Abstract
Objective
Studies have linked vasomotor symptoms to markers of cardiovascular disease risk, yet few have considered clinical cardiovascular events. Data suggest that associations may depend upon the age that symptoms occur. We examined associations between vasomotor symptoms and cardiovascular events and endothelial function, considering age of symptom onset.
Methods
The Women’s Ischemia Syndrome Evaluation enrolled women referred for coronary angiography for suspected myocardial ischemia. 254 women aged >50 years, postmenopausal, with both ovaries, not taking hormone therapy underwent a baseline evaluation, were followed annually (median=6.0 years), and the National Death Index was searched to ascertain cardiovascular disease mortality (median=9.3 years). A subset of participants underwent brachial artery ultrasound for flow mediated dilation. Receiver operating curve analysis was used to determine vasomotor symptom groups [symptoms beginning <age 42 (early onset), beginning ≥42 (later onset), never] which were examined in relation to cardiovascular events and flow mediated dilation in Cox proportional hazard and linear regression models.
Results
Women reporting early onset vasomotor symptoms (HR=3.35, 95%CI=1.23, 7.86, p=.005) and women who never had vasomotor symptoms (HR=2.17, 95%CI=1.02, 4.62, p=.05) had higher cardiovascular disease mortality than women with later onset symptoms (multivariable models). Women with early onset vasomotor symptoms had lower flow mediated dilation than women with later onset symptoms (b=-4.31, SE=2.10, p=.04, multivariable).
Conclusions
Women with signs and symptoms of ischemia who had vasomotor symptoms beginning early in midlife had higher cardiovascular disease mortality and reduced endothelial function relative to women with later onset symptoms. Future research should evaluate the vascular phenotype of women with early midlife vasomotor symptoms.
Keywords: vasomotor symptoms, menopausal symptoms, hot flashes cardiovascular disease, endothelial dysfunction, mortality
Introduction
Eighty percent of women experience menopausal symptoms, particularly vasomotor symptoms (VMS; hot flashes, night sweats) at some point during the menopause transition.1,2 While traditionally believed to persist for several years around the final menstrual period, newer data indicate that VMS start earlier in life, in many cases in the late reproductive years when a woman is still menstruating.3 These symptoms also appear to persist much longer than previously thought; on average a decade.4,5
VMS are well-known to be associated with poorer quality of life, mental health, and functioning during the menopause transition.6,7 However, recent data also suggest a potential relationship between VMS and physical health, particularly indicators of cardiovascular disease (CVD) risk. More frequent or severe VMS have been linked to adverse CVD risk factors8-10 and subclinical CVD, such as increased intima media thickness,11 aortic calcification,12 and reduced brachial artery flow mediated dilation (FMD),12,13 a marker of endothelial dysfunction.
As existing studies were largely conducted among midlife women, few have examined relationships between VMS and clinical CVD events. The few studies examining clinical CVD events yield mixed and inconclusive findings.14-16 Further, emerging data indicate that associations between VMS and CVD risk may depend on the age at which VMS occur,15,17 yet an age-dependence of VMS-CVD risk associations is not routinely examined. Thus, there is an important gap in knowledge about age-dependent relationships between VMS and adverse CVD events.
Accordingly, we investigated associations between the history of menopausal symptoms, principally VMS, and risk for non-fatal CVD events and CVD mortality among women participating in the National Heart Lung and Blood Institute (NHLBI) Women’s Ischemia Syndrome Evaluation (WISE). We considered the age at which symptoms began, as well as the role of CVD risk factors, in VMS-CVD risk associations. Further, as VMS have been hypothesized to be associated with endothelial dysfunction,12 we additionally tested relations between these menopausal symptoms and brachial artery FMD.
Methods
Study Participants
The WISE is an NHLBI-sponsored four-center prospective cohort study of 936 women undergoing clinically ordered coronary angiography for further evaluation of symptoms and signs of suspected myocardial ischemia described elsewhere.18 Briefly, exclusion criteria included emergency referral, pregnancy, cardiomyopathy, New York Heart Association class IV congestive heart failure (CHF), acute ischemic syndrome (defined as acute myocardial infarction (MI) or unstable angina) within one month prior to study entry, coronary revascularization within 6-months prior to entry, conditions other than ischemic heart disease likely to be fatal or requiring frequent hospitalization within 4-years, contraindication to provocative myocardial stress testing, and a condition likely to affect study retention (alcoholism, drug abuse, or severe psychiatric illness). Among women screened and meeting criteria, 50% agreed to participate. All women provided written, informed consent in accordance with institutional guidelines. The study was approved by the institutional review board at each WISE clinical site and the data coordinating center.
The analytic sample was comprised of 254 WISE participants who were postmenopausal (determined by methods previously described)19 and over the age of 50 (to allow adequate time for a woman to report on her history of VMS); who had both ovaries; and who were not currently taking hormone therapy (HT). Additional analyses of brachial artery FMD included 104 women in that substudy. Women undergoing FMD measurement were more likely to be white (p=0.003), to have lower SBP (p=0.01), and were less likely to have used HT (p=0.02), but did not differ on any other characteristic from the larger WISE sample.
Procedures
At the time of coronary angiography, baseline evaluation was completed that included collection of demographic, medical history, detailed reproductive history and exogenous hormone use, psychosocial and symptom data from 1998 to 2002, and a fasting blood sample as described previously (cross-sectional phase).18 Participants were then followed annually for fatal and nonfatal CVD events for a median of 6 years; subsequently, the National Death Index was searched to ascertain CVD mortality, which extended follow up time for mortality to a total (median) of 9.3 years.
Menopausal Symptoms
Menopausal symptom history was obtained in the reproductive questionnaire. Women were asked “Have you ever had menopausal symptoms, such as hot flashes or night sweats?” (yes/no). If yes, women were queried about the age of first symptom onset.
CVD Mortality and Non-Fatal CVD Events
Method of assessing CVD mortality and non-fatal CVD events (non-fatal myocardial infarction, congestive heart failure, stroke) are described in detail elsewhere.20 Briefly, women were contacted at 3 months and annually thereafter regarding adverse CVD events or hospitalizations for up to 9 years (median, 6.0 [interquartile range, 3.2-7.1]). For deaths, we obtained a death certificate, records of any related CVD hospitalizations during the preceding follow-up time period, and/or description by a primary relative regarding the circumstances surrounding the death. A National Death Index search was subsequently conducted and death certificates obtained which extended the follow-up, for mortality only, to a median of 9.3 years (interquartile range 8.4-10.3 years). The cause of death was reviewed by two cardiologist investigators masked to the clinical and angiographic data; a third reviewer was used in discrepant death classification.
Flow Mediated Dilation (FMD)
A sub-sample of WISE participants at the four clinical sites participated in the brachial artery ultrasound sub-study,21,22 104 of whom met criteria for inclusion in the present analysis. All short and long-acting vasoactive medications were withheld for 24-48 hours, respectively. Resting brachial artery diameter was measured with B-mode ultrasonography with a 7.5-MHz probe. A blood pressure cuff placed distal to the brachial artery was inflated to 40 mm Hg greater than systolic blood pressure for 4 minutes. Brachial artery diameter was reassessed for 2 minutes after cuff deflation. Quantitative analysis was performed at a core laboratory (University of Pittsburgh) by investigators masked to subject identifiers. Images were digitized, and calibrated electronic calipers were used to measure brachial artery diameter with the intima-media interface to define arterial borders. Three measurements were made in each analyzed frame and averaged (intraclass correlation coefficient between 2 independent observers =0.94).22 FMD was calculated as FMD=100x (peak diameter after cuff deflation – resting diameter)/resting diameter.
Covariates
Covariates derived from the baseline WISE examination included demographic, medical history (e.g., hypertension, diabetes, dyslipidemia), and current and prior smoking. Steroid and protein assay methods were used by the WISE hormone core laboratory to determine estradiol and follicle stimulating hormone levels.23 The WISE reproductive status questionnaire includes a detailed history of menarche, date of last menstrual period, current and prior menstrual cycling patterns, prior reproductive events (pregnancy, hysterectomy, oophorectomy), and current and prior oral contraceptive or HT use. Documentation of type of gynecological surgery was obtained when the reproductive hormone levels in the blood were not consistent with the reported status during expert consensus review for menopausal status determination. A core laboratory (blinded to historical or clinical data) used in previous NHLBI-sponsored multicenter trials analyzed baseline coronary angiography for CAD presence and severity,24 including quantitative assessment of the presence of obstructive CAD (defined as 50% or more luminal diameter stenosis in one or more epicardial coronary artery), and the WISE CAD severity score using previously published methods.24 The WISE CAD severity score was based on percent stenosis adjusted for any complete collateral vessels, with the possible range of score being 5 (no detectable stenosis) to 100 (multiple severe lesions).
Statistical Analysis
For women with a history of VMS, we used ROC analysis to estimate the optimal VMS age cut-points, yielding three groups: VMS occurring before age 42 (“early onset VMS”), VMS occurring at age 42 or later (“later onset VMS”), or never experiencing VMS. In descriptive comparisons, baseline data are reported as frequencies or means ± standard deviation. Highly skewed variables are reported as medians (interquartile range). Because of the significant differences in age among the three groups, all p-values were age-adjusted using analysis of variance. VMS groups were examined in relation to the outcomes of CVD mortality, non-fatal CVD events, and combined fatal and non-fatal CVD events using the Kaplan-Meier method to estimate rates and generate p-values using the log-rank statistic. For combined non-fatal CVD events and CVD mortality, only the first event was used. For multivariate modeling, we fitted Cox proportional hazards models to test the association of the three VMS groups with CVD mortality after adjusting sequentially for other baseline variables. Covariates were chosen among standard coronary risk factors and other baseline variables shown in univariate analysis to predict these outcomes. These were added in incremental steps: (1) Unadjusted; (2) adjusted for demographic factors; (3) further adjusted for presence vs. absence of obstructive CAD; (4) further adjusted for traditional CVD risk factors; (5) same as model 4 but substituting the CAD severity score (log transformed because of skewed distribution) for the simple presence vs. absence of obstructive CAD. At each step, we re-examined the association between the VMS group and CV mortality.
In additional models, we evaluated the VMS onset groups in relation to the FMD in linear regression models. Covariates were selected whose univariate association with FMD resulted in a p-value of <0.10. Only one variable, race, qualified, yet we additionally forced age and CAD into the model. Analyses were conducted with SAS v9.3 (SAS Institute, Cary, NC). All analyses were two-tailed and alpha=0.05.
Results
The mean age of the women at entry was 66 ± 8 years. The majority (82%) were non-Hispanic White, over a third (37%) had a BMI>30, 56% had obstructive CAD, and 33% had diabetes. Of the 254 participants, 93 (37%) reportedly never had VMS, 40 (16%) had VMS that started before age 42 (early onset VMS), and 121 (47%) reported VMS that began after age 42 (later onset VMS). Both groups of women with VMS were younger and more often reported a history of hypertension than women never reporting VMS, and women with early onset VMS had a higher BMI than women with later onset VMS. No other risk factors varied between groups (Table 1).
Table 1.
Sample characteristics by vasomotor symptom (VMS) onset group
| VMS Group
|
|||
|---|---|---|---|
| Never (N=93) | Onset < age 42 (N=40) | Onset ≥ age 42 (N=121) | |
| Age, years, M (SD)abc | 69 ± 8 | 65 ± 8 | 65 ± 8 |
| Race/ethnicity (% White) | 77 (83) | 32 (80) | 23 (81) |
| Education (n, % ≥high school) | 68 (73) | 25 (62) | 95 (79) |
| Body mass index, M (SD)d | 29.0 ± 6.9 | 31.2 ± 6.8 | 28.9 ± 5.6 |
| Smoker (n, % ever) | 42 (45) | 27 (68) | 61 (51) |
| Presence of obstructive CAD (n, %) | 59 (63) | 18 (45) | 65 (54) |
| CAD severity score, Median (IQR) | 18.8 (5.0, 33.0) | 10.0 (6.2, 25.5) | 10.4 (5.0, 23.8) |
| Diabetes history (n, %) | 32 (34) | 15 (38) | 36 (30) |
| Dyslipidemia history (n, %) | 44 (52) | 21 (58) | 71 (64) |
| Hypertension history (n, %)abc | 48 (53) | 28 (70) | 83 (69) |
| Ever hormone therapy use (n, %) | 7 (8) | 15 (39) | 30 (25) |
p<.05 overall difference between groups,
p<.05 Never VMS vs. VMS<Age 42,
p<.05 Never VMS vs. VMS≥Age 42,
p<.05 VMS<Age 42 vs. VMS≥Age 42; age-adjusted
VMS=vasomotor symptoms; M=mean; SD=standard deviation; CAD=coronary artery disease;; IQR=interquartile range
Among the 254 women, 40 (16%) had at least one nonfatal CVD event (over a median of 6 years) and 56 (22%) had a fatal CVD event (over a median of 9.3 years). Of the 40 women with nonfatal CV events, 16 (40%) eventually died of cardiovascular causes. Women with early-onset VMS and to a lesser extent women who never reported VMS had significantly higher CVD mortality than women who had later onset VMS (Table 2, Figure 1). Non-fatal CVD events did not significantly differ among VMS groups. Relations between VMS onset group and CVD mortality persisted, and for early onset VMS strengthened, when adjusted for multiple covariates (Table 3), even as the overall sample size and number of events was reduced due to missing values in covariates (e.g., from 254 women/56 events to 216 women/44 events).
Table 2.
Number and Kaplan-Meier estimated 7-year rates of cardiovascular disease (CVD) events by vasomotor symptom (VMS) onset group
| Event | VMS group
|
P | ||
|---|---|---|---|---|
| Never (n=93) | Onset < 42 (n=40) | Onset ≥ 42 (n=121) | ||
| CVD death | 16 (.18) | 11 (.31) | 12 (.11) | 0.02 |
| Nonfatal CVD eventa | 17 (.20) | 4 (.11) | 18 (.17) | 0.57 |
| Nonfatal CVD event or CVD deathb | 28 (.32) | 13 (.36) | 26 (.23) | 0.27 |
Nonfatal myocardial infarction, congestive heart failure, or stroke
Nonfatal myocardial infarction, congestive heart failure, stroke, or CVD death
VMS=vasomotor symptom; CVD=cardiovascular disease
Figure 1.
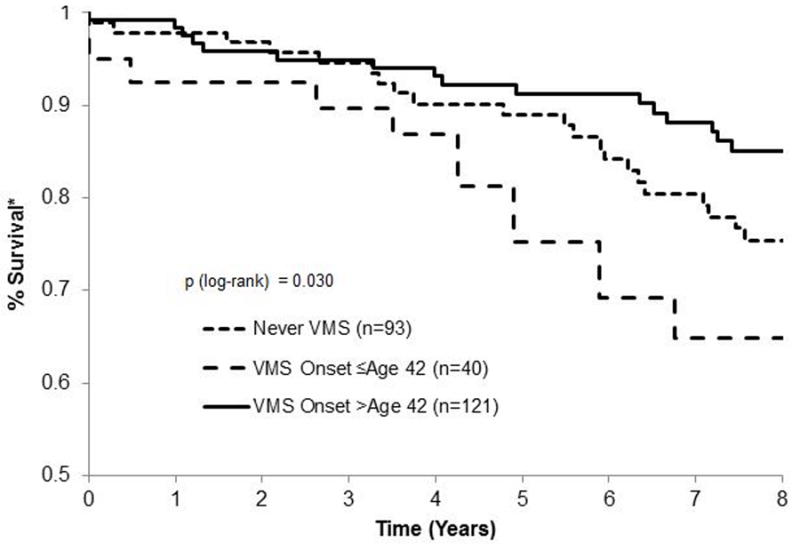
Kaplan Meier curves for relations between vasomotor symptom (VMS) onset group and cardiovascular disease (CVD) mortality
*Survival from CVD-related death
Table 3.
Association between vasomotor symptom (VMS) group and cardiovascular disease (CVD) mortality
| N (no. of events) | HR | 95% CI | P Value | |
|---|---|---|---|---|
| Model 1. Unadjusted | 254 (56) | |||
| VMS ≥ 42 onset | 1.00 | - | - | |
| Never VMS | 1.96 | 1.07, 3.57 | 0.03 | |
| VMS < 42 onset | 2.34 | 1.13, 4.86 | 0.02 | |
| Model 2. Adjusted for demographic factorsa | 254 (56) | |||
| VMS ≥ 42 onset | 1.00 | - | - | |
| Never VMS | 1.86 | 1.01, 3.44 | 0.05 | |
| VMS < 42 onset | 2.42 | 1.16, 5.02 | 0.02 | |
| Model 3. Further adjusted for obstructive CAD | 254 (56) | |||
| VMS ≥ 42 onset | 1.00 | - | - | |
| Never VMS | 1.82 | 0.98, 1.29 | 0.06 | |
| VMS < 42 onset | 2.70 | 1.29, 5.65 | 0.008 | |
| Model 4. Further adjusted for CVD risk factors and past HT useb | 223 (46) | |||
| VMS ≥ 42 onset | 1.00 | - | - | |
| Never VMS | 2.41 | 1.15, 5.11 | 0.02 | |
| VMS < 42 onset | 2.83 | 1.68, 8.96 | 0.002 | |
| Model 5. Model 4 with CAD severityc | 216 (44) | |||
| VMS ≥ 42 onset | 1.00 | - | - | |
| Never VMS | 2.17 | 1.02, 4.62 | 0.05 | |
| VMS < 42 onset | 3.35 | 1.23, 7.86 | 0.005 |
Age, Race (white vs. non-white)
History of hypertension, body mass index, diabetes, ever smoker, dyslipidemia, ever hormone therapy use
CAD severity score (log transformed) instead of presence of obstructive CAD (yes/no)
VMS=vasomotor symptoms; CVD=cardiovascular disease; HR=hazard ratio; CI=confidence interval; CAD=coronary artery disease
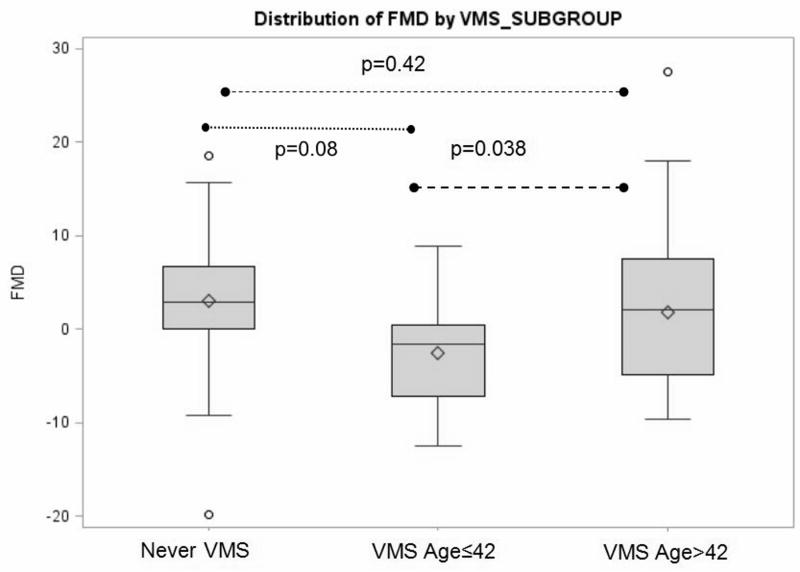
Women with early onset VMS also had a lower FMD [M(SD) = -2.6% (5.7%)] than either the women who never had VMS [M(SD) = 3.0% (6.8%)] or who had later onset VMS [M(SD) = 1.8% (7.7%), p’s<0.05]. Notably, the average FMD was negative in the early onset VMS group, indicating that on average these women had constriction, instead of dilation as expected in response to release of occlusion in response to shear-mediated nitric oxide release. Associations persisted when adjusted for covariates (Table 4, Figure 2).
Table 4.
Association between vasomotor symptom (VMS) group and flow mediated dilation
| Model 1 | Model 2 | |
|---|---|---|
|
| ||
| Beta (SE) | ||
| VMS group | ||
| ≥ 42 onset | Ref. | Ref. |
| Never | 1.21 (1.51) | 1.16 (1.54) |
| < 42 onset | -4.43 (2.10)b | -4.31 (2.10)b |
| White | - | -4.03 (2.37)a |
| Age | - | -0.04 (0.09) |
| Presence of CAD | - | 0.74 (1.46) |
p<.10,
p<.05,
N=104
VMS=vasomotor symptoms; CAD=coronary artery disease
Figure 2.
Distribution of flow mediated dilation (FMD) by vasomotor symptom (VMS) onset group
We conducted several sensitivity analyses. First, we excluded women who ever had a history of HT use, reducing our analytic sample to N=198; despite the reduced sample size, conclusions on the relationship of VMS to CVD mortality remained unchanged (data not shown). Second, we restricted all models to women non-missing on all covariates (N=216) and findings were unchanged (data not shown). Finally, we considered the additional covariates endogenous estradiol, follicle stimulating hormone, education, age at menopause, and hysterectomy status in primary multivariable models and findings were unchanged (data not shown).
Discussion
We found that women with a history of early onset VMS (first VMS occurring before age 42 years) and to a lesser extent women who never had VMS had higher CVD mortality than women who had later onset VMS. Women with early onset VMS also had lower FMD, indicating reduced endothelial function, relative to women with later onset VMS. Associations were not explained by demographic factors or by standard CVD risk factors.
Emerging data suggests links between VMS and CVD risk. Initial investigations were motivated by posthoc findings from large trials of HT showing that the CVD impact of HT use may vary as a function of VMS presence.25,26 Subsequent findings from observational cohort studies indicated that VMS were associated with a poorer CVD risk factor profile8-10 and higher subclinical CVD controlling for CVD risk factors.11-13 However, few observational studies have included adverse clinical CVD events, in part due to the long latency between the onset of VMS, which typically occur at midlife, and the emergence of clinical events in women, that typically occur decades later. The few studies examining clinical events or mortality have produced mixed findings, some showing positive associations,14 others showing inverse associations largely explained by CVD risk factors,16 and still others showed VMS-CVD relations depending on when in a woman’s lifespan the VMS occurred.15
In the present study, VMS showed differential relations with CVD mortality depending on the age at which the VMS first occurred. The women who experienced early onset VMS were at the highest CVD mortality risk. Notably, several cohort studies indicate that many women start their VMS earlier than previously thought. In the Penn Ovarian Aging Study over 30% of women reported having VMS at study entry, in their late reproductive years when they were still cycling regularly.3 In the Study of Women’s Health Across the Nation (SWAN), VMS trajectories constructed from VMS assessed prospectively over 13 years of the menopause transition were examined in relation to subclinical CVD,17 finding similar to the present results, that it was women with early onset VMS who had the highest subclinical CVD. Conversely, in the WHI observational study, it was the women with later onset VMS (reported at entry) who showed the highest risk.15 The precise nature of the age dependence of VMS and the emergence of CVD risk in midlife women requires further investigation. However, there is increasing appreciation that the impact of related phenomena (e.g., exogenous hormones) on CVD risk may vary by the age or the stage of the woman.27,28
We additionally studied the outcome of brachial artery FMD, a marker of endothelial function. Similar to the findings for CVD mortality, the women experiencing early onset menopausal symptoms also showed lower FMD, or poorer endothelial function, than women with later onset symptoms. Several other reports have found that VMS are associated with lower FMD12,13 yet generally did not test an age dependence of these associations and largely studied women early in the transition. Similar to clinical events, the impact of exogenous hormones on the endothelium appears to vary by age.29 The endothelium is critical to multiple aspects of vascular tone and function and has long been known to be affected by reproductive factors (e.g., hormones, pregnancy events) in women.30,31 Its potential role in VMS and the implications of VMS for vascular health offers an important and understudied area of future investigation.
Several additional findings deserved mention. Women who never experienced VMS showed elevated CVD mortality risk relative to women who had later onset VMS. Although the reason for this finding is not immediately clear, in the present study women who never had VMS were older than the other groups, either decreasing the precision in their recall of VMS or increasing the opportunity for these women to die of CVD during the follow up period (although age was controlled in all analyses). Other studies indicate that women never experiencing VMS are a small minority,2 although in the present investigation these women comprised 38% of the sample, differences that may be due to the specific characteristics of the target cohort or the retrospective nature of the symptom reporting, with the recall of symptoms over long periods of time vulnerable to memory biases. The present findings of increased risk among women never having menopausal symptoms do point to the potential value in better understanding characteristics of the women who do not experience this highly prevalent symptom. Further, findings were observed for CVD mortality but not non-fatal CVD events. The reason for this difference is not clear, but non-fatal CVD events were collected over a shorter follow up period than fatal events, with correspondingly smaller numbers of these events.
The mechanisms that might underlie associations between early onset symptoms and increased mortality risk require elucidation. In the present study, women with earlier onset VMS had a higher BMI than their later onset counterparts. This finding is consistent with other studies, such as the Penn Ovarian Aging Study or the Study of Women’s Health Across the Nation that show a higher BMI with VMS among younger midlife women.3,32 Women with VMS also had a greater history of hypertension, consistent with other reports.8,33 However, BMI, hypertension history, and other key covariates such as race/ethnicity or history of HT use were all controlled in the present investigation. Reproductive hormones did not significantly differ between VMS onset groups and did not explain these associations. Other potential pathways such as the autonomic nervous system34 or hypothalamic pituitary adrenal axis35 should be investigated.
Several limitations deserve mention. First, as noted above, our symptom measure asked women to recall their symptoms over many years, reports that may be influenced by recall biases. The symptom question specified menopausal symptoms more generically, with VMS provided as an example. Although VMS are the classic and most prevalent menopausal symptom, it is possible that some participants were recalling symptoms other than VMS. The sample size was relatively modest, particularly for the women with early onset symptoms and for FMD models. The sample was comprised of women with signs and symptoms of ischemia sufficient to be referred for coronary angiography and primarily white women, and thereby findings may not generalize to other groups.
This study had several strengths. Clinical CVD events, the focus here, are under-studied in the VMS-CVD risk literature. The cardiovascular health of the sample was rigorously quantified. Evaluating the age at which their symptoms first occurred, a factor rarely considered with respect to VMS, is important as aging effects are increasingly understood to be important for reproductive factors in relation to women’s cardiovascular health.27,28 Finally, FMD was assessed, showing parallel results as those for CVD and further supporting the validity of study findings.
Conclusions
In conclusion, among women with symptoms and signs of ischemic heart disease referred for coronary angiography, early onset VMS (before age 42) had elevated CVD mortality compared to women with later onset VMS. These associations were not explained by CVD risk factors or by demographic factors. Viewed in concert with findings from other cohorts, the present findings emphasize the need for further investigation of the cardiovascular health of women experiencing menopausal symptoms early in midlife.
Acknowledgments
Sources of Funding
This work was supported by contracts from the National Heart, Lung and Blood Institutes (NHLBI), nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, RO1-HL-073412-01, grants U0164829,U01 HL649141, U01 HL649241, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, New Jersey, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania, and QMED, Inc., Laurence Harbor, New Jersey, and the Edythe L. Broad Endowment, the Barbra Streisand Women’s Cardiovascular Research and Education Program, the Linda Joy Pollin Women’s Heart Health Program, and the Constance Austin Fellowship Endowment, Cedars-Sinai Medical Center, Los Angeles and the Erika Glazer Women’s Heart Health Project, Cedars-Sinai Medical Center, Los Angeles, California, and by an NHLBI grant K24HL123565 to Thurston.
This work is solely the responsibility of the authors. The content of this article does not necessarily represent the views of the NHLBI or the Department of Health and Human Services.
Footnotes
Conflicts of Interest
G Braunstein is a consultant for Merck (Merck Manual), Takada Pharmaceuticals, Amgen, Allergan, Synagena, Sanofi, and Johnson and Johnson. G Braunstein owns stock in Pfizer and is a part-time employee of Pathway Genomics. F Stanczyk has consulted with Merck & Co., Agile Therapeutics, TherapeuticsMD, and Abbvie. For the remaining authors none were declared.
References
- 1.Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118(Suppl 12B):14–24. doi: 10.1016/j.amjmed.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 2.Gold E, Colvin A, Avis N, et al. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation (SWAN) Am J Public Health. 2006;96(7):1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman EW, Grisso JA, Berlin J, Sammel M, Garcia-Espana B, Hollander L. Symptom reports from a cohort of African American and white women in the late reproductive years. Menopause. 2001;8(1):33–42. doi: 10.1097/00042192-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095–1104. doi: 10.1097/AOG.0b013e318214f0de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avis N, Crawford S, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531–539. doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avis NE, Colvin A, Bromberger JT, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women’s Health Across the Nation. Menopause. 2009;16(5):860–869. doi: 10.1097/gme.0b013e3181a3cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bromberger JT, Assmann SF, Avis NE, Schocken M, Kravitz HM, Cordal A. Persistent mood symptoms in a multiethnic community cohort of pre- and perimenopausal women. Am J Epidemiol. 2003;158(4):347–356. doi: 10.1093/aje/kwg155. [DOI] [PubMed] [Google Scholar]
- 8.Gast GC, Grobbee DE, Pop VJ, et al. Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;51(6):1492–1498. doi: 10.1161/HYPERTENSIONAHA.107.106526. [DOI] [PubMed] [Google Scholar]
- 9.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet Gynecol. 2012;119(4):753–761. doi: 10.1097/AOG.0b013e31824a09ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Vasomotor symptoms and insulin resistance in the Study of Women’s Health Across the Nation. J Clin Endocrinol Metab. 2012;97(10):3487–3494. doi: 10.1210/jc.2012-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Powell LH, Matthews KA. Hot flashes and carotid intima media thickness among midlife women. Menopause. 2011;18(4):352–358. doi: 10.1097/gme.0b013e3181fa27fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: Findings from the Study of Women’s Health Across the Nation Heart Study. Circulation. 2008;118(12):1234–1240. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bechlioulis A, Kalantaridou SN, Naka KK, et al. Endothelial function, but not carotid intima-media thickness, is affected early in menopause and is associated with severity of hot flushes. J Clin Endocrinol Metab. 2010;95(3):1199–1206. doi: 10.1210/jc.2009-2262. [DOI] [PubMed] [Google Scholar]
- 14.Gast GC, Pop VJ, Samsioe GN, et al. Vasomotor menopausal symptoms are associated with increased risk of coronary heart disease. Menopause. 2011;18(2):146–151. doi: 10.1097/gme.0b013e3181f464fb. [DOI] [PubMed] [Google Scholar]
- 15.Szmuilowicz ED, Manson JE, Rossouw JE, et al. Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause. 2011;18(6):603–610. doi: 10.1097/gme.0b013e3182014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svartberg J, von Muhlen D, Kritz-Silverstein D, Barrett-Connor E. Vasomotor symptoms and mortality: the Rancho Bernardo Study. Menopause. 2009;16(5):888–891. doi: 10.1097/gme.0b013e3181a4866b. [DOI] [PubMed] [Google Scholar]
- 17.Thurston R, El Khoudary S, Tepper P, et al. Trajectories of vasomotor symptoms and carotid intima media thickness in the Study of Women’s Health Across the Nation. Stroke. 2016;47(1):12–17. doi: 10.1161/STROKEAHA.115.010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merz CN, Kelsey SF, Pepine CJ, et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: Protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33(6):1453–1461. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 19.Johnson BD, Merz CN, Braunstein GD, et al. Determination of menopausal status in women: The NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J Womens Health (Larchmt) 2004;13(8):872–887. doi: 10.1089/jwh.2004.13.872. [DOI] [PubMed] [Google Scholar]
- 20.Sharaf B, Wood T, Shaw L, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166(1):134–141. doi: 10.1016/j.ahj.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sedlak TL, Johnson BD, Pepine CJ, Reis SE, Bairey Merz CN. Brachial artery constriction during brachial artery reactivity testing predicts major adverse clinical outcomes in women with suspected myocardial ischemia: results from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. PLoS One. 2013;8(9):e74585. doi: 10.1371/journal.pone.0074585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holubkov R, Karas RH, Pepine CJ, et al. Large brachial artery diameter is associated with angiographic coronary artery disease in women. Am Heart J. 2002;143(5):802–807. doi: 10.1067/mhj.2002.121735. [DOI] [PubMed] [Google Scholar]
- 23.Anderson DC, Hopper BR, Lasley BL, Yen SS. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976;28(2):179–196. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- 24.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory) Am J Cardiol. 2001;87(8):937–941. doi: 10.1016/s0002-9149(01)01424-2. A933. [DOI] [PubMed] [Google Scholar]
- 25.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 26.Huang AJ, Sawaya GF, Vittinghoff E, Lin F, Grady D. Hot flushes, coronary heart disease, and hormone therapy in postmenopausal women. Menopause. 2009;16(4):639–643. doi: 10.1097/gme.0b013e31819c11e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenfant F, Tremollieres F, Gourdy P, Arnal JF. Timing of the vascular actions of estrogens in experimental and human studies: Why protective early, and not when delayed? Maturitas. 2011;68(2):165–173. doi: 10.1016/j.maturitas.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Clarkson TB, Melendez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: Its origin, current status, and future. Menopause. 2013;20(3):342–353. doi: 10.1097/GME.0b013e3182843aad. [DOI] [PubMed] [Google Scholar]
- 29.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol. 2007;27(8):1782–1787. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- 30.Kim KH, Young BD, Bender JR. Endothelial estrogen receptor isoforms and cardiovascular disease. Mol Cell Endocrinol. 2014;389(1-2):65–70. doi: 10.1016/j.mce.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissgerber TL. Flow-mediated dilation: can new approaches provide greater mechanistic insight into vascular dysfunction in preeclampsia and other diseases? Curr Hypertens Rep. 2014;16(11):487. doi: 10.1007/s11906-014-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152(5):463–473. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 33.Gerber LM, Sievert LL, Warren K, Pickering TG, Schwartz JE. Hot flashes are associated with increased ambulatory systolic blood pressure. Menopause. 2007;14(2):308–315. doi: 10.1097/01.gme.0000236938.74195.c6. [DOI] [PubMed] [Google Scholar]
- 34.Thurston R, Barinas-Mitchell E, Jennings JR, et al. Physiologically monitored hot flashes and subclinical cardiovascular disease among midlife women (abstract) Menopause. 2015;22(12):1371. [Google Scholar]
- 35.Reed SD, Newton KM, Larson JC, et al. Daily salivary cortisol patterns in midlife women with hot flashes. Clin Endocrinol (Oxf) 2015 doi: 10.1111/cen.12995. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]