Abstract
Background
HIV may amplify immunologic, physiologic, and functional changes of aging. We determined associations of frailty phenotype, a T-cell senescence marker (p16INK4a expression), age, and demographics with exposures of the intracellular metabolites (IM) and endogenous nucleotides (EN) of tenofovir/emtricitabine (TFV/FTC), efavirenz (EFV), atazanavir (ATV), and ritonavir (RTV).
Materials and Methods
Plasma and PBMC samples for drug, IM, and EN concentrations were collected at 4 time points in HIV+ adults receiving TFV/FTC with EFV or ATV/RTV. Subjects underwent frailty phenotyping and p16INK4a expression analysis. Noncompartmental analysis generated an area under the curve (AUC) for each analyte. Spearman rank correlation and Kruskal-Wallis tests were used to assess associations between AUC, demographics, and aging markers, adjusting for multiple comparisons with the Holm procedure.
Results
Subjects (n=79) ranged in age from 22–73yr (median 48yr). Forty-eight were African-American, 24 were female, 54 received EFV. Three subjects (range 51–60yr) demonstrated frailty, with 17 subjects (range 26–60yr) demonstrating pre-frailty. Negative associations were observed between p16INK4a expression and each of FTC-triphosphate (r= −0.45), deoxyadenosine triphosphate (dATP) (r= −0.47), and deoxycytidine triphosphate (dCTP) (r= −0.57) AUCs (p-values<0.02). TFV and FTC AUCs were larger among subjects with lower renal function or higher chronologic age (p-values ≤0.05). No associations were observed for EFV, ATV, or RTV AUCs.
Conclusions
Associations of IM/EN exposure and p16INK4a expression observed here suggest that senescence may alter drug phosphorylation, metabolism, or transport. This finding warrants further mechanistic study to ensure optimal treatment in the aging HIV+ population.
Introduction
Older HIV-infected adults (≥50 years) may experience increased morbidity/mortality due to overlapping effects of HIV infection and aging.(1, 2) Optimal antiretroviral (ARV) therapy is critical to the health of this growing HIV sub-population. Despite this, little is known regarding how the physiologic and immunologic processes of aging may affect ARV pharmacokinetics. As cohort studies demonstrate, chronologic age is an imperfect marker of aging, particularly in HIV-infected subjects, (3, 4) and measuring other biomarkers of aging, such as frailty and cellular senescence, should be undertaken.
Nucleoside reverse transcriptase inhibitors (NRTIs), particularly tenofovir (TFV) and emtricitabine (FTC), form the backbone of recommend ARV regimens,(5) and undergo metabolism in immune cells to their active phosphorylated forms.(6) These intracellular metabolites (IM) compete with endogenous nucleotides (EN) during reverse transcription. The IM:EN ratio may be important for virologic efficacy,(7, 8) although little is known about the concentrations of EN in HIV-infected patients receiving ARVs, or effects of cellular senescence on IM/EN concentrations.
Here, we present the results of ARV, IM, and EN area under the curve (AUC; a measure of exposure) in HIV-infected subjects, and their associations with aging biomarkers.
Methods
Clinical Study Conduct
HIV-infected adults (≥18 years) receiving TFV/FTC 300/200mg with efavirenz 600mg (EFV) or atazanavir/ritonavir 300/100mg (ATV/r) daily for ≥two weeks were recruited from the UNC HealthCare Infectious Diseases Clinic (Chapel Hill, NC) and the Cone Health Regional Center for Infectious Diseases (Greensboro, NC). The study protocol was approved by the Institutional Review Boards of both institutions (Clinicaltrials.gov NCT01180075).
Subjects underwent eligibility screening prior to providing 4 timed blood samples (pre-dose, and 2, 4–6, and 10–14 hours post-dose). Sampling times were optimized based on a previous intensive PK study in older HIV-infected adults.(9) Subjects completed the protocol in 1–3 visits, providing 1–2 blood samples per visit; subjects underwent frailty phenotyping and provided blood for p16INK4a analysis once.
Subjects were included in the study if they: demonstrated adherence, defined as ≤1 missed dose in the last week and no missed doses in the 3 days prior to sampling; were not anemic (hemoglobin <10 mg/dL); were not receiving co-medications expected to alter ARV drug exposures by ≥30% or alter intracellular nucleotide pools; had no unstable, acute, medical conditions, and no DAIDS Grade ≥2 lab abnormalities, with the exception of total bilirubin for subjects receiving ATV/r. Subjects were included if their creatinine clearance (CrCL), as calculated by the Cockroft-Gault formula,(10) was >30 mL/min. Women of childbearing potential underwent urine pregnancy testing prior to sampling, as pregnancy was exclusionary.
Frailty phenotyping was conducted per Fried et al(11). Three positive markers of the frailty phenotype defined frailty, while 1–2 positive markers defined pre-frailty. Testing was conducted by the NC TraCS Institute Bionutrition Core.
Analytical Methods
Blood collected in K2EDTA tubes was kept on ice and centrifuged at 3000g for 15 minutes at 4C within 30 minutes of collection to recover plasma, and stored at −80C until analysis. Total concentrations of TFV, FTC, EFV, ATV, and ritonavir (RTV) were analyzed using validated methods.(12, 13) Additional File 1 contains brief methods for unbound EFV, ATV, and RTV concentrations.
Blood collected in 8mL CPT tubes was stored at room temperature for ≤4 hours prior to centrifugation. Internal testing demonstrated that samples could undergo centrifugation at 1300g for 30 minutes, the resulting cell layer and plasma transferred to a 15mL conical tube, and stored on ice for ≤8 hours from collection without significant decreases in cell count or drug concentrations (data not shown). Samples were then processed to recover peripheral blood mononuclear cells (PBMCs) for IM and EN concentrations, as previously described.(9) Brief methods of the tenofovir diphosphate (TFV-dp), emtricitabine triphosphate (FTC-tp), deoxyadenosine triphosphate (dATP), and deoxycytidine triphosphate (dCTP) assay are provided in Additional File 1. Drug concentrations were determined in the UNC Center for AIDS Research Clinical Pharmacology and Analytical Chemistry Laboratory.
Blood collected in K2EDTA tubes was used to isolate T-cells and provide cellular RNA for PCR-based determination of p16INK4a expression, using previously published methods.(14)
Pharmacokinetic and Statistical Analysis
Subjects who completed the study per protocol were included. To construct an AUC, the four samples were assumed to occur in the same dosing interval. Pre-dose samples were assigned to the end, rather than the beginning, of the dosing interval to capture exposure from 2–24 hours. Phoenix Win Nonlin 6.3 (Pharsight, A Certara Company, St. Louis, MO) was used to calculate AUC, using the linear up/log down method and oral dosing model. Exact Wilcoxon rank-sum tests were used to test the IM and EN AUCs for differences between regimens.
Associations between AUC and subject demographics, including positive frailty components, CD4:CD8 ratio and p16INK4a expression, were assessed using Spearman’s rank correlation test (continuous variables) and an exact Kruskal-Wallis test (categorical variables). Because age and CrCL were significantly correlated, multiple linear regression of AUC was used to assess adjusted associations for these variables. For statistical analyses, AUC values were log10-transformed and p16INK4a values were log2-transformed. A two-sided p-value ≤0.05 was considered statistically significant; the Holm (stepdown Bonferroni) procedure was used to adjust for multiple comparisons (174 tests); reported p-values are the Holm-adjusted values unless otherwise noted. Analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC) or R 3.1.2 (r-project.org).
Results
Subject Demographics and Safety
Fifty-four subjects receiving TFV/FTC/EFV and twenty-five receiving TFV/FTC/ATV/r were enrolled and provided ≥1 blood sample. Subject demographics are provided in Table 1. Seventy-three of 79 subjects completed the study per protocol; the remaining 6 did not provide 4 blood samples and were excluded. Three subjects demonstrated frailty; 1 receiving TFV/FTC/ATV/r and 2 receiving TFV/FTC/EFV. No serious adverse events were reported. Concentration-time plots are provided in Additional File 2.
Table 1.
Demographic data of study subjects. Data are presented as median (min, max) or number (percent). BMI: body mass index; CrCL: creatinine clearance.
| Characteristic | Total (n=79) | Efavirenz Group (n=54) | Atazanavir/ritonavir Group (n=25) |
|---|---|---|---|
| Age (years) | 48 (22, 73) | 48 (22, 73) | 48 (24, 61) |
| HIV Duration (years) | 10 (1, 31) | 10 (1, 31) | 11 (1, 24) |
| BMI (kg/m2) | 28.1 (17.6, 44.3) | 27.1 (17.6, 44.3) | 29.8 (20.2, 40.4) |
| CrCL (mL/min) | 108 (43, 228) | 109 (43, 200) | 100 (67, 228) |
| Log2(p16INK4a) | 2.03 (−1.1, 3.91) | 1.93 (−1.14, 2.81) | 2.23 (0.16, 3.91) |
| CD4 Count (cell/mm3) | 732 (125, 1724) | 736 (126, 1724) | 692 (375, 1436) |
| CD4:CD8 ratio | 0.9 (0.3, 2.8) | 0.9 (0.3, 2.8) | 0.9 (0.3, 1.4) |
| Female | 24 (30%) | 14 (26%) | 10 (40%) |
| Race | |||
| African American | 48 (61%) | 32 (59%) | 16 (64%) |
| Caucasian | 26 (33%) | 19 (35%) | 7 (28%) |
| Other/Unknown | 5 (6%) | 3 (6%) | 2 (8%) |
| Total Frailty Markers | n=75 | n=50 | n=25 |
| 0 | 55 (73%) | 40 (80%) | 15 (60%) |
| 1–2 (pre-frail) | 17 (23%) | 8 (16%) | 9 (36%) |
| 3 (frail) | 3 (4%) | 2 (4%) | 1 (4%) |
Associations of Exposure with Aging Markers and Demographics
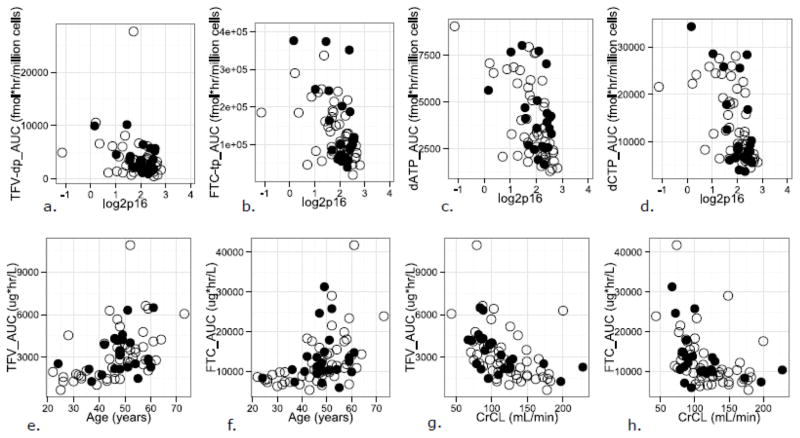
Results of the Spearman correlation analyses are presented in Table 2. No statistically significant differences were observed for the IM/EN AUCs between regimens (unadjusted p>0.1); all association testing was done using pooled AUCs. Figure 1a–h shows scatter plots of IM/EN AUCs vs. demographics shown in Table 2.
Table 2.
Spearman rho (r) and Holm adjusted p-values for associations between demographic variables and analyte area under the curve (AUC), with the number of subjects who completed per protocol. CrCL: creatinine clearance; dATP: deoxyadenosine triphosphate; dCTP: deoxycytidine triphosphate; TFV-dp: tenofovir diphosphate; FTC-tp: emtricitabine triphosphate; TFV: tenofovir; FTC: emtricitabine.
| Analyte AUC tested | Age r (adjusted p-value) n=73 |
Calculated CrCL r (adjusted p-value) n=73 |
Log2 p16INK4a r (adjusted p-value) n=70 |
|---|---|---|---|
| dATP | −0.09 (1) | −0.06 (1) | −0.47 (0.0066) |
| dCTP | −0.14 (1) | 0.17 (1) | −0.57 (<0.0001) |
| TFV-dp | 0.23 (1) | −0.26 (1) | −0.33 (0.8105) |
| FTC-tp | 0.02 (1) | −0.009 (1) | −0.45 (0.0179) |
| TFV | 0.49 (0.0017) | −0.57 (<0.0001) | 0.047 (1) |
| FTC | 0.54 (<0.0001) | −0.56 (0.0001) | 0.36 (0.4302) |
Figure 1a–h.
Scatter plots of tenofovir disphosphate (TFV-tp), emtricitabine triphosphate (FTC-tp), deoxyadenosine triphosphate (dATP), and deoxycytidine triphosphate (dCTP) area under the curve (AUC) values vs. log2p16INK4a (log2p16) expression (a–d); TFV and FTC AUC values vs. age (e, f); and TFV and FTC AUC values vs. creatinine clearance (CrCL; g,h), by regimen. Subjects receiving TFV/FTC with efavirenz are shown in the open circles; those receiving TFV/FTC with atazanavir/ritonavir are shown in the closed circles.
Age and CrCL were significantly associated with FTC and TFV AUC, with the Spearman rho (r) demonstrating relationships of similar strength, but in opposite direction, i.e., a positive association between AUC and age (r= 0.54, 0.49 for FTC and TFV, respectively), and a negative association between AUC and CrCL (r= −0.56, −0.57 for FTC and TFV, respectively). Age remained significantly associated with TFV AUC and FTC AUC after adjusting for CrCL, and vice versa (p<0.05).
Negative associations between p16INK4a and AUC were observed for FTC-tp, dATP, and dCTP (r= −0.45, −0.47, −0.57, p= 0.018, 0.007, and <0.001, respectively).
After adjustment for multiple comparisons, no significant associations between EFV, ATV, and RTV total or unbound AUCs with any of the tested demographics, including frailty and p16INK4a expression, were observed (not shown).
Discussion
In this study, an association between increasing age/decreasing CrCL and TFV/FTC exposures, and an association of higher p16INK4a expression and lower intracellular exposures of FTC-tp, dATP, and dCTP were observed. The association between CrCL and TFV/FTC AUC is expected (15), however, the association with chronologic age remained significant after controlling for CrCL. The negative associations between p16INK4a expression and FTC-TP and ENs were unexpected, and may suggest senescence-altered cellular transport of nucleotides, and/or alterations of kinase/phosphorylase activity involved in their metabolism. Inflammation, part of the senescent-cell phenotype, may affect hepatic drug metabolizing enzymes,(16) and could affect enzyme function at other sites, such as in PBMCs.(17) The drugs themselves may alter p16INK4a expression; aging patients and/or patients with persistent immune activation may possess different cellular subpopulations within PBMC samples, affecting apparent phosphorylation. The clinical implications and mechanisms of these findings remain to be elucidated.
Several investigations have suggested that TFV/TFV-dp concentrations are increased in subjects receiving protease-inhibitors, likely due P-glycoprotein inhibition in PBMCs.(18, 19) However, we and others (20) have not observed this in our data.
Strengths of this work include measuring parent, IM, and EN concentrations for TFV/FTC, and total/unbound EFV, ATV, and RTV concentrations in 73 HIV-infected adults, along with measuring frailty and senescence markers. Limitations include the small number of frail subjects, and a moderate sample size relative to the number of hypothesis tests. Nonetheless, this work provides a basis for further investigation of intracellular pharmacology and effects of senescence on NRTI metabolism, efficacy, and toxicity.
Supplementary Material
Acknowledgments
The authors wish to thank the IRB 09-2120 study participants, as well as the staffs of the UNC Clinical and Translational Research Center (UL1RR02574), the UNC Health Care Infectious Diseases Clinic, and the Cone Health Regional Center for Infectious Diseases. Phoenix Win Nonlin software is generously provided to the Division of Pharmacotherapy and Experimental Therapeutics in the UNC Eshelman School of Pharmacy through the Certara Center of Excellence program. We also thank Dr. Angela Kashuba for oversight of drug concentration analysis in the UNC Center for AIDS Research Clinical Pharmacology and Analytical Chemistry Laboratory, and Cynthia Lee, Jordan Messer, and Jingxian Chen for assisting with AUC calculations.
JBD is supported by K23AI093156; funding for this work was provided in part by UL1RR02574.
OF, MC, HMAP, KM, CS, NW, SM, RW, MGH are supported in part by the UNC Center for AIDS Research (P30AI050410).
MC is supported by T32-GM086330.
C. Torrice and NES are supported by R01-AG024379-10.
Footnotes
Disclosures
Versions of this work were presented at both the 16th International Workshop on the Clinical Pharmacology of HIV and Hepatitis C Treatment (May 26–28, 2015, Alexandria, VA, Poster 34) and AIDS 2014 (July 20–25, 2014, Melbourne, Australia, Poster THPDB0105).
C. Trezza is now an employee of ViiV Healthcare and owns stock in GlaxoSmithKline.
References
- 1.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, Bertisch B, Bernasconi E, Weber R Study tSHC. Morbidity and Aging in HIV-Infected Persons: The Swiss HIV Cohort Study. Clinical Infectious Diseases. 2011;53:1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 2.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, Berti A, Rossi E, Roverato A, Palella F. Premature Age-Related Comorbidities Among HIV-Infected Persons Compared With the General Population. Clinical Infectious Diseases. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 3.Margolick J, Li X, Detels R, Phair J, Rinaldo C, Jacobson L Study MAC. Earlier Occurrence of the Frailty Phenotype in HIV+ Men than in HIV– Men: The MACS Cohort. 18th Conference on Retroviruses and Opportunistic Infections Paper #794.Feb, 2011. [Google Scholar]
- 4.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Margolick JB for the Multicenter ACS. HIV-1 Infection Is Associated With an Earlier Occurrence of a Phenotype Related to Frailty. 2007;62:1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Apr 8, 2015. [PubMed] [Google Scholar]
- 6.Anderson PL, Kakuda TN, Lichtenstein KA. The Cellular Pharmacology of Nucleoside- and Nucleotide-Analogue Reverse-Transcriptase Inhibitors and Its Relationship to Clinical Toxicities. Clinical Infectious Diseases. 2004;38:743–753. doi: 10.1086/381678. [DOI] [PubMed] [Google Scholar]
- 7.Amie SM, Noble E, Kim B. Intracellular nucleotide levels and the control of retroviral infections. Virology (New York, NY) 2013;436:247–254. doi: 10.1016/j.virol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Lerma JG, W A, MEC, Q Z, ASY, J M, A H, A M, S K, W L, CYL, DLH, E K, CPP, ASR, JFR, WAL, WH Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. Journal of virology. 2011;85:6610–6617. doi: 10.1128/JVI.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumond JB, Adams JL, Prince HMA, Kendrick RL, Wang R, Jennings SH, Malone S, White N, Sykes C, Corbett AH, Patterson KB, Forrest A, Kashuba ADM. Pharmacokinetics of two common antiretroviral regimens in older HIV-infected patients: a pilot study. HIV Medicine. 2013;14:401–409. doi: 10.1111/hiv.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cockcroft D, Gault M. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in Older Adults: Evidence for a Phenotype. 2001;56:M146–157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 12.Rezk NL, Crutchley RD, Kashuba ADM. Simultaneous quantification of emtricitabine and tenofovir in human plasma using high-performance liquid chromatography after solid phase extraction. Journal of Chromatography B. 2005;822:201–208. doi: 10.1016/j.jchromb.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Rezk NL, Tidwell RR, Kashuba ADM. High-performance liquid chromatography assay for the quantification of HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors in human plasma. Journal of Chromatography B. 2004;805:241–247. doi: 10.1016/j.jchromb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Nelson JA, Krishnamurthy J, Menezes P, Liu Y, Hudgens MG, Sharpless NE, Eron JJ., Jr Expression of p16(INK4a) as a biomarker of T-cell aging in HIV-infected patients prior to and during antiretroviral therapy. Aging cell. 2012;11:916–918. doi: 10.1111/j.1474-9726.2012.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilead Sciences. Truvada (emtricitabne/tenofovir) Full US Prescribing Information. Foster City, CA: 2009. [Google Scholar]
- 16.Fu ZD, Csanaky IL, Klaassen CD. Effects of Aging on mRNA Profiles for Drug-Metabolizing Enzymes and Transporters in Livers of Male and Female Mice. Drug Metabolism and Disposition. 2012;40:1216–1225. doi: 10.1124/dmd.111.044461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, McMahan V, Bushman LR, Casapía M, Montoya-Herrera O, Veloso VG, Mayer KH, Chariyalertsak S, Schechter M, Bekker LG, Kallás EG, Grant RM iPrEx Study Team. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Science translational medicine. 2012;4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahiri CD, Tao S, Jiang Y, Sheth AN, Acosta EP, Marconi VC, Armstrong WS, Schinazi RF, Vunnava A, Sanford S, Ofotokun I. Impact of protease inhibitors on intracellular concentration of tenofovir-diphosphate among HIV-1 infected patients. Aids. 2015;29:1113–1115. doi: 10.1097/QAD.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janneh O, Anwar T, Jungbauer C, Kopp S, Khoo SH, Back DJ, Chiba P. P-glycoprotein, multidrug resistance-associated proteins and human organic anion transporting polypeptide influence the intracellular accumulation of atazanavir. Antivir Ther. 2009;14:965–974. doi: 10.3851/IMP1399. [DOI] [PubMed] [Google Scholar]
- 20.Kiser JJ, Aquilante CL, Anderson PL, King TM, Carten ML, Fletcher CV. Clinical and Genetic Determinants of Intracellular Tenofovir Diphosphate Concentrations in HIV-Infected Patients. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2008;47:298–303. doi: 10.1097/qai.0b013e31815e7478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.