Abstract
Background
Autism spectrum disorders (ASD) are a growing concern with more than 1 in every 68 children affected in the United States by age 8. Limited scientific advances have been made regarding the etiology of autism, with general agreement that both genetic and environmental factors contribute to this disorder.
Objective
To explore the link between exposure to PBDE, mitochondrial dysfunction and autism risk.
Results
Perinatal exposures to PBDEs may contribute to the etiology or morbidity of ASD including mitochondrial dysfunction based on (i) their increased environmental abundance and human exposures, (ii) their activity towards implicated in neuronal development and synaptic plasticity including mitochondria, and (iii) their bioaccumulation in mitochondria.
Conclusions
In this review, we propose that PBDE, and possibly other environmental exposures, during child development can induce or compound mitochondrial dysfunction, which in conjunction with a dysregulated antioxidant response, increase a child’s susceptibility of autism.
Keywords: Autism risk, mitochondrial dysfunction, neuronal development, PBDE exposure, antioxidant response, oxidative stress
Introduction
Autism spectrum disorders (ASD) are a growing concern, with more than 1 in every 68 children affected in the United States by the age of eight years. Complex interactions between genes and environmental factors are thought to contribute to ASD risk. Based on a study on identical twins, exposure to shared environmental factors seems to play a more critical role than genetic heritability in autism [1]. Evidence is accumulating for a potentially large role in ASD etiology and/or morbidity for the early in-utero environment, including environmental exposures. Among these, polybrominated biphenyl ethers (PBDE) exposure is a potential risk factor based on (i) their increased environmental abundance and human exposures [2], (ii) their activity towards targets implicated in neuronal development and synaptic plasticity [3], including mitochondria [4–16], (iii) their higher accumulation in children than adults living in the same quarters [17], and (iv) the demonstrated association between PBDE perinatal exposure and developmental/delayed neurotoxicity [3, 18, 19]. This study explores the potential detrimental role of PBDE exposures contributing to mitochondrial dysfunction and autism risk.
Mitochondria and autism
Given the critical role of mitochondria in bioenergetics [20–24] and immunity [25], it is not surprising that mitochondrial dysfunction could contribute to the etiology and/or severity of neurological disorders including autism [26–28]. One of the most prevalent metabolic disorders associated with ASD is mitochondrial dysfunction. A meta-analysis [20] showed that 5% of children with ASD met the criteria for a mitochondrial respiratory chain disorder (MRCD) as judged by the modified Walker criterion [29]. This well-established approach relies on significant decreases in mitochondrial electron transport Complex activities (e.g., 30% or less of control values in cultured cells), clinical outcomes (e.g., learning disabilities) and/or the occurrence of known pathogenic mitochondrial DNA mutations [29]. When less stringent criteria are used, >30% of children in the general ASD population exhibit metabolic biomarkers representative of mitochondrial dysfunction [30]. A study [31] reported that up to 50% of children with ASD have at least one biomarker of mitochondrial dysfunction. Our work showed that 80% of children with autism with high severity scores (8 and above) demonstrated lower than normal electron transport chain function in lymphocytes when compared to neurotypical controls [23]. Our studies have also shown that children with autism are more likely to have mtDNA overreplication and mtDNA deletions than typically neurodeveloping children [23, 32], indicating that their mtDNA is more damaged as a result of an imbalance between increased reactive species production and antioxidant responses. The higher incidence of high mtDNA copy number and deletions seems to reflect the fact that lymphocytic mitochondria from children with autism produced more reactive oxygen species than those from typically neurodeveloping children [23], and that oxidative stress enhances mtDNA replication [33, 34]. Evidence for a compromised mitochondrial function (altered mitochondrial dynamics) and intracellular redox status in pyramidal neurons in ASD brains was provided when analyzing post-mortem BA21 temporal cortex samples [35]. Furthermore, a higher mtDNA copy number was also observed in a pilot study performed on post-mortem samples from brain regions of control and children with autism (Table 1). Frontal and temporal cortex from cases exhibited mtDNA over-replication compared to typically neurodeveloping children (1.6- and 1.14-fold; p = 0.004 and 0.04; Table 1) and at similar ratios than those obtained with PBMC and in brain structures that had been implicated in autism [36, 37]. These data indicate that PBMC possess biomarkers of mitochondrial dysfunction found in brain tissues, providing strong rationale for launching systematic studies of mitochondrial dysfunction in autism using readily available PBMC.
Table 1.
mtDNA copy number in brain regions from control children and children with autism.*
| Cortex region | mtDNA copy number | |
|---|---|---|
| Typically developing | Autism | |
| Frontal | 2803 ± 92 | 4414 ± 241* |
| Temporal | 3706 ± 103 | 4232 ± 124* |
Samples obtained from the Autism Tissue Program brain bank were collected with a post-mortem interval of 24-h or less. Ages ranged from 6–15 years for both groups. Causes of death were multisystem organ failure, drowning, smoke inhalation, and gunshot.
p <0.05.
Some children with ASD have increased activities of certain Complexes within the mitochondrial electron transport chain rather than deficits [23, 38]; however, this situation is also interpreted as a mitochondrial dysfunction given that the appropriate ratio of Complexes allows the correct oxidation of substrates for obtaining ATP. Some of the ASD cases with reported mitochondrial dysfunction present higher lactate-to-pyruvate ratios in plasma, which indicates higher fluxes of glucose going through glycolysis than via mitochondria [23, 24], and another study presented evidence of higher lactate in brain of a subset of subjects with autism [39]. The finding that not all individuals with mitochondrial dysfunction show high lactate-to-pyruvate ratios is not surprising considering that increases in this ratio in plasma usually reflect a significant co-occurrence of a myopathy [23, 38, 40], which may not be necessarily present in some ASD children. Even when a child presents a typical mitochondrial respiratory chain disorder, its diagnosis still constitutes a challenge to clinicians, especially because the clinical presentation in children shows an enormous variation [41]. Further evidence of mitochondrial dysfunction in ASD has demonstrated in human studies of genetic disorders associated with ASD and animal models, including fragile X disorders [42–44], phosphatase and tensin homolog (PTEN) haploinsufficiency [45] or mutations [45], Rett syndrome [46–48], succinic semialdehyde dehydrogenase deficiency [49, 50], 15q11–q13 duplication syndrome [51, 52], Down’s syndrome [53, 54], among others [55, 56]. Taken together, these studies suggest that mitochondrial dysfunction may be present in a considerable number of children with ASD and, based on the broad phenotype of mitochondrial chain respiratory disorders, that such dysfunction might be manifested as a spectrum of clinical outcomes.
Evidently the 7- to 8-fold increase in the incidence of autism in California from the early 1990s through the present [57] cannot be attributed solely to changes in diagnostic criteria, the inclusion of milder cases, an earlier age at diagnosis or genetic causes suggesting that yet unidentified environmental exposures could contribute to the escalating diagnostic risks. The etiology of mitochondrial dysfunction in ASD is unknown with limited evidence for a contribution from pathogenic mtDNA mutations [58–61]. This suggests that mitochondrial dysfunction in ASD may be de novo or acquired. In this regard, it has been proposed that ASD may arise from environmental triggers [1] in genetically predisposed subpopulations [62, 63]. This notion is supported by a study of dizygotic twins that estimated that the environment contributed more to the risk of developing autism (55%) than that attributed solely to genetic factors (37%) with these factors contributing about equally for the broader ASD diagnosis [1]. Mitochondria are central to this concept since mtDNA polymorphisms can result in increased disease predisposition [64, 65]. However, mitochondrial dysfunction can also result from dietary habits such as maternal folate [66, 67] and iron [68–70] status or environmental exposures previously implicated in ASD including heavy metals [71–74], chemicals [75], polychlorinated biphenyls [76], pollution [77–79], pesticides [80, 81] or maternal infection during pregnancy [28, 82–89].
Among these exposures, PBDEs may be viewed as suitable candidates to promote or enhance adverse outcomes of subclinical conditions based on (i) their increased environmental abundance and human exposures [2], (ii) their activity towards targets implicated in neuronal development and synaptic plasticity [3] including mitochondria [4–16], (iii) their higher accumulation in children than adults living within the same quarters [17], (iv) the association between developmental/delayed neurotoxicity and perinatal exposure to PBDEs [3, 18, 19], and (v) the relatively high intracellular and mitochondrial bioaccumulation [7]. Although autism is a complex neurobehavioral syndrome with many risk genes [90–98], current data indicates that over-excitation of local networks is a common etiologic factor [99, 100]; however, the prevalence of mitochondrial dysfunction [20, 22–24, 98, 101] and increased oxidative stress [32, 45, 101–105] observed in autism may also set the basis for a disrupted network, and evidenced more upon exposure to environmental triggers with a neurotoxic component. If perinatal PBDE exposure were one of the precipitating factors in autism -in line with the “second-hit stress hypothesis”- the severity of this background would set the perinatal oxidative phosphorylation capacity, and thus, the relative severity of the disease at birth. Individuals with initially high oxidative phosphorylation capacities would require multiple exposures (or a combination of triggers) to cross oxidative phosphorylation thresholds and thus remain asymptomatic until late in life. Individuals starting with a lower initial capacity and requiring fewer exposures (or combinations) to have the same effect would develop symptoms early in life. This differential effect of the PBDE-induced bioenergetic decline could be further accentuated in individuals with partial oxidative phosphorylation defects as reported in autism [20–24, 32, 38, 98, 101, 105–109]. This concept is supported by the findings that mitochondrial dysfunction in neurons with PTEN deficiency, a genetic background shared by a subset of children with autism [27], in significantly enhanced by nanomolar concentrations of BDE-49, one of the least abundant PBDEs.
General background on PBDEs
PBDEs represent an important group of high volume chemicals extensively used in plastics, textiles, furniture, and electronic devices [110]. Global production of PBDEs has reached approximately 148 million lb/year [110]. PBDEs are used as additive flame-retardants in plastics to which they are not chemically bound and can thus leach from polymers and pervasively accumulate in the built environment and ecosystem [110–113]. PBDEs share structural similarity to the persistent non-coplanar polychlorinated biphenyls and have high heat stability, high lipid solubility, and low vapor pressure, which contribute to their environmental persistence and bioaccumulation [114] impacting individual- [115] and population-level health outcomes [116]. The extent of toxicity by PBDE congeners can be dependent upon conformational differences, position and degree of halogenation and hydroxylation [117].
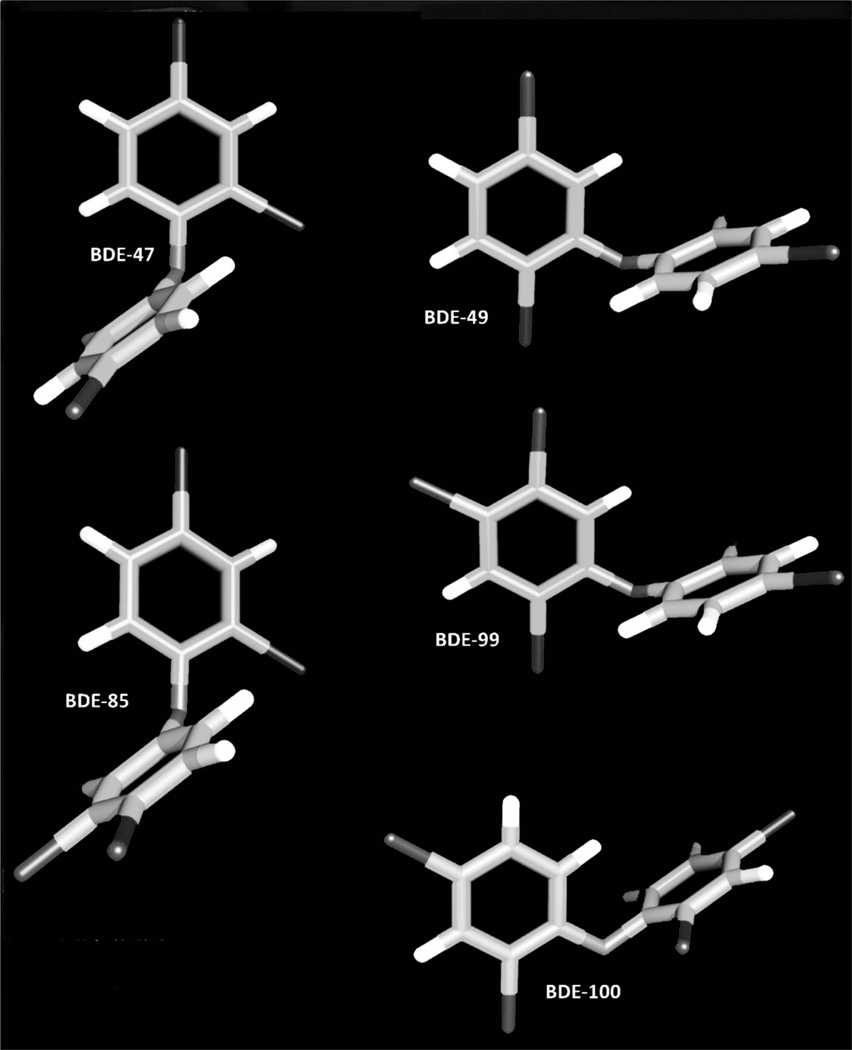
In contrast to the polychlorinated biphenyls, whose levels in environmental samples are slowly decreasing [118], PBDE residues in environmental media and in human tissues appear to be increasing [119]. Recent studies are demonstrating a world-wide increase in PBDEs’ concentrations in the human diet [120–137], especially in seafood and fish [120–122, 125, 126, 130–134, 138], regardless of the cooking method [139], which may result in dietary exposures and PBDE body burdens in humans [110, 111, 131, 132]. For example, PBDE congeners in human breast milk from Swedish women have increased exponentially over the last two decades [140, 141], and studies in US populations have demonstrated the presence of PBDEs in human breast milk, adipose tissue, and blood [142, 143]. Interestingly, the levels of PBDEs in breast milk of US women reflect a body burden that far exceeds that reported in the Scandinavian studies [140–142]. In particular, PBDE levels in northern California women are among the highest levels reported to date [144, 145], as expected for the San Francisco Bay area, one of the most contaminated regions worldwide [146–149]. PBDE levels in breast adipose tissue from women living in this area were 3- to 25-times higher than those in other regions of the world [144, 145]. The average ∑PBDEs was 86 ng/g fat with BDE-47, -154, -153, -99, and -100 as the major congeners in 1990 [150]. Data collected from women from 1995–1998 showed that the total level was 2-times higher than that from 1990, with 2- to 3-fold higher concentrations of congeners -47, -99, and -100 [145]. Figure 1 depicts the three-dimensional chemical structures of some PBDEs, such as BDE-47, -49, -85, -99, and -100.
Figure 1.
Three-dimensional chemical structures of selected PBDEs
Circulating levels of PBDEs in children aged 2 to 5 years living in northern California from the Childhood Autism Risks from Genetics and the Environment (CHARGE) Study at the University of California Davis were reported to be 10-to 1000-fold higher than similar aged populations in Mexico and Europe, 5-times higher than similar aged children across the U.S., and 2- to 10-fold higher than U.S. adults [151]. This higher exposure may be partially explained by the fact that infants can accumulate 2- to 4-times more PBDEs than adults within the same geographical area [152]. In addition, California regulations require all furnishings to pass flammability tests for fire safety [153]. Although no specific flame-retardants are mandated, it is quite likely that PBDEs are added to polyurethane foam used in furnishings [2]. Then the main source for PBDE exposure in California compared to that of other regions would be hand-to-mouth contact with consumer products and ingestion/inhalation of dust in indoor microenvironments. In support of this argument, a study performed with women living in northern California, indicated that individual PBDE congeners correlated with each other, but correlations across PBDE and polychlorinated biphenyls congeners were modest [145], suggesting that maternal exposures to PBDEs came primarily from non-dietary sources [2, 150]. However, processed foods (especially pork and chicken products) and exposure to new upholstered furniture were the major predictors of blood levels of PBDEs in 2–5 year olds from CHARGE Study [151] suggesting that both diet and environmental exposure might be relevant in this population of children from northern California.
Reports using animal models, as well as epidemiological and human tissue studies, indicate that certain environmental chemicals and drugs can cross the placenta during pregnancy and interact with fetal cell targets leading to disorders, which arise later in development [154–156]. PBDE concentrations in maternal blood predict the level of fetal exposures for some BDE congeners [157], suggesting maternal transfer to the developing fetus during pregnancy. Studies demonstrating induction of cytochrome P4501A in rat fetal livers whose mothers underwent PBDE exposures [158] and the presence of several PBDE congeners in human fetal liver [19] substantiates transplacental exposure to PBDEs in rodents and humans. The maternal transfer of both lipophilic PBDEs and their less lipophilic hydroxylated congeners are likely to cause developmental neurotoxicity [3, 18, 159–162]. For instance, BDE-49 and its hydroxylated metabolite, not typically measured in human samples, have been recently detected in gestational tissues from women in Michigan at levels comparable to commonly detected BDE-47 (17% of total PBDEs; [163]). This observation is consistent with reports identifying BDE-49 as a major contributor to PBDE load in fish [164, 165], including one study on Great Lakes fish that identified BDE-49 as the most abundant congener [166]. These data significantly underscore the importance of meta- and para-bromination substitutions in determining the bioaccumulation of highly neurotoxic congeners during gestation, and the possible contribution of hydroxylated metabolites to adverse outcomes. Similar to structurally related non-coplanar polychlorinated biphenyls [162], PBDEs have a stringent structure-activity relationship towards altering Ca2+ signaling pathways via interactions with microsomal ryanodine receptors, with BDE-49 and hydroxylated metabolites being most active [161]. Chronic, low-level maternal and fetal exposures to specific PBDE congener profiles during pregnancy could affect signaling systems essential for activity dependent dendritic growth and proper development of excitatory and inhibitory networks in the fetus [3, 161]. An imbalance of excitatory and inhibitory neurotransmission has been implicated in the etiology of a number of syndromic and idiopathic developmental disorders, including autism [100].
PBDEs, mitochondria, and autism
Several key factors could relate PBDE exposure to autism susceptibility. Among them, maternal transfer of PBDEs to the fetus transplacentally during gestation, early postnatal exposure to PBDEs via maternal milk (especially those highly hydrophobic) and exposure to PBDEs during early postnatal development. Although the mechanisms responsible for PBDE-induced injury are not well understood, recent research has focused on the ability of PBDEs to disrupt thyroid hormone status, leading to abnormalities in fetal growth and development in laboratory animals [18, 167–170] as well as disrupting intracellular Ca2+ homeostasis especially in excitable cells [4, 5, 8, 9, 11, 14–16, 171]. In this regard, BDE-47 and hydroxylated derivatives had been shown to release Ca2+ from or inhibit calcium uptake by endoplasmic reticulum and mitochondrial stores in PC12 cells [5, 9], human neuroblastoma cell line SH-N-SH [11], cerebellar fractions and cerebellar granule cells [8, 15], exhibiting a preferential effect on mitochondria [4, 8, 15, 171]. A growing body of evidence suggests that PBDE or their hydroxylated metabolites can induce mitochondrial dysfunction by promoting inhibition of the electron transport chain or uncoupling electron transport with ATP synthesis [9, 172], mitochondrial depolarization [6, 10, 173], altered mitochondrial morphology [174], release of cytochrome c and apoptosis [10, 11], and increased oxidative stress [6, 7, 10, 11, 173, 175] in vivo [173, 176, 177] or in vitro [5, 6, 9, 10, 14–16] in a variety of biological systems. mitochondrial dysfunction has been reported in individuals with autism or ASD [23, 24, 38, 98, 101, 105, 107–109, 178, 179]. Our studies showed that Complex IV and V are inhibited by BDE-49 at low nM concentrations and that these effects are enhanced in the presence of PTEN deficiency, background shared by a subset of children with autism [27]. Given that the levels of PBDEs in blood samples from children aged 2–5 years from CHARGE were not significantly different than those from age-matched typically neurodeveloping children [180], it is tempting to propose that the response to a perinatal PBDE exposure differs between these diagnostic groups, compounded by the bioaccumulation of PBDE in mitochondria [7]. This bioaccumulation of PBDEs implicated in neurotoxicity [3, 161] may enhance the pre-existing mitochondrial dysfunction and/or initiate it, contributing to the onset or morbidity of ASD.
Antioxidant responses and autism
The capacity of cells to maintain homeostasis during oxidative stress resides in the induction of protective enzymes, as well as non-enzymatic defenses such as glutathione [181–186], playing Nuclear Factor, Erythroid 2-Like 2 (Nrf2) as an important role in the regulation of these processes [187–189]. Nrf2 induces antioxidant and detoxifying enzymes through its binding to the antioxidant response element (ARE) [190, 191]. Nrf2 is sequestered in the cytoplasm as an inactive complex with its cytosolic repressor Kelch-like ECH associated protein-1 (Keap-1). The dissociation of Nrf2 from Keap-1 is crucial for its nuclear translocation, followed by binding to DNA and activation of cytoprotective genes [191]. Nrf2 phosphorylation has been described as a critical event for the nuclear translocation of this transcription factor and its transcriptional activity [191, 192]. To date, multiple signaling kinases related to cell survival/proliferation have been reported to regulate Nrf2, including extracellular signal-regulated kinase (ERK), c-jun NH2-terminal kinase (JNK), phosphatidylinositol-3-kinase (PI3K) and protein kinase C (PKC) [191, 193]. Indeed, the phosphorylation of Nrf2 by these different kinases at multiple sites seems to be an important mechanism in Nrf2-mediated ARE activation and in regulating the stability of this transcription factor [194]. Post-translational modification of Nrf2 by various protein-kinase signaling pathways can affect its nuclear translocation. Some of the kinases identified as responsible for Nrf2 phosphorylation are ERK, JNK, PI3K and PKC [191].
Nrf2 has an important role in the protection against induced-organ injury [191] by regulating the response to cellular stress and cell survival/proliferation [188, 195–197]. Therefore, the Nrf2-ARE pathway could act as a sensor and respond to chemical stress before the onset of cytotoxicity. In line with this, Nrf2 could be activated in response to PBDE exposure as an adaptive response against oxidative and inflammatory cell damage; however, a dysregulated Nrf2-mediated response might not be enough to overcome PBDE-mediated mitochondrial damage, considering the high susceptibility to oxidative stress by certain complexes and mitochondrial enzymes [198–200]. In support of this concept, lower gene expression of Nrf2 has been reported in granulocytes of children with autism suggesting lower response to activate the antioxidant response capacity and possibly linked to the increased mtDNA deletions [201]. Nrf2 may also define the initial threshold for toxicity by controlling, at least in part, constitutive aspects of cell defense [190, 195, 196]. In this regard, it has been described that an agent could stimulate the nuclear accumulation of Nrf2 at non-cytotoxic concentrations or after a short time of incubation, although at longer times of exposure, it could induce significant cytotoxicity [195].
Several studies have shown mitochondrial dysfunction reported in PBMC from children with autism [23], deficits accompanied by increased oxidative stress, evidenced by higher rates of hydrogen peroxide production [23] and increased mtDNA deletions [32]. The mitochondrial electron transport chain is the major intracellular source of reactive oxygen species, and as such, mtDNA becomes oxidatively modified as it is evidenced by its relatively high mutation rate [202] and accumulation of deletions with age [203, 204]. Mitochondria can compensate for these damages by responding with increased mtDNA replication without increases in oxidative phosphorylation [34, 205–209]; however, increases in copy number have also been associated with defective transcription, respiratory chain deficiency, and age-related accumulation of mtDNA deletions [210]. Not only the production of reactive oxygen species is higher in samples from ASD cases but also evidence of lower antioxidant defenses has been presented. Glutathione deficits have been reported in plasma, immune cells and post-mortem brain from ASD children [105, 211–213]. A deficit in glutathione antioxidant capacity may limit the ability to catabolize hydrogen peroxide efficiently, increasing both oxidative stress-mediated damage and the vulnerability to subsequent pro-oxidant environmental exposures [214, 215]. Thus, exposure to environmental stressors could be further compounded (second hit hypothesis) in the presence of a pre-existent mitochondrial dysfunction. This is demonstrated by the enhanced neurotoxic effect of excitotoxic amino acids when oxidative phosphorylation is inhibited [216–219] or the exacerbated neuronal mitochondrial toxicity to PBDEs in the presence of an autistic-like background (PTEN deficiency) [27]. In this regard, oxidative stress may be a key mechanism by which mitochondria are negatively influenced by exposures to pro-oxidant environmental triggers [71–76, 80, 81] and/or by medical conditions coexisting with ASD diagnosis such as immune dysregulation [201, 220]. Free radicals, when not accompanied by appropriate antioxidant defenses, can initiate a cascade of deleterious events, which can promote or perpetuate mitochondrial and cellular damage [211, 221].
Concluding remarks
Finally, more research needs to be done to understand the risk factors for autism, specifically how environmental exposures impact redox homeostasis and mitochondrial function, and how these exposures unveil functionally deficient backgrounds contributing to a feed-forward cycle of damage. Although a growing body of evidence suggests that PBDE can induce mitochondrial dysfunction by a variety of mechanisms, limited effort has been devoted to find the differential susceptibility of autism to those most biologically active PBDEs, not typically measured, but clearly implicated in neurotoxicity. Therapies seeking to decrease oxidative stress-mediated damage, improve mitochondrial function or minimize symptoms observed in some ASD cases need to be carefully evaluated if a careful biochemical and metabolic characterization of the subject has not been done to avoid deleterious side effects or refractory outcomes [222, 223]. This is relevant considering that reactive oxygen species do not solely elicit damage to biomolecules but also exhibit a role in signal transduction pathways significant to bioenergetics and cellular metabolism [224–226].
Acknowledgments
Sarah Wong: performed all experiments pertaining to mtDNA, contributed to the writing of this review, and approved the final manuscript being submitted.
Cecilia Giulivi: Conceptualized and designed the study, wrote the manuscript, performed some of the statistical analyses, and approved the final manuscript as submitted.
Funding Source: This work was supported by a grant from Autism Speaks, Simons Foundation, NIEHS R01-ES011269, R01-ES015359 and R01-ES020392. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
List of Abbreviations
- ASD
Autism Spectrum Disorder
- PBDE
polybrominated diphenyl ethers
- PTEN
phosphatase and tensin homolog
- ARE
antioxidant response element
- Nrf2
Nuclear Factor, Erythroid 2-Like 2
- PBMC
peripheral blood monocytic cells
- mtDNA
mitochondrial DNA
- CHARGE
CHildhood Autism Risks from Genetics and Environment Study
Footnotes
Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ Sci Technol. 2008;42(21):8158–8164. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]
- 3.Dingemans MM, van den Berg M, Westerink RH. Neurotoxicity of Brominated Flame Retardants: (In-)Direct Effects of Parent and Hydroxylated Polybrominated Diphenyl Ethers on the (Developing) Nervous System. Environ Health Perspect. 2011 doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coburn CG, Curras-Collazo MC, Kodavanti PRS. In vitro effects of environmentally relevant polybrominated diphenyl ether (PBDE) congeners on calcium buffering mechanisms in rat brain. Neurochem. Res. 2008;33(2):355–364. doi: 10.1007/s11064-007-9430-x. [DOI] [PubMed] [Google Scholar]
- 5.Dingemans MML, Heusinkveld HJ, Bergman A, van den Berg M, Westerink RHS. Bromination Pattern of Hydroxylated Metabolites of BDE-47 Affects Their Potency to Release Calcium from Intracellular Stores in PC12 Cells. Environ. Health Perspect. 2010;118(4):519–525. doi: 10.1289/ehp.0901339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu X, Hu D, Xu Y. Effects of tetrabrominated diphenyl ether and hexabromocyclododecanes in single and complex exposure to hepatoma HepG2 cells. Environ. Toxicol. Pharmacol. 2009;27(3):327–337. doi: 10.1016/j.etap.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Huang SC, Giordano G, Costa LG. Comparative cytotoxicity and intracellular accumulation of five polybrominated diphenyl ether congeners in mouse cerebellar granule neurons. Toxicol. Sci. 2010;114(1):124–132. doi: 10.1093/toxsci/kfp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kodavanti PRS. Differential effects of polybrominated diphenyl ethers and polychlorinated biphenyls on intracellular signaling in rat neuronal cultures. Organohalogen Compd. 2003;65:1–4. [Google Scholar]
- 9.Dingemans MML, de Groot A, van Kleef RGDM, Bergman A, van den Berg M, Vijverberg HPM, et al. Hydroxylation increases the neurotoxic potential of BDE-47 to affect exocytosis and calcium homeostasis in PC12 cells. Environ. Health Perspect. 2008;116(5):637–643. doi: 10.1289/ehp.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao J, White CC, Dabrowski MJ, Kavanagh TJ, Eckert ML, Gallagher EP. The role of mitochondrial and oxidative injury in BDE 47 toxicity to human fetal liver hematopoietic stem cells. Toxicol. Sci. 2008;101(1):81–90. doi: 10.1093/toxsci/kfm256. [DOI] [PubMed] [Google Scholar]
- 11.Yu K, He Y, Yeung LWY, Lam PKS, Wu RSS, Zhou B. DE-71-induced apoptosis involving intracellular calcium and the Bax-mitochondria-caspase protease pathway in human neuroblastoma cells in vitro. Toxicol. Sci. 2008;104(2):341–351. doi: 10.1093/toxsci/kfn088. [DOI] [PubMed] [Google Scholar]
- 12.He P, Wang A-g, Xia T, Gao P, Niu Q, Guo L-j, et al. Mechanisms underlying the developmental neurotoxic effect of PBDE-47 and the enhanced toxicity associated with its combination with PCB153 in rats. NeuroToxicology. 2009;30:1088–1095. doi: 10.1016/j.neuro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Yan C, Huang D, Zhang Y. The involvement of ROS overproduction and mitochondrial dysfunction in PBDE-47-induced apoptosis on Jurkat cells. Exp. Toxicol. Pathol. 2011;63:413–417. doi: 10.1016/j.etp.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Dingemans MML, van den Berg M, Bergman A, Westerink RHS. Calcium-related processes involved in the inhibition of depolarization-evoked calcium increase by hydroxylated PBDEs in PC12 cells. Toxicol. Sci. 2010;114(2):302–309. doi: 10.1093/toxsci/kfp310. [DOI] [PubMed] [Google Scholar]
- 15.Kodavanti PRS, Ward TR. Differential effects of commercial polybrominated diphenyl ether and polychlorinated biphenyl mixtures on intracellular signaling in rat brain in vitro. Toxicol. Sci. 2005;85(2):952–962. doi: 10.1093/toxsci/kfi147. [DOI] [PubMed] [Google Scholar]
- 16.Reistad T, Fonnum F, Mariussen E. Neurotoxicity of the pentabrominated diphenyl ether mixture, DE-71, and hexabromocyclododecane (HBCD) in rat cerebellar granule cells in vitro. Arch. Toxicol. 2006;80(11):785–796. doi: 10.1007/s00204-006-0099-8. [DOI] [PubMed] [Google Scholar]
- 17.Lunder S, Hovander L, Athanassiadis I, Bergman Ö. Significantly Higher Polybrominated Diphenyl Ether Levels in Young U.S. Children than in Their Mothers. Environ. Sci. Technol. 2010;44(13):5256–5262. doi: 10.1021/es1009357. [DOI] [PubMed] [Google Scholar]
- 18.Branchi I, Capone F, Alleva E, Costa LG. Polybrominated diphenyl ethers: Neurobehavioral effects following developmental exposure. NeuroToxicology. 2003;24(3):449–462. doi: 10.1016/S0161-813X(03)00020-2. [DOI] [PubMed] [Google Scholar]
- 19.Schecter A, Johnson-Welch S, Tung KC, Harris TR, Papke O, Rosen R. Polybrominated diphenyl ether (PBDE) levels in livers of U.S. human fetuses and newborns. J Toxicol Environ Health A. 2007;70(1):1–6. doi: 10.1080/15287390600748369. [DOI] [PubMed] [Google Scholar]
- 20.Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2012;17(3):290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauhan A, Gu F, Essa MM, Wegiel J, Kaur K, Brown WT, et al. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J Neurochem. 2011;117(2):209–220. doi: 10.1111/j.1471-4159.2011.07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas RH. Autism and mitochondrial disease. Dev Disabil Res Rev. 2010;16(2):144–153. doi: 10.1002/ddrr.112. [DOI] [PubMed] [Google Scholar]
- 23.Giulivi C, Zhang Y-F, Omanska-Klusek A, Ross-Inta CM, Wong S, Hertz-Picciotto I, et al. Mitochondrial dysfunction in autism. JAMA. 2010;304:2389–2396. doi: 10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira G, Diogo L, Grazina M, Garcia P, Ataide A, Marques C, et al. Mitochondrial dysfunction in autism spectrum disorders: a population-based study. Dev Med Child Neurol. 2005;47(3):185–189. doi: 10.1017/s0012162205000332. [DOI] [PubMed] [Google Scholar]
- 25.Arnoult D, Soares F, Tattoli I, Girardin SE. Mitochondria in innate immunity. EMBO Rep. 2011;12(9):901–910. doi: 10.1038/embor.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26(3):383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Napoli E, Hung C, Wong S, Giulivi C. Toxicity of the flame-retardant BDE-49 on brain mitochondria and neuronal progenitor striatal cells enhanced by a PTEN-deficient background. Toxicol Sci. 2013;132(1):196–210. doi: 10.1093/toxsci/kfs339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giulivi C, Napoli E, Schwartzer J, Careaga M, Ashwood P. Gestational exposure to a viral mimetic poly(i:C) results in long-lasting changes in mitochondrial function by leucocytes in the adult offspring. Mediators Inflamm. 2013;2013:609602. doi: 10.1155/2013/609602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernier FP, Boneh A, Dennett X, Chow CW, Cleary MA, Thorburn DR. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology. 2002;59(9):1406–1411. doi: 10.1212/01.wnl.0000033795.17156.00. [DOI] [PubMed] [Google Scholar]
- 30.Hardan AY, Fung LK, Libove RA, Obukhanych TV, Nair S, Herzenberg LA, et al. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol Psychiatry. 2012;71(11):956–961. doi: 10.1016/j.biopsych.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frye R. Biomarker of abnormal energy metabolism in children with autism spectrum disorder. N Am J Med Sci Monit. 2012;5:141–147. [Google Scholar]
- 32.Napoli E, Wong S, Giulivi C. Evidence of reactive oxygen species-mediated damage to mitochondrial DNA in children with typical autism. Mol Autism. 2013;4(1):2. doi: 10.1186/2040-2392-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HC, Yin PH, Chi CW, Wei YH. Increase in mitochondrial mass in human fibroblasts under oxidative stress and during replicative cell senescence. J Biomed Sci. 2002;9(6 Pt 1):517–526. doi: 10.1007/BF02254978. [DOI] [PubMed] [Google Scholar]
- 34.Liu CS, Tsai CS, Kuo CL, Chen HW, Lii CK, Ma YS, et al. Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic Res. 2003;37(12):1307–1317. doi: 10.1080/10715760310001621342. [DOI] [PubMed] [Google Scholar]
- 35.Tang G, Gutierrez Rios P, Kuo SH, Akman HO, Rosoklija G, Tanji K, et al. Mitochondrial abnormalities in temporal lobe of autistic brain. Neurobiol Dis. 2013;54:349–361. doi: 10.1016/j.nbd.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeVito TJ, Drost DJ, Neufeld RW, Rajakumar N, Pavlosky W, Williamson P, et al. Evidence for cortical dysfunction in autism: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 2007;61(4):465–473. doi: 10.1016/j.biopsych.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 37.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31(3):137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Graf WD, Marin-Garcia J, Gao HG, Pizzo S, Naviaux RK, Markusic D, et al. Autism associated with the mitochondrial DNA G8363A transfer RNA(Lys) mutation. J Child Neurol. 2000;15(6):357–361. doi: 10.1177/088307380001500601. [DOI] [PubMed] [Google Scholar]
- 39.Goh S, Dong Z, Zhang Y, DiMauro S, Peterson BS. Mitochondrial dysfunction as a neurobiological subtype of autism spectrum disorder: evidence from brain imaging. JAMA Psychiatry. 2014;71(6):665–671. doi: 10.1001/jamapsychiatry.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frye RE, Rossignol DA. Mitochondrial dysfunction can connect the diverse medical symptoms associated with autism spectrum disorders. Pediatr Res. 2011;69(5 Pt 2):41R–47R. doi: 10.1203/PDR.0b013e318212f16b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scaglia F, Towbin JA, Craigen WJ, Belmont JW, Smith EO, Neish SR, et al. Clinical spectrum, morbidity, and mortality in 113 pediatric patients with mitochondrial disease. Pediatrics. 2004;114(4):925–931. doi: 10.1542/peds.2004-0718. [DOI] [PubMed] [Google Scholar]
- 42.Hagerman R, Hoem G, Hagerman P. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol Autism. 2010;1(1):12. doi: 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zingerevich C, Greiss-Hess L, Lemons-Chitwood K, Harris SW, Hessl D, Cook K, et al. Motor abilities of children diagnosed with fragile X syndrome with and without autism. J Intellect Disabil Res. 2009;53(1):11–18. doi: 10.1111/j.1365-2788.2008.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, et al. Autism profiles of males with fragile X syndrome. Am J Ment Retard. 2008;113(6):427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Napoli E, Ross-Inta C, Wong S, Hung C, Fujisawa Y, Sakaguchi D, et al. Mitochondrial dysfunction in Pten haplo-insufficient mice with social deficits and repetitive behavior: interplay between Pten and p53. PLoS One. 2012;7(8):e42504. doi: 10.1371/journal.pone.0042504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grosser E, Hirt U, Janc OA, Menzfeld C, Fischer M, Kempkes B, et al. Oxidative burden and mitochondrial dysfunction in a mouse model of Rett syndrome. Neurobiol Dis. 2012;48(1):102–114. doi: 10.1016/j.nbd.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Gibson JH, Slobedman B, K NH, Williamson SL, Minchenko D, El-Osta A, et al. Downstream targets of methyl CpG binding protein 2 and their abnormal expression in the frontal cortex of the human Rett syndrome brain. BMC Neurosci. 2010;11:53. doi: 10.1186/1471-2202-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Condie J, Goldstein J, Wainwright MS. Acquired microcephaly, regression of milestones, mitochondrial dysfunction, and episodic rigidity in a 46,XY male with a de novo MECP2 gene mutation. J Child Neurol. 2010;25(5):633–636. doi: 10.1177/0883073809342004. [DOI] [PubMed] [Google Scholar]
- 49.Knerr I, Gibson KM, Jakobs C, Pearl PL. Neuropsychiatric morbidity in adolescent and adult succinic semialdehyde dehydrogenase deficiency patients. CNS Spectr. 2008;13(7):598–605. doi: 10.1017/s1092852900016874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim KJ, Pearl PL, Jensen K, Snead OC, Malaspina P, Jakobs C, et al. Succinic semialdehyde dehydrogenase: biochemical-molecular-clinical disease mechanisms, redox regulation, and functional significance. Antioxid Redox Signal. 2011;15(3):691–718. doi: 10.1089/ars.2010.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frye RE. 15q11.2–13 duplication, mitochondrial dysfunction, and developmental disorders. J Child Neurol. 2009;24(10):1316–1320. doi: 10.1177/0883073809333531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Filipek PA, Juranek J, Smith M, Mays LZ, Ramos ER, Bocian M, et al. Mitochondrial dysfunction in autistic patients with 15q inverted duplication. Ann Neurol. 2003;53(6):801–804. doi: 10.1002/ana.10596. [DOI] [PubMed] [Google Scholar]
- 53.Pagano G, Castello G. Oxidative stress and mitochondrial dysfunction in Down syndrome. Adv Exp Med Biol. 2012;724:291–299. doi: 10.1007/978-1-4614-0653-2_22. [DOI] [PubMed] [Google Scholar]
- 54.Pallardo FV, Lloret A, Lebel M, d'Ischia M, Cogger VC, Le Couteur DG, et al. Mitochondrial dysfunction in some oxidative stress-related genetic diseases: Ataxia-Telangiectasia, Down Syndrome, Fanconi Anaemia and Werner Syndrome. Biogerontology. 2010;11(4):401–419. doi: 10.1007/s10522-010-9269-4. [DOI] [PubMed] [Google Scholar]
- 55.Frye RE. Mitochondrial disease in 22q13 duplication syndrome. J Child Neurol. 2012;27(7):942–949. doi: 10.1177/0883073811429858. [DOI] [PubMed] [Google Scholar]
- 56.Su H, Fan W, Coskun PE, Vesa J, Gold JA, Jiang YH, et al. Mitochondrial dysfunction in CA1 hippocampal neurons of the UBE3A deficient mouse model for Angelman syndrome. Neurosci Lett. 2011;487(2):129–133. doi: 10.1016/j.neulet.2009.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology. 2009;20(1):84–90. doi: 10.1097/EDE.0b013e3181902d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marazziti D, Baroni S, Picchetti M, Landi P, Silvestri S, Vatteroni E, et al. Mitochondrial alterations and neuropsychiatric disorders. Curr Med Chem. 2011;18(30):4715–4721. doi: 10.2174/092986711797379221. [DOI] [PubMed] [Google Scholar]
- 59.Alvarez-Iglesias V, Mosquera-Miguel A, Cusco I, Carracedo A, Perez-Jurado LA, Salas A. Reassessing the role of mitochondrial DNA mutations in autism spectrum disorder. BMC Med Genet. 2011;12:50. doi: 10.1186/1471-2350-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Persico AM, Napolioni V. Autism genetics. Behav Brain Res. 2013;251:95–112. doi: 10.1016/j.bbr.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Hadjixenofontos A, Schmidt MA, Whitehead PL, Konidari I, Hedges DJ, Wright HH, et al. Evaluating mitochondrial DNA variation in autism spectrum disorders. Ann Hum Genet. 2013;77(1):9–21. doi: 10.1111/j.1469-1809.2012.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herbert MR, Russo JP, Yang S, Roohi J, Blaxill M, Kahler SG, et al. Autism and environmental genomics. NeuroToxicology. 2006;27(5):671–684. doi: 10.1016/j.neuro.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 63.Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmun Rev. 2004;3(7–8):557–562. doi: 10.1016/j.autrev.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 64.Bayona-Bafaluy MP, Lopez-Gallardo E, Montoya J, Ruiz-Pesini E. Maternally inherited susceptibility to cancer. Biochim Biophys Acta. 2011;1807(6):643–649. doi: 10.1016/j.bbabio.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Nishigaki Y, Fuku N, Tanaka M. Mitochondrial haplogroups associated with lifestyle-related diseases and longevity in the Japanese population. Geriatr Gerontol Int. 2010;10(Suppl 1):S221–S235. doi: 10.1111/j.1447-0594.2010.00599.x. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hartiala J, Allayee H, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96(1):80–89. doi: 10.3945/ajcn.110.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol. 2014;43(2):443–464. doi: 10.1093/ije/dyt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmidt RJ, Tancredi DJ, Krakowiak P, Hansen RL, Ozonoff S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am J Epidemiol. 2014;180(9):890–900. doi: 10.1093/aje/kwu208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harvey L, Boksa P. Additive effects of maternal iron deficiency and prenatal immune activation on adult behaviors in rat offspring. Brain Behav Immun. 2014;40:27–37. doi: 10.1016/j.bbi.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Nuttall JR. The plausibility of maternal toxicant exposure and nutritional status as contributing factors to the risk of autism spectrum disorders. Nutr Neurosci. 2015 doi: 10.1080/1028415X.2015.1103437. [DOI] [PubMed] [Google Scholar]
- 71.Fowler BA, Woods JS. Ultrastructural and biochemical changes in renal mitochondria during chronic oral methyl mercury exposure: the relationship to renal function. Exp Mol Pathol. 1977;27(3):403–412. doi: 10.1016/0014-4800(77)90010-7. [DOI] [PubMed] [Google Scholar]
- 72.Shenker BJ, Guo TL, O I, Shapiro IM. Induction of apoptosis in human T-cells by methyl mercury: temporal relationship between mitochondrial dysfunction and loss of reductive reserve. Toxicol Appl Pharmacol. 1999;157(1):23–35. doi: 10.1006/taap.1999.8652. [DOI] [PubMed] [Google Scholar]
- 73.Pourahmad J, Mihajlovic A, O'Brien PJ. Hepatocyte lysis induced by environmental metal toxins may involve apoptotic death signals initiated by mitochondrial injury. Adv Exp Med Biol. 2001;500:249–252. doi: 10.1007/978-1-4615-0667-6_38. [DOI] [PubMed] [Google Scholar]
- 74.Goyer RA. Toxic and essential metal interactions. Annu Rev Nutr. 1997;17:37–50. doi: 10.1146/annurev.nutr.17.1.37. [DOI] [PubMed] [Google Scholar]
- 75.Hiura TS, Li N, Kaplan R, Horwitz M, Seagrave JC, Nel AE. The role of a mitochondrial pathway in the induction of apoptosis by chemicals extracted from diesel exhaust particles. J Immunol. 2000;165(5):2703–2711. doi: 10.4049/jimmunol.165.5.2703. [DOI] [PubMed] [Google Scholar]
- 76.Wong PW, Garcia EF, Pessah IN. ortho-substituted PCB95 alters intracellular calcium signaling and causes cellular acidification in PC12 cells by an immunophilin-dependent mechanism. J Neurochem. 2001;76(2):450–463. doi: 10.1046/j.1471-4159.2001.00022.x. [DOI] [PubMed] [Google Scholar]
- 77.Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119(6):873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry. 2013;70(1):71–77. doi: 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Volk HE, Kerin T, Lurmann F, Hertz-Picciotto I, McConnell R, Campbell DB. Autism spectrum disorder: interaction of air pollution with the MET receptor tyrosine kinase gene. Epidemiology. 2014;25(1):44–47. doi: 10.1097/EDE.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sherer TB, Richardson JR, Testa CM, Seo BB, Panov AV, Yagi T, et al. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson's disease. J Neurochem. 2007;100(6):1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamano T, Morita S. Effects of pesticides on isolated rat hepatocytes, mitochondria, and microsomes II. Arch Environ Contam Toxicol. 1995;28(1):1–7. doi: 10.1007/BF00213961. [DOI] [PubMed] [Google Scholar]
- 82.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17(7):389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130(6):e1447–e1454. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10(11):643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- 85.Poletaev AB, Poletaeva AA, Pukhalenko AI, Zamaleeva RS, Cherepanova NA, Frizin DV. Adaptive maternal immune deviations as a ground for autism spectrum disorders development in children. Folia Med (Plovdiv) 2014;56(2):73–80. doi: 10.2478/folmed-2014-0011. [DOI] [PubMed] [Google Scholar]
- 86.Onore CE, Schwartzer JJ, Careaga M, Berman RF, Ashwood P. Maternal immune activation leads to activated inflammatory macrophages in offspring. Brain Behav Immun. 2014;38:220–226. doi: 10.1016/j.bbi.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mazina V, Gerdts J, Trinh S, Ankenman K, Ward T, Dennis MY, et al. Epigenetics of autism-related impairment: copy number variation and maternal infection. J Dev Behav Pediatr. 2015;36(2):61–67. doi: 10.1097/DBP.0000000000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee BK, Magnusson C, Gardner RM, Blomstrom A, Newschaffer CJ, Burstyn I, et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun. 2015;44:100–105. doi: 10.1016/j.bbi.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord. 2015;45(12):4015–4025. doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anon Possible link between the contactin 4 gene and autism spectrum disorder identified. Future Neurol. 2008;3(3):225–227. [Google Scholar]
- 91.Buxbaum JD, Silverman J, Keddache M, Smith CJ, Hollander E, Ramoz N, et al. Linkage analysis for autism in a subset families with obsessive-compulsive behaviors: evidence for an autism susceptibility gene on chromosome 1 and further support for susceptibility genes on chromosome 6 and 19. Mol Psychiatry. 2004;9(2):144–150. doi: 10.1038/sj.mp.4001465. [DOI] [PubMed] [Google Scholar]
- 92.Feuk L. Copy number variation in the autism genome. Expert Opin. Med. Diagn. 2008;2(4):417–428. doi: 10.1517/17530059.2.4.417. [DOI] [PubMed] [Google Scholar]
- 93.Kim SJ, Cox N, Courchesne R, Lord C, Corsello C, Akshoomoff N, et al. Transmission disequilibrium mapping at the serotonin transporter gene (SLC6A4) region in autistic disorder. Mol Psychiatry. 2002;7(3):278–288. doi: 10.1038/sj.mp.4001033. [DOI] [PubMed] [Google Scholar]
- 94.Li H, Yamagata T, Mori M, Momoi MY. Absence of causative mutations and presence of autism-related allele in FOXP2 in Japanese autistic patients. Brain Dev. 2005;27(3):207–210. doi: 10.1016/j.braindev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 95.Nagarajan RP, Patzel KA, Martin M, Yasui DH, Swanberg SE, Hertz-Picciotto I, et al. MECP2 promoter methylation and X chromosome inactivation in autism. Autism Res. 2008;1(3):169–178. doi: 10.1002/aur.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petit E, Herault J, Raynaud M, Cherpi C, Perrot A, Barthelemy C, et al. X chromosome and infantile autism. Biol Psychiatry. 1996;40(6):457–464. doi: 10.1016/0006-3223(96)85270-X. [DOI] [PubMed] [Google Scholar]
- 97.Ramanathan S, Woodroffe A, Flodman PL, Mays LZ, Hanouni M, Modahl CB, et al. A case of autism with an interstitial deletion on 4q leading to hemizygosity for genes encoding for glutamine and glycine neurotransmitter receptor sub-units (AMPA 2, GLRA3, GLRB) and neuropeptide receptors NPY1R, NPY5R. BMC Med Genet. 2004;5:10. doi: 10.1186/1471-2350-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ramoz N, Reichert JG, Smith CJ, Silverman JM, Bespalova IN, Davis KL, et al. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am J Psychiatry. 2004;161(4):662–669. doi: 10.1176/appi.ajp.161.4.662. [DOI] [PubMed] [Google Scholar]
- 99.Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468(7321):263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci. 2006;9(10):1221–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- 101.Zecavati N, Spence SJ. Neurometabolic disorders and dysfunction in autism spectrum disorders. Curr Neurol Neurosci Rep. 2009;9(2):129–136. doi: 10.1007/s11910-009-0021-x. [DOI] [PubMed] [Google Scholar]
- 102.Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Dalla Bernardina B, et al. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic Biol Med. 2012;52(10):2128–2141. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 103.Obrenovich ME, Shamberger RJ, Lonsdale D. Altered heavy metals and transketolase found in autistic spectrum disorder. Biol Trace Elem Res. 2011;144(1–3):475–486. doi: 10.1007/s12011-011-9146-2. [DOI] [PubMed] [Google Scholar]
- 104.Ming X, Johnson WG, Stenroos ES, Mars A, Lambert GH, Buyske S. Genetic variant of glutathione peroxidase 1 in autism. Brain Dev. 2010;32(2):105–109. doi: 10.1016/j.braindev.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 105.James SJ, Rose S, Melnyk S, Jernigan S, Blossom S, Pavliv O, et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. Faseb J. 2009 doi: 10.1096/fj.08-128926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Palmieri L, Persico AM. Mitochondrial dysfunction in autism spectrum disorders: cause or effect? Biochim Biophys Acta. 2010;1797(6–7):1130–1137. doi: 10.1016/j.bbabio.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 107.Clark-Taylor T, Clark-Taylor BE. Is autism a disorder of fatty acid metabolism? Possible dysfunction of mitochondrial beta-oxidation by long chain acyl-CoA dehydrogenase. Med Hypotheses. 2004;62(6):970–975. doi: 10.1016/j.mehy.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 108.Fillano JJ, Goldenthal MJ, Rhodes CH, Marin-Garcia J. Mitochondrial dysfunction in patients with hypotonia, epilepsy, autism, and developmental delay: HEADD syndrome. J Child Neurol. 2002;17(6):435–439. doi: 10.1177/088307380201700607. [DOI] [PubMed] [Google Scholar]
- 109.Lombard J. Autism: a mitochondrial disorder? Med Hypotheses. 1998;50(6):497–500. doi: 10.1016/s0306-9877(98)90270-5. [DOI] [PubMed] [Google Scholar]
- 110.McDonald TA. A perspective on the potential health risks of PBDEs. Chemosphere. 2002;46(5):745–755. doi: 10.1016/s0045-6535(01)00239-9. [DOI] [PubMed] [Google Scholar]
- 111.Sjodin A, Patterson DG, Jr, Bergman A. A review on human exposure to brominated flame retardants--particularly polybrominated diphenyl ethers. Environ Int. 2003;29(6):829–839. doi: 10.1016/S0160-4120(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 112.De Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46(5):583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- 113.Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193(Pt 3):199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 114.Darnerud PO. Toxic effects of brominated flame retardants in man and in wildlife. Environ Int. 2003;29(6):841–853. doi: 10.1016/S0160-4120(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 115.Park HY, Hertz-Picciotto I, Sovcikova E, Kocan A, Drobna B, Trnovec T. Neurodevelopmental toxicity of prenatal polychlorinated biphenyls (PCBs) by chemical structure and activity: a birth cohort study. Environ Health. 2010;9:51. doi: 10.1186/1476-069X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, Von Gunten U, et al. The challenge of micropollutants in aquatic systems. Science. 2006;313(5790):1072–1077. doi: 10.1126/science.1127291. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, Zhao C, Ma W, Liu H, Wang T, Jiang G. Quantitative structure-activity relationship for prediction of the toxicity of polybrominated diphenyl ether (PBDE) congeners. Chemosphere. 2006;64(4):515–524. doi: 10.1016/j.chemosphere.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 118.Noren K, Meironyte D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere. 2000;40(9–11):1111–1123. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- 119.Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG. Polybrominated diphenyl ether flame retardants in the North American environment. Environ Int. 2003;29(6):771–779. doi: 10.1016/S0160-4120(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 120.Bakker MI, De Winter-Sorkina R, De Mul A, Boon PE, Van Donkersgoed G, Van Klaveren JD, et al. Dietary intake of polybrominated diphenylethers in the Netherlands. Organohalogen Compd. 2006;68:387–390. [Google Scholar]
- 121.Bocio A, Domingo JL, Marti-Cid R, Mata E, Teixido A, Llobet JM. Dietary intake of PBDEs by the population of Catalonia, Spain. Temporal trend. Organohalogen Compd. 2007;69:327/1–327/3. [Google Scholar]
- 122.Bocio A, Llobet JM, Domingo JL, Corbella J, Teixido A, Casas C. Polybrominated Diphenyl Ethers (PBDEs) in Foodstuffs: Human Exposure through the Diet. J. Agric. Food Chem. 2003;51(10):3191–3195. doi: 10.1021/jf0340916. [DOI] [PubMed] [Google Scholar]
- 123.Dirtu AC, Covaci A. Daily intake of OCPs, PCBs and PBDEs from food consumption and indoor dust ingestion in Romania. Organohalogen Compd. 2008;70:562–565. [Google Scholar]
- 124.Dirtu AC, Covaci A. Estimation of Daily Intake of Organohalogenated Contaminants from Food Consumption and Indoor Dust Ingestion in Romania. Environ. Sci. Technol. 2010;44(16):6297–6304. doi: 10.1021/es101233z. [DOI] [PubMed] [Google Scholar]
- 125.Fernandes A, White S, D'Silva K, Rose M. Simultaneous determination of PCDDs, PCDFs, PCBs and PBDEs in food. Talanta. 2004;63(5):1147–1155. doi: 10.1016/j.talanta.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 126.Gensler M, Schwind K-H, Jira W. Polybrominated diphenylether (PBDE) in animal food. Fleischwirtschaft. 2009;89(5):105–110. [Google Scholar]
- 127.Huwe JK, Larsen GL. Polychlorinated Dioxins, Furans, and Biphenyls, and Polybrominated Diphenyl Ethers in a U.S. Meat Market Basket and Estimates of Dietary Intake. Environ. Sci. Technol. 2005;39(15):5606–5611. doi: 10.1021/es050638g. [DOI] [PubMed] [Google Scholar]
- 128.Kim M, Kim D-G, Choi SW, Bong Y-H, Jang J-H, Chung G-S. Levels and dietary intakes of polybrominated diphenyl ethers and hexachlorobenzene in meat in Korea. Organohalogen Compd. 2008;70:1780–1783. [Google Scholar]
- 129.Kiviranta H, Hallikainen A, Ruokojarvi P, Rantakokko P, Vartiainen T. PCDD/F, PCB, and PBDE levels in finnish foodstuffs in 2003–2005. Organohalogen Compd. 2006;68:1898–1901. [Google Scholar]
- 130.Ohta S, Ishizuka D, Nishimura H, Nakao T, Aozasa O, Shimidzu Y, et al. Comparison of polybrominated diphenyl ethers in fish, vegetables, and meats and levels in human milk of nursing women in Japan. Chemosphere. 2002;46(5):689–696. doi: 10.1016/s0045-6535(01)00233-8. [DOI] [PubMed] [Google Scholar]
- 131.Schecter A, Paepke O, Ryan JJ, Rosen R, Tung KC, Pavuk M, et al. PBDEs in U.S. milk, blood, and food, and temporal trends for PBDEs, PCDDs, and PCBs in US blood. Organohalogen Compd. 2004;66:2800–2806. (Dioxin 2004) [Google Scholar]
- 132.Schecter A, Paepke O, Tung K-C, Staskal D, Birnbaum L. Polybrominated Diphenyl Ethers Contamination of United States Food. Environ. Sci. Technol. 2004;38(20):5306–5311. doi: 10.1021/es0490830. [DOI] [PubMed] [Google Scholar]
- 133.Seo J, Lee N-H, Shin J-H, Han S-Y, Jung K-K, Kang I-H, et al. Dietary PBDE intake: a market-basket study in Korea. Organohalogen Compd. 2008;70:2147–2150. [Google Scholar]
- 134.Shen H-t, Han J-l, Ren Y-p. Preliminary evaluation of PCDD-Fs in agricultural products and human intake. Huanjing Huaxue. 2007;26(2):269–270. [Google Scholar]
- 135.Sun S-J, Zhao J-H, Leng J-H, Wang P-Y, Wang Y, Fukatsu H, et al. Levels of dioxins and polybrominated diphenyl ethers in human milk from three regions of northern China and potential dietary risk factors. Chemosphere. 2010;80(10):1151–1159. doi: 10.1016/j.chemosphere.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 136.Tlustos C, Fernandes A, White S, Rose M. PBDEs, PBDD/Fs and PBBs in carcass fat, liver, eggs and milk produced in Ireland. Organohalogen Compd. 2008;70:209–212. [Google Scholar]
- 137.Yu Y-X, Li J-L, Zhang X-Y, Yu Z-Q, Van de Wiele T, Han S-Y, et al. Assessment of the Bioaccessibility of Polybrominated Diphenyl Ethers in Foods and the Correlations of the Bioaccessibility with Nutrient Contents. J. Agric. Food Chem. 2010;58(1):301–308. doi: 10.1021/jf9036358. [DOI] [PubMed] [Google Scholar]
- 138.Hites RA, Foran JA, Schwager SJ, Knuth BA, Hamilton MC, Carpenter DO. Global assessment of polybrominated diphenyl ethers in farmed and wild salmon. Environ Sci Technol. 2004;38(19):4945–4949. doi: 10.1021/es049548m. [DOI] [PubMed] [Google Scholar]
- 139.Perello G, Marti-Cid R, Castell V, Llobet JM, Domingo JL. Concentrations of polybrominated diphenyl ethers, hexachlorobenzene and polycyclic aromatic hydrocarbons in various foodstuffs before and after cooking. Food Chem. Toxicol. 2009;47(4):709–715. doi: 10.1016/j.fct.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 140.Sjodin A, Hagmar L, Klasson-Wehler E, Kronholm-Dlab K, Jakobsson E, Bergman O. Flame retardant exposure: Polybrominated diphenyl ethers in blood from Swedish workers. Environ. Health Perspect. 1999;107(8):643–648. doi: 10.1289/ehp.107-1566483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lind Y, Darnerud PO, Atuma S, Aune M, Becker W, Bjerselius R, et al. Polybrominated diphenyl ethers in breast milk from Uppsala County, Sweden. Environmental Research. 2003;93(2):186–194. doi: 10.1016/s0013-9351(03)00049-5. [DOI] [PubMed] [Google Scholar]
- 142.Schecter A, Pavuk M, Paepke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers' milk. Environ. Health Perspect. 2003;111(14):1723–1729. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Betts KS. Rapidly rising PBDE levels in North America. Environ Sci Technol. 2002;36(3):50A–52A. doi: 10.1021/es022197w. [DOI] [PubMed] [Google Scholar]
- 144.She J, Petreas M, Winkler J, Visita P, McKinney M, Kopec D. PBDEs in the San Francisco Bay Area: measurements in harbor seal blubber and human breast adipose tissue. Chemosphere. 2002;46(5):697–707. doi: 10.1016/s0045-6535(01)00234-x. [DOI] [PubMed] [Google Scholar]
- 145.Petreas M, Nelson D, Brown FR, Goldberg D, Hurley S, Reynolds P. High concentrations of polybrominated diphenylethers (PBDEs) in breast adipose tissue of California women. Environ Int. 2011;37(1):190–197. doi: 10.1016/j.envint.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Davis JA, Hetzel F, Oram JJ, McKee LJ. Polychlorinated biphenyls (PCBs) in San Francisco Bay. Environ Res. 2007;105(1):67–86. doi: 10.1016/j.envres.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 147.Flegal AR, Brown CL, Squire S, Ross JR, Scelfo GM, Hibdon S. Spatial and temporal variations in silver contamination and toxicity in San Francisco Bay. Environ Res. 2007;105(1):34–52. doi: 10.1016/j.envres.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 148.Luengen AC, Friedman CS, Raimondi PT, Flegal AR. Evaluation of mussel immune responses as indicators of contamination in San Francisco Bay. Mar Environ Res. 2004;57(3):197–212. doi: 10.1016/S0141-1136(03)00070-9. [DOI] [PubMed] [Google Scholar]
- 149.Oros DR, Ross JR, Spies RB, Mumley T. Polycyclic aromatic hydrocarbon (PAH) contamination in San Francisco Bay: a 10-year retrospective of monitoring in an urbanized estuary. Environ Res. 2007;105(1):101–118. doi: 10.1016/j.envres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 150.Petreas M, She J, Brown FR, Winkler J, Windham G, Rogers E, et al. High body burdens of 2,2',4,4'-tetrabromodiphenyl ether (BDE-47) in California women. Environ Health Perspect. 2003;111(9):1175–1179. doi: 10.1289/ehp.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rose M, Bennett DH, Bergman A, Fangstrom B, Pessah IN, Hertz-Picciotto I. PBDEs in 2–5 year-old children from California and associations with diet and indoor environment. Environ. Sci. Technol. 2010;44(7):2648–2653. doi: 10.1021/es903240g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Toms LM, Harden F, Paepke O, Hobson P, Ryan JJ, Mueller JF. Higher accumulation of polybrominated diphenyl ethers in infants than in adults. Environ Sci Technol. 2008;42(19):7510–7515. doi: 10.1021/es800719v. [DOI] [PubMed] [Google Scholar]
- 153.Affairs DoC, editor. 1992. Bureau of Home Furnishings and Thermal Insulation; p. 133. [Google Scholar]
- 154.Alexander FE, Patheal SL, Biondi A, Brandalise S, Cabrera ME, Chan LC, et al. Transplacental chemical exposure and risk of infant leukemia with MLL gene fusion. Cancer Res. 2001;61(6):2542–2546. [PubMed] [Google Scholar]
- 155.Ma X, Buffler PA, Gunier RB, Dahl G, Smith MT, Reinier K, et al. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environ. Health Perspect. 2002;110(9):955–960. doi: 10.1289/ehp.02110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Woodruff TJ, Axelrad DA, Kyle AD, Nweke O, Miller GG, Hurley BJ. Trends in environmentally related childhood illnesses. Pediatrics. 2004;113(4 II):1133–1140. [PubMed] [Google Scholar]
- 157.Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ. Health Perspect. 2003;111(9):1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001;61(1):76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
- 159.Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardants: A novel class of developmental neurotoxicants in our environment? Environ. Health Perspect. 2001;109(9):903–908. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gill U, Chu I, Ryan JJ, Feeley M. Polybrominated diphenyl ethers: human tissue levels and toxicology. Rev Environ Contam Toxicol. 2004;183:55–97. doi: 10.1007/978-1-4419-9100-3_3. [DOI] [PubMed] [Google Scholar]
- 161.Kim KH, Bose DD, Ghogha A, Riehl J, Zhang R, Barnhart CD, et al. Para- and ortho-substitutions are key determinants of polybrominated diphenyl ether activity toward ryanodine receptors and neurotoxicity. Environ Health Perspect. 2011;119(4):519–526. doi: 10.1289/ehp.1002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther. 2010;125(2):260–285. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miller MF, Chernyak SM, Batterman S, Loch-Caruso R. Polybrominated diphenyl ethers in human gestational membranes from women in southeast Michigan. Environ Sci Technol. 2009;43(9):3042–3046. doi: 10.1021/es8032764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mariottini M, Corsi I, Della Torre C, Caruso T, Bianchini A, Nesi I, et al. Biomonitoring of polybrominated diphenyl ether (PBDE) pollution: a field study. Comp Biochem Physiol C Toxicol Pharmacol. 2008;148(1):80–86. doi: 10.1016/j.cbpc.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 165.Roosens L, Dirtu AC, Goemans G, Belpaire C, Gheorghe A, Neels H, et al. Brominated flame retardants and polychlorinated biphenyls in fish from the river Scheldt, Belgium. Environ Int. 2008;34(7):976–983. doi: 10.1016/j.envint.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 166.Manchester-Neesvig JB, Valters K, Sonzogni WC. Comparison of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in Lake Michigan salmonids. Environ Sci Technol. 2001;35(6):1072–1077. doi: 10.1021/es001422b. [DOI] [PubMed] [Google Scholar]
- 167.Birnbaum LS, Staskal DF. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004;112(1):9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Meerts IATM, van Zanden JJ, Luijks EAC, van Leeuwen-Bol I, Marsh G, Jakobsson E, et al. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56(1):95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- 169.Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002;66(1):105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
- 170.Stoker TE, Laws SC, Crofton KM, Hedge JM, Ferrell JM, Cooper RL. Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol Sci. 2004;78(1):144–155. doi: 10.1093/toxsci/kfh029. [DOI] [PubMed] [Google Scholar]
- 171.Kodavanti PRS, Royland JE, Ramabhadran R, Osorio C, Alzate O. Changes in proteomic profiles of cerebellum following developmental exposure to aroclor 1254 or DE-71. Organohalogen Compd. 2007;69:494/1–494/4. [Google Scholar]
- 172.van Boxtel AL, Kamstra JH, Cenijn PH, Pieterse B, Wagner MJ, Antink M, et al. Microarray Analysis Reveals a Mechanism of Phenolic Polybrominated Diphenylether Toxicity in Zebrafish. Environ. Sci. Technol. 2008;42(5):1773–1779. doi: 10.1021/es0720863. [DOI] [PubMed] [Google Scholar]
- 173.Tseng L-H, Lee C-W, Pan M-H, Tsai S-S, Li M-H, Chen J-R, et al. Postnatal exposure of the male mouse to 2,2',3,3',4,4',5,5',6,6'-decabrominated diphenyl ether: Decreased epididymal sperm functions without alterations in DNA content and histology in testis. Toxicology. 2006;224(1–2):33–43. doi: 10.1016/j.tox.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 174.Talsness CE, Shakibaei M, Kuriyama SN, Grande SW, Sterner-Kock A, Schnitker P, et al. Ultrastructural changes observed in rat ovaries following in utero and lactational exposure to low doses of a polybrominated flame retardant. Toxicol. Lett. 2005;157(3):189–202. doi: 10.1016/j.toxlet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 175.Nie F, Qu J, Yang G, Wu W. Oxidative damage of decabromodiphenyl ether to the mitochondria from the liver of Carassius auratus in vitro. Anquan Yu Huanjing Xuebao. 2008;8(5):5–8. [Google Scholar]
- 176.Hsu PC, Tseng LH, Lee CW. Effects of prenatal exposure of decabrominated diphenyl ether (PCBDE 209) on reproductive system in male mice. Organohalogen Compd. 2006;68:1547–1550. [Google Scholar]
- 177.Talsness CE, Shakibaei M, Kuriyama S, de Souza C, Chahoud I. Ultrastructural changes in the ovaries of adult offspring following a single maternal exposure to low dose 2,2',4,4',5-pentabromodiphenyl ether. Organohalogen Compd. 2003;61:88–91. [Google Scholar]
- 178.Blaylock RL. A possible central mechanism in autism spectrum disorders, part 2: immunoexcitotoxicity. Altern Ther Health Med. 2009;15(1):60–67. [PubMed] [Google Scholar]
- 179.Lerman-Sagie T, Leshinsky-Silver E, Watemberg N, Lev D. Should autistic children be evaluated for mitochondrial disorders? J Child Neurol. 2004;19(5):379–381. doi: 10.1177/088307380401900510. [DOI] [PubMed] [Google Scholar]
- 180.Hertz-Picciotto I, Bergman A, Fangstrom B, Rose MB, Krakowiak P, Pessah IN, et al. Polybrominated diphenyl ethers in relation to autism and developmental delay: A case-control study. Environ Health. 2011;10(1):1. doi: 10.1186/1476-069X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Valdameri G, Trombetta-Lima M, Worfel PR, Pires AR, Martinez GR, Noleto GR, et al. Involvement of catalase in the apoptotic mechanism induced by apigenin in HepG2 human hepatoma cells. Chem Biol Interact. 2011;193(2):180–189. doi: 10.1016/j.cbi.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 182.Chang YF, Hsu YC, Hung HF, Lee HJ, Lui WY, Chi CW, et al. Quercetin induces oxidative stress and potentiates the apoptotic action of 2-methoxyestradiol in human hepatoma cells. Nutr Cancer. 2009;61(5):735–745. doi: 10.1080/01635580902825571. [DOI] [PubMed] [Google Scholar]
- 183.Lima CF, Valentao PC, Andrade PB, Seabra RM, Fernandes-Ferreira M, Pereira-Wilson C. Water and methanolic extracts of Salvia officinalis protect HepG2 cells from t-BHP induced oxidative damage. Chem Biol Interact. 2007;167(2):107–115. doi: 10.1016/j.cbi.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 184.García-Alonso J, Ros G, Jesús Periago M. Antiproliferative and cytoprotective activities of a phenolic-rich juice in HepG2 cells. Food Research International. 2006;39(9):982–991. [Google Scholar]
- 185.Alia M, Ramos S, Mateos R, Granado-Serrano AB, Bravo L, Goya L. Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol Appl Pharmacol. 2006;212(2):110–118. doi: 10.1016/j.taap.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 186.Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16(10):577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 187.Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-κB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol. Pharmacol. 2006;69(3):1033–1040. doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]
- 188.Ding M, Zhao J, Bowman L, Lu Y, Shi X. Inhibition of AP-1 and MAPK signaling and activation of Nrf2/ARE pathway by quercitrin. Int J Oncol. 2010;36(1):59–67. [PubMed] [Google Scholar]
- 189.Tanigawa S, Fujii M, Hou DX. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic Biol Med. 2007;42(11):1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 190.Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol. 2006;69(3):1033–1040. doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]
- 191.Eggler AL, Gay KA, Mesecar AD. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res. 2008;52(Suppl 1):S84–S94. doi: 10.1002/mnfr.200700249. [DOI] [PubMed] [Google Scholar]
- 192.Nebreda AR, Porras A. p38 MAP kinases: beyond the stress response. Trends Biochem Sci. 2000;25(6):257–260. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- 193.Wang X, Ye XL, Liu R, Chen HL, Bai H, Liang X, et al. Antioxidant activities of oleanolic acid in vitro: possible role of Nrf2 and MAP kinases. Chem Biol Interact. 2010;184(3):328–337. doi: 10.1016/j.cbi.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 194.Pi J, Bai Y, Reece JM, Williams J, Liu D, Freeman ML, et al. Molecular mechanism of human Nrf2 activation and degradation: role of sequential phosphorylation by protein kinase CK2. Free Radic Biol Med. 2007;42(12):1797–1806. doi: 10.1016/j.freeradbiomed.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Copple IM, Goldring CE, Jenkins RE, Chia AJ, Randle LE, Hayes JD, et al. The hepatotoxic metabolite of acetaminophen directly activates the Keap1-Nrf2 cell defense system. Hepatology. 2008;48(4):1292–1301. doi: 10.1002/hep.22472. [DOI] [PubMed] [Google Scholar]
- 196.Goldring CE, Kitteringham NR, Elsby R, Randle LE, Clement YN, Williams DP, et al. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology. 2004;39(5):1267–1276. doi: 10.1002/hep.20183. [DOI] [PubMed] [Google Scholar]
- 197.Naidu S, Vijayan V, Santoso S, Kietzmann T, Immenschuh S. Inhibition and genetic deficiency of p38 MAPK up-regulates heme oxygenase-1 gene expression via Nrf2. J Immunol. 2009;182(11):7048–7057. doi: 10.4049/jimmunol.0900006. [DOI] [PubMed] [Google Scholar]
- 198.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757(5–6):509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 199.Szeto HH. Mitochondria-targeted peptide antioxidants: Novel neuroprotective agents. AAPS Journal. 2006;8(3):521–531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee JM, Shih AY, Murphy TH, Johnson JA. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278(39):37948–37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- 201.Napoli E, Wong S, Hertz-Picciotto I, Giulivi C. Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics. 2014;133(5):e1405–e1410. doi: 10.1542/peds.2013-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Linnane AW, Ozawa T, Marzuki S, Tanaka M. Mitochondrial-DNA Mutations as an Important Contributor to Ageing and Degenerative Diseases. Lancet. 1989;1(8639):642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- 203.Lee HC, Pang CY, Hsu HS, Wei YH. Differential accumulations of 4,977 bp deletion in mitochondrial DNA of various tissues in human ageing. Biochim Biophys Acta. 1994;1226(1):37–43. doi: 10.1016/0925-4439(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 204.Fukui H, Moraes CT. Mechanisms of formation and accumulation of mitochondrial DNA deletions in aging neurons. Hum Mol Genet. 2009;18(6):1028–1036. doi: 10.1093/hmg/ddn437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lee HC, Yin PH, Lu CY, Chi CW, Wei YH. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem J. 2000;348(Pt 2):425–432. [PMC free article] [PubMed] [Google Scholar]
- 206.Chou YF, Huang RF. Mitochondrial DNA deletions of blood lymphocytes as genetic markers of low folate-related mitochondrial genotoxicity in peripheral tissues. Eur J Nutr. 2009;48(7):429–436. doi: 10.1007/s00394-009-0031-0. [DOI] [PubMed] [Google Scholar]
- 207.Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- 208.Lee HC, Lu CY, Fahn HJ, Wei YH. Aging- and smoking-associated alteration in the relative content of mitochondrial DNA in human lung. FEBS Lett. 1998;441(2):292–296. doi: 10.1016/s0014-5793(98)01564-6. [DOI] [PubMed] [Google Scholar]
- 209.Moreno-Loshuertos R, Acin-Perez R, Fernandez-Silva P, Movilla N, Perez-Martos A, Rodriguez de Cordoba S, et al. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat Genet. 2006;38(11):1261–1268. doi: 10.1038/ng1897. [DOI] [PubMed] [Google Scholar]
- 210.Ylikallio E, Tyynismaa H, Tsutsui H, Ide T, Suomalainen A. High mitochondrial DNA copy number has detrimental effects in mice. Hum. Mol. Genet. 2010;19(13):2695–2705. doi: 10.1093/hmg/ddq163. [DOI] [PubMed] [Google Scholar]
- 211.Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, et al. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry. 2012;2:e134. doi: 10.1038/tp.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141b(8):947–956. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Melnyk S, Fuchs GJ, Schulz E, Lopez M, Kahler SG, Fussell JJ, et al. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J Autism Dev Disord. 2012;42(3):367–377. doi: 10.1007/s10803-011-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Finkelstein JD. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin Chem Lab Med. 2007;45(12):1694–1699. doi: 10.1515/CCLM.2007.341. [DOI] [PubMed] [Google Scholar]
- 215.Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. 2008;13(3):216–226. [PubMed] [Google Scholar]
- 216.Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet. 1992;2(4):324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- 217.Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci. 2007;27(27):7310–7317. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Noch E, Khalili K. Molecular mechanisms of necrosis in glioblastoma: the role of glutamate excitotoxicity. Cancer Biol Ther. 2009;8(19):1791–1797. doi: 10.4161/cbt.8.19.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kraft AD, Harry GJ. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int J Environ Res Public Health. 2011;8(7):2980–3018. doi: 10.3390/ijerph8072980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ashwood P, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen RL, Croen LA, et al. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008;204(1–2):149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Frye RE, Delatorre R, Taylor H, Slattery J, Melnyk S, Chowdhury N, et al. Redox metabolism abnormalities in autistic children associated with mitochondrial disease. Transl Psychiatry. 2013;3:e273. doi: 10.1038/tp.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Napoli E, Duenas N, Giulivi C. Potential therapeutic use of the ketogenic diet in autistic spectrum disorders. Frontiers in Pediatrics. 2014;2 doi: 10.3389/fped.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gargus JJ. Mitochondrial energy-deficiency endophenotypes in autism. J Neurochem. 2008;104:16. [Google Scholar]
- 224.Huang J, Lam GY, Brumell JH. Autophagy signaling through reactive oxygen species. Antioxid Redox Signal. 2011;14(11):2215–2231. doi: 10.1089/ars.2010.3554. [DOI] [PubMed] [Google Scholar]
- 225.Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20(7):332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 226.Luckhart S, Pakpour N, Giulivi C. Host-pathogen interactions in malaria: cross-kingdom signaling and mitochondrial regulation. Curr Opin Immunol. 2015;36:73–79. doi: 10.1016/j.coi.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]