Abstract
Early-life stress is thought to increase later vulnerability for developing depressive illness by sensitizing underlying stress-responsive systems. Guinea pig pups separated from their mother and isolated in a novel cage for 3 hr exhibit a sensitized depressive-like behavioral response when separated again the following day as well as weeks later. The behavioral response and its sensitization appear to be mediated by inflammatory factors. To determine if this sensitization is specific to the separation response or if it reflects a broader underlying depressive-like state, guinea pig pups that had either been separated for 3 hr or remained with their mothers were observed in the forced swim test the following 3 days. Earlier separation was found to increase the duration of immobility, a measure sensitive to antidepressant treatment. These results support the use of the guinea pig as a model for examining mechanisms of inflammatory-mediated sensitization of depression following stress in early life.
Keywords: depressive-behavior, early experience, early-life stress, forced swim, guinea pig, maternal separation
1 | INTRODUCTION
It is now indisputable that early trauma increases vulnerability for developing depression and other forms of psychopathology for many individuals in later life (e.g., Heim & Binder, 2012; Mandelli, Petrelli, & Serretti, 2015). It appears that social or attachment-related stressors, such as neglect or abuse, may be particularly potent (Brown, Harris, & Copeland, 1977; Mandelli et al., 2015; McCory, De Brito, & Viding, 2011). The mechanisms underlying these effects are only beginning to be understood. However, the process commonly is conceived as involving sensitization of some stress-related physiological system(s) (e.g., hypothalamic-pituitary-adrenal, inflammatory), with the early trauma increasing responsiveness so that exposure to additional stressors in adulthood elicits enhanced and/or unregulated stress responses that precipitate, or actually constitute, the depressive episode (Gold, Goodwin, & Chrousos, 1988; Heim, Newport, Mletzko, Miller, & Nemeroff, 2008).
The guinea pig offers a potential animal model for examining such sensitization effects. Guinea pigs exhibit evidence of filial attachment (Hennessy & Ritchey, 1987; Jäckel & Trillmich, 2003; Porter, Berryman, & Fullerton, 1973). When separated from the attachment figure in a novel environment, pups initially vocalize to re-establish contact with the mother, but after an hour or more, begin to quiet and adopt a characteristic crouched posture with closed eyes and extensive piloerection (Hennessy, Long, Nigh, Williams, & Nolan, 1995). This response is reminiscent of the depressive or “despair” response evinced by human or macaque infants separated for prolonged periods (Kaufman & Rosenblum, 1967; Spitz, 1946). Moreover, the response sensitizes so that pups exhibit more of the depressive-like behavior when a second separation is imposed 24 hr after the first. Once established, the enhanced response persists days or even weeks past weaning (Schneider, Schiml, Deak, & Hennessy, 2012; and unpublished observations).
Although we have demonstrated that the depressive-like response to separation sensitizes with repeated separation, the model would be strengthened if we could show that the sensitized response generalizes to other depression-related paradigms. Human depression is not a single behavior pattern, but rather an internal state manifested by a variety of behaviors. It should be possible, therefore, to show that previous separation increases some other depression-related behavior. Furthermore, our previous findings lacked what has been termed “trans-situationality” (Maier & Watkins, 2005). That is, because we assessed sensitization in the same context as that in which the previous separation occurred, we cannot be sure that the increased later depressive-like behavior fully reflected a change in internal state, as opposed to conditioning to the environmental surroundings in which the previous separation occurred. The present study addressed these issues by asking whether previous maternal separation would increase depressive-like responding in an entirely different context. A number of depression-related paradigms have been developed for rodents, but these have been limited largely to rats and mice. The most widely used of these is the forced swim test (Porsolt, Le Pichon, & Jalfre, 1977). When placed in a vessel of water from which they cannot escape, rats and mice eventually stop swimming and attempting to climb the walls, and instead become immobile and float. Immobility in this test (either increased duration or reduced latency) has high predictive validity in that it is selectively responsive to a wide-range of antidepressant compounds (Cryan, Valentino, & Lucki, 2005; Czéh, Fuchs, Wiborg, & Simon, 2016). The forced swim test recently has been validated in the guinea pig by two studies demonstrating delayed onset of immobility following administration of a variety of antidepressants but not control substances (Rex, Voight, Wicke, & Fink, 2008; Wicke, Rex, Jongen-Relo, Groth, & Gross, 2007). Therefore, in the present experiment, we examined whether the separation procedure known to increase depressive-like behavior in guinea pig pups during a second separation the following day would also increase immobility during later testing in the forced swim.
2 | MATERIALS AND METHODS
2.1 | Animals and experimental conditions
Albino Hartley guinea pigs (Cavia porcellus) were bred and housed in our laboratory from breeders purchased from Hilltop Lab Animal, Inc., Scottdale, PA. Following birth (Day 0) mother and litter were maintained in an opaque plastic cage (73 × 54 × 24 cm) with a wire front and sawdust bedding. Food and water were available ad libitum. The colony room was kept at ~70°F on a 12:12 light/dark schedule with lights on at 0700 hr. Pups were housed continuously with the mother with the exception of behavioral testing to be described below. Sixteen pups (8 male, 8 female) were assigned to each of two conditions: separated (SEP) and non-separated (NSEP). No more than one pup from a litter was assigned to either condition. All procedures were in compliance with PHS guidelines and were approved by the Wright State University Laboratory Animal Care and Use Committee.
2.2 | Behavioral testing
2.2.1 | Separation
Consistent with past studies (e.g., Hennessy, Paik, Caraway, Schiml, & Deak, 2011), SEP pups were isolated in an empty, clear, plastic test cage (47 × 24 × 20 cm) for 3 hr on one occasion between 21 and 23 days of age. The pup was gently removed from the home cage and transported to a test room in the same laboratory suite, where it was monitored from behind one-way glass during Min 0–30, 60–90, and 150–180 by an observer trained to 85% inter-observer reliability. Behaviors recorded included the number of 1-min intervals the pup engaged in any of the three passive, depressive-like behavioral categories characteristic of separated pups: (1) a distinctive crouched stance with feet pulled close to the body—sometimes transitioning into lying on the cage floor with the trunk supporting the body (crouch); (2) complete or near complete closure of one or both eyes for greater than 1 s (eye-close); (3) piloerection over more than half of the body (piloerection). The number of 1-min intervals in which a pup displayed all three passive behaviors was scored as “full passive” behavior. In addition, “whistle” vocalizations, typical of the initial active phase of separation, were tallied using a handheld counter. A microphone positioned within 15 cm of the test cage broadcast the sound to headphones to assist the observer. NSEP pups were simply left in their home cages. All separations were begun between 0800 and 0900 hr.
2.2.2 | Forced swim test
Animals in both SEP and NSEP conditions underwent three forced-swim trials at 24-hr intervals. The first trial occurred 24 hr following initiation of separation for SEP pups and at a comparable age for NSEP animals (M age = 22.7 days for SEP and 23.0 days for NSEP). A clear, glass cylinder (20 × 45 cm) was filled to a depth of 21 cm with 30 ± 1 °C fresh water. At this depth, no pup could touch bottom with its nose above water. The test procedures were based on those described for the guinea pig (Wicke et al., 2007), but with modification. Those investigators exposed the animals to the water for 5 min on a training day 24 hr prior to a single 5-min test session. However, because immobility during an initial trial has been shown to be sensitive to antidepressants in rats and mice (e.g., Overstreet, Keeney, & Hogg, 2004; Parale & Kulkarni, 1986), we scored behavior during the first as well as second 5-min trial. Further, because pilot testing revealed that duration of immobility was substantially lower in our guinea pigs than in those of Wicke et al. (2007), but that immobility increased over days, we also scored behavior during a third, 5-min trial 24 hr after the second. During these three trials, behavior was recorded with a Logitech c920 webcam positioned directly above the cylinder and later scored with a custom behavior observation program. The primary behavior of interest was immobility, defined as the guinea pig maintaining a near stationary posture with its front paws no more than slightly moving so as to keep its nose above water and its hind legs spread so as to maintain balance. Both the total duration of immobility and latency to onset were scored. Total climbing time and swimming time also were monitored. Climbing was defined as the pup actively attempting to scale the glass walls with its front paws extending above the water. Swimming was identified as the pup pedaling through the water with all four paws under the surface. Immediately following each forced swim test, the pup was placed in a drying cage on a heating pad for 30 min before being returned to the home cage. The drying cage contained two clean, dry towels positioned under a 120-W infrared heat lamp.
2.3 | Data analysis
For duration of immobility, climbing, and swimming, data were analyzed with 2 (Condition) × 2 (Sex) × 3 (Trial) analyses of variance (ANOVAs) with Trial treated as a repeated measure. Due to lack of normal distribution of latency scores, non-parametric tests were used. Mann–Whitney U tests examined differences across variables of Condition and Sex on each trial. Friedman ANOVA then assessed effects of Day. Spearman-rho correlation coefficients were used to assess the relation between the number of 1-min intervals full passive behavior was observed during separation and duration of immobility during each day of forced swim testing, as well as during all days combined. A probability level of p < .05 was accepted throughout.
3 | RESULTS AND DISCUSSION
During separation, pups showed the typical pattern of initially vocalizing at a high rate before quieting and increasingly exhibiting the passive, depressive-like behaviors (Table 1).
TABLE 1.
Median levels of behavior during separation
| Min 0–30 | Min 60–90 | Min 150–180 | |
|---|---|---|---|
| Vocalizationa | 704 | 106 | 0 |
| Crouchb | 1 | 0 | 28.5 |
| Eye-closeb | 0 | 0 | 18.5 |
| Piloerectionb | 12.5 | 17.5 | 30 |
| Full passiveb | 0 | 0 | 15 |
Frequency.
Number of 1 min intervals.
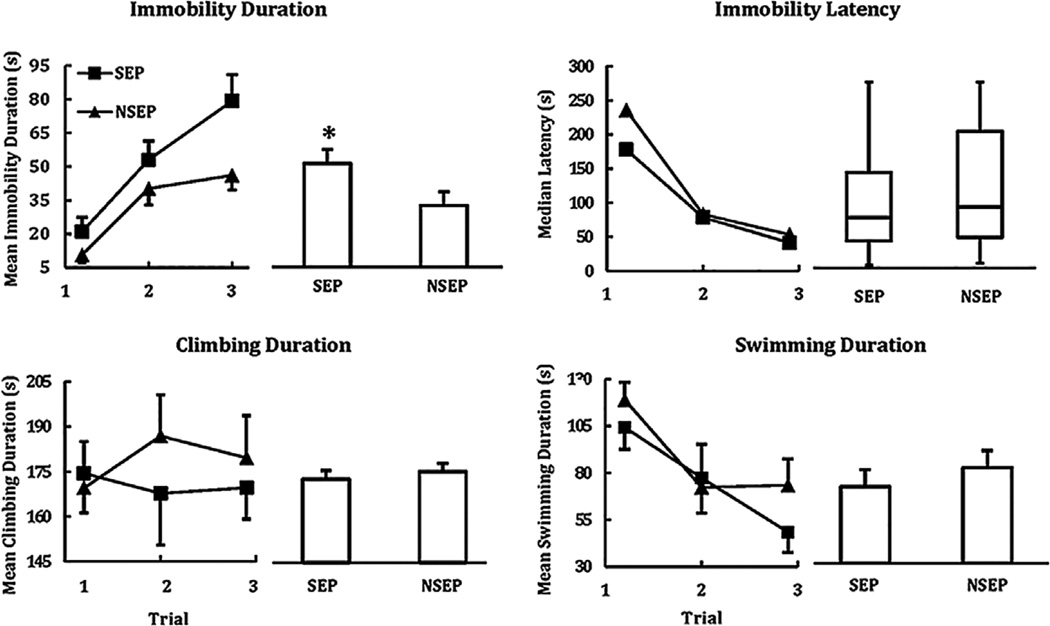
For forced swim, changes across trials (main effects) were seen for three of the four measures (Figure 1). Time (s) spent immobile increased, F (2, 56) = 35.34, p < .001, whereas latency to onset of immobility decreased, p < .001. Of the two active behavior measures, time spent swimming also declined with repeated testing, F (2, 56) = 14.66, p < .001. Of most interest here, however, was a main effect of Condition for immobility duration, F (1, 28) = 4.59, p < .05. Pups that previously had been separated were immobile longer than NSEP pups across trials. No other main or interaction effects were significant. There was no significant correlation between the duration of passive behavior during separation and immobility duration during forced swim.
FIGURE 1.
Mean duration in s that previously separated and non-separated guinea pig pups spent immobile, climbing, and swimming; and median latency in s for pups to become immobile, during each of 3 days of forced swim, as well as averaged across all 3 days (histograms). Standard errors of the means are indicated with vertical lines. Box and whisker plots indicate the 10th, 25th, 50th, 75th, and 90th percentiles. Changes across days were significant for immobility duration, immobility latency, and swimming duration. *p < .05 versus NSEP
In this experiment, a single 3-hr period of isolation in a novel cage increased immobility during forced swim across the following 3 days. This effect does not appear to have been due to physical exertion during the separation period. First, pups were relatively inactive during separation, spending considerable time exhibiting the passive, depressive-like behaviors. Second, the difference between groups did not diminish with greater time since the separation period. Rather the difference was maintained across the 3 days of forced swim testing and, while the interaction between Condition and Trial was not significant, the absolute difference between the two groups actually increased with repeated forced swim trials.
In altricial rats and mice, effects of early manipulations of pups can often be traced to changes in treatment of the pup by the mother upon return to the nest (e.g., Parent et al., 2005). By contrast, maternal behavior in the precocial guinea pig is extremely passive, with negligible active behavior directed toward the pup beyond one week of age (Hennessy & Jenkins, 1994; König, 1985). Thus, at the age of pups tested here, the chance that maternal treatment of the pup following return to the home cage affected forced swim performance seems remote.
It appears instead that the stress of the separation procedure affected later forced swim performance. Separation of the sort conducted here is known to increase hypothalamic-pituitary-adrenal, sympathetic, and amygdala activity as well as central monoamine turnover related to stress (Harvey, Moore, Lucot, & Hennessy, 1994; Hennessy, Tamborski, Schiml, & Lucot, 1989; Maken, Weinberg, Cool, & Hennessy, 2010; Tamborski, Lucot, & Hennessy, 1990). Earlier studies of the passive, depressive-like response of separated guinea pig pups indicate that the mechanism involves stress-induced inflammatory activity. Stressors can induce a systemic inflammatory response (Maier & Watkins, 1998; Wohleb, McKim, Sheridan, & Godbout, 2015), and anti-inflammatory treatment of guinea pig pups reduces the depressive-like response to an initial separation that occurs immediately following administration (Hennessy et al., 2015; Perkeybile, Schiml-Webb, O’Brien, Deak, & Hennessy, 2009) as well as during sensitization of the response at the time of additional separations a day or more later (Hennessy et al., 2011; Hennessy et al., 2015). These findings jibe well with those of recent human studies. Inflammatory activity has not only been found to induce depressive symptomology (Bull et al., 2009), but may play a key role in the depression of those who were exposed to neglect, abuse, and other severe stressors in childhood (Slavich & Irwin, 2014).
Interpretation of results in the forced swim has been a subject of considerable debate. The idea often promoted in the literature that immobility reflects something akin to despair, or represents a true animal model of depression, has come under considerable recent criticism (Czéh et al., 2016; Molendijk & de Kloet, 2015). What is not debated is that the forced swim is an effective antidepressant screen with good predictive validity (Cryan et al., 2005; Hendriksen & Groenink, 2015). Thus, our results are best interpreted as generalization of the depressive-like response that guinea pig pups exhibit during separation to another depression-related paradigm, or to a behavioral state associated with depression, but not to another animal model of depression. In the current study, we used guinea pig pups close to the natural weaning age (~25 days), whereas the forced swim is most often examined with older rats and mice. Yet, the two extant studies demonstrating selective responsiveness to antidepressants in guinea pigs both used prepubertal animals. In one, males as young as 50 days were tested (Rex et al., 2008). In the other, ages were not provided, but the weights of the animals fell in the range of guinea pigs of the age tested here (Hennessy, Becker, & O’Neil, 1991; Wicke et al., 2007).
We saw no indication of sex differences in the forced swim or in the response to separation, which is consistent with the great majority of our previous, related studies. However, it would be premature to conclude that sex differences do not exist in the responses of guinea pig pups to separation, or the sensitization of these responses to a depressive-like state. The current experiment's sample sizes of eight males and eight females are among the largest we have included in such studies. More typically, sample sizes have been limited to five or six animals of each sex. While a greater focus on sex-differences is a goal for future work, studies to date have generally been underpowered to sensitively detect differences between males and females.
While much has been learned about the relation of early-life stress, inflammation, and later depression, much more remains hypothetical and in need of further study. The guinea pig may be particularly well-suited for this task. The evidence of filial attachment from an early age, the similarity of many of its responses during separation with those of non-human primates (Hennessy, 2003), and the sensitization of depressive-like behavior during repeated separations all favor the guinea pig over traditional rat and mouse models. The present results indicate that the sensitization of passive, depressive-like behavior following maternal separation in the guinea pig is not specific to future separation events, but instead generalizes to an entirely different context that is selectively sensitive to antidepressant treatments. This finding provides a critical, additional piece of evidence supporting the validity of guinea pig studies in pursuing the developmental processes by which early-life stress enhances susceptibility for depressive illness at later ages.
Acknowledgments
The authors would like to thank Brittany Price and Kate Berberich for help with data collection. The work was supported by grant MH068228 from the National Institute of Mental Health.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
REFERENCES
- Brown GW, Harris T, Copeland JR. Depression and loss. British Journal of Psychiatry. 1977;130:1–18. doi: 10.1192/bjp.130.1.1. [DOI] [PubMed] [Google Scholar]
- Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, Pariante CM. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-α and ribavirin treatment. Molecular Psychiatry. 2009;14:1095–1104. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified forced swim test. Neuroscience and Biobehavioral Reviews. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Czéh B, Fuchs E, Wiborg O, Simon M. Animal models of major depression and their clinical implications. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2016;64:293–310. doi: 10.1016/j.pnpbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression: Relation to the neurobiology of stress (Part 2) New England Journal of Medicine. 1988;319:413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- Harvey AT, Moore H, Lucot JB, Hennessy MB. Monoamine activity in anterior hypothalamus of guinea pig pups separated from their mothers. Behavioral Neuroscience. 1994;108:171–176. doi: 10.1037//0735-7044.108.1.171. [DOI] [PubMed] [Google Scholar]
- Hendriksen H, Groenink L. Back to the future of psychopharmacology: A perspective on animal models in drug discovery. European Journal of Pharmacology. 2015;759:30–41. doi: 10.1016/j.ejphar.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental Neurology. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Enduring maternal influences in a precocial rodent. Developmental Psychobiology. 2003;42:225–236. doi: 10.1002/dev.10095. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Becker LA, O'Neil DR. Peripherally-administered CRH suppresses the vocalizations of isolated guinea pig pups. Physiology and Behavior. 1991;50:17–22. doi: 10.1016/0031-9384(91)90492-7. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Jenkins R. A descriptive analysis of nursing behavior in the guinea pig (Cavia porcellus) Journal of Comparative Psychology. 1994;108:23–28. doi: 10.1037/0735-7036.108.1.23. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Long SJ, Nigh CK, Williams MT, Nolan D. Effects of peripherally administered corticotropin-releasing factor (CRF) and a CRF antagonist: Does peripheral CRF activity mediate behavior of guinea pig pups during isolation? Behavioral Neuroscience. 1995;109:1137–1145. doi: 10.1037//0735-7044.109.6.1137. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Paik KD, Caraway JD, Schiml PA, Deak T. Proinflammatory activity and the sensitization of depressive-like behavior during maternal separation. Behavioral Neuroscience. 2011;125:426–433. doi: 10.1037/a0023559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Ritchey RL. Hormonal and behavioral attachment responses in infant guinea pigs. Developmental Psychobiology. 1987;20:613–625. doi: 10.1002/dev.420200607. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Stafford NP, Yusko-Osborne B, Schiml PA, Xanthos ED, Deak T. Naproxen attenuates sensitization of depressive-like behavior and fever during maternal separation. Physiology and Behavior. 2015;139:34–40. doi: 10.1016/j.physbeh.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Tamborski A, Schiml P, Lucot J. The influence of maternal separation on plasma concentrations of ACTH, epinephrine, and norepinephrine in guinea pig pups. Physiology and Behavior. 1989;45:1147–1152. doi: 10.1016/0031-9384(89)90101-7. [DOI] [PubMed] [Google Scholar]
- Jäckel M, Trillmich F. Olfactory individual recognition of mothers by young guinea-pigs (Cavia porcellus) Ethology. 2003;109:197–208. [Google Scholar]
- Kaufman IC, Rosenblum LA. The reaction to separation in infant monkeys: Anaclitic depression and conservation withdrawal. Psychosomatic Medicine. 1967;29:648–675. doi: 10.1097/00006842-196711000-00010. [DOI] [PubMed] [Google Scholar]
- König B. Maternal activity budget during lactation in two species of Caviidae (Cavia porcellus and Galea musteloides) Zeitschrift für Tierpsychologie. 1985;68:215–230. [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience and Biobehavioral Reviews. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maken DS, Weinberg J, Cool D, Hennessy MB. An investigation of the effects of maternal separation and novelty on central mechanisms mediating pituitary-adrenal activity in infant guinea pigs. Behavioral Neuroscience. 2010;124:800–809. doi: 10.1037/a0021465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli L, Petrelli C, Serretti A. The role of early trauma in adult depression: A meta-analysis of published literature. Childhood trauma and adult depression. European Psychiatry. 2015;30:665–680. doi: 10.1016/j.eurpsy.2015.04.007. [DOI] [PubMed] [Google Scholar]
- McCory E, De Brito SA, Viding E. The impact of childhood maltreatment: A review of neurobiological and genetic factors. Frontiers in Psychiatry. 2011;2:1–14. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk ML, de Kloet ER. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology. 2015;62:389–391. doi: 10.1016/j.psyneuen.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Keeney A, Hogg S. Antidepressant effects of citalopram and CRF receptor antagonist CP-154,526 in a rat model of depression. European Journal of Pharmacology. 2004;492:195–201. doi: 10.1016/j.ejphar.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Parale MP, Kulkarni SK. Clonidine-induced behavioural despair in mice: Reversal by antidepressants. Psychopharmacology. 1986;89:171–174. doi: 10.1007/BF00310623. [DOI] [PubMed] [Google Scholar]
- Parent C, Tie-Yuan Z, Caldji C, Bagot R, Champagne FA, Pruessner J, Meaney MJ. Maternal care and individual differences in defensive responses. Current Directions in Psychological Science. 2005;14:229–233. [Google Scholar]
- Perkeybile AM, Schiml-Webb PA, O’Brien E, Deak T, Hennessy MB. Anti-inflammatory influences on behavioral, but not cortisol, responses during maternal separation. Psychoneuroendocrinology. 2009;34:1101–1108. doi: 10.1016/j.psyneuen.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Porter RH, Berryman JC, Fullerton C. Exploration and attachment behavior in infant guinea pigs. Behaviour. 1973;45:312–322. doi: 10.1163/156853974x00705. [DOI] [PubMed] [Google Scholar]
- Rex A, Voigt JP, Wicke KM, Fink H. In vivo/ex vivo and behavioural study of central effects of 5-HT1B/1D and 5-HT1A antagonists in guinea pigs. Pharmacology, Biochemistry, and Behavior. 2008;88:196–204. doi: 10.1016/j.pbb.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Schneider RL, Schiml PA, Deak T, Hennessy MB. Persistent sensitization of depressive-like behavior and thermogenic response during maternal separation in pre- and post-weaning guinea pigs. Developmental Psychobiology. 2012;54:514–522. doi: 10.1002/dev.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz RA. Anaclitic depression: An inquiry into the genesis of psychiatric conditions in early childhood: II. Psychoanalytical Study of the Child. 1946;2:313–342. [PubMed] [Google Scholar]
- Tamborski A, Lucot JB, Hennessy MB. Central dopamine turnover in guinea pig pups during separation from their mothers in a novel environment. Behavioral Neuroscience. 1990;104:607–611. doi: 10.1037//0735-7044.104.4.607. [DOI] [PubMed] [Google Scholar]
- Wicke KM, Rex A, Jongen-Relo A, Groth I, Gross G. The guinea pig forced swim test as a new behavioral despair model to characterize potential anti-depressants. Psychopharmacology. 2007;195:95–102. doi: 10.1007/s00213-007-0874-0. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Sheridan JF, Godbout J. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Frontiers in Neuroscience. 2015;8:1–17. doi: 10.3389/fnins.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]