ABSTRACT
Purpose
To investigate whether cysts in diabetic macular edema are better visualized in the red channel of color fundus camera images, as compared with the green channel, because color fundus camera screening methods that emphasize short-wavelength light may miss cysts in patients with dark fundi or changes to outer blood retinal barrier.
Methods
Fundus images for diabetic retinopathy photoscreening were acquired for a study with Aeon Imaging, EyePACS, University of California Berkeley, and Indiana University. There were 2047 underserved, adult diabetic patients, of whom over 90% self-identified as a racial/ethnic identify other than non-Hispanic white. Color fundus images at nominally 45 degrees were acquired with a Canon Cr-DGi non-mydriatic camera (Tokyo, Japan) then graded by an EyePACS certified grader. From the 148 patients graded to have clinically significant macular edema by the presence of hard exudates in the central 1500 μm of the fovea, we evaluated macular cysts in 13 patients with cystoid macular edema. Age ranged from 33 to 68 years. Color fundus images were split into red, green, and blue channels with custom Matlab software (Mathworks, Natick, MA). The diameter of a cyst or confluent cysts was quantified in the red-channel and green-channel images separately.
Results
Cyst identification gave complete agreement between red-channel images and the standard full-color images. This was not the case for green-channel images, which did not expose cysts visible with standard full-color images in five cases, who had dark fundi. Cysts appeared more numerous and covered a larger area in the red channel (733 ± 604 μm) than in the green channel (349 ± 433 μm, P < .006).
Conclusions
Cysts may be underdetected with the present fundus camera methods, particularly when short-wavelength light is emphasized or in patients with dark fundi. Longer wavelength techniques may improve the detection of cysts and provide more information concerning the early stages of diabetic macular edema or the outer blood retinal barrier.
Key Words: diabetic retinopathy, diabetic macular edema, clinically significant macular edema, retina imaging, light tissue interactions, cystoid macular edema, color fundus photography, diabetic retinal screening
Diabetic macular edema has received considerable attention as a chief cause of loss of visual acuity in working adults, with a variety of new treatments and imaging modalities emerging to address this problem.1–5 Approximately 21 million people worldwide are estimated to have diabetic macular edema.
The pattern of diabetic macular edema varies across individuals, with cystoid macular edema being a major cause of severe central vision loss in which a degenerative change of the neural retina occurs. Cystoid macular edema is associated with a wide variety of ocular or systemic conditions, including not only diabetes, vein occlusion, and other retinal vascular diseases but also inflammatory diseases, age-related macular degeneration, inherited retinal dystrophies including retinitis pigmentosa, tractional maculopathies and retinal detachment, optic nerve head abnormalities, a consequence of cataract surgery, choroidal melanoma, and others.6–13 Cystoid macular edema usually consists of clusters of radial cystoid spaces filled with extracellular fluid as a result of damage to the blood-retinal barriers.2,3 Cysts usually appear in the center of the macula and occupy places in which cells have been displaced.7,12 Cysts appear in many layers of the retina1,3,7–14 and commonly appear at the level of outer plexiform layer and inner nuclear layer. Histological findings indicate swelling and necrosis of Müller cell cytoplasm, retinal vascular abnormalities, and neuronal degeneration associated with cystoid macular edema.2,15
The superficial layers of the retina were previously the main target of detection and treatment of diabetic macular edema, with leakage from retinal vessels such as from changes to endothelial cells emphasized in the detection of diabetic macular edema and cystoid macular edema.1,3,7,14,15 However, the mechanisms leading to diabetic macular edema are complex, and both inner retinal and outer blood-retinal barriers can be involved. More recent studies have emphasized the role of the outer blood-retinal barrier, including the retinal pigment epithelium.16–23 These findings have led to the proposed new therapeutic targets. Further, the significant impact to sight is related to the structures in deeper layers, including the deeper cysts being related to worse visual acuity,9 and photoreceptor integrity indicating which eyes had worse visual acuity.24,25
In assessing diabetic retinas for clinically significant macular edema, the presence of cysts must be judged with respect to the central macula. Clinically, there are several methods to detect the macular edema or cystoid spaces. Fluorescein angiography can provide the documentation of distinct cysts with petaloid shapes,11,13 with more distinct cysts seen with confocal, scanning laser techniques.26 Detection of cysts in eyes with cataract and leakage from retinal vessels can be challenging with slit-lamp biomicroscopy and ophthalmoscopy, and therefore several newer techniques have been used. These include fundus autofluorescence,27 optical coherence tomography (OCT),1–3,10–13,19,22–25 imaging with a scanning laser ophthalmoscope to increase contrast,8,9 and fundus cameras.9,13,25,27
All methods used to detect the macular edema or cystoid spaces are affected by the absorbers in the eye. The major absorbing substances are the crystalline lens, macular pigments, melanin in the pigment epithelium and choroid, H2O, and blood components, both oxygenated hemoglobin and hemoglobin.23,28–30 Melanin granules in the fundus are an important factor in light absorption. The concentration of melanin in the retinal pigment epithelium and choroid plays a major role in the amount of absorption. The interaction of the human ocular fundus tissues with light of differing wavelength has been modeled extensively,28,30 taking into account the reflectivity and multiple scattering properties of the multilayered fundus. Human reflectometry data show that dark fundi absorb more relatively short-wavelength light than light fundi do.28
The appearance of the macula can change greatly in narrow band illumination.28 With near infrared light and long-wavelength visible light, it is possible to see deeper fundus structures such as drusen. As the wavelength decreases, the appearance of the deeper structures of the retina is degraded. The use of intermediate wavelengths improves the appearance of intermediate and superficial vessels. The appearance of blood vessels does not necessarily change monotonically with wavelength because there are several inflections in the absorption curves for hemoglobin and deoxygenated hemoglobin that can become significant when imaging retinal arteries.28 With the use of shorter wavelength illumination, the macular pigment degrades the view of the fovea.28 The fundus often looks reddish in ophthalmoscopy or broadband imaging devices.28,29 This is because many of the major fundus absorbers have a tendency to absorb short wavelengths that would provide a blue fundus appearance much more than long wavelengths that provide a red appearance. Therefore, with longer wavelengths, the total reflectance increases and also the fraction of light reflected from the deeper layers. Furthermore, using long wavelength and infrared illumination in imaging devices has led to success in detecting subretinal pathology that may be seen using in vivo angiographic dye studies, with long wavelength and infrared illumination providing useful contrast despite ocular opacities or hemorrhage.8,9,28
Improving the imaging and image grading of dark fundi are important because many of the patients who undergo digital retinal imaging for diabetic retinopathy screening are in populations having mainly dark fundi.23,31 We are performing a study of digital retinal imaging that includes EyePACS standardized, non-mydriatic color fundus images and human grading of full-color images, using store and forward methodology.23,31 We selected a feature that we had previously shown to be associated with severe visual impairment and not always readily detected, cystoid macular edema, and for which the impairment is particularly dependent upon size and location near the central fovea.8,9 The use by EyePACS and other grading systems of full-color images has the disadvantage of the potential problem that large differences in individual pigmentation may interfere with judging the presence of pathological features. However, use of full-color images has the advantage that it does not suffer from the potential problem of poor penetration into the fundus of darker eyes if only red-free images are used. The purpose of this study was threefold. First, we demonstrated the visibility of cystoid macular edema in longer wavelength light in our sample of mainly dark-eyed patients who did not regularly receive eye examinations, using a camera and conditions that are typical in a clinical setting. We provided examples for comparison of cystoid macular edema as seen in the red channel, the green channel, and optical coherence tomography (OCT). Additional examples showing lesions in dark eyes compare OCT with fluorescein angiography.
Second, we tested whether the classification in the red channel and the green channel for each eye agreed with the standardized EyePACS outcome used in diabetic retinopathy screening. A classification of cystoid macular edema generates a referral, and agreement for diabetic retinopathy screening is by eye, not lesion by lesion. We hypothesized that examining the longer wavelength images of an RGB image could provide advantages to seeing deeper fundus structures in a manner similar to using longer wavelength illumination, and that this would be beneficial for detecting cystoid macular edema. We demonstrated complete agreement between classification for cystoid macular edema of the red-channel images and the standardized full-color images, but the green-channel images did not show some of the cysts.
Third, we determined whether the detection of cystoid macular edema in the red channel of color fundus images provides the same or better quantification of cystoid macular edema compared with the green channel. The cysts were compared in the same red and green images from the same color fundus image to eliminate the possibility of variability among separate images. Our sample included patients with cysts in diabetic macular edema in a racially and ethnically diverse population with a large proportion of dark fundi to determine whether cystoid macular edema is more readily visible in the red-channel image, as compared to the green-channel image. We found that the green-channel images failed to provide sufficient contrast to quantify cysts in some of the dark eyes. Our results have implications for improving the acquisition and analysis of images used in diabetic retinopathy screening, in that methodology using only red-free imaging acquisition, grading, or software algorithms may miss sight-threatening pathology.
METHODS
The fundus images in this study were from patients who participated in a prospective diabetic retinopathy photoscreening study with Aeon Imaging, EyePACS, University of California Berkeley, and Indiana University. There were 2047 underserved, adult diabetic patients, of whom over 90% self-identified as a racial/ethnic identity other than non-Hispanic white, and therefore these patients generally have darker fundi. At the time of study, many of these patients did not have access to routine eye care. They participated in our diabetic retinopathy screening study to determine if they needed referral for ocular conditions and assigning a priority for one of the limited number of appointments. Specialty imaging studies such as fluorescein angiography were not available at our study site. Despite the recommendation of annual eye examinations or at least one examination every 2 years, more than 66% of these diabetic patients reported that their last eye examination was conducted more than 3 years previously.23 Recruitment included several services that provided care for diabetic patients, including the endocrinology and cardiology services in addition to the eye care services. Patients underwent screening for diabetic retinopathy at the following four Alameda County Medical Center community clinics: Eastmont Wellness Center, Newark Wellness Center, Winton Wellness Center, and Highland Hospital. Screening was performed with a laser scanning digital camera, a Canon Cr-DGi non-mydriatic camera (Tokyo, Japan), nominally 45 degrees visual angle. Three sites also included an iVue OCT (Optovue Inc, Fremont, CA), with datasets from Highland generally not having the OCT measurements. De-identified imaging session data were transferred for review and analysis to EyePACS, a web-based teleophthalmology platform developed by UC Berkeley (EyePACS)23,31 and used in over 190 US and international sites. The tenets of the Declaration of Helsinki were followed; informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study. The research was approved by the Institutional Review Boards of Indiana University, Alameda Health, and University of California Berkeley.
The color fundus images were graded by EyePACS-certified graders, according to the International Classification for Diabetic Retinopathy.31,32 The criterion for diagnosis with clinically significant macular edema is the presence of hard exudates in the central 1500 μm of the fovea,32,33 a method validated by a dilated clinical examination.33 Two of the authors led the image grading (TVL and TJG). Two additional authors (SGB and SBY) transferred the image and clinical data, located patients with clinically significant macular edema, verified that the SD-OCT data were sufficient for further study, and prepared the data for further grading. From 148 patients with clinically significant macular edema, we evaluated macular cysts in 13 patients with cystoid macular edema, age range 33 to 68 years. Therefore, all patients in this study had cysts identified in the full-color images, without any color separation or filtration. Stereo fundus photographs were not available to be used to judge thickening. Table 1 shows the proportions of patients with clinically significant macular edema in terms of fundus color, gender, and age.
TABLE 1.
Characteristics and proportion of patients with clinically significant macular edema
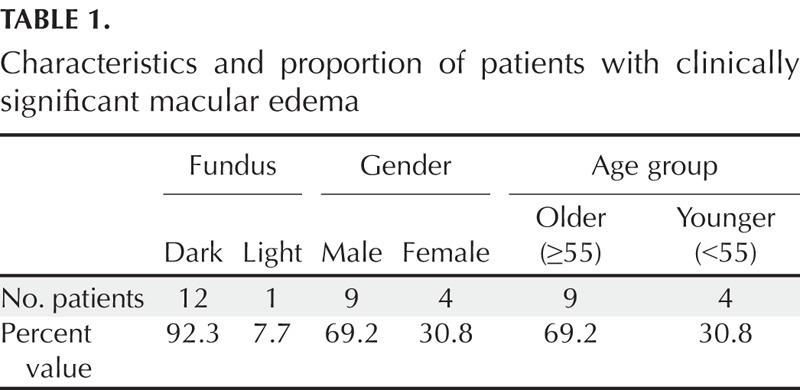
Custom Matlab (Mathworks, Natick, MA) software was used to split the color fundus images (Fig. 1A) into their three color components red, green (Fig. 1B, C, with enlargements D, F), and blue (not shown). Only red- and green-channel images converted to grayscale were used to evaluate the appearance of macular cysts in red-channel images and green-channel images. The blue-channel images when converted to grayscale were often quite dark and provided information similar to that found more readily in the green-channel images. A composite map of the red- or green-channel image plus a scale showing the grids from the OCT retinal thickness map results was used to quantify the region of each cyst and compare it to OCT scans (Adobe Photoshop CS5.1, San Jose, CA). Only cysts touching the central 1.0 mm around the fixation from the SD-OCT scans were sampled (Fig. 1G, H). Before further data analysis, two additional graders (CAC, VEM) determined from the SD-OCT data that the clinically significant macular edema was not merely due to vitreoretinal traction, which in fact was seen in some patients. There was a single grade for each subject, with the data reviewed in batches as it was acquired and the rare disagreement resolved by the senior author (AEE) and the two graders, both experienced optometrists (VEM and CAC). Not every cyst in the region of interest could be identified by SD-OCT because there were only seven b-scans available in full resolution from the iVue instrument. Further, as is typical in diabetic retinopathy screening, angiography was not available. Therefore, the 13 of 2047 cases with cystoid macular edema in this study may underrepresent the numbers of cases that might be found with other methods.
FIGURE 1.
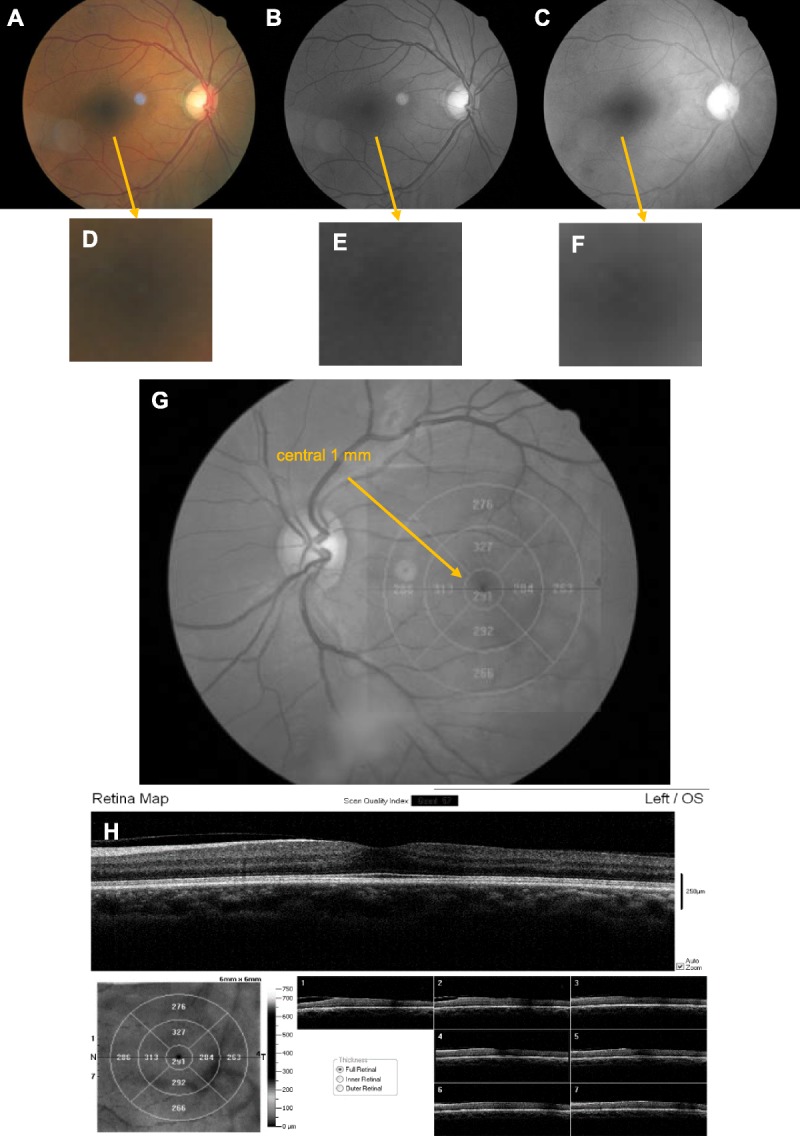
Method for the separation of the red and green color channels from the full-color fundus image in a diabetic patient without large cysts, hard exudates, or retinal layer disruption. (A) Color fundus image generated with white light illumination. (B) Grayscale image from the green channel of the image in (A), similar to a red-free image. (C) Grayscale image from the red channel of the image in (A). Note the higher contrast of retinal blood vessels in the green-channel image in (B) than in the red-channel image in (C). Also, an unwanted corneal reflection temporal to the fovea is more visible in the color image in (A) and the green-channel image in (B) than in the red-channel image in (C), indicating chromatic aberration. (D, E, F) Enlargement of foveal region for (A), (B), and (C), respectively. (G) Diagram showing the central 1 mm of the macula at which the cysts were evaluated, with a lack of significantly thickened quadrants. (H) Top—OCT b-scan of the central 1 mm of the macula, showing clear-cut photoreceptor layers, fairly well-organized inner retinal layers, a lack of large cysts or hard exudates, and a partial vitreous detachment. Bottom left—retinal thickness map from the OCT, plotted in microns on the computed en face image from the inner retinal OCT data. Bottom right—7 OCT b-scans at full resolution, horizontally across the fundus. The location of each scan is shown on the left side of the computed image.
Manual grading was performed on the split images using Photoshop and any grayscale adjustments desired (MA). First, for all red-channel images, the largest diameter of each cyst or region was covered by contiguous cysts, and points were selected on the opposite sides of this diameter. Next, we quantified the pixel values for each point (x1, y1) and (x2, y2). Then we calculated the Euclidean distance between those points in pixels. Finally, we converted to microns, using 5.88 μm per pixel. If there were two or more distinct cysts, we calculated the average sizes of all cysts. If there was a region with overlapping or contiguous multiple cysts, sometimes including brighter and darker regions, the overall cyst area was used. The same procedure was repeated on green-channel images (Fig. 2). The red-channel images were lighter, had whiter and more saturated optic nerve heads, and most strikingly had retinal veins that were more prominent than retinal arteries (Fig. 1A–C, also Figs. 3, 4, 5, and 6 panels A vs. B). The red-channel images may look unusual because they have so few retinal vessels that are visible. In some eyes, the choroidal vasculature was more visible in the red-channel images, Fig. 4 in particular, but also Fig. 3. A trained grader would likely know which grayscale images were derived from the red channel versus green channel, particularly from the fainter appearance of arteries in the red channel. A paired t-test was performed to compare the mean cyst sizes between the red and green images.
FIGURE 2.
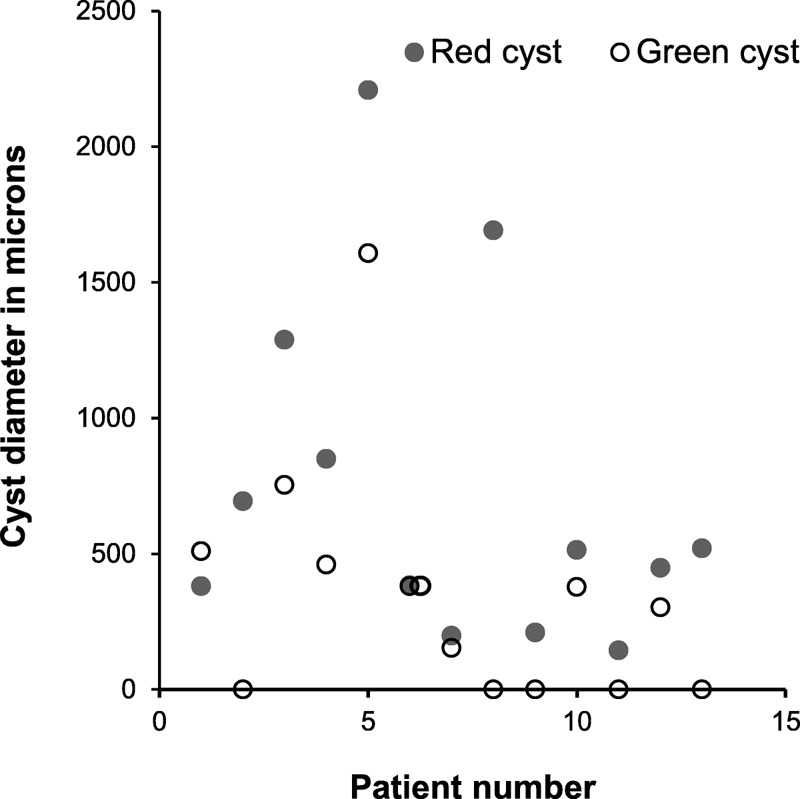
Average width, in microns on the retina, of macular cysts in 16 patients with cystoid macular edema, showing that cysts appeared larger and more numerous in the red-channel images. When cysts could not be seen in the green-channel image for a subject, a value of 0 is shown.
FIGURE 3.
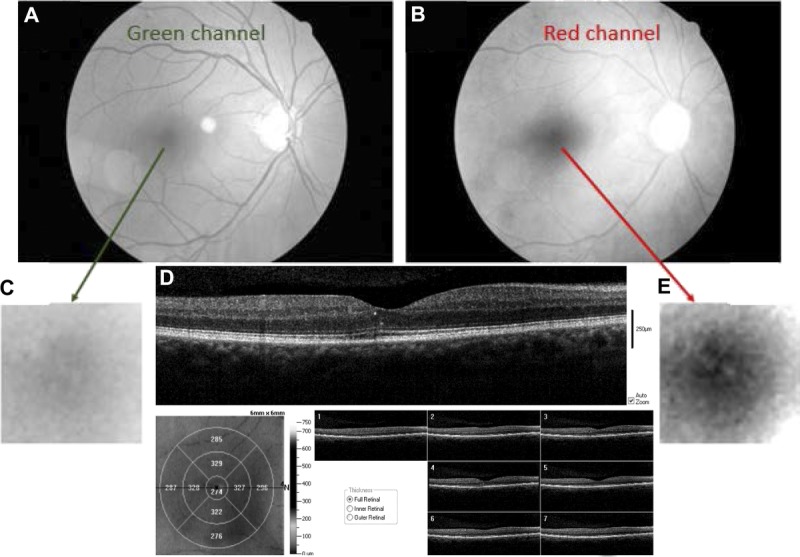
Fundus image and OCT scans of the right eye in a patient with dark iris color and hyperreflective foci, demonstrating outer retinal damage. (A) Grayscale image from the green channel. (B) Grayscale image from the red channel of the same image, showing focal regions of hyperreflectivity within the fovea. (C) Enlarged image of the central fovea from the green-channel image. (D) OCT cross-section showing hard exudates to the temporal side of the fovea, and corresponding retinal thickness map and the regions of hyperreflectivity. One of the focal hyperreflective areas is within the foveal pit in the outer nuclear layer just above the external limiting membrane, and the other is at the border of the outer nuclear layer and inner plexiform layer. (E) Enlarged image of the central fovea taken from red channel image, showing the hyperreflectivity in the central region more clearly.
FIGURE 4.
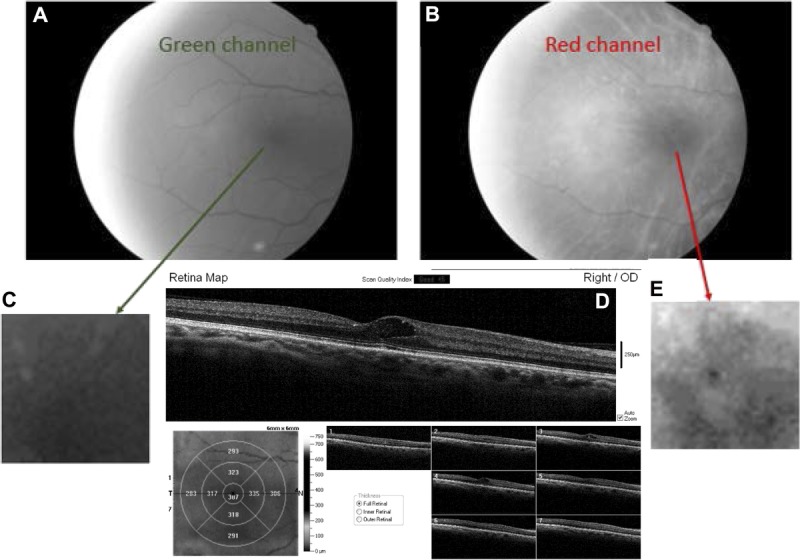
Fundus image of the right eye in a patient with ocular media changes typical of lens changes in a diabetic patient, with hard exudates in the fovea and large cysts just temporal to the foveal pit involving both the inner and outer retina. (A) Grayscale image from the green channel, with poorer contrast than the corresponding images in Fig. 1 or Fig. 3. (B) Grayscale image from the red channel of the same image, showing clearer reflectivity changes. (C) Enlarged image of the central fovea from the green-channel image. (D) OCT cross-section showing hard exudates and cysts in the fovea, and corresponding retinal thickness map. The photoreceptor layers are disrupted in the fovea. (E) Enlarged image of the central fovea taken from the red-channel image, showing the hyperreflectivity and hyporeflectivity in the central region more clearly.
FIGURE 5.

A fundus image of the right eye in a patient with damage at the level of the retinal pigment epithelium. (A) Grayscale image from the green channel, showing little detail. (B) Grayscale image from the red channel of the same image, demonstrating the reflectivity changes better than with the green-channel image. (C) Enlarged image of the central fovea from the green-channel image. (D) OCT cross-section showing hard exudates to the nasal side of the fovea and disruption to the outer retina and to a lesser extent the inner retina, and corresponding retinal thickness map. (E) Enlarged image of the central fovea taken from the red-channel image, showing the hyperreflectivity in the central region more clearly, demonstrating the reflectivity changes related to the disrupted outer retinal layers seen on OCT.
FIGURE 6.

A fundus image of the right eye in a patient with light iris color with extensive inner and outer retina damage, showing numerous areas of damage in all imaging modalities. This is subject 4 in Fig. 2. (A) Grayscale image from the green channel, showing multiple cysts and hard exudates, along with hemorrhage. (B) Grayscale image from the red channel of the same image, again showing multiple cysts and hard exudates but with less emphasis on the hemorrhages. (C) Top—OCT cross-section showing extensive cysts and hard exudates. Numerous cysts are seen in multiple layers of the inner retina, particularly visible in the ganglion cell layer and the inner plexiform layers. The hyperreflective hard exudates are more visible in the layers that are dark on OCT, i.e. the outer nuclear layer and the myoid zone, which are outer retinal structures. Bottom left—enlarged image of the retinal thickness map, indicating extensive retinal thickening. Bottom right—all 7 horizontal b-scans showing extensive retinal damage, with the scan from the locations indicated on the left side of the thickness map. Very large hard exudates are seen nasal to the fovea in panel C2, and considerable numbers of hyperreflective structures are seen in the myoid and ellipsoid zones in panel C3.
For comparison among imaging modalities available in a tertiary referral center, we retrospectively selected imaging data from two Japanese patients with dark fundi and clinically significant macular edema. Tertiary eye centers routinely provide dilated examinations, in contrast to non-mydriatic diabetic retinopathy screening. Pupil dilation may help reduce the problems in images that occur when too little light returns from a dark fundus through a small pupil and ocular media changes. These patients underwent SD-OCT with color fundus photography (OCT 2000; Topcon Corporation, Tokyo, Japan) and fluorescein angiography (SLO F-10; Nidek Co., Aichi, Japan).
RESULTS
Cysts were identified in each eye for the patients with cystoid macular edema in the red-channel images, indicating complete agreement of red-channel images and the standard full-color images when classifying a patient for referral in diabetic retinopathy screening. However, the agreement of the green-channel images and standard full-color images was poor. Entire cysts could not be seen in 5 of 13 eyes in the green-channel images (Fig. 2).
As expected, the average size of retinal cysts in the red channel images, 125 ± 103 pixels or 733 ± 604 μm, was significantly larger than in the green channel, 59.4 ± 73.7 pixels or 349 ± 433 μm (P < .006). For a probability of a Type I error (α) = 0.05, and a sample size of 13, the power (1 − β) would be 0.810. Decision making in diabetic retinopathy screening differs from some types of medical decision making in that a weighting towards over-referral confers little risk to the patient is preferable to under-referral, which may be associated with severe visual impairment. Therefore, a method that is statistically better than another should be used when there is little risk conferred in adopting the new method.
Interestingly, the five patients in whom the cysts were not seen have dark color irides, which is typical in an underserved population, and none had Non-Hispanic white ethnicity (Fig. 3). In contrast, the cysts are visible in both the red-channel and the green-channel images in a patient with a light iris (Fig. 6).
Figs. 3, 4, and 5 support the quantitative findings that the red-channel images showed the macular cysts and additional damage to the deeper structures of the retina better than the green channel images did. The OCT images indicate the level of the damage to retinal layers, which included both inner and outer retinal damage for some patients. In poor ocular media, the red channel image gave more information about the deeper structure of the retina than did the green channel image (Fig. 4). The damage at the level of retinal pigment epithelium was easier to detect in the red channel image, as compared with the green channel image in which the damage was not detectable (Fig. 5).
The OCT b-scans were in good agreement with the findings of the red-channel images from the fundus camera, despite being different instruments and different methodology. Specifically, cystoid macular edema was seen in both imaging modalities, and where OCT b-scans were available, cysts were visualized in retinal regions corresponding to reflectivity changes in the red images. However, the retinal thickness data from the OCT did not always indicate that pathology was present. For instance, there are clear cut exudates (Fig. 3) and outer retinal changes (Fig. 5C, particularly the left side of scan 3 and the right side of scan 5), but the corresponding retinal thickness maps indicated CMT values were only 274 and 257 μm, respectively.
In diabetic patients with clinically significant macular edema, the hard exudates, larger hemorrhages and microaneurysms, and vessel remodeling features were seen on dilated color fundus photography and the red-channel and green-channel images (Figs. 7 and 8). The fundus photographs of these patients did not have the small pupil artifact found in some of the non-mydriatic photographs, which were missing a ring of image data in the periphery (Figs. 4 and 5). Retinal arteries were less well visualized than veins in the red-channel images. Although the central macular cysts were visible in all fundus images, the extent and number of the cystic spaces seen on OCT were not readily appreciated with the other methods. With fluorescein angiography, the cysts appeared coalesced and were low in contrast, whereas the leaking retinal vessels were in high contrast and covered large areas. The hyperreflective foci34 in the OCT b-scan, which have been shown to be predictive of visual acuity in cystoid macular edema, appeared dark because of the inverted contrast. The OCT data clearly showed outer retinal involvement: there were hyperreflective foci in the photoreceptor and the RPE layers (Figs. 7 and 8). The bright hard exudates in the color images had more detail in the OCT b-scan. The hyperreflective foci were coalesced in the central macular in Fig. 7 and temporal to the central macular in Fig. 8. There was extensive subretinal fluid and cysts at multiple retinal levels seen in the OCT b-scan, whereas the fluorescein angiography in the central macula showed irregularity of perfusion and a petaloid appearance. As with the underserved patients, these patients seen in a tertiary center also have dark choroidal pigmentation and had outer retinal pathology that was not obvious from color fundus photographs.
FIGURE 7.
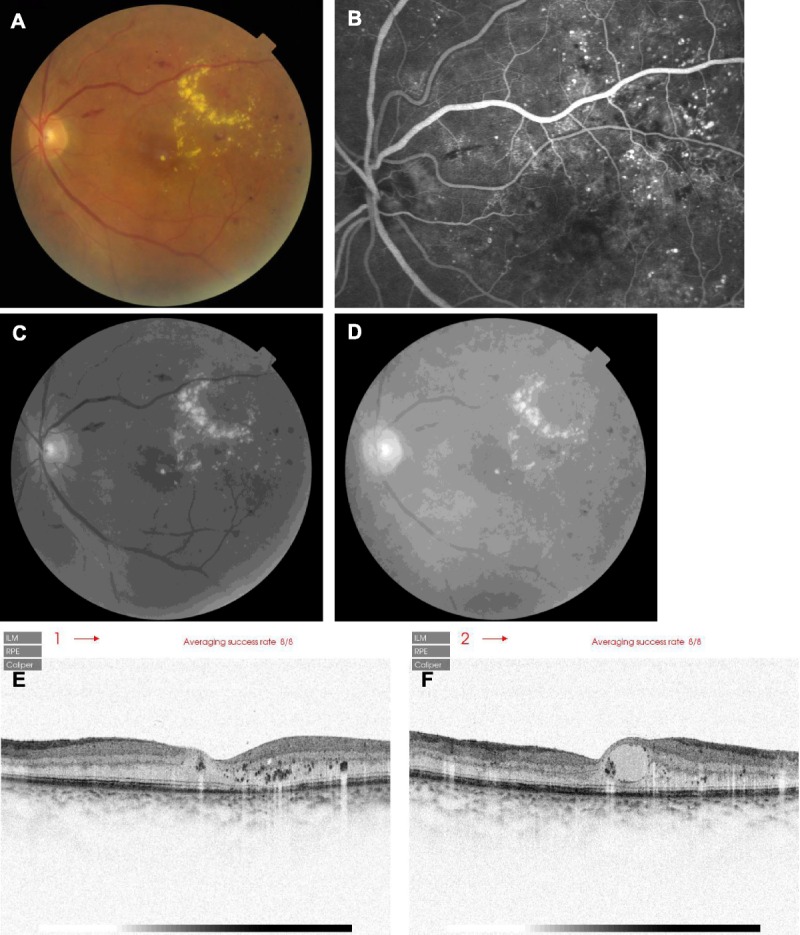
Comparison of retinal imaging modalities in a 67-year-old male diabetic patient with mild cataract and cystoid macular edema, from Japan. (A) Color fundus photograph, showing a hard exudate in the center of the macula, and hard exudates covering much of the superior temporal macular, and numerous hemorrhages. The large cystic region in the center of the macula was visible, but the contrast is not high. (B) Fluorescein angiography showing numerous microaneurysms, abnormal vessel patterns particularly superior temporal to the fovea, and blockage of fluorescence hemorrhages, such as from the flame hemorrhage that is superior temporal to the optic nerve head. There are numerous regions in which perfusion was not seen, not limited to the blockage of fluorescence from the overlying pathological features. The cystic region in the central macula is visualized on fluorescein angiography, but the cysts were not well delineated. (C) Grayscale image from the green channel of the fundus image, showing hard exudates and the cyst, but fewer of the hemorrhages and vascular abnormalities. (D) Grayscale image from the red channel of the fundus image, with similar or better visualization of hard exudates and the cyst, but less visibility of the retinal arteries. (E) SD-OCT scan, acquired horizontally near the fovea. The contrast is inverted, so that the hyperreflective foci, including hard exudates, appear dark. Cysts appear whitish. Note numerous hyperreflective foci in the photoreceptor and RPE layers, including deeper than the outer nuclear layer. Hyperreflective material is clumped in the cyst just nasal to the fovea pit, which is not separated from the outer nuclear layer. (F) Similar SD-OCT, but acquired vertically near the fovea, emphasizing large central macular cysts that may be above the outer nuclear layer.
FIGURE 8.
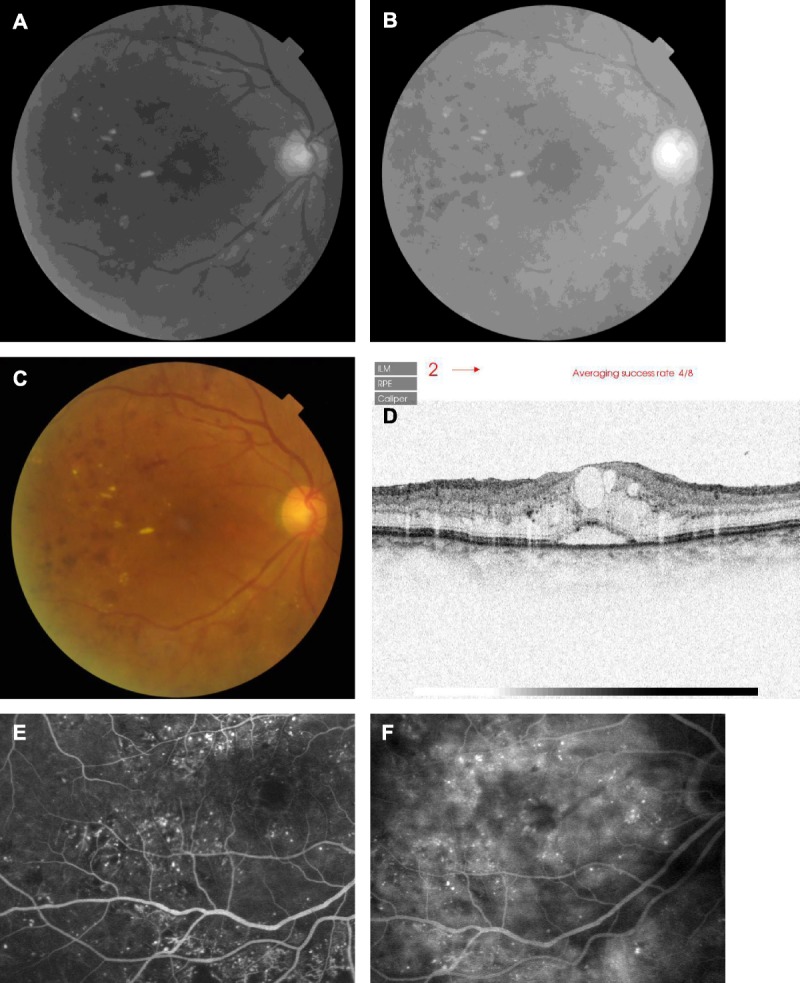
Comparison of retinal imaging modalities in a 71-year-old female diabetic patient with mild cataract and cystoid macular edema, from Japan. (A) Grayscale image from the green channel of the fundus image, showing hard exudates and hemorrhages, but with the cysts in the central macular barely visualized. (B) Grayscale image from the red channel of the fundus image, with similar or better visualization of hard exudates and the cyst, good visualization of the hemorrhages, but less visibility of the retinal arteries and saturation of the optic nerve head. (C) Color fundus photograph, for comparison to (A) and (B). (D) SD-OCT scan, acquired vertically near the fovea. The contrast is inverted, and the subretinal fluid and numerous cysts appear whitish. Hyperreflective foci are clearly seen in many fundus layers, including beneath the photoreceptor layers over the subretinal fluid. (E) Early venous phase fluorescein angiography, showing numerous microaneurysms and areas of dye leakage and abnormal perfusion. Abnormal patterns of retinal vessels are seen, particularly in the inferior retina. The delineation of the central macular cysts is poor. (F) Late-phase fluorescein angiography, showing continued focal leakage from microaneurysms, and better delineation in the central macular of irregular fluorescence that is consistent with cysts. Perfusion is patchy.
DISCUSSION
We investigated the advantages of using the red-channel image over the green-channel image in viewing macular cysts in diabetic macular edema, using a non-mydriatic fundus camera. Modern color fundus cameras typically operate with a broadband light source and a sensor with red, green, and blue channels. It has long been known that wavelength influences the visibility of retinal blood vessels when flood illuminated photography is performed.35 The higher potential contrast in the green channel for the blood contained within retinal vessels may be of particular interest for the detection of diabetic retinopathy lesions such as microaneurysms, hemorrhage, intra-retinal microvascular abnormalities, new vessels on the disc, and new vessels elsewhere. However, there is not necessarily an advantage for the detection of the leakage of lipids and proteins such as albumin, which are not detected by the increased absorption of light as compared with adjacent retina, but rather have a higher index of refraction change as compared with other features nearby and therefore appear brighter.1
The problem with the low intensity of fundus images from patients with small pupils or dark fundi is made worse when only short wavelength illumination is used. In cases in which the cysts were not seen in the green-channel images, then cysts likely would have been missed in red-free only images or images with color balances that emphasize the green information. Our images showed that cysts in darkly pigmented eyes could be seen in the red-channel images (Fig. 3) and in both the red-channel images and green-channel images in light eyes (Fig. 6). In dark eyes, there is greater absorption by hemoglobin and choroidal melanin, and in some cases by macular pigment, in the wavelength range corresponding to the green channel. The result is that less light penetrates through the many layers of retinal capillaries into the deeper layers to reflect off cysts, and less light returns from the choroid to retro-illuminate cysts. The OCT data show the complexity of the pathological retinal changes: there are many small cysts, exudates, and changes to the structure of individual layers throughout the layers of the retina that scatter light. This scattered light is readily absorbed by the blood in the retinal vasculature on the way out of the retina, particularly for the light in the green channel. Thus, although the green channel images may look dark, the retinal vessel contrast is higher than in the red-channel images, which can be brighter because of the increased light that has been multiply scattered but is not absorbed by hemoglobin. Thus, although the red-channel images and green-channel images may emphasize different specific lesions, cysts can be seen in both channels in light eyes but may not be detectable in green-channel images in dark eyes. Another advantage of using long-wavelength light and infrared light is that transmission through the ocular media is relatively more independent of age, with improved transmission of light spectra when the wavelength is greater than 650 μm.36 Moreover, diabetic patients are more susceptible to cataract than non-diabetic patients.37–39 Fig. 4 shows an example of macular fundus images in a patient with cataract. The red image shows a view of the retina that can be used to detect macular cysts. With flood illumination, images of the retinal vessels are not as distinct as when using a scanning laser ophthalmoscope, and therefore the longer wavelength illumination may miss retinal vascular lesions. Further using red-channel images in patients with light fundi may give bright images but with less detail of the retinal structures as compared to green-channel images (Fig. 6). With modern fundus cameras, the red and green channel may be available for further manipulation either during data acquisition or via image processing to enhance the detection of macular edema.
Our finding of a clear-cut advantage of red-channel images over the green-channel images was in an underserved population in which the proportion of patients with dark eyes is quite high and the prevalence of retinal complications from diabetes is also expected to be high.40 In a population with less risk of diabetic complications because of better blood glucose control or regular eye care, there could be a lower prevalence of cystoid macular edema.
Several emerging methods used to detect cystoid macular edema are not necessarily a part of screening of underserved populations because color fundus photography is lower in cost than OCT or examination by a specialist and less invasive than fluorescein angiography.23 Several exciting new developments are found in higher end instrumentation, which may eventually find their way into management of cystoid macular edema and other ocular complications of diabetes.41–44 The highly magnified images from adaptive optics scanning laser ophthalmoscope devices can pinpoint vessel remodeling far earlier than is expected clinically, along with more extensive fluid leakage and hard exudates.41 The same instrument can also be used in a scattered light imaging mode, similar to wider field scanning laser ophthalmoscopes,8 visualizing small cysts in diabetic macular edema. The variation over time of the light scattered from blood particles maps out the capillaries and larger retinal vessels, demonstrating areas of poor perfusion. Image processing of high-speed OCT data can also provide maps of capillaries and larger retinal vessels, similarly documenting areas of poor perfusion.42–44
The use of full-color fundus images that has long been the standard is established in the main classification systems for diabetic eyes32 and has the advantage of direct correspondence with clinical experience during fundus examination. However, there is a need for improvement from many standpoints. As described above, the large variations in ocular pigmentation are exaggerated when short-wavelength light is used, making it difficult to have a constant criterion in detecting diabetic macular edema. There are several factors that are problematic regardless of ocular pigmentation. First, the spatial resolution and contrast in the image are decreased when both long and short wavelength light is used because of transverse chromatic aberration, and the image information for red and blue can be separated laterally by more than a typical pixel.23 Further quantification using full-color information is more difficult than with grayscale information from a single color band at a time because of the complex multidimensional judgments. An underlying model of color discrimination is required that depends upon not only the wavelengths used but also the spatial frequency content because color appearance depends upon size and context, e.g. the size of the lesions and viewing distance.45–47 Finally, all of these factors interact with differences in ocular pigmentation, cameras, techniques, or monitor systems. With the definition of diabetic macular edema changing because of the adoption of newer imaging modalities, the problem remains even if longer wavelength techniques are used: in an underserved environment referral for very limited treatment resources for sight-threatening retinopathy needs to be provided judiciously because of the extremely high demand.
The clear-cut damage to the outer retinal structures in the patients in this study is in agreement with the histological and animal model work showing that the outer blood-retinal barrier function is damaged in diabetes.16–22 The use of the longer wavelength illumination techniques, including fundus photography and OCT, may provide earlier detection and better management of the new therapeutic regimes for diabetic macular edema. It is not yet known whether cysts in different retinal layers indicate one mechanism that can lead to edema being more important than another, but damage to and early detection in the deeper layers might be enhanced by using longer wavelength techniques.44–47
Ann E. Elsner
Indiana University School of Optometry
800 East Atwater Ave
Bloomington, IN 47405
e-mail: aeelsner@indiana.edu
ACKNOWLEDGMENTS
This study was supported by grant EY020017 from the National Eye Institute, National Institutes of Health. This study was presented in the form of a Scientific Paper (140042) at the annual meeting of the American Academy of Optometry in Denver, CO, November 13, 2014.
The authors declare no conflicts of interest.
Received August 3, 2015; accepted August 11, 2016.
REFERENCES
- 1.Bhagat N, Grigorian RA, Tutela A, et al. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol 2009;54:1–32. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Zeng H, Bao S, et al. Diabetic macular edema: new concepts in patho-physiology and treatment. Cell Biosci 2014;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology 2015;122:1375–94. [DOI] [PubMed] [Google Scholar]
- 4.Simó R, Hernandez C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retin Eye Res 2015;48:160–80. [DOI] [PubMed] [Google Scholar]
- 5.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanoff M, Fine BS, Brucker AJ, et al. Pathology of human cystoid macular edema. Surv Ophthalmol 1984;28:505–11. [DOI] [PubMed] [Google Scholar]
- 7.Quinn CJ. Cystoid macular edema. Optom Clin 1996;5:111–30. [PubMed] [Google Scholar]
- 8.Remky A, Beausencourt E, Hartnett ME, et al. Infrared imaging of cystoid macular edema. Graefes Arch Clin Exp Ophthalmol 1999;237:897–901. [DOI] [PubMed] [Google Scholar]
- 9.Beausencourt E, Remky A, Elsner AE, et al. Infrared scanning laser tomography of macular cysts. Ophthalmology 2000;107:375–85. [DOI] [PubMed] [Google Scholar]
- 10.Rotsos TG, Moschos MM. Cystoid macular edema. Clin Ophthalmol 2008;2:919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson MW. Etiology and treatment of macular edema. Am J Ophthalmol 2009;147:11–21. [DOI] [PubMed] [Google Scholar]
- 12.Scholl S, Augustin A, Loewenstein A, et al. General pathophysiology of macular edema. Eur J Ophthalmol 2011;21:S10–9. [DOI] [PubMed] [Google Scholar]
- 13.Tomkins-Netzer O, Ismetova F, Bar A, et al. Functional outcome of macular edema in different retinal disorders. Prog Retin Eye Res 2015;48:119–36. [DOI] [PubMed] [Google Scholar]
- 14.Tso MO. Pathology of cystoid macular edema. Ophthalmology 1982;89:902–15. [DOI] [PubMed] [Google Scholar]
- 15.Fine BS, Brucker AJ. Macular edema and cystoid macular edema. Am J Ophthalmol 1981;92:466–81. [DOI] [PubMed] [Google Scholar]
- 16.Vinores SA, Campochiaro PA, Lee A, et al. Localization of blood-retinal barrier breakdown in human pathologic specimens by immunohistochemical staining for albumin. Lab Invest 1990;62:742–50. [PubMed] [Google Scholar]
- 17.Vinores SA, McGehee R, Lee A, et al. Ultrastructural localization of blood-retinal barrier breakdown in diabetic and galactosemic rats. J Histochem Cytochem 1990;38:1341–52. [DOI] [PubMed] [Google Scholar]
- 18.Xu HZ, Le YZ. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci 2011;52:2160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahrouj M, Alsarraf O, Liu Y, et al. C-type natriuretic peptide protects the retinal pigment epithelium against advanced glycation end product-induced barrier dysfunction. J Pharmacol Exp Ther 2013;344:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahrouj M, Alsarraf O, McMillin JC, et al. Vascular endothelial growth factor modulates the function of the retinal pigment epithelium in vivo. Invest Ophthalmol Vis Sci 2014;55:2269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Ramírez M, Hernández C, Palomer X, et al. Fenofibrate prevents the disruption of the outer blood retinal barrier through downregulation of NF-κB activity. Acta Diabetol 2016;53:109–18. [DOI] [PubMed] [Google Scholar]
- 22.Abozaid MA, Scoles D, Goldberg M, et al. En face optical coherence tomography of outer retinal discontinuity and fan-shaped serous macular detachment in diabetic macular edema. JAMA Ophthalmol 2015;133:961–3. [DOI] [PubMed] [Google Scholar]
- 23.Elsner AE, King BJ. Screening for macular disorders: the optometrist’s perspective. Clin Optom 2015;7:15–38. [Google Scholar]
- 24.Otani T, Yamaguchi Y, Kishi S. Correlation between visual acuity and foveal microstructural changes in diabetic macular edema. Retina 2010;30:774–80. [DOI] [PubMed] [Google Scholar]
- 25.Shen Y, Liu K, Xu X. Correlation between visual function and photoreceptor integrity in diabetic macular edema: spectral-domain optical coherence tomography. Curr Eye Res 2016;41:391–9. [DOI] [PubMed] [Google Scholar]
- 26.Arend O, Remky A, Elsner AE, et al. Quantification of cystoid changes in diabetic maculopathy. Invest Ophthalmol Vis Sci 1995;36:608–13. [PubMed] [Google Scholar]
- 27.McBain VA, Forrester JV, Lois N. Fundus autofluorescence in the diagnosis of cystoid macular oedema. Br J Ophthalmol 2008;92:946–9. [DOI] [PubMed] [Google Scholar]
- 28.Elsner AE, Burns SA, Weiter JJ, et al. Infrared imaging of sub-retinal structures in the human ocular fundus. Vision Res 1996;36:191–205. [DOI] [PubMed] [Google Scholar]
- 29.Van Norren D, Tiemeijer L. Spectral reflectance of the human eye. Vision Res 1986;26:313–20. [DOI] [PubMed] [Google Scholar]
- 30.Delori FC, Pflibsen KP. Spectral reflectance of the human ocular fundus. Appl Opt 1989;28:1061–77. [DOI] [PubMed] [Google Scholar]
- 31.Cuadros J, Bresnick G. EyePACS: an adaptable telemedicine system for diabetic retinopathy screening. J Diabetes Sci Technol 2009;3:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–82. [DOI] [PubMed] [Google Scholar]
- 33.Litvin TV, Ozawa GY, Bresnick GH, et al. Utility of hard exudates for the screening of macular edema. Optom Vis Sci 2014;91:370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang JW, Chung H, Chan Kim H. Correlation of optical coherence tomographic hyperreflective foci with visual outcomes in different patterns of diabetic macular edema. Retina 2016;36:1630–9. [DOI] [PubMed] [Google Scholar]
- 35.Delori FC, Gragoudas ES, Francisco R, et al. Monochromatic ophthalmoscopy and fundus photography. The normal fundus. Arch Ophthalmol 1977;95:861–8. [DOI] [PubMed] [Google Scholar]
- 36.Boettner EA, Wolter JR. Transmission of the ocular media. Invest Ophthalmol Vis Sci 1962;1:776–83. [Google Scholar]
- 37.Klein BE, Klein R, Moss SE. Prevalence of cataracts in a population-based study of persons with diabetes mellitus. Ophthalmology 1985;92:1191–6. [DOI] [PubMed] [Google Scholar]
- 38.Saaddine JB, Honeycutt AA, Narayan KM, et al. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005–2050. Arch Ophthalmol 2008;126:1740–7. [DOI] [PubMed] [Google Scholar]
- 39.Klein BE, Klein R, Moss SE. Incidence of cataract surgery in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Am J Ophthalmol 1995;119:295–300. [DOI] [PubMed] [Google Scholar]
- 40.Varma R, Torres M, Peña F, et al. Los Angeles Latino Eye Study Group. Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology 2004;111:1298–306. [DOI] [PubMed] [Google Scholar]
- 41.Burns SA, Elsner AE, Chui TY, et al. In vivo adaptive optics microvascular imaging in diabetic patients without clinically severe diabetic retinopathy. Biomed Opt Express 2014;5:961–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miura M, Hong YJ, Yasuno Y, et al. Three-dimensional vascular imaging of proliferative diabetic retinopathy by Doppler optical coherence tomography. Am J Ophthalmol 2015;159:528–38. e3. [DOI] [PubMed] [Google Scholar]
- 43.Hwang TS, Gao SS, Liu L, et al. Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol 2016;134:367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Q, Lee CS, Chao J, et al. Wide-field optical coherence tomography based microangiography for retinal imaging. Sci Rep 2016;6:22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burns SA, Elsner AE, Pokorny J, et al. The Abney effect: chromaticity coordinates of unique and other constant hues. Vision Res 1984;24:479–89. [DOI] [PubMed] [Google Scholar]
- 46.Elsner AE, Pokorny J, Burns SA. Chromaticity discrimination: effects of luminance contrast and spatial frequency. J Opt Soc Am A 1986;3:916–20. [DOI] [PubMed] [Google Scholar]
- 47.Elsner AE, Burns SA, Pokorny J. Changes in constant-hue loci with spatial-frequency. Color Res Appl 1987;12:42–50. [Google Scholar]


