Abstract
OBJECTIVE
In postmenopausal Black women in the Women’s Health Initiative (WHI) randomized trial, estrogen alone reduced breast cancers but its comprehensive influence on health outcomes in Black women is unknown. Therefore, we examined this issue in the WHI overall and by African ancestry.
METHODS
1,616 Black women with prior hysterectomy, including 1061 with percent African ancestry determination, at 40 US centers were randomly assigned to conjugated equine estrogen (0.625 mg/d) or placebo for 7.2 years (median) intervention with 13 years cumulative follow-up. Coronary heart disease (CHD) and breast cancer were primary efficacy and safety outcomes, respectively. A global index also included stroke, colorectal cancer, hip fracture, pulmonary embolism and death.
RESULTS
Black women in the estrogen alone group compared to Black women in the placebo group had fewer breast cancers (17 vs. 40, hazard ratio [HR] 0.47 95% confidence interval [CI] 0.26–0.82). In women with >80% African ancestry, breast cancer HR was lower (0.32 95% CI 0.12–0.86, trend p=0.04 for ancestry effect). Most other outcomes including CHD, stroke, hip fracture and the global index were null with estrogen use in Black women; a global index effect was more favorable in younger Black women (HR 0.65 95% CI 0.43–0.98).
CONCLUSIONS
In Black postmenopausal women with prior hysterectomy, estrogen alone significantly reduced breast cancer incidence with no adverse influence on CHD, venous thromboembolism or all-cause mortality. Favorable estrogen alone global index effects in younger Black women warrant further study.
Keywords: Estrogen alone, randomized trial, Women’s Health Initiative, Black women, African ancestry
Introduction
Following reports from the Women’s Health Initiative (WHI) (1, 2) and the Million Women’s Study (3), menopausal hormone therapy use decreased by about 50 percent in the US (4, 5) and in other places around the world (6, 7). Nonetheless, estrogen plus progestin and estrogen alone (for women with prior hysterectomy) remain frequently prescribed medications as they are the optimal approach to climacteric symptom management. As a result, there is a need for reliable information regarding the risks and benefits of their use.
While Black women have more severe climacteric symptoms than White women (8, 9, 10) and are more likely to have higher risk of stroke and heart disease death than White women (11,12), the role of menopausal hormone therapy on chronic disease outcomes among Black women been sparse. For example, in early observational studies of coronary heart disease (CHD) and menopausal hormone therapy from 1966 through 1996, Black women comprised only 173 of 148,437 participants (0.1%) (13). More recently, excluding the two WHI Hormone Therapy trials, the seven largest randomized clinical trials evaluating estrogen plus progestin or estrogen alone for any clinical outcome have enrolled a total of 13,942 women. Of these, only 333 Black women were enrolled, 2.4% (14–19).
Against this background, the two WHI randomized, controlled hormone clinical trials with their diverse racial/ethnic study populations provide a unique opportunity to assess the relationships among menopausal hormone therapy and health outcomes in Black women (1, 20). In the WHI hormone therapy trial evaluating estrogen alone in postmenopausal women with prior hysterectomy, in analyses including all participants, estrogen alone use significantly reduced breast cancer incidence (21, 22). Recently, the 1,616 Black women participating in the trial were also seen to have a significantly decreased breast cancer incidence with estrogen alone use (hazard ratio [HR] 0.47 95% confidence interval [CI] 0.26–0.82) (23).
As a comprehensive overview of the long term effects of estrogen alone use on chronic disease outcomes for Black women in this trial have not been previously reported, we examined the cumulative risks and benefits for estrogen alone use in postmenopausal Black women participating in the WHI randomized trial overall and by African ancestry. The major objective was to determine if the substantial reduction in breast cancer risk seen with estrogen alone in Black occurs within a context of overall safety as measured by a global index of health outcomes under potential hormone influence.
Methods
Design Overview including Setting and Participants
Details of the design and implementation of the WHI trial evaluating estrogen alone have been described elsewhere (24, 25). Postmenopausal women between 50–79 years of age with anticipated survival > three years without a breast cancer history were eligible. Between 1993 and 1998, 10,739 women, including 1,616 Black women, were entered from 40 clinical centers in the US. A three month washout period was required for those using hormone therapies. The trial was approved by institutional review boards at the clinical centers and the participants provided informed written consent.
Baseline Information was collected using standardized questionnaires. Medication use was collected by review of participants’ medication containers. A mammogram non-suspicious for cancer was required for entry and annual mammography was a pre-requisite for ongoing study pill distribution. Body weight and height, determined using standardized methods, were used to calculate body mass index (BMI).
Race/ethnicity was by self-report. Women who self-reported themselves as Black had determination of African ancestry (available in 66% [n=1061] of Black women) using genetic information from 656,852 single nucleotide polymorphisms (SNPs). The admixture contribution (a proportion ranging from 0–100%) of four ancestral populations (European, African, East Asian, Native American) for each self-identified Black women was estimating using Frappe software as previously described (26). Three ordinal groups of African ancestry were created. Estimates of African ancestry were used to subdivide Black cases into two groups: African Americans with < 80% African ancestry and African Americans with ≥ 80% African ancestry (cut point representing median African ancestry among Black women in the trial). The third group comprised White women who were assumed to have the least amount of African ancestry.
Randomization and Intervention
In the estrogen alone trial, women were randomized to daily conjugated equine estrogen (CEE) (0.625 mg/d) alone (Premarin ®) or an identical appearing placebo. Randomization was performed by the WHI Clinical Coordinating Center using a computerized, stratified, permuted block algorithm. Coded study pills were distributed with both staff and participants blinded to group assignment.
Outcomes and Follow-up
Clinical outcomes were reported at six month intervals and were confirmed by medical record review by local physician with final adjudication at the Clinical Coordinating Center. All self-reported strokes received central adjudication by trained neurologist reviewers (27).
Intervention ended on February 29, 2004 after 7.2 years median follow-up based on no favorable risk-to-benefit ratio and increased stroke risk (25). Per protocol follow-up continued through the original specified completion date March 31, 2005. Continued follow-up required additional written consent, obtained in 78% of surviving participants.
Statistical Analyses
The analyses described in this study were not protocol pre-specified. They were conducted to determine whether the reduction in breast cancer seen in Black women with estrogen alone use in this trial occurs within a context of overall safety considering the balance of health outcomes under potential hormone influence.
The trial monitoring outcomes included a global index representing the earliest time-to-event of seven major clinical outcomes felt to be under hormone influence and have impact on survival including coronary heart disease (myocardial infarction or death from heart disease), invasive breast cancer, stroke, pulmonary emboli, colorectal cancer, hip fracture and death from all other causes. For the current analyses by race/ethnicity, an expanded venous thromboembolism category that added deep venous thrombosis to pulmonary embolism was also considered. However, the global index was calculated as in prior WHI reports from this trial (8, 18).
Outcomes were assessed with time-to-event methods based on the intention-to-treat principal. Hazard ratios by race/ethnicity were estimated from Cox proportional-hazard analyses that included indicator variables for randomization group and the interaction with race ethnicity. The analyses were stratified by race/ethnicity, age, prior disease, randomized assignment in the WHI dietary-modification trial; statistical significance was based on the test of interaction. Comparisons of findings in Black and White women are shown for the intervention phase as well as intervention and post-intervention phases combined.
Additionally, HRs were estimated in case-only analyses using logistic regression of randomization assignment stratified by the three ordinal groups of African admixture. For rare outcome (e.g. < 5% incidence during study follow-up) case-only analyses provide HRs and corresponding CIs for subgroups that are essentially equivalent to those that would arise if the subgroups were available on the entire randomized cohort (28 Moreover, under a Cox model that stratifies on the baseline classification variable, the treatment HR can be calculated using logistic regression of the randomization indicator on subset indicator variables with “offset” determined by the randomization fraction of the trial cohort as a whole and genotyping rates.
All statistical analyses were conducted using SAS software version 9.3 (SAS Institute Inc.) and R software version 2.15 (R Foundation for Statistical Computing).
Results
Baseline characteristics for the two randomization groups (estrogen alone vs. placebo) in both Black and White women were well balanced (Table 1). However, substantial differences are seen when comparing characteristics of Black to White trial participants regardless of randomization group. Black women were younger and gave birth to their first child at a younger age, less commonly had prior hormone therapy use, and more commonly had moderate/severe vasomotor symptoms. In addition, Black women were heavier and were more likely to have diabetes, hypertension and a history of myocardial infarction or stroke.
Table 1.
Baseline Characteristics of Participants in the Women’s Health Initiative Trial of Evaluating Estrogen Alone by Randomization Group and Stratified by Race/Ethnicity
| Whites | Blacks | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Active (n=4,009) |
Placebo (n=4,075) |
Active (n=781) |
Placebo (n=835) |
||||
| N | % | N | % | N | % | N | % | |
| Age at screening, y, mean (SD) | 64.3 | (7.2) | 64.3 | (7.3) | 61.7 | (7.0) | 61.5 | (7.1) |
| Menopausal hormone therapy use status | ||||||||
| Never used | 1985 | 49.5 | 1975 | 48.5 | 488 | 62.6 | 508 | 61.0 |
| Past user | 1497 | 37.3 | 1536 | 37.7 | 216 | 27.7 | 245 | 29.4 |
| Current user1 | 527 | 13.1 | 562 | 13.8 | 76 | 9.7 | 80 | 9.6 |
| Baseline vasomotor symptoms, % | ||||||||
| None | 2420 | 60.8 | 2460 | 60.9 | 286 | 37.3 | 296 | 36.1 |
| Mild | 1007 | 25.3 | 1049 | 26.0 | 239 | 31.2 | 263 | 32.0 |
| Moderate/severe | 551 | 13.9 | 529 | 13.1 | 242 | 31.6 | 262 | 31.9 |
| Body mass index, kg/m2, median (IQR) | 29.0 (25.3, 33.3) | 28.7 (25.4, 32.9) | 31.2 (27.4, 35.9) | 30.9 (27.5, 35.8) | ||||
| Systolic BP, mm Hg, mean (SD) | 129.7 | (17.3) | 129.3 | (17.5) | 134.4 | (17.5) | 134.2 | (17.6) |
| Diastolic BP, mm Hg, mean (SD) | 76.0 | (9.0) | 75.9 | (9.2) | 79.3 | (9.4) | 79.2 | (9.4) |
| Smoking. % | ||||||||
| Never | 2018 | 50.8 | 2012 | 49.9 | 394 | 51.5 | 389 | 47.6 |
| Past | 1566 | 39.4 | 1606 | 39.8 | 268 | 35.0 | 322 | 39.4 |
| Current | 391 | 9.8 | 416 | 10.3 | 103 | 13.5 | 107 | 13.1 |
| Bilateral oophorectomy, % | 1513 | 40.2 | 1620 | 42.2 | 260 | 37.8 | 304 | 42.6 |
| Medical treatment, % | ||||||||
| Treated for diabetes | 249 | 6.2 | 247 | 6.1 | 108 | 13.8 | 107 | 12.9 |
| Treated for hypertension or BP ≥140/90 | 1874 | 50.1 | 1849 | 49.3 | 534 | 71.8 | 548 | 69.5 |
| Elevated cholesterol levels requiring medication | 554 | 13.8 | 605 | 14.8 | 123 | 15.7 | 131 | 15.7 |
| Statin use at baseline | 304 | 7.6 | 335 | 8.2 | 56 | 7.2 | 57 | 6.8 |
| Aspirin use (≥80mg/d) at baseline | 872 | 21.8 | 903 | 22.2 | 104 | 13.3 | 115 | 13.8 |
| Medical History, % | ||||||||
| Myocardial infarction | 124 | 3.1 | 130 | 3.2 | 34 | 4.4 | 34 | 4.1 |
| Angina | 290 | 7.3 | 280 | 6.9 | 74 | 9.6 | 75 | 9.1 |
| CABG/PCI2 | 94 | 2.4 | 88 | 2.2 | 19 | 2.5 | 16 | 2.0 |
| Stroke | 45 | 1.1 | 58 | 1.4 | 21 | 2.7 | 23 | 2.8 |
| Deep vein thrombosis or pulmonary embolism | 75 | 1.9 | 73 | 1.8 | 6 | 0.8 | 9 | 1.1 |
| Family history of breast cancer | 719 | 19.0 | 672 | 17.5 | 110 | 15.5 | 127 | 16.6 |
| > High school degree/GED | 2691 | 67.6 | 2833 | 69.9 | 518 | 67.8 | 574 | 69.8 |
| Family income ≥ $50,000, % | 924 | 24.2 | 954 | 24.7 | 130 | 18.0 | 133 | 17.0 |
| Number of term pregnancies | ||||||||
| Never pregnant/never had term pregnancy | 316 | 7.9 | 304 | 7.5 | 133 | 17.2 | 111 | 13.4 |
| 1 – 2 | 1071 | 26.9 | 1138 | 28.1 | 259 | 33.5 | 288 | 34.9 |
| 3 – 4 | 1719 | 43.2 | 1754 | 43.3 | 214 | 27.7 | 248 | 30.0 |
| 5+ | 877 | 22.0 | 859 | 21.2 | 166 | 21.5 | 179 | 21.7 |
| Age at first birth3 | ||||||||
| <20 | 827 | 24.6 | 838 | 24.4 | 248 | 48.2 | 281 | 46.8 |
| 20 – 29 | 2375 | 70.8 | 2384 | 69.5 | 237 | 46.1 | 293 | 48.8 |
| 30+ | 153 | 4.6 | 209 | 6.1 | 29 | 5.6 | 26 | 4.3 |
| Gail 5 year risk of breast cancer, mean (SD) | 1.8 | (1.0) | 1.8 | (1.0) | 0.9 | (0.5) | 0.9 | (0.6) |
Required a 3-month washout prior to randomization
CABG/PCI=coronary artery bypass graft/percutaneous coronary intervention
Among those with term pregnancy.
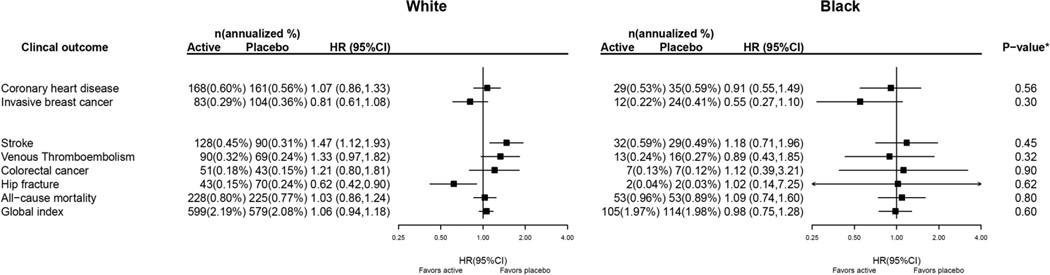
Study results for invasive breast cancer, CHD and other clinical outcomes during the 7.2 years (median) intervention are presented in Figure 1. Among White women, those in the estrogen alone group had fewer hip fractures and somewhat fewer breast cancers compared to those in the placebo group. Among Black women, there were also somewhat fewer breast cancers in the estrogen alone compared to the placebo group. In neither White nor Black women was the difference in breast cancer incidence by randomization group statistically significant. Race/ethnicity did not significantly modify the effect of estrogen alone on any of the clinical outcomes separately, or combined (global index) (p-interaction in all cases >= 0.30).
Figure 1. Clinical Outcomes in the Women’s Health Initiative CEE–alone Trial during the intervention phase According to Race.
* P–value corresponds to a test of the interaction between randomization arm and race.
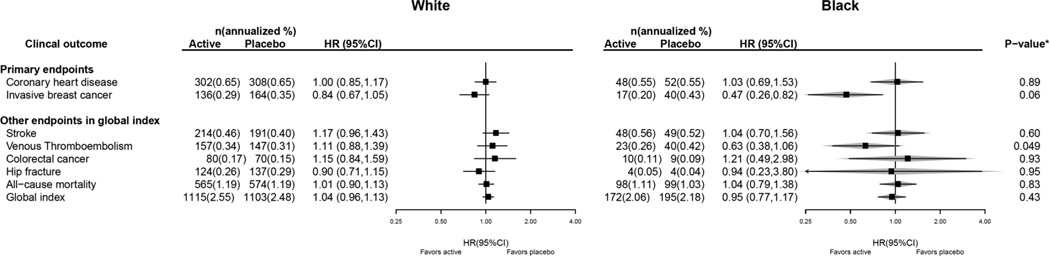
Cumulative study results, after 13 years (median) follow-up incorporating both intervention and post-intervention events, are presented in Figure 2. The 53% reduction in breast cancer incidence with estrogen alone use in Black women (HR 0.47 95% CI 0.26–0.82) was somewhat greater than that seen in White women (interaction P = 0.06) and was associated with no adverse influence on the global index (HR 0.95 95% CI 0.77–1.17). There were somewhat fewer venous thromboembolic events in Black women in the estrogen alone group (HR 0.63 95% CI 0.38–1.06) with a significantly greater effect in Black compared to White women (interaction P=0.049). Other outcomes, including CHD and all-cause mortality were null with no differences between Black and White women. Summary statistics from case-only analyses of Blacks, with genetic data, are overlaid to demonstrate the similarity of the case-only estimates with those from full cohort analyses (Figure 2).
Figure 2. Clinical Outcomes in the Women’s Health Initiative CEE–alone Trial for the Overall Combined Phases (Cumulative Follow–up) According to Race.
To demonstrate the validity of the case-only analysis, HR (95%CI) estimated from only Black cases that had genetic data available are also displayed, and represented by the gray diamonds. * P–value corresponds to a test of the interaction between randomization arm and race.
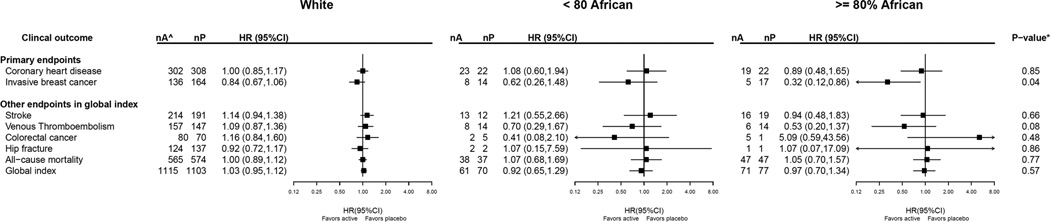
Baseline characteristics by percent African ancestry, including breast cancer risk factors are outlined In Table 2. The Black women with > 80% African ancestry, compared to Black women with less African ancestry, tended to be younger and younger at first birth, less educated, heavier, have lower income, less commonly had prior hormone therapy use, have had more term pregnancies and were at slightly lower 5 year Gail breast cancer risk. Intervention results for the case-only analyses by African ancestry are presented in Figure 3. In White women, the cumulative estrogen alone influence on almost all clinical outcomes was not significantly different from zero. The reduction in breast cancer incidence with estrogen alone use was significantly modified by African ancestry, where the largest benefit was observed among Black women with ≥ 80% African ancestry (trend P= 0.04 for effect modification by ancestry). A somewhat lower incidence in venous thromboembolism with estrogen alone use was also seen in Black women with ≥ 80% African ancestry (trend P=0.08).
Table 2.
Baseline Characteristics of Participants in the Women’s Health Initiative Trial Evaluating Estrogen Alone by Race and by Percent African Ancestry
| Black: ≥ 80% African (n=531) |
Black: < 80% African (n=530) |
White (n=8084) |
||||
|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % |
| Age at screening, y, mean (SD) | 61.0 | (6.8) | 62.4 | (7.2) | 64.3 | (7.2) |
| Menopausal hormone therapy use status | ||||||
| Never used | 357 | 67.2 | 311 | 58.8 | 3960 | 49.0 |
| Past user | 132 | 24.9 | 160 | 30.2 | 3033 | 37.5 |
| Current user4 | 42 | 7.9 | 58 | 11.0 | 1089 | 13.5 |
| Baseline vasomotor symptoms, % | ||||||
| None | 180 | 34.2 | 208 | 39.9 | 4880 | 60.9 |
| Mild | 165 | 31.4 | 164 | 31.5 | 2056 | 25.6 |
| Moderate/severe | 181 | 34.4 | 149 | 28.6 | 1080 | 13.5 |
| Body mass index, kg/m2, median (IQR) | 31.5(27.9, 36.8) | 30.7(26.9, 35.1) | 28.8(25.4, 33.1) | |||
| Systolic BP, mm Hg, mean (SD) | 134.8 | (17.9) | 134.4 | (17.7) | 129.5 | (17.4) |
| Diastolic BP, mm Hg, mean (SD) | 79.5 | (9.2) | 79.1 | (9.4) | 75.9 | (9.1) |
| Smoking, % | ||||||
| Never | 267 | 51.3 | 232 | 44.5 | 4030 | 50.3 |
| Past | 180 | 34.6 | 217 | 41.7 | 3172 | 39.6 |
| Current | 73 | 14.0 | 72 | 13.8 | 807 | 10.1 |
| Bilateral oophorectomy | 177 | 39.4 | 185 | 40.2 | 3133 | 41.2 |
| Medical treatment, % | ||||||
| Treated for diabetes | 76 | 14.4 | 63 | 11.9 | 496 | 6.1 |
| Treated for hypertension or BP ≥140/90 | 357 | 69.7 | 356 | 68.2 | 3723 | 49.7 |
| Elevated cholesterol levels requiring medication | 93 | 17.5 | 93 | 17.5 | 1159 | 14.3 |
| Statin use at baseline | 41 | 7.7 | 42 | 7.9 | 639 | 7.9 |
| Aspirin use (≥80mg/d) at baseline | 64 | 12.1 | 75 | 14.2 | 1775 | 22.0 |
| Medical History, % | ||||||
| Myocardial infarction | 22 | 4.1 | 21 | 4.0 | 254 | 3.1 |
| Angina | 46 | 8.8 | 49 | 9.3 | 570 | 7.1 |
| CABG/PCI5 | 10 | 2.0 | 14 | 2.7 | 182 | 2.3 |
| Stroke | 12 | 2.3 | 18 | 3.4 | 103 | 1.3 |
| Deep vein thrombosis or pulmonary embolism | 5 | 0.9 | 5 | 0.9 | 148 | 1.8 |
| Family history of breast cancer | 80 | 16.9 | 89 | 18.2 | 1391 | 18.2 |
| > High school degree/GED | 328 | 63.0 | 389 | 75.0 | 5524 | 68.8 |
| Family income ≥ $50,000, % | 73 | 14.7 | 108 | 21.7 | 1878 | 24.5 |
| Number of term pregnancies | ||||||
| Never pregnant/never had term pregnancy | 92 | 17.5 | 77 | 14.7 | 620 | 7.7 |
| 1 – 2 | 165 | 31.4 | 203 | 38.7 | 2209 | 27.5 |
| 3 – 4 | 137 | 26.0 | 159 | 30.3 | 3473 | 43.2 |
| 5+ | 132 | 25.1 | 86 | 16.4 | 1736 | 21.6 |
| Age at first birth6 | ||||||
| <20 | 199 | 55.9 | 152 | 40.5 | 1665 | 24.5 |
| 20 – 29 | 139 | 39.0 | 207 | 55.2 | 4759 | 70.1 |
| 30+ | 18 | 5.1 | 16 | 4.3 | 362 | 5.3 |
| Gail 5 year risk of breast cancer, mean (SD) | 0.9 | (0.5) | 1.0 | (0.7) | 1.8 | (1.0) |
Required a 3-month washout prior to randomization
CABG/PCI=coronary artery bypass graft/percutaneous coronary intervention
Among those with term pregnancy.
Figure 3. Clinical Outcomes in the Women’s Health Initiative CEE–alone Trial for the Overall Combined Phases (Cumulative Follow–up) According to African Ancestry: Case–only Analysis.
^ nA and nP are the number of cases in active arm and placebo arm, respectively. * P–value corresponds to a 1 degree–of–freedom test for trend of the interaction between randomization arm and race.
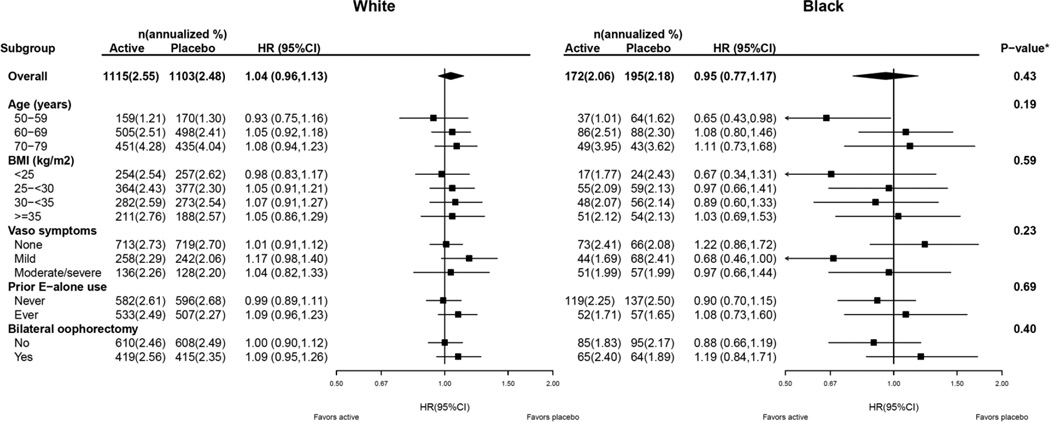
The effects of estrogen alone on the global index in subgroups by age, BMI, vasomotor symptoms at baseline, and prior estrogen alone use, are further stratified by race/ethnicity, and presented in Figure 4. In these analyses, subgroup interactions did not vary by race. For example, for both White and Black women, the HRs were more favorable in younger women regardless of race (p-3-way interaction = 0.19). In Black women, HRs were < 1 in the clinically relevant subgroups of age (50–59 years (HR 0.65 95% CI 0.43–0.98), experiencing vasomotor symptoms, and BMI (<25 kg/m2). The global index HRs were essentially null in overweight and obese Black women.
Figure 4. Risk of Global Index in the Women’s Health Initiative CEE-alone Trial for the Overall Combined Phases (Cumulative Follow-up) According to Race Stratified by Select Subgroups.
The p-value for the overall effect of CEE on global index corresponds to a test of the interaction between randomization arm and race. For the subgroup analysis, the p-value corresponds to a three-way interaction between randomization group, race and subgroup.
Discussion
During long term, cumulative follow-up of the WHI trial, estrogen alone use significantly reduced breast cancer incidence in Black women with no adverse influence on CHD, the global index or all-cause mortality with a suggestion of reduced venous thromboembolism risk as well. The favorable effects of estrogen alone on the global index in Black women beginning use in the fifth decade and those with vasomotor symptoms are noteworthy as such women also would be most likely to climacteric symptom benefit from hormone use.
Prior to this report, information on menopausal hormone therapy influence on any clinical outcome in Black women has been limited. The current study, with randomized clinical trial findings based on 1,616 Black women, addresses an unmet need by providing the first comprehensive, reliable information on estrogen alone influence on long term chronic disease risk in this population. The findings are of particular clinical relevance since, compared to White women, Black women are more likely to have had a hysterectomy, making them more commonly candidates for estrogen alone use (29, 30, 31).
Both in the general population (11,12) and in participants in the current clinical trial, Black women had substantially more risk factors for CHD and stroke compared to White women. However, in the WHI estrogen alone trial, no increase in coronary heart disease (CHD) (32) was seen among Black women during the 7.2 years intervention. We now report that no increase in CHD emerged during 6.8 years of additional post-intervention follow-up. While no overall increase in stroke incidence was seen in Black women during the same period (48 vs 49 cases for estrogen and placebo groups, respectively, HR 1.04 95% CI 0.70–1.56), the null result appears to differ from a HR for stroke of 1.61 (95% CI 0.90–2.90) for estrogen alone previously reported for Black women during the intervention period. However, that subgroup analysis was limited to ischemic rather than total stroke incidence (33). Nonetheless, as estrogen alone significantly increased stroke risk in White women during the intervention period (1.47 95% CI 1.12–1.93) with no interaction by race/ethnicity seen (33), the estrogen result on stroke risk in Black women requires cautious interpretation and confirmation from additional studies.
Several estrogen alone effects may have mitigated the anticipated increase in cardiovascular disease based on the higher incidence of CHD risk factors in Black women. Recently, an interaction between estrogen alone and race/ethnicity on systolic blood pressure was reported. After one year in the WHI trial, estrogen alone increased mean blood pressure for White women (1.07 mmHg 95% CI 0.54–1.59) but not for Black women (−0.17 mmHg 95% CI −1.44–1.09) (interaction P< 0.001) (34). An additional factor may have been the effect of estrogen alone in reducing fasting glucose levels and moderately decreasing diabetes risk (35). In this regard, two reports have suggested most/all of the adverse cardiovascular disease risk seen in racial/ethnic minorities may be related to the higher diabetes incidence found in those groups (11, 36), pointing to the potential importance of diabetes prevention interventions in minority populations.
The estrogen alone effect in reducing breast cancer incidence in Black women in the current report parallel findings in the entire WHI study population where a statistically significant decrease in breast cancer incidence was seen (21, 22, 37). The suggestion of greater reduction in breast cancer incidence with estrogen alone use in Black women with higher percentage of African ancestry supports a genetic contribution to the finding. Potential mediating mechanisms could include differences in reproductive hormone metabolism and/or differences in gene expression related to estrogen receptor function in White and Black women (38). Ongoing studies are exploring these potential mechanisms.
In the WHI hormone therapy trials, the estrogen alone effect on breast cancer differs markedly from the estrogen plus progestin effect on this outcome. In the WHI trial evaluating estrogen plus progestin, a statistically significantly increase in breast cancer incidence was seen overall, as well as in Black women (HR 1.38 95% CI 0.77–2.48) (39).
Few prior observational studies have examined associations of menopausal hormone therapy and breast cancer in Black women with mixed results seen. Interpretation of these studies is clouded by analyses combining results from estrogen alone and estrogen plus progestin use where no increase (40, 41), increase (42), or decrease (43) in breast cancer risk have been suggested for hormone therapy use. In the Million Women Study cohort, with 4919 Black women and 180 breast cancer cases, use of menopausal hormone therapy was associated with somewhat less breast cancer risk than seen for White women (HR 0.87 95% CI 0.75–1.00). However, the analyses combined findings for estrogen alone with those for estrogen plus progestin use, precluding direct comparison to the current WHI results (44). As most Black participants in the Million Women Study are first generation migrants, differences could be anticipated in comparison to findings in US women. Additional information comes from a case-control study from the African American Breast Cancer Epidemiology and Risk (AMBER) cohort. With 1,644 breast cancer cases, estrogen plus progestin use was associated with a significant increase in receptor positive breast risk. However, estrogen alone use was not associated with either an increase or decrease in risk (45), a result which differs from the WHI randomized clinical trial result where a reduction in risk with estrogen alone is seen overall and in Black women (21, 22).
A reduction in breast cancer with estrogen alone use differs from the preponderance of even recent cohort studies which consistently associate estrogen alone use, especially longer term use, with higher breast cancer risk (46, 47, 48). For example, in the Nurse’s Health Study cohort, an increase in breast cancer with estrogen alone use was only seen after 20+ years use (46). Another observational study versus randomized trial difference is the short time-from-menopause to hormone therapy initiation found in most observational studies as there was little or no increase in breast cancer risk when estrogen alone use was begun 5 years or more after menopause (49, 50). Nonetheless, the concept of an actual reduction in risk with estrogen alone use has received suggestive support from findings in other randomized trials. In the Estrogen for the Prevention of Re-Infarction Trial (ESPRIT) which entered 1,017 women post myocardial infarction, those assigned to the unopposed estrogen (estradiol valerate) had somewhat fewer breast cancers (HR 0.47 95% CI 0.19–1.15) (51). Similarly, in a small trial in Denmark, 192 women randomized to daily 17-Beta-estradiol had a significant reduction in a combined endpoint of mortality or breast cancer (HR 0.42 95% CI 0.18–0.97) (52). The remaining differences regarding estrogen alone influence on breast cancer in randomized trials compared to observational cohort studies may represent confounding by currently unrecognized variables.
Study strengths include the randomized double-blind, placebo-controlled design, the number of Black participants, baseline and ongoing mammography screening, long post-intervention follow-up, and high-quality outcome assessment. Limitations include those associated with post-hoc analyses and the modest number of events in some disease outcome categories, especially in analyses by African ancestry. While African ancestry was not assessed in White women participating in this trial, in a recent study, only 1.4% of self-reported White women in the US were found to carry ≥ 2% African ancestry (53).
Conclusion
In summary, estrogen alone use in Black postmenopausal women with prior hysterectomy significantly reduced breast cancer incidence with no apparent adverse influence on coronary heart disease, venous thromboembolism or all-cause mortality. The favorable effects of estrogen alone on the global index in younger Black women, those beginning use in the fifth decade and those with vasomotor symptoms are noteworthy and warrant further study. After full consideration of risks and benefits, the current findings provide reassurance for Black women with prior hysterectomy who are close to menopause considering estrogen alone use for climacteric symptom management.
Acknowledgments
Funding/Support: The Women’s Health Initiative is funded by the National Heart, Lung, and Blood Institute at the National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 321115, 32118-32119, 32122, 42107-26, 42129-32 and 44221. Wyeth-Ayerst donated the study drugs.
Role of the Sponsors: The Women’s Health Initiative (WHI) project office at the National Heart, Lung, and Blood Institute (NHLBI), which was the sponsor, had a role in the design and conduct of the study; interpretation of the data; review and approval of the manuscript; and decision to submit the manuscript for publication. Decisions concerning the above, as well as data collection, management, and analysis, resided with committees composed of WHI Investigators and included NHLBI representatives.
Additional Contributions: We thank the Women’s Health Initiative investigators, staff, and the trial participants for their outstanding dedication and commitment.
A Short List of Women’s Health Initiative Investigators
Program Office: Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, Maryland). Clinical Coordinating Center: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, Washington). Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts); Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, California); Rebecca Jackson (Ohio State University, Columbus); Cynthia A. Thomson (University of Arizona, Tucson/Phoenix); Jean Wactawski-Wende (State University of New York, Buffalo); Marian Limacher (University of Florida, Gainesville/Jacksonville); Robert Wallace (University of Iowa, Iowa City/Davenport); Lewis Kuller (University of Pittsburgh, Pittsburgh, Pennsylvania); Rowan T. Chlebowski, (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center); Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, North Carolina).
Women’s Health Initiative Memory Study: Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, North Carolina).
Footnotes
REGISTRATION: clinical trials.gov Identifier: NCT00000611
Conflict of Interest Disclosures: Dr. Chlebowski reported receiving consulting fees or honoraria from Novartis, Amgen, Genentech and Genomic Health; fees for participation in review activities for Pfizer and Novo Nordisk; payment for lectures from Novartis and Genentech; and payment for educational activities from Educational Concepts Group. No other authors have conflicts of interest.
These data have been previously published in oral abstract format at the ASCO Annual Meeting in Chicago, Illinois, May 29 – June 2, 2015. These data and results, however, have not been previously published in manuscript format.
Author contributions: Dr. Chlebowski and Mr. Aragaki had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: Chlebowski, Barrington, Manson, O’Sullivan, Wallace, Prentice. Acquisition of data: Chlebowski, Manson, Sato, Sullivan, Cauley, Wallace, Qi, Prentice. Analysis and interpretation of data: Chlebowski, Barrington, Aragaki, Manson, Sarto, Sullivan, Qi, Sarto, O’Sullivan, Wu, Cauley, Wallace, Prentice. Drafting of the manuscript: Chlebowski. Critical revision of the manuscript for important intellectual content: Chlebowski, Barrington, Aragaki, Manson, O'Sullivan, Wu, Cauley, Qi, Wallace, Prentice. Administrative, technical or material support: Chlebowski, Manson, O'Sullivan, Cauley, Wallace, Prentice.
Additional Information: A full list of all the investigators who have contributed to Women’s Health Initiative science appears at https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
References
- 1.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT, Hendrix SL, Langer RD, et al. Estrogen plus progestin influence on breast cancer and mammography in healthy postmenopausal women: The Women’s Health Initiative randomized trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 3.Beral V Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. Aug 9;362(9382):419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 4.Hersh AL, Stefanick MI, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2005;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 5.Steinkeller AR, Denison SE, Eldridge SL, et al. A decade of postmenopausal hormone therapy prescribing in the United States long-term effects of the Women’s Health Initiative. Menopause. 2012;19:616–621. doi: 10.1097/gme.0b013e31824bb039. [DOI] [PubMed] [Google Scholar]
- 6.Watson J, Wise L, Green J. Prescribing of hormone therapy for menopause, tibolone, and bisphosphonates in women in the UK between 1991 and 2005. Eur J Clin Pharmacol. 2007 Sep;63(9):843–849. doi: 10.1007/s00228-007-0320-6. Epub 2007 Jun 28. [DOI] [PubMed] [Google Scholar]
- 7.Huet L, Couris CM, Tainturier V, Jaglal S, Colin C, Schott AM. Trends in HRT and anti-osteoporosis medication prescribing in a European population after the WHI study. Osteoporosis Int. 2008 Jul;19(7):1047–1054. doi: 10.1007/s00198-008-0587-1. Epub 2008 Mar 29. [DOI] [PubMed] [Google Scholar]
- 8.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health across the nation. Am J Public Health. 2006;96(7):1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grisso JA1, Freeman EW, Maurin E, et al. Racial differences in menopause information and the experience of hot flashes. J Gen Intern Med. 1999;14(2):98–103. doi: 10.1046/j.1525-1497.1999.00294.x. [DOI] [PubMed] [Google Scholar]
- 10.Reed SD, Lampe JW, Qu C, et al. Premenopausal vasomotor symptoms in an ethnically diverse population. Menopause. 2014;21(2):153–158. doi: 10.1097/GME.0b013e3182952228. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Hebert JR, Balasubramanian R, Wieck NM, et al. All-cause, cardiovascular, and cancer mortality rates in postmenopausal white, black, Hispanic, and Asian women with and without diabetes in the United States: the Women's Health Initiative, 1993–2009. Am J Epidemiology. 2013;178(10):1533–1541. doi: 10.1093/aje/kwt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jha AK, Varosy PD, Kanaya AM, et al. Differences in medical care and disease outcomes among Black and white women with heart disease. Circulation. 2006;108:1089–1094. doi: 10.1161/01.CIR.0000085994.38132.E5. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson WK, Brown AF, Gathe J, et al. Hormone replacement therapy for African American women: missed opportunities for effective intervention. Menopause. 1999;6(2):147–155. [PubMed] [Google Scholar]
- 14.The Writing Group for the PEPI. Hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA. 1996 Nov 6;276(17):1389–1396. [PubMed] [Google Scholar]
- 15.Grady D, Applegate W, Bush T, Furberg C, Riggs B, Hulley SB. Heart and Estrogen/progestin Replacement Study (HERS): design, methods, and baseline characteristics. Control Clin Trials. 1998 Aug;19(4):314–335. doi: 10.1016/s0197-2456(98)00010-5. [DOI] [PubMed] [Google Scholar]
- 16.Mosekilde L, Hermann AP, Beck-Nielsen H, Charles P, Nielsen SP, Serensen OH. The Danish Osteoporosis Prevention Study (DOPS): project design and inclusion of 2000 normal perimenopausal women. Maturitas. 1999 Mar 15;31(3):207–219. doi: 10.1016/s0378-5122(99)00006-7. [DOI] [PubMed] [Google Scholar]
- 17.Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effects of lower dose conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002 May 22–29;287(20):2668–2676. doi: 10.1001/jama.287.20.2668. [DOI] [PubMed] [Google Scholar]
- 18.Veerus P, Hovi SL, Fischer K, Rahu M, Hakama M, Hemminki E. Results from the Estonian postmenopausal hormone therapy trial [ISRCTN35338757] Maturitas. 2006 Sep 20;55(2):162–173. doi: 10.1016/j.maturitas.2006.01.012. Epub 2006 Feb 28. [DOI] [PubMed] [Google Scholar]
- 19.Vickers MR, MacLennan AH, Lawton B, et al. Main morbidities recorded in the women’s international study of long duration oestrogen after menopause (WISDOM): a randomized controlled trial of hormone replacement therapy in postmenopausal women. BMJ. 2007 Aug 4;335(7613):239. doi: 10.1136/bmj.39266.425069.AD. Epub 2007 Jul 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 21.Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized trial. Lancet. 2012;13(5):476–486. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone. JAMA Oncol. doi: 10.1001/jamaoncol.2015.0494. Published online April 16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chlebowski RT, Anderson GL, Aragaki A, Prentice R. Breast cancer and menopausal hormone therapy by race/ethnicity and body mass index. J Natl Cancer Inst. 2016;108(2) doi: 10.1093/jnci/djv327. pii djv327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 25.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 26.Tang H, Peng J, Wang P, Risch NJ. Estimation of individual admixture: Analytical and study design considerations. Genet Epidemiology. 2005;28(4):289–301. doi: 10.1002/gepi.20064. [DOI] [PubMed] [Google Scholar]
- 27.Wassertheil-Smoller S, Kaplan RC, Salazar CR. Stroke in the Women’s Health Initiative. Semin Reprod Med. 2014 Nov;32(6):438–446. doi: 10.1055/s-0034-1384627. Epub 2014 Oct 16. Review. [DOI] [PubMed] [Google Scholar]
- 28.Self SG, Longton G, Kopecky KJ, Liang KY. On estimating HLA/disease association with application to a study of aplastic anemia. Biometrics. 1991;47:53–61. [PubMed] [Google Scholar]
- 29.Brown AF, Perez-Stable EJ, Whitaker EE, et al. Ethnic differences in hormone replacement prescribing patterns. J Gen Intern Med. 1999;14(11):663–669. doi: 10.1046/j.1525-1497.1999.10118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Ins. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 31.Ganesan K, Teklehaimanot S, Asunion M. The associations of hormone replacement therapy and preventive practices in minority women. J Natl Med Assoc. 2005;97(1):68–73. [PMC free article] [PubMed] [Google Scholar]
- 32.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 33.Hendrix SL, Wassertheil-Smoller S, Johnson KC, et al. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113(20):2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 34.Shimbo D, Wang L, Lamonte MJ, et al. The effect of hormone therapy on mean blood pressure and visit-to-visit blood pressure variability in postmenopausal women: results from the Women’s Health Initiative randomized controlled trials. J Hypertens. 2014;32(10):2071–2081. doi: 10.1097/HJH.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonds DE, Lasser N, Qi L, et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women's Health Initiative randomized trial. Diabetologia. 2006;49(3):459–468. doi: 10.1007/s00125-005-0096-0. [DOI] [PubMed] [Google Scholar]
- 36.Henderson SO, Haiman CA, Wilkens LR, et al. Established risk factors account for most of the racial differences in cardiovascular disease mortality. PLoS One. 2007;2(4):e377. doi: 10.1371/journal.pone.0000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal therapy and health outcomes during the intervention and extended post stopping of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quan L, Hong CC, Ziproli G, et al. Variants of estrogen-related genes and breast cancer risk in European and African American women. Endocr Relat Cancer. 2014;21(6):853–864. doi: 10.1530/ERC-14-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou N, Hong S, Wang W, et al. Hormone replacement therapy and breast cancer: Heterogeneous risks by race, weight, and breast density. J Natl Cancer Inst. 2013;105:1365–1372. doi: 10.1093/jnci/djt207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell Jenkins BW, Addison C, Wilson G, et al. Association of the Joint Effect of Menopause and Hormone Replacement therapy and cancer in African American women: The Jackson Heart Study. Int J Environ Res Public Health. 2011;8:2491–2504. doi: 10.3390/ijerph8062491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg L, Palmer JR, Wise LA, Adams-Campbell LL. A prospective study of female hormone use and breast cancer among black women. Arch Intern Med. 2006;166(7):760–765. doi: 10.1001/archinte.166.7.760. [DOI] [PubMed] [Google Scholar]
- 43.Hall IJ, Moorman PG, Millikan RC, Newman B. Comparative analysis of breast cancer risk factors among African-American women and White women. Am J Epidemiol. 2005;161:40–51. doi: 10.1093/aje/kwh331. [DOI] [PubMed] [Google Scholar]
- 44.Gathani T, Ali R, Balkwill A, et al. Ethnic differences in breast cancer incidnce in England are due to differences in known risk factos for the disease» prospective study. British J Ca. 2014;110:224–229. doi: 10.1038/bjc.2013.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg L, Bethea TN, Viscidi E, et al. Postmenopausal female hormone use and estrogen receptor-positive and negative breast cancer in African American women. J Natl Cancer Inst. 2015;108(4) doi: 10.1093/jnci/djv361. pii:djv361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166(9):1027–1032. doi: 10.1001/archinte.166.9.1027. [DOI] [PubMed] [Google Scholar]
- 47.Bakken K, Fournier A, Lund E, et al. Menopausal hormone therapy and breast cancer risk: impact of different treatments. The European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2011;128:144–156. doi: 10.1002/ijc.25314. [DOI] [PubMed] [Google Scholar]
- 48.Fournier A, Mesrine S, Dossus L, et al. Risk of breast cancer after stopping menopausal hormone therapy in the E3N cohort. Breast Cancer Res Treat. 2014;145(2):535–543. doi: 10.1007/s10549-014-2934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prentice RL, Chlebowski RT, Stefanick ML, et al. Conjugated equine estrogens and breast cancer risk in the Women’s Health Initiative clinical trial and observational study. Am J Epidemiol. 2008;167(12):1407–1415. doi: 10.1093/aje/kwn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beral V, Reeves G, Bull D, Green J Million Women Study Collaborators. Breast cance risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103(4):296r–305r. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomized trial. BMJ. 2012;345:e6409. doi: 10.1136/bmj.e6409. [DOI] [PubMed] [Google Scholar]
- 52.Cherry N, McNamee R, Heagerty A, et al. Long-term safety of unopposed estrogen used by women surviving myocardial infarction: 14-year follow-up of the ESPRIT randomized controlled trial. BJOG. 2014;121(6):700–705. doi: 10.1111/1471-0528.12598. [DOI] [PubMed] [Google Scholar]
- 53.Bryc K, Durand EY, Macpherson M, et al. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet. 2015;96:1–17. doi: 10.1016/j.ajhg.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]