesicle pool, in intestinal Caco-2 cells.(33)
It was recently reported that ATP11C is acutely regulated at the post-translational level by caspase-mediated cleavage of the protein; ATP11C contains caspase-sensitive sites that are cleaved upon an apoptotic stimulus, which leads to inactivation of the protein.(21) The researchers also showed that this cleavage can be mediated by caspase 3, 6, and 7. This report, combined with our present data, support the hypothesis for a role of ATP11C in the protection of hepatocytes to bile salt overload. Indeed, in WT mice and cholestatic ATP8B1-deficient mice, we observed equal hepatic Atp11c mRNA levels, but we found reduced ATP11C protein levels that coincided with reduced OATP1B2 protein levels. It is well established that hydrophobic bile salts can induce apoptosis in hepatocytes involving activation of caspases(35) and that this process involves, among others, caspase 6.(36) Hence, bile-salt-induced activation of hepatic caspases may cleave ATP11C to restrict delivery of OATP1B2 to the basolateral plasma membrane and thereby limit uptake of bile salts in pericentral hepatocytes. This represents a novel level of protection of hepatocytes against bile salt overload.
In conclusion, we have characterized ATP11C as an important mediator of basolateral localization of the bile salt transport proteins, OATP1B2, OATP1A1, OATP1A4, and NTCP, in central hepatocytes of the liver lobule. In addition, ATP11C is identified as a possible gatekeeper to prevent bile-salt-induced hepatotoxicity and as a novel gene that may predispose to Rotor syndrome-like phenotypes in men.
Supplementary Material
Acknowledgments
We are grateful to Drs. B. Beutler and O. Siggs (The Scripps Research Institute, La Jolla, CA) for providing us with ATP11C-deficient mice. We thank Dr. Wout Lamers for helpful discussions.
Abbreviations
- AAV8
adeno-associated virus serotype 8
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AMC
Academic Medical Center
- AST
aspartate aminotransferase
- BDG
bilirubin diglucuronide
- BMG
bilirubin monoglucuronide
- BSEP
bile salt export pump
- CA
cholate
- CLF
cholyl-L-lysyl-fluorescein
- ER
endoplasmic reticulum
- GLUT1
glucose transporter 1
- GS
glutamine synthetase
- HPLC
high-performance liquid chromatography
- mRNA
messenger RNA
- MRP2
multidrug resistance-associated protein 2
- NTCP
Na+-taurocholate-cotransporting polypeptide
- OATP
organic anion-transporting polypeptide
- PFIC1
progressive familial intrahepatic cholestasis type 1
- shRNA
short hairpin RNA
- TC
taurocholate
- TGN
trans-Golgi network
- UCB
unconjugated bilirubin
- WT
wild type
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.28522/suppinfo.
References
- 1.Paulusma CC, Elferink RP. P4 Atpases—the physiological relevance of lipid flipping transporters. FEBS Lett. 2010;584:2708–2716. doi: 10.1016/j.febslet.2010.04.071. [DOI] [PubMed] [Google Scholar]
- 2.Sebastian TT, Baldridge RD, Xu P, Graham TR. Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim Biophys Acta. 2012;1821:1068–1077. doi: 10.1016/j.bbalip.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takatsu H, Baba K, Shima T, Umino H, Kato U, Umeda M, et al. Atp9b, a P4-Atpase (a putative aminophospholipid translocase), localizes to the trans-Golgi network in a Cdc50 protein-independent manner. J Biol Chem. 2011;286:38159–38167. doi: 10.1074/jbc.M111.281006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryde S, Hennrich H, Verhulst PM, Devaux PF, Lenoir G, Holthuis JC. Cdc50 Proteins are critical components of the human class-1 P4-Atpase transport machinery. J Biol Chem. 2010;285:40562–40572. doi: 10.1074/jbc.M110.139543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman JA, Molday RS. Critical role of the beta-subunit Cdc50a in the stable expression, assembly, subcellular localization, and lipid transport activity of the P4-Atpase Atp8a2. J Biol Chem. 2011;286:17205–17216. doi: 10.1074/jbc.M111.229419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenoir G, Williamson P, Puts CF, Holthuis JC. Cdc50p plays a vital role in the Atpase reaction cycle of the putative aminophospholipid transporter Drs2p. J Biol Chem. 2009;284:17956–17967. doi: 10.1074/jbc.M109.013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siggs OM, Schnabl B, Webb B, Beutler B. X-linked cholestasis in mouse due to mutations of the P4-Atpase Atp11c. Proc Natl Acad Sci U S A. 2011;108:7890–7895. doi: 10.1073/pnas.1104631108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siggs OM, Arnold CN, Huber C, Pirie E, Xia Y, Lin P, et al. The P4-type Atpase Atp11c is essential for B lymphopoiesis in adult bone marrow. Nat Immunol. 2011;12:434–440. doi: 10.1038/ni.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yabas M, Teh CE, Frankenreiter S, Lal D, Roots CM, Whittle B, et al. Atp11c is critical for the internalization of phosphatidylserine and differentiation of B lymphocytes. Nat Immunol. 2011;12:441–449. doi: 10.1038/ni.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Der Woerd WL, Van Mil SWE, Stapelbroek JM, Klomp LW, Van De Graaf SF, Houwen RH. Familial cholestasis: progressive familial intrahepatic cholestasis, benign recurrent intrahepatic cholestasis and intrahepatic cholestasis of pregnancy. Best Pract Res Clin Gastroenterol. 2010;24:541–553. doi: 10.1016/j.bpg.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Pawlikowska L, Groen A, Eppens EF, Kunne C, Ottenhoff R, Looije N, et al. A mouse genetic model for familial cholestasis caused by Atp8b1 mutations reveals perturbed bile salt homeostasis but no impairment in bile secretion. Hum Mol Genet. 2004;13:881–892. doi: 10.1093/hmg/ddh100. [DOI] [PubMed] [Google Scholar]
- 12.Paulusma CC, De Waart DR, Kunne C, Mok KS, Elferink RP. Activity of the bile salt export pump (Abcb11) is critically dependent on canalicular membrane cholesterol content. J Biol Chem. 2009;284:9947–9954. doi: 10.1074/jbc.M808667200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folmer DE, Mok KS, De Wee SW, Duijst S, Hiralall JK, Seppen J, et al. Cellular localization and biochemical analysis of mammalian Cdc50a, a glycosylated beta-subunit for P4 Atpases. J Histochem Cytochem. 2012;60:205–218. doi: 10.1369/0022155411435705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaher H, Meyer Zu Schwabedissen HE, Tirona RG, Cox ML, Obert LA, Agrawal N, et al. Targeted disruption of murine organic anion-transporting polypeptide 1b2 (Oatp1b2/Slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol Pharmacol. 2008;74:320–329. doi: 10.1124/mol.108.046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenderink JB, Geibel S, Grabsch E, De Pont JJ, Bamberg E, Friedrich T. Electrophysiological analysis of the mutated Na, K-Atpase cation binding pocket. J Biol Chem. 2003;278:51213–51222. doi: 10.1074/jbc.M306384200. [DOI] [PubMed] [Google Scholar]
- 16.Shah S, Sanford UR, Vargas JC, Xu H, Groen A, Paulusma CC, et al. Strain background modifies phenotypes in the Atp8b1-deficient mouse. Plos One. 2010;5:E8984. doi: 10.1371/journal.pone.0008984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunne C, Acco A, Hohenester S, Duijst S, De Waart DR, Zamanbin A, et al. Defective bile salt biosynthesis and hydroxylation in mice with reduced cytochrome P450 activity. Hepatology. 2013;57:1509–1517. doi: 10.1002/hep.26133. [DOI] [PubMed] [Google Scholar]
- 18.Van De Steeg E, Wagenaar E, Van Der Kruijssen CM, Burggraaff JE, De Waart DR, Elferink RP, et al. Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest. 2010;120:2942–2952. doi: 10.1172/JCI42168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Waart DR, Hausler S, Vlaming ML, Kunne C, Hanggi E, Gruss HJ, et al. Hepatic transport mechanisms of cholyl-L-lysyl-fluorescein. J Pharmacol Exp Ther. 2010;334:78–86. doi: 10.1124/jpet.110.166991. [DOI] [PubMed] [Google Scholar]
- 20.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344:1164–1168. doi: 10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- 22.Shah S, Sanford UR, Vargas JC, Xu H, Groen A, Paulusma CC, et al. Strain background modifies phenotypes in the Atp8b1-deficient mouse. Plos One. 2010;5:e8984. doi: 10.1371/journal.pone.0008984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuzaka Y, Hayashi H, Kusuhara H. Impaired hepatic uptake by organic anion-transporting polypeptides is associated with hyperbilirubinemia and hypercholanemia in Atp11c mutant mice. Mol Pharmacol. 2015;88:1085–1092. doi: 10.1124/mol.115.100578. [DOI] [PubMed] [Google Scholar]
- 24.Csanaky IL, Lu H, Zhang Y, Ogura K, Choudhuri S, Klaassen CD. Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: studies in Oatp1b2-null mice. Hepatology. 2011;53:272–281. doi: 10.1002/hep.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Limaye PB, Lehman-Mckeeman LD, Klaassen CD. Dysfunction of organic anion transporting polypeptide 1a1 alters intestinal bacteria and bile acid metabolism in mice. Plos One. 2012;7:e34522. doi: 10.1371/journal.pone.0034522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Csanaky IL, Selwyn FP, Lehman-Mckeeman LD, Klaassen CD. Organic anion-transporting polypeptide 1a4 (Oatp1a4) is important for secondary bile acid metabolism. Biochem Pharmacol. 2013;86:437–445. doi: 10.1016/j.bcp.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van De Steeg E, Stranecky V, Hartmannova H, Noskova L, Hrebicek M, Wagenaar E, et al. Complete Oatp1b1 And Oatp1b3 deficiency causes human rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J Clin Invest. 2012;122:519–528. doi: 10.1172/JCI59526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui Y, Konig J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter Slc21a6. J Biol Chem. 2001;276:9626–9630. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- 29.Yabas M, Coupland LA, Cromer D, Winterberg M, Teoh NC, D’rozario J, et al. Mice deficient in the putative phospholipid flippase Atp11c exhibit altered erythrocyte shape, anemia, and reduced erythrocyte life span. J Biol Chem. 2014;289:19531–19537. doi: 10.1074/jbc.C114.570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolpert E, Pascasio FM, Wolkoff AW, Arias IM. Abnormal sulfobromophthalein metabolism in rotor’s syndrome and obligate heterozygotes. N Engl J Med. 1977;296:1099–1101. doi: 10.1056/NEJM197705122961907. [DOI] [PubMed] [Google Scholar]
- 31.Van Der Velden LM, Wichers CG, Van Breevoort AE, Coleman JA, Molday RS, Berger R, et al. Heteromeric interactions required for abundance and subcellular localization of human Cdc50 proteins and class 1 P4-Atpases. J Biol Chem. 2010;285:40088–40096. doi: 10.1074/jbc.M110.139006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulusma CC, Folmer DE, Ho-Mok KS, De Waart DR, Hilarius PM, Verhoeven AJ, et al. Atp8b1 requires an accessory protein for endoplasmic reticulum exit and plasma membrane lipid flippase activity. Hepatology. 2008;47:268–278. doi: 10.1002/hep.21950. [DOI] [PubMed] [Google Scholar]
- 33.Van Der Mark VA, De Waart DR, Ho-Mok KS, Tabbers MM, Voogt HW, Oude Elferink RP, et al. The lipid flippase heterodimer Atp8b1-Cdc50a is essential for surface expression of the apical sodium-dependent bile acid transporter (Slc10a2/Asbt) in intestinal Caco-2 cells. Biochim Biophys Acta. 2014;1842(12 Pt A):2378–2386. doi: 10.1016/j.bbadis.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Moreno-Smith M, Halder JB, Meltzer PS, Gonda TA, Mangala LS, Rupaimoole R, et al. Atp11b mediates platinum resistance in ovarian cancer. J Clin Invest. 2013 May 1;123(5):2119–2130. doi: 10.1172/JCI65425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon Jh, Gores Gj. Death Receptor-Mediated Apoptosis And The Liver. J Hepatol. 2002 Sep;37(3):400–410. doi: 10.1016/s0168-8278(02)00209-x. [DOI] [PubMed] [Google Scholar]
- 36.Rust C, Wild N, Bernt C, Vennegeerts T, Wimmer R, Beuers U. Bile acid-induced apoptosis in hepatocytes is caspase-6-dependent. J Biol Chem. 2009 Jan 30;284(5):2908–2916. doi: 10.1074/jbc.M804585200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.