Abstract
Objective
Activation of STAT1 is directly downstream of cytokine receptors that signal from specific pro-inflammatory cytokines known to be dysregulated in schizophrenia, such as IFNγ, IL6, IL2, and IL10, as well as hypoxia, viral/bacterial infections and peptide growth factors. If the increased cytokine protein levels repeatedly observed in schizophrenia have biological consequences, then the measurement of pSTAT1 is a logical step forwards.
Methods
Peripheral blood mononuclear cells (PBMCs) from Controls (CON; n=13) and subjects with schizophrenia (SZ; n=22) were extracted using the Ficoll method. Participants with schizophrenia were diagnosed using the SCID, clinical symptomology was measured using the Positive and Negative Syndrome Scale (PANSS), and cognitive functioning was measured using the MATRICS Consensus Cognitive Battery. Levels of activated STAT1 (Y701; phosphorylated STAT1; pSTAT1) were measured by a commercially available ELISA in nuclear extracts from PBMCs.
Results
There was a significant bimodal distribution in the sample, with a subgroup of schizophrenia patients expressing significantly greater levels of activated pSTAT1 than the remainder of participants. In this SZ subgroup, levels of pSTAT1 were 68% higher than the control group, and 62% higher than the remainder of SZ subjects. Furthermore, this subsample of patients manifested significantly poorer cognitive performance on several measures of the MATRICS.
Discussion
pSTAT1 levels may provide a measure of the biological relevance of widely reported elevations in levels of cytokines in SZ over the past several decades. Activation of kinase cascades can be used to partition or disassemble the composite immune signal in living patients with SZ.
Keywords: pSTAT1, Peripheral blood mononuclear cells (PBMCs), lymphocytes, schizophrenia, kinases, phosphorylation, cytokines
1. INTRODUCTION
A subclinical immunoreactive state, broadly characterized by elevated levels of serum/plasma cytokines, as well as several parameters of immune cellular functioning, has been widely reported in schizophrenia (SZ) subjects [1,2]. Both genetic genome-wide association studies (GWAS) [3] and an increased prevalence of autoimmune conditions in the disorder [4,5] provide further support for a role of immunoreactivity in schizophrenia. However, there is substantial variability in published findings, which could be due to the multiple sources that contribute to blood cytokine levels, such as adipose tissue, liver and muscle. There is also no clear understanding of the biological impact of these subclinical elevated levels of cytokine proteins in participants with schizophrenia.
STAT1 is a member of the signal transducer and activator of transcription (STAT) family of transcription factors, capable of carrying a signal from a membrane receptor directly to the nucleus and a DNA cognate sequence. STAT proteins are dormant in the cytoplasm until activated by tyrosine phosphorylation. Multiple signals can activate pSTAT1 using the JAK/STAT axis, including growth factors (EFG), cytokines (INFγ, IL6, IL2, IL3, IL12, and IL22), and hypoxia [6,7]. Cytokine receptors of the type gp130 and IFNGR are associated with JAK kinases that induce tyrosine phosphorylation of cytoplasmic STAT1 (Y701), and pSTAT1 is especially strongly activated in CD14+ monocytes stimulated with IFNγ or IFNα [8]. Because a given cell can integrate cytokine signals from multiple pathways, different cytokines such as IFNγ and IL-6 (often implicated in schizophrenia) can both induce STAT1 activation upon binding to their specific membrane receptors [9]. A recurrent theme in schizophrenia research is the coordination of increased immunoreactivity, as measured by serum cytokines, and cognitive measures [10].
Levels of pSTAT1 can be measured using biochemical methods in peripheral blood mononuclear cells (PBMCs), as demonstrated by a number of previously published human studies. These include the measurement of immune states in rheumatoid arthritis (IL6) [11], as well as in response to corticosteroids in active asthma [12], and to cytokines in hepatitis C (IFNα) [8]. We hypothesized that measurement of an intracellular signaling pathway downstream of a cytokine receptor could aggregate the inputs of multiple immune stimuli and provide a more consistent measure of immune activation in peripheral blood samples. This study reports the analyses for a novel investigation into the molecular, clinical and cognitive interface.
2. METHODS
2.1 Subject Recruitment
Subjects were recruited from the University of Illinois at Chicago (UIC) Medical Center and surrounding urban community mental health clinics. The study was approved by UIC Institutional Review Board and all subjects provided written informed consent before participating in any study procedures. General exclusion criteria for all subjects were: 1) reported infections, 2) neurological disease or head trauma, 3) current substance dependence, 4) substance abuse within the past two months, and 5) pregnancy. Diagnosis was established using a SCID/DSM-IV interview [13], and available collateral information. Illicit substance use was obtained as part of the SCID diagnostic interview although we did not apply urine toxicology. The non-clinical control sample were defined as persons that did not meet criteria for current or past psychiatric diagnosis as evaluated by the SCID/DSM-IV. Clinical symptomology was measured using the Positive and Negative Syndrome Scale (PANSS), and cognition was measured using the MATRICS Consensus Cognitive Battery [14,15].
2.2 Subject Descriptives
A total of 35 subjects participated in the study of which 22 were participants with schizophrenia (SZ; 17 males and 5 females) and 13 were control subjects with no major Axis 1 disorder (CON; 5 males and 8 females). Of the 22 participants with SZ, 16 participants were evaluated on the inpatient psychiatric unit and 6 participants were evaluated in the outpatient clinic. The duration of illness mean score of the SZ group was 20 years. Additionally, 11/22 participants with SZ reported nicotine use compared to 5/13 non-clinical participants. Out of twenty-two participants with SZ, 18 were reported as being on atypical antipsychotic medication, 3 were reported as taking typical antipsychotic medication, and one was reported as un-medicated at the time of the blood draw. No CON subjects reported taking any antipsychotic medications. In addition, no relationship was found between pSTAT1 levels and CPZ equivalents of these medications [16,17]. Further, we cannot easily ascertain duration of pharmacological treatment due to multiple changes in psychotropic regimen that are difficult and unreliable to track, but if we approximate this parameter using ‘duration of the illness’, we do not observe a significant relationship.
2.3 PBMC Collection
All peripheral blood sample collections were performed under non-fasting conditions in the early morning between 07:00 and 09:00h to avoid circadian fluctuations. PBMCs were extracted using standard Ficoll gradients [18,19].
2.4 Nuclear Protein Extraction and Measurement
Nuclear protein extraction was performed as previously published [20] and protein levels were quantified using the Bradford method.
The pSTAT1 modification was measured utilizing the enzyme-linked immunosorbent assay (ELISA) kit commercially available from Abcam (#126456). Levels of total Histone 3 (H3) protein for each sample were also measured for normalization purposes using an aliquot of the identical sample utilized for the pSTAT1 modification. Total H3 protein was measured using the ActiveMotif (#53110) kit as previously described [21].
2.5 Clinical Measures
The primary clinical measures for this study were the Positive and Negative Syndrome Scale (PANSS) and the MATRICS Consensus Cognitive Battery [14,15]. PANSS items were scored along a continuum of severity between 1 (asymptomatic) to 7 (extreme symptom severity). Coefficient alpha, for inter-rater reliability was between 0.83 and 0.87. With regard to the MATRICs, we analyzed the cognitive domain scores rather than the individual test scores, which are considered a composite and reliable assessment of particular cognitive abilities [22,23,24]. These six domain scores include Processing speed, Attention, Working Memory, Verbal Memory, Visual Memory and Reasoning.
2.6 Statistical Analysis
SPSS (version 15.0 for Windows) was used for all statistical analyses. Data are presented as mean values ± standard error. A probability level of p<0.05 was the criterion to achieve statistical significance. Demographic and clinical data were analyzed by using Chi Square and analysis of variance (ANOVA). Scheffe post hoc tests were used to identify significant pair-wise group differences. Bimodality was tested assuming mixtures of normal distributions [25,26].
3. RESULTS
3.1 Descriptive Demographics
We found no significant effect of age, sex, race or BMI on pSTAT1 levels (Table 1). There was no significant difference between groups in nicotine use. Additionally, there was no significant difference in duration of illness between the SZ-LOW group compared to the SZ-HIGH group. We also found no effect of length of sample storage time, or number of freeze-thaw events. There were no significant differences in total H3 levels between controls and participants with schizophrenia.
Table 1. Demographics and Descriptives and pSTAT1 levels in CON-LOW, SZ-LOW and SZ-HIGH groups.
Demographics and Mean pSTAT1 for Participant Sub-Groups
| CON-LOW pSTAT1 (N=13) |
SZ-LOW pSTAT1 (N=13) |
SZ-HIGH pSTAT1 (N=9) |
P-value | ||||
|---|---|---|---|---|---|---|---|
| Sex | N | N | N | n.s | |||
| Males | 5 | 10 | 7 | ||||
| Females | 8 | 3 | 2 | ||||
| Age (Mean±SD) | 38.1 ±11.6 | 39.5 ±12.5 | 38.2 ±11.9 | n.s | |||
| Race | N | N | N | n.s | |||
| Caucasian, non-Hispanic | 1 | 0 | 1 | ||||
| Black, non-Hispanic | 8 | 10 | 8 | ||||
| Asian or other pacific Islander | 2 | 0 | 0 | ||||
| Hispanic | 2 | 3 | 0 | ||||
| BMI (Mean±SD) | 29.8 ±7.5 | 35.1 ±8.6 | 31.2 ±9.6 | n.s | |||
| Medication | Yes | No | Yes | No | Yes | No | |
| Typical Antipsychotics | 0 | 13 | 3 | 10 | 0 | 9 | |
| Atypical Antipsychotics | 0 | 13 | 11 | 2 | 8 | 1 | |
|
Mean pSTAT1 OD Levels/Total H3 |
0.14 | 0.08 | 0.46 | P<0.001 | |||
| Standard Deviation | 0.10 | 0.04 | 0.18 | ||||
| Standard Error | 0.03 | 0.01 | 0.06 | ||||
3.2 Bimodal Distribution and Diagnostic Differences
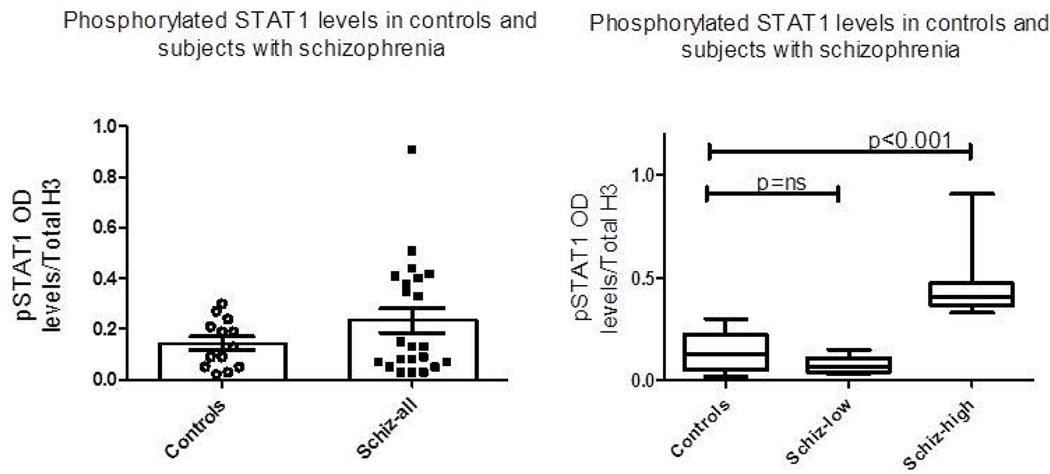
There was a trend towards increased pSTAT1 levels when comparing control (n=13) and schizophrenia (n=22) groups (0.1431 vs 0.2330, df 33, p<0.09). However, as seen in Figure 1a, the data suggested a bimodal distribution within the group of participants with schizophrenia, thus bimodality was tested assuming a mixture of two normal distributions [25,26]. The samples from participants with schizophrenia had a significantly bimodal distribution (μ1=0.015, σ1=0.124; μ2=0.310, σ2=0.206; p<0.001). Data were then analyzed based on this result, and participants with schizophrenia with a measurement above the μ2=0.310 value were coded as SZ-HIGH pSTAT1 activation while the remainder of the SZ sample was coded as SZ-LOW pSTAT1 activation (μ2<0.310). As noted in Table 1 there was no significant difference in gender, race or age between groups.
Figure 1.
(a) Scatterbar plot of control and all schizophrenia subjects on measures of pSTAT1 normalized to Total H3 protein from nuclear extracts in PBMCs. No significant difference (p<0.09) between control group and all participants with schizophrenia group. (b) Schizophrenia participants divided along a significant bimodal distribution as noted in results into SZ-LOW pSTAT1 and SZ-HIGH pSTAT1 groups. Box-whisker plots demonstrating median of the three groups. Significant differences as indicated. No difference between CON-LOW pSTAT1 vs SZ-LOW pSTAT1 groups.
We were thus able to characterize the participants into three groups; CON-LOW pSTAT1 (μ2<0.310), SZ-LOW pSTAT1 (μ2<0.310), and SZ-HIGH pSTAT1 (μ2≥0.310). Levels of pSTAT1 were now analyzed using a one-way ANOVA (Figure 1b). In the SZ-HIGH pSTAT1 subgroup, levels of pSTAT1 were 69% higher when compared to the CON-LOW pSTAT1 subjects, and 62% higher when compared to the SZ-LOW pSTAT1 subjects (Table 1).
3.3 Clinical and Cognitive Correlations
Our primary objective was to distinguish schizophrenia subjects according to whether they had activated pSTAT1 or not, i.e. subjects designated as SZ-HIGH pSTAT1 vs SZ-LOW pSTAT1. An ANOVA, with post-hoc Scheffe test, found that the total PANSS score as a measure of psychopathology was virtually identical in the two schizophrenia groups (104.54 vs. 104.44), and both were significantly higher than the control group (34.92). Furthermore a t-test indicated that there were no significant differences between the SZ-HIGH and SZ-LOW groups on the PANSS positive, negative or general subscales. There was also no significant correlation between pSTAT1 levels and PANSS scores in the SZ-HIGH pSTAT1 group.
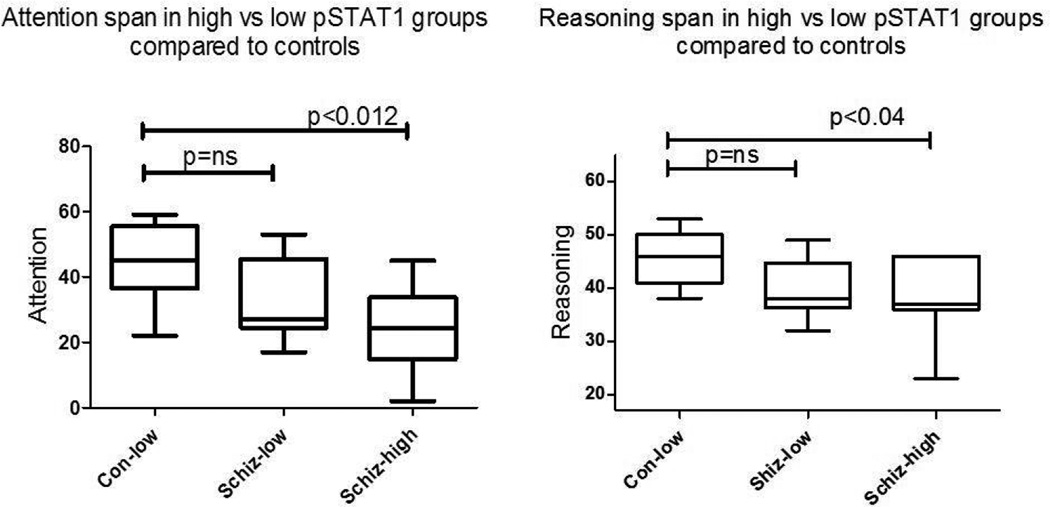
To continue this focus of analysis in the area of cognition, we initially compared all subjects with LOW-pSTAT1 vs HIGH-pSTAT1 on the six domain scores. Processing speed (t=2.2, p<0.01) and Attention (t=2.3, p<0.03) were significantly worse in subjects with HIGH-pSTAT1 when compared to all subjects with a LOW-pSTAT1 (CON-LOW + SZ-LOW). To identify cognition correlates of HIGH pSTAT1 in the schizophrenia subgroups, we next performed an ANOVA with the three groups, CON-LOW, SZ-LOW and SZ-HIGH, on the six domains. Attention and Reasoning scores were significantly lower in the SZ-HIGH group compared to either of the low groups, i.e. CON-LOW or SZ-LOW. Participants with schizophrenia with elevated pSTAT1 (SZ-HIGH) levels scored significantly worse on both Attention-scores (p<0.01) and Reasoning scores (p<0.04) than either the CON-LOW pSTAT1 or SZ-LOW pSTAT1 groups. On either of these two measures, the SZ-LOW group was not different from the CON-LOW group. A similar trend was observed for Processing Speed, but did not reach significance. Thus, SZ-HIGH pSTAT1 subjects showed poorer performance on these specific cognitive measures than the remainder of the sample. Though these particular cognitive measures were significantly worse in the SZ-HIGH group, we did not demonstrate a correlation between pSTAT1 levels and MATRICs domain scores.
4. DISCUSSION
Given the known heterogeneity of the schizophrenia diagnosis, as well as the multiple sources of immunoreactivity, the presence of a subgroup of participants with schizophrenia with a heightened level of pSTAT1 in their nuclear protein extracts may not be surprising, but remains an intriguing finding. The presence of the CON-LOW pSTAT1 group and the SZ-LOW pSTAT1 group provided a naturalistic subject comparison to further distinguish schizophrenia subjects with SZ-HIGH pSTAT1 activation.
STAT1 is phosphorylated by the JAK/STAT cascade emanating from membrane cytokine receptors that are targets of pro-inflammatory cytokines such as IFNγ and IL6. These cytokines are elevated in schizophrenia, as summarized by several meta-analyses [1,2]. As an example, IFNγ will induce STAT1 activation and sustained occupancy of the pSTAT1 cistrome [27,28], resulting in further upregulation of additional pro-inflammatory cytokines such as TNFα, IL6 and IL12β. pSTAT1 occupancy of its cistrome is facilitated by epigenetic chromatin modifications resulting in accessible chromatin [29,28]. This is consistent with our recent report of elevated histone 3 phosphorylated at serine 10 (H3S10phos) [21] in PBMCs from patients with schizophrenia. Furthermore, activation of the pSTAT1 pathway by IFNγ or IL6 can upregulate expression of Toll-like receptors (TLRs) in a variety of immune cells including monocytes [30,31], and consequently can prime these cells to amplify signals along the NF-κB pathway. TLR signals emanate from exogenous and endogenous TLR ligands, which are comprised of bacterial elements (lipopolysaccharides) as well as complexes of fatty acids and acute reactive proteins [32] resulting in what is sometimes termed ‘sterile’ inflammation.
Associations between immune functioning and cognition are increasingly reported. In rodents, increases in peripheral pro-inflammatory cytokines can induce changes in immune signaling in the hippocampus [33]. Recently, Fillman et al have also identified a subgroup of patients with schizophrenia that have elevated cytokines who demonstrate decreases in cognition (verbal fluency) combined with decreases in specific brain volume [34]. Specifically, elevated IL-1β significantly predicted lower scores in Verbal Fluency. Another study using plasma from patients with first episode psychosis, found that the expression of MCP-1, a chemokine involved in initiation of the inflammatory process, negatively correlated with learning and memory measures [35]. Furthermore, a recent analysis of transcriptomic data from PBMCs of patients with schizophrenia and healthy controls found altered expression of genes in the antigen presentation pathway that was more pronounced in patients with cognitive deficits as opposed to those with no cognitive deficits or healthy controls [36].
Limitations of this study are clearly the small sample size, particularly the limited number of subjects in the SZ-HIGH group. Other limitations include the absence of serum levels of cytokines to contextualize the immune environment. Finally, the use of PBMCs does not allow us to focus the findings in a given peripheral blood cell type, a process that would be best accomplished using flow cytometry.
A subclinical inflammatory state characterized by chronically increased pro-inflammatory cytokines, is a perennial finding in schizophrenia. The intracellular events that results from this endogenous cytokine production have not been studied in schizophrenia. The results reported provide a strategy to pursue these signals into the cell, and because intracellular phosphorylation pathways serve as aggregators of composite incoming stimuli, could identify the downstream target of immunoreactive stimuli such as bacterial infections, adiposity, pharmacology, and drugs of abuse.
Figure 2.
1) Attention span as measured by the MATRICS battery is significantly worse in the schizophrenia group characterized by high levels of pSTAT1 (SZ-HIGH). No difference between CON-LOW pSTAT1 vs SZ-LOW pSTAT1 groups. 2) Reasoning as measured by the MATRICS battery is worse in schizophrenia subjects with activated pSTAT1 levels (SZ-HIGH) when compared to controls (CON-LOW). No difference between CON-LOW pSTAT1 vs SZ-LOW pSTAT1 groups.
Acknowledgments
The authors would like to thank the research subjects who participated in this study. Role of funding source: This work was supported in part by PHS grant (NIH) R01MH094358 (R.P.S.)
Footnotes
Conflict of interest statement: There are no conflicts of interest.
Financial disclosure statement: There are no financial interests to disclose.
Ethical conduct of research: The authors state that they have obtained appropriate institutional review board approval from the University of Illinois at Chicago IRB. In addition, informed consent was obtained from the participants involved prior to the initiation of research procedures.
References
- 1.Potvin S, Stip E, Sepehry Aa, Gendron A, Bah R, Kouassi E. Inflammatory Cytokine Alterations in Schizophrenia: A Systematic Quantitative Review. Biol Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S-J, Chao Y-L, Chen C-Y, Chang C-M, Wu EC-H, Wu C-S, et al. Prevalence of autoimmune diseases in in-patients with schizophrenia: nationwide population-based study. Br J Psychiatry. 2012;200:374–380. doi: 10.1192/bjp.bp.111.092098. [DOI] [PubMed] [Google Scholar]
- 5.Benros ME, Mortensen PB, Eaton WW. Autoimmune diseases and infections as risk factors for schizophrenia. Ann N Y Acad Sci. 2012;1262:56–66. doi: 10.1111/j.1749-6632.2012.06638.x. [DOI] [PubMed] [Google Scholar]
- 6.Terui K, Haga S, Enosawa S, Ohnuma N, Ozaki M. Hypoxia/re-oxygenation-induced, redox-dependent activation of STAT1 (signal transducer and activator of transcription 1) confers resistance to apoptotic cell death via hsp70 induction. Biochem J. 2004;380:203–209. doi: 10.1042/BJ20031891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khodarev NN, Roizman B, Weichselbaum RR. Molecular Pathways: Interferon/Stat1 Pathway: Role in the Tumor Resistance to Genotoxic Stress and Aggressive Growth. Clin Cancer Res. 2012;18:3015–3021. doi: 10.1158/1078-0432.CCR-11-3225. [DOI] [PubMed] [Google Scholar]
- 8.Vakkila J, Nieminen U, Siitonen S, Turunen U, Halme L, Nuutinen H, et al. A novel modification of a flow cytometric assay of phosphorylated STAT1 in whole blood monocytes for immunomonitoring of patients on IFNα regimen. Scand J Immunol. 2008;67:95–102. doi: 10.1111/j.1365-3083.2007.02028.x. [DOI] [PubMed] [Google Scholar]
- 9.Murray PJ. The JAK-STAT Signaling Pathway: Input and Output Integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 10.Frydecka D, Misiak B, Pawlak-Adamska E, Karabon L, Tomkiewicz A, Sedlaczek P, Kiejna A, Beszłej JA. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265:449–459. doi: 10.1007/s00406-014-0533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz Ma, Diaz-Torné C, Hernández MV, Reina D, de la Fuente D, Castellví I, et al. IL-6 blockade reverses the abnormal STAT activation of peripheral blood leukocytes from rheumatoid arthritis patients. Clin Immunol. 2015;158:174–182. doi: 10.1016/j.clim.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 12.Gernez Y, Tirouvanziam R, Nguyen KD, Herzenberg La, Krensky AM, Nadeau KC. Altered phosphorylated signal transducer and activator of transcription profile of CD4+CD161+ T cells in asthma: modulation by allergic status and oral corticosteroids. J Allergy Clin Immunol. 2007;120:1441–1448. doi: 10.1016/j.jaci.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.First MB, Spitzer RL, Gibbon M, Williams JB. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P) [Google Scholar]
- 14.Kay SR, Fiszbein A, Opler LA. The Positive And Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 15.Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery. Los Angeles: MATRICS Assessment Inc; 2006. [Google Scholar]
- 16.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 17.Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: Comparing apples and oranges! Indian J Psychiatry. 2013;55:207–208. doi: 10.4103/0019-5545.111475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavin DP, Rosen C, Chase K, Grayson DR, Tun N, Sharma RP. Dimethylated lysine 9 of histone 3 is elevated in schizophrenia and exhibits a divergent response to histone deacetylase inhibitors in lymphocyte cultures. J Psychiatry Neurosci. 2009;34:232–237. [PMC free article] [PubMed] [Google Scholar]
- 19.Chase KA, Sharma RP. Nicotine induces chromatin remodelling through decreases in the methyltransferases GLP, G9a, Setdb1 and levels of H3K9me2. Int J Neuropsychopharmacol. 2012;2:1–10. doi: 10.1017/S1461145712001101. [DOI] [PubMed] [Google Scholar]
- 20.Sharma RP, Rosen C, Kartan S, Guidotti A, Costa E, Grayson DR, et al. Valproic acid and chromatin remodeling in schizophrenia and bipolar disorder: preliminary results from a clinical population. Schizophr Res. 2006;88:227–231. doi: 10.1016/j.schres.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Sharma RP, Feiner B, Chase KA. Histone H3 phosphorylation is upregulated in PBMCs of schizophrenia patients in comparison to healthy controls. Schizophr Res. 2015;169:498–499. doi: 10.1016/j.schres.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen DN, Randall C, Bello D, Armstrong C, Frantom L, Cross C, et al. Are working memory deficits in bipolar disorder markers for psychosis? Neuropsychology. 2010;24:244–254. doi: 10.1037/a0018159. [DOI] [PubMed] [Google Scholar]
- 23.Bora E, Vahip S, Akdeniz F, Gonul AS, Eryavuz A, Ogut M, et al. The effect of previous psychotic mood episodes on cognitive impairment in euthymic bipolar patients. Bipolar Disord. 2007;9:468–477. doi: 10.1111/j.1399-5618.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 24.Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, et al. The Neurocognitive Signature of Psychotic Bipolar Disorder. Biol Psychiatry. 2007;62:910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Holzmann H, Vollmer S. A likelihood ratio test for bimodality in two-component mixtures. AStA Advances in Statistical Analysis. 2008;92:57–69. [Google Scholar]
- 26.Chen J, Li P. Hypothesis test for normal mixture models: The em approach. Ann Stat. 2009;37:2523–2542. [Google Scholar]
- 27.Qiao Y, Giannopoulou EG, Chan CH, Park S-H, Gong S, Chen J, et al. Synergistic activation of inflammatory cytokine genes by interferon-γ-induced chromatin remodeling and toll-like receptor signaling. Immunity. 2013;39:454–469. doi: 10.1016/j.immuni.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satoh JI, Tabunoki H. A comprehensive profile of ChIP-Seq-based STAT1 target genes suggests the complexity of STAT1-mediated gene regulatory mechanisms. Gene Regul Syst Bio. 2013;2013:41–56. doi: 10.4137/GRSB.S11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Ivashkiv LB. IFN-γ abrogates endotoxin tolerance by facilitating Toll-like receptor-induced chromatin remodeling. Proc Natl Acad Sci U S A. 2010;107:19438–19443. doi: 10.1073/pnas.1007816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosisio D, Polentarutti N, Sironi M, Bernasconi S, Miyake K, Webb GR, et al. Stimulation of toll-like receptor 4 expression in human mononuclear phagocytes by interferon-gamma: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood. 2002;99:3427–3431. doi: 10.1182/blood.v99.9.3427. [DOI] [PubMed] [Google Scholar]
- 31.Yamada T, Fischle W, Sugiyama T, Allis CD, Grewal SIS. The Nucleation and Maintenance of Heterochromatin by a Histone Deacetylase in Fission Yeast. Mol Cell. 2005;20:173–185. doi: 10.1016/j.molcel.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 33.Terrando N, Monaco C, Ma D, Foxwell BMJ, Feldmann M, Maze M. Tumor necrosis factor- triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fillman SG, Weickert TW, Lenroot RK, Catts SV, Bruggemann JM, Catts VS, et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca’s area volume. Mol Psychiatry. 2015:1–9. doi: 10.1038/mp.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martínez-Cengotitabengoa M, Mac-Dowell KS, Leza JC, Micó JA, Fernandez M, Echevarría E, et al. Cognitive impairment is related to oxidative stress and chemokine levels in first psychotic episodes. Schizophr Res. 2012;137:66–72. doi: 10.1016/j.schres.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Wu JQ, Green MJ, Gardiner EJ, Tooney Pa, Scott RJ, Carr VJ, et al. Altered neural signaling and immune pathways in peripheral blood mononuclear cells of schizophrenia patients with cognitive impairment: A transcriptome analysis. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.12.010. [DOI] [PubMed] [Google Scholar]