Abstract
Objective
The current study examined the effects of pharmacologic dopaminergic manipulations on working memory-related brain activation in postmenopausal women to further understand the neurochemistry underlying cognition after menopause.
Method
Eighteen healthy postmenopausal women, mean age 55.21 years, completed three study days with dopaminergic drug challenges during which they performed an fMRI visual verbal N-back test of working memory. Acute stimulation with 1.25 mg oral D2 agonist bromocriptine, acute blockade with 1.5 mg oral haloperidol, and matching placebo were administered randomly and blindly on three study days.
Results
We found that dopaminergic stimulation increased activation primarily in the posterior regions of the working memory network compared to dopaminergic blockade using a whole brain cluster-level corrected analysis. The dopaminergic medications did not affect working memory performance.
Conclusions
Patterns of increased BOLD signal activation after dopaminergic stimulation were found in this study in posterior brain regions with no effect on working memory performance. Further studies should examine specific dopaminergic contributions to brain functioning in healthy postmenopausal women in order to determine the effects of the increased brain activation on cognition and behavior.
Keywords: menopause, dopamine, working memory, fMRI
The brain is a major target for circulating gonadal steroids and the change in hormone levels after menopause is likely to have implications for cognitive functioning. Clinical and preclinical studies have linked gonadal steroids and cognition (e.g.1,2) and it has been hypothesized that menopause has detrimental effects on cognition that are over and above the expected effects of normal aging. However, evidence for changes in cognition after menopause is equivocal. Some studies found decreased cognitive performance post menopause in domains such as memory, attention, problem solving, and motor skills (e.g.3–5). Other studies have not found changes in cognition post menopause (e.g.6–8). One way to begin to understand these individual differences in cognition post menopause is to examine the underlying neurobiological processes that are affected by menopause.
Subjective reports of changes in executive functioning at mid-life are a concern for many women. Studies have shown as many as 60% of women reported undesirable memory changes at mid-life9. One mechanism hypothesized to be responsible for cognitive changes post menopause is the decrease in estradiol and its effects on the functioning of the prefrontal cortex10. Additionally, the estradiol change post menopause has been shown to affect the functioning of neurotransmitter systems in the prefrontal cortex that support cognition across a number of model systems from rats2 to non-human primates11 to humans12. Particular focus has been given to executive functioning changes after menopause and one component that is often examined is working memory13. Working memory is the ability to hold and manipulate a small amount of information over a short period of time14. Longitudinal studies of cognition as women move across the menopause transition indicate that working memory is not impaired by menopause1,3. However, studies have shown that working memory was improved by postmenopausal estrogen15,16. Thus, working memory systems are modifiable in postmenopausal women.
Working memory is also modulated by dopaminergic systems through the striatal-frontal pathway (e.g.17) and it has been hypothesized that age changes in the frontal lobe dopaminergic system are responsible for cognitive aging18. Studies have shown that there is a linear age-related decrease in dopamine receptor availability19,20. Additionally, there are sex differences in D2 receptor binding particularly in the frontal cortex19 with one study showing greater binding for women compared to men21, thus implicating a role for gonadal steroid modulation of dopaminergic functioning.
A handful of prior studies have examined dopaminergic functioning in postmenopausal women. Craig and colleagues22 found that long term postmenopausal estrogen treatment enhanced dopaminergic responsivity as measured by the growth hormone response to apomorphine challenge compared to women not taking estrogen therapy. Gardiner et al.23 found an increase in dopamine transporter relative to baseline in the putamen in women who took 0.625 oral conjugated equine estrogen (CEE) per day for four weeks and then another two weeks of CEE plus 10 mg oral medroxyprogesterone acetate (MPA). Epperson et al.24 examined the effects of atomoxetine, a selective norepinephrine reuptake inhibitor that increases extracellular norepinephrine and dopamine, in peri- and postmenopausal women with subjective cognitive complaints. They found that peri- and postmenopausal women showed improvement in subjective but not objective cognition after atomoxetine compared to placebo.
The prior literature shows that dopaminergic systems remain responsive in postmenopausal women and are involved in cognitive processes that are affected by estrogen22–24. However, it is difficult to isolate the contribution of the dopaminergic system independent of estrogen treatment or norepinephrine modulation in the studies described above. The current study examined direct stimulation and blockade of the dopaminergic system in healthy postmenopausal women during a functional magnetic resonance imaging (fMRI) working memory task. Working memory tasks during fMRI activate a network of bilateral frontal, parietal, and cerebellar regions25. In postmenopausal women, estrogen treatment compared to placebo has been shown to increase frontal activation during working memory tasks26,27. Studies in younger adults have shown frontal lobe modulation of working memory networks after dopaminergic manipulations (i.e.28,29) and decreased frontal activation was observed when performance was improved suggesting increased dopaminergic efficiency. No studies thus far have examined dopaminergic manipulations in postmenopausal women during an fMRI working memory task to examine the direct influence of dopaminergic modulation on working memory networks.
The aim of the study was to examine the independent contribution of direct dopaminergic manipulations on brain functioning in postmenopausal women. As much of the literature reviewed above examined estrogen-dopamine interactions after menopause it is important to understand the independent dopaminergic contribution. We hypothesized that dopaminergic stimulation would increase frontal activation in the working memory network and improve working memory performance compared to dopaminergic blockade.
Method
Participants
Participants were 18 cognitively normal postmenopausal women, aged 52–59 years, M(SD) = 55.21(2.3; See Table 1 for demographic information). Sixteen participants were STRAW+10 Stage +1 early postmenopause and two were Stage +2 late postmenopause based on their years since their final menstrual period. Participants were recruited with media advertisements in the Burlington, VT region. Four additional participants passed the screening but withdrew before beginning the study days because of the time commitment for the study. Participants were required to be postmenopausal, without menses for one year and without surgically-induced menopause. Medical exclusion criteria were similar to our prior studies (e.g.30) and included smoking, a history of breast cancer, use of hormone therapy during the last year, medications that have CNS effects, known intolerance to ergots, and contraindications for MRI. All participants met these criteria. No participants had a prior history of postmenopausal hormone use. Medication use by women in this study was as follows: two women took medications for hypertension, two took cholesterol lowering medications, two took levothyroxine for hypothyroidism, and three reported taking migraine medication as needed but not 48 hours before any study day.
Table 1.
Demographic data (means and standard deviations) for the postmenopausal women.
| N = 18 | |
|---|---|
| Age (y) | 55.21 (2.3) |
| BMI | 25.01 (3.0) |
| Education (y) | 15.44 (2.8) |
| Years since menopause (y) | 5.50 (3.3) |
| Ethnicity (N) | |
| Hispanic/Non-Hispanic | 2/16 |
| Race (N) | |
| White | 18 |
After passing the telephone screening, participants came to the University of Vermont (UVM) Clinical Research Center (CRC) for a medical and psychological screening. As in our prior medication challenge studies12,30, after signing informed consent documents, participants provided a medical history, underwent physical and laboratory tests assessing hematopoietic, renal, hepatic, and hormonal function. No women had any major medical illness as confirmed by the physical exam. Participants provided a blood sample that was used to ensure postmenopausal status of FSH > 20 IU/L. Participants were cognitively evaluated using the Mini Mental State Exam (MMSE;31), Brief Cognitive Rating Scale32, and the Mattis Dementia Rating Scale (DRS,33) to establish a Global Deterioration Scale score (GDS) which rated the degree of cognitive impairment32. Participants were required to have an MMSE score greater than or equal to 27, a DRS score greater than or equal to 123, and a GDS score of 1 or 2.
Behavioral screening consisted of a partial Structured Clinical Interview for DSM-IV-TR (SCID;34) to establish the presence/absence major depression, mania or dysthymia. Participants were also screened with the Beck Depression Inventory-II (BDI-II;35). A cut off score of 10 was used for the BDI, and participants scoring over this criterion were discontinued from further participation. Five out of 18 women had remitted major depressive disorder (MDD) and no women had current MDD. All participants met these criteria for the cognitive and behavioral screening.
Challenge Procedure
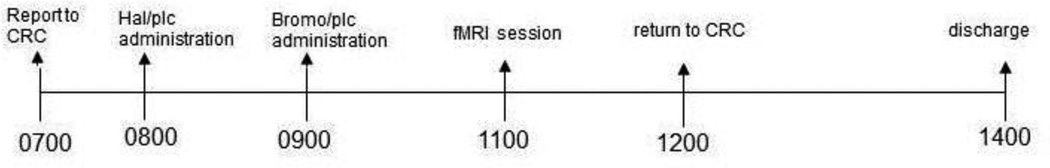
After passing the medical and psychological screening, participants came to the UVM CRC for three dopaminergic challenge days. The medication on one day was the agonist bromocriptine (BROMO), on a second day it was the dopaminergic antagonist haloperidol (HAL), and the third day was placebo (PLC). On each challenge day, participants reported to the UVM CRC by 0700 (see Figure 1). Similar to our prior medication challenge studies12,30, each woman performed a baseline motor skill sobriety test to serve as a comparison to a second test before discharge in the afternoon. An intravenous line (IV) was inserted and blood was drawn for estradiol (E2), estrone (E1), and testosterone (T) assays. At the end of the study all assays were run in one batch for a radioimmunoassay at the Reproductive Endocrine Research Laboratory at the University of Southern California.
Figure 1.
Study design.
A double-blind, double-dummy method of administration of the challenge drugs was followed. Participants received 1.25 mg bromocriptine orally, 1.5 mg haloperidol orally, or matching oral placebo. Participants took one pill 180 minutes before the MRI exam that was either haloperidol or placebo. Then at 120 minutes before the MRI they took another pill that was either bromocriptine or placebo. On each day only one of the pills was active drug or both pills were placebo. Thus, one study day was bromocriptine challenge, one day was haloperidol challenge, and one day was placebo. These times are similar to what has been shown for bromocriptine36 and haloperidol37 to have their maximum effects on cognition. The half-life of bromocriptine has been shown to be 4.85 hours and oral haloperidol is between 14 and 36 hours. Thus, study days occurred at least one week apart. Drug order was fully counterbalanced across participants. The drug order was developed by the CRC Informaticist and delivered directly to the research pharmacy so that study personnel remained blinded to drug order. Nausea was reported in 10% of our participants but it occurred after the MRI session and did not impact data collection. After the fMRI session that took approximately 70 minutes, participants were given lunch. Vital signs and pupil diameter were assessed at six time points during the study day. At the end of the study day, participants were discharged after passing the sobriety test to the satisfaction of the research nurse and covering physician.
fMRI Working Memory Task
The fMRI task was our standard30,38 visually presented verbal N-back task to probe working memory circuitry. Participants saw a string of consonants (except L, W, and Y), presented in upper case letters, one every three seconds. Four conditions were presented: 0-back, 1-back, 2-back, and 3-back. The 0-back control condition had a minimal working memory load; participants were asked to decide if the current letter matched a single target letter that was specified before the epoch began. In the 1-, 2-, and 3-back conditions, participants indicated whether the current letter on the screen matched a letter that was either 1, 2 or 3 back in the sequence.
The 0-, 1-, 2-, and 3-back conditions were repeated three times in a counterbalanced order such that the same condition was not repeated two times in a row. In this block design task, participants responded to nine items in each block that took 27 seconds. A rest break followed with a plus sign (+) fixation for 12 seconds. The total time of the task was 8 minutes 12 seconds. Participants practiced the N-back task before drug dosing began on each challenge day to ensure that they understood task instructions.
Participants responded to all items indicating whether it was a match or mismatch by pressing a button on an MRI compatible fiber optic button response system (Psychology Software Tools, Pittsburgh, PA). Stimuli were delivered through an MR-safe computer monitor. Experimental tasks were programmed using the E-prime software package and presented by PC; the PC recorded participant responses.
Behavioral Measures
At the beginning of each challenge day, participants completed the Profile of Mood States (POMS;39), BDI-II35, and Beck Anxiety Inventory (BAI;40) to obtain a baseline measure of mood before the testing procedures began. After the cognitive battery was completed, participants completed the POMS a second time as well as the Stanford Sleepiness Scale41, Subjective Visual Analogue Scale (SVAS;42), and a Physical Symptom Checklist (PSCL). The experimenter completed the Brief Psychiatric Rating Scale (BPRS;43) and Objective Visual Analogue Scale (OVAS;42).
fMRI Scan Procedure
The MRI procedures were similar to our prior studies in postmenopausal women30,38. All participants were scanned on a Philips 3T Achieva scanner and received the following MR sequences as part of the imaging protocol: (1) A sagittal T1-weighted spoiled gradient volumetric sequence oriented perpendicular to the anterior commissure (AC)-posterior commissure (PC) plane using a repetition time (TR) of 9.9 ms, echo time (TE) of 4.6 ms, flip angle of 8 degrees, number signal averages (NSA) 1, field of view (FOV) of 256 mm, 256 × 256 matrix, and 1 mm slice thickness with no gap for 140 contiguous slices. (2) An axial T2-weighted gradient spin echo (GRASE) sequence using the AC-PC line for slice positioning. Twenty-eight contiguous slices 5 mm thick and no gap were acquired using TR 2466 ms, TE 80 ms, NSA 3 and FOV of 230 mm. All images were reviewed by a board-certified neuroradiologist to exclude intracranial pathology. fMRI was performed using EpiBOLD (echoplanar blood oxygenation level dependent) imaging using a single-shot sequence (TR 2500 ms, TE 35 ms, flip angle 90 degrees, 1 NSA for 197 volumes). Resolution was 2.5 mm × 2.8 mm × 4 mm. Thirty-four contiguous slices 4 mm thick with no gap were obtained in the axial oblique plane parallel to the AC-PC plane using a FOV of 240 mm and a matrix size of 128 × 96. Field map correction for magnetic inhomogeneities was accomplished by acquiring images with offset TE at the end of the functional series.
fMRI Analyses
Statistical analyses were performed using a 3 (Drug: BROMO, HAL, PLC) × 4 (Working Memory Load: 0-, 1-, 2-, 3-back) random effects ANOVA using standard ANOVA procedures in Brain Voyager (Brain Voyager QX, The Netherlands). We hypothesized that drug effects on working memory-related activation would increase as the working memory load increased; thus the design matrix included all N-back conditions. The contrast vector for the overall interaction was as follows: −6, +1, +2, +3 for the 0-, 1-, 2- and 3-back conditions for the BROMO challenge day, +6, −1, −2, −3 for the 0-, 1-, 2-, 3-back conditions for the HAL challenge day, and 0, 0, 0, 0 for the placebo challenge day. This contrast allowed for the comparison of bromocriptine and haloperidol while including placebo information in the model. The hemodynamic response function was accounted for in these models. To probe the basis for the interaction between drug and working memory load, we examined drug effects in the following comparisons: 3-back minus 0-back, 2-back minus 0-back, and 1-back minus 0-back conditions. To examine the main effect of drug, we examined each drug compared to placebo across the increasing working memory load.
To correct for multiple comparisons, we used the cluster-level statistical threshold estimator from Brain Voyager QX to estimate a minimum cluster size threshold based on the approach of Forman et al.44.This procedure estimated a minimum cluster size of 9 voxels in functional space (3×3×3) at an alpha level of 0.005 for the fMRI analyses described below.
Working Memory Performance Analysis
Working memory performance during the N-back task was examined using the signal detection measures of sensitivity (d’) and bias (C;45) as we have done in our prior studies30,38. Sensitivity is a measure of how different two classes of items are as measured by d’ and is represented in standard deviation units. In the N-back task, the two classes of items are matches and mismatches for each of the working memory load conditions. Larger d’s represent greater sensitivity and greater accuracy. Bias (C) is the tendency for a participant to endorse a letter as a match or mismatch also represented in standard deviation units. Liberal response bias indicates that a participant calls a large number of responses matches in contrast to conservative bias indicating that the participant makes many mismatch responses. Bias scores of greater than 0 are conservative while bias scores less than 0 are liberal.
Results
Activation Data
First, we examined working memory-related brain activation during the N-back task to demonstrate the expected task effect on the placebo challenge day. Second, we examined the dopaminergic modulation of the working memory network after the bromocriptine compared to the haloperidol challenge day.
Working Memory Activation
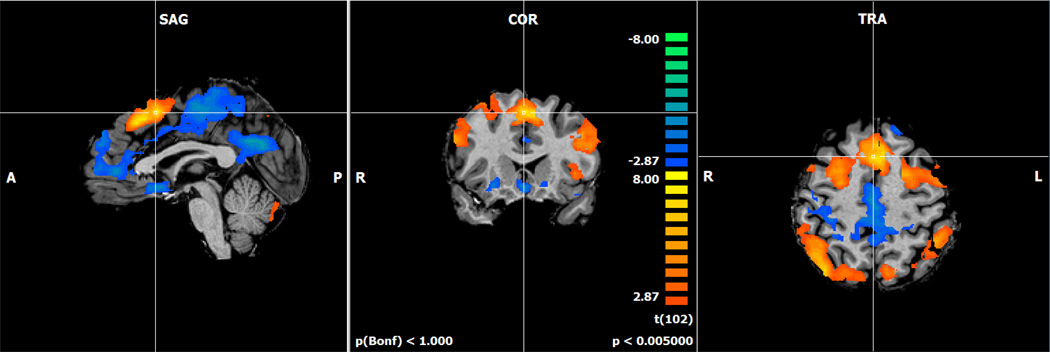
In our sample of healthy postmenopausal women, when we examined the activation related to increasing working memory load, we found the expected bilateral frontal, parietal, and cerebellar working memory network on the placebo challenge day (Figure 2;46,47).
Figure 2.
Activation map for the increasing working memory load contrast form the N-back task on the placebo challenge day (p < .005). The N-back task activated the expected bilateral frontal, parietal, and cerebellar regions during the placebo challenge day. Orange colors represent regions where the activation is increasing as the working memory loar (N) increases. Blue colors represent activation that is decreasing as the N increases.
Dopaminergic Modulation of Working Memory Activation
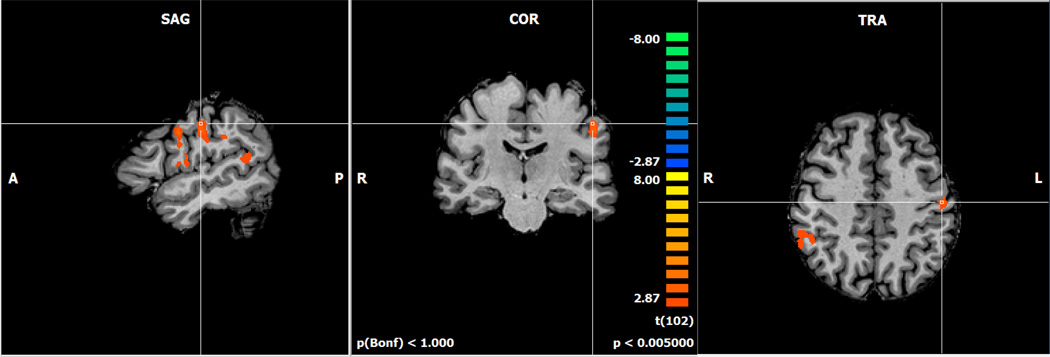
Second, we examined brain activation for the effects of the dopaminergic manipulations on increasing working memory load during the N-back task. Specifically, we examined bromocriptine minus haloperidol as working memory load increased (Figure 3). Increased activation for bromocriptine compared to haloperidol was found in the left precentral gyrus (BA 6), and bilateral inferior parietal lobules (BA 40; Table 2).
Figure 3.
Activation map for the bromocriptine minus haloperidol challenges during increasing working memory load contrast. Orange colors represent greater activation for the bromocriptine compared to haloperidol challenge.
Table 2.
Effects of bromocriptine compared to haloperidol placebo during increasing working memory load contrast including Talairach coordinates, cluster size, region descriptions (Brodmann’s areas, BA), t values, and uncorrected voxel-level p values.
| Contrast | Coordinates | Cluster Extent |
Region Description |
t value |
p value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| BROMO-HAL | |||||||
| Increasing WM load |
|||||||
| −49 | −2 | 33 | 1823 | Left precentral gyrus (BA 6) | 4.29 | <.001 | |
| −58 | −32 | 36 | 398 | Left inferior parietal lobule (BA 40) | 4.60 | <.001 | |
| 50 | −41 | 36 | 391 | Right inferior parietal lobule (BA 40) | 3.42 | <.001 | |
| 3-back – 0-back | |||||||
| −49 | −2 | 33 | 1033 | Left precentral gyrus (BA 6) | 4.57 | <.001 | |
| −55 | −14 | 29 | 797 | Left precentral gyrus (BA 3) | 3.79 | <.001 | |
| 2-back – 0-back | |||||||
| 50 | −41 | 39 | 905 | Right inferior parietal lobule (BA 40) | 3.83 | <.001 | |
| −49 | −35 | 30 | 335 | Left inferior parietal lobule (BA 40) | 4.25 | <.001 | |
| −52 | −11 | 30 | 432 | Left precentral gyrus (BA 4) | 4.01 | <.001 | |
To probe this interaction and further understand how the drug effects changed as the working memory load increased, we examined drug differences at each of the working memory load condition minus the 0-back match condition. First, for the 3-back minus 0-back comparison, greater activation was seen for the bromocriptine minus haloperidol comparison in regions similar to the activation observed for the overall interaction described above (Table 2). Specifically, increased activation was seen in the left precentral gyrus (BA 6 and 3). Second, similar regions also showed increased activation for bromocriptine minus haloperidol comparison on the 2-back minus 0-back comparison in the left precentral gyrus (BA 4) and the left and right inferior parietal lobules (BA 40). Finally, for 1-back compared to the 0-back condition no activation differences were across challenge conditions.
To examine the drug effect, we compared bromocriptine to placebo and haloperidol to placebo separately. The results showed increased activation for the bromocriptine minus placebo condition that were similar to the whole model in the left precentral gyrus and bilateral inferior parietal lobes. The haloperidol compared to placebo showed no differences at the alpha level used.
Working Memory Performance
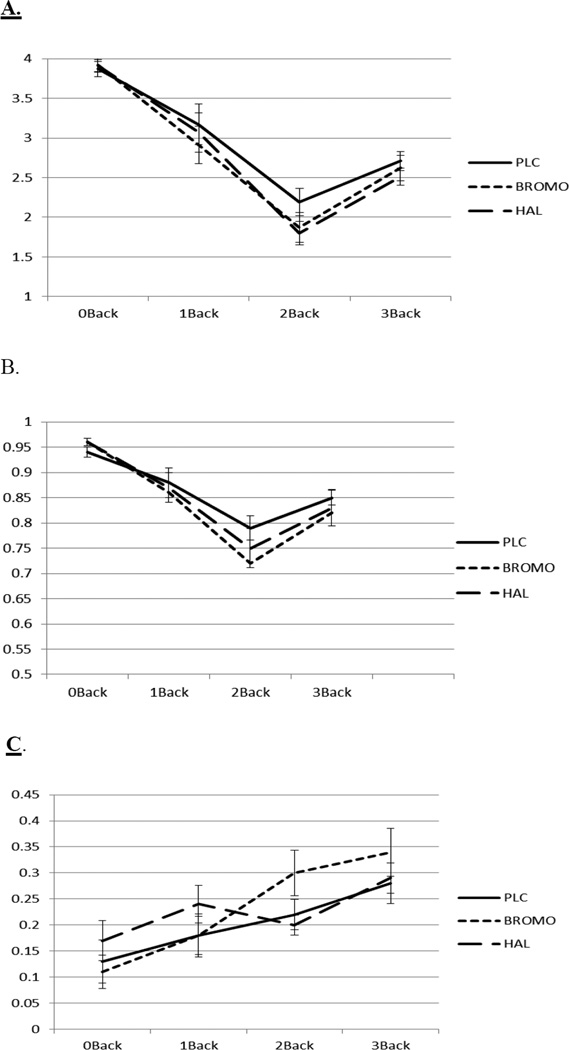
Data were analyzed with a 3 (Drug: BROMO, HAL, PLC) × 4 (Working Memory Load: 0-, 1-, 2-, 3-back) mixed model ANOVA for d’, proportion correct, and C (Figures 4a, 4b, 4c). Challenge drug and working memory load were within-subjects factors.
Figure 4.
Sensitivity (d’ Figure 4a), proportion correct (Figure 4b), and bias (C, Figure 4c) with standard errors on the 0-, 1-, 2-, and 3-back conditions on the bromocriptine, haloperidol, and placebo challenge days.
The analysis of d’ showed a main effect of working memory load (F(3,48)=478.62, p<.001). Performance was best on the 0-back and worst on the 2-back condition. There was no main effect or interaction involving challenge drug (ps > .36). The data pattern for the percent correct measure was similar with a main effect of working memory load (F(3,48)=48.08, p<.001).
For the bias measure C there was also a main effect of working memory load (F(3,48)=14.00, p<.001) that showed that as the working memory load increases participants became more conservative with their responding. There was no main effect or interaction involving drugs for the bias measure C.
Behavioral Measures
At the beginning of each study day, participants completed the POMS, BDI, and BAI questionnaires. There were no differences on mood ratings on these measures before each of the study days began (ps>.14). Mood and physical symptoms were assessed after the MRI when participants returned to the CRC to examine the effects of the challenge drugs on mood and physical symptoms. No differences were found between the bromocriptine, haloperidol, and placebo study days on any of these measures.
Vital Signs and Hormone Values
Blood pressure, pulse, and pupil diameter were monitored at six time points throughout the challenge day. Analyses were conducted on the maximum change score from the baseline measurement for each variable. Overall, there were no main effects or interactions involving bromocriptine or haloperidol challenges over time on any of the vital signs measures.
Blood samples were obtained for hormone assays at the beginning of each study day before any other study procedures. As expected, we found no differences in E1, E2, or T values across the three drug challenge days.
Discussion
The current study was the first to examine the working memory-related functional brain circuitry affected by direct dopaminergic stimulation and blockade in postmenopausal women. The results showed that the D2 agonist bromocriptine increased brain activation primarily in posterior regions of the working memory network compared to the antagonist haloperidol. In addition, the post hoc analysis of the two medications separately compared to placebo showed that the increased activation appeared to be driven by bromocriptine rather than haloperidol. However, neither bromocriptine nor haloperidol affected working memory performance. These findings highlight that the dopaminergic system is responsive to manipulations in healthy postmenopausal women and emphasize the need for further studies to examine how these brain activation effects may influence cognition and behavior.
We hypothesized that dopaminergic stimulation would increase frontal lobe activation and improve performance in postmenopausal women. The data showed that activation was increased after bromocriptine compared to haloperidol in posterior regions of the working memory network, but not in the frontal regions as predicted. In addition, there were no effects of either medication on performance. Prior studies using bromocriptine to examine N-back activation during fMRI found decreased frontal activation and improved performance, but the participants were younger and the samples were mixed with regard to sex (e.g.28,48). Thus, perhaps sex and menopausal status affect the BOLD signal after dopaminergic manipulations and these effects are observed in more posterior working memory regions. fMRI studies of working memory in postmenopausal women during estrogen treatment (for a review see26) have also shown increased BOLD activation. Studies examining estrogen compared to placebo treatment in postmenopausal women found increased frontal activation and no effect on performance during working memory tasks27,49,50. While the prior studies of estrogen treatment and fMRI differ with regard to design, hormone treatments, and neuropsychological tests it appears that an increase in the BOLD signal measured during fMRI is common across estrogen treatment studies26. Our dopaminergic manipulation also showed an increase in BOLD signal during the stimulation compared to the blockade condition although it was in parietal and posterior frontal regions. This pattern of results leads to the hypothesis that dopaminergic stimulation may have similar effects on brain functioning as estrogen treatment in postmenopausal women.
Epperson and colleagues24,51 have used a dopamine stimulation method to examine effects on subjective and objective cognitive performance in peri- and postmenopausal women in two studies. They found that atomoxetine treatment for six weeks compared to placebo improved subjective reports of memory and attention but had no effect on objective performance24. They also found lisdexamphetamine for four weeks compared to placebo improved subjective cognition as well as delayed recall in postmenopausal with menopause related subjective cognitive decline. Thus, studies are beginning to examine methods other than hormonal treatment after menopause to affect brain functioning and methods that affect the dopaminergic system may be useful in this endeavor. However, further work is needed to examine the relationship of the increased BOLD signal found in the current study as well as improved subjective cognition found in Epperson et al.24 to objective cognitive performance in postmenopausal women.
There are some caveats about the current study that should be considered when interpreting these data. First, we did not find any effect of bromocriptine or haloperidol on working memory performance. Prior studies have also found minimal effects of the 1.25 mg dose of bromocriptine on cognitive performance but similarly observed effects on brain activation (e.g.28,48,52). In addition, we chose a low dose of haloperidol in our healthy participants so as to not produce excessive side effects and we may not have observed any effects of haloperidol on its own as a result. In addition, haloperidol at higher doses has a less specific pharmacologic profile. It has been advised that modest doses are used in pharmacological imaging to avoid confounds of task specific effects of drugs with secondary influences of altered arousal or other systemic effects53. Our examination of vital signs, mood, and behavioral measures indicated that our fMRI findings were not affected by these variables. However, a larger dose of the medications may reveal bromocriptine and/or haloperidol effects on working memory performance.
Additionally, our sample size was small and thus affected our ability to use the most conservative correction for multiple comparisons in the imaging analysis. We were not able to correct at a FWE level for the whole brain analysis. We believe the whole brain analysis was necessary to examine the influence of direct dopaminergic modulation effects on working memory-related brain networks in postmenopausal women which had not yet been examined. We did use a cluster level correction in this initial study of dopaminergic modulation of working memory in postmenopausal women. Thus, we take these data patterns to be suggestive of the functioning of the dopaminergic system in postmenopausal women and the relationship between dopaminergic functioning and cognitive performance warrants further study with larger samples.
Conclusion
Overall, these data showed that a dopaminergic agonist increased posterior activation during a working memory task in healthy postmenopausal women compared to a dopaminergic antagonist. However, performance was not affected by the medication challenges. We propose that while the functioning of the dopaminergic system is influenced by circulating estrogen before menopause, dopaminergic system functioning was still modifiable after menopause in our sample. The structural19,20 as well as functional54 changes in the dopaminergic system continue into old age. However, in early postmenopause the dopaminergic system appears to continue to respond to pharmacologic manipulations. Further studies are needed to determine whether and what kind of dopaminergic manipulation may benefit cognition in healthy postmenopausal aging.
Acknowledgments
This work was supported by NIA K01 AG 030380, NCRR-00109, DoE SC 0001753. The authors wish to thank the research nursing staff of the University of Vermont CRC for their hard work and support of this study and our volunteers for their dedication to clinical research. We would also like to thank Jay Gonyea, Scott Hipko, Trevor Andrews, Ph.D., and Richard Watts, D.Phil. from the University of Vermont MRI Center for Biomedical Imaging for their help in MRI data acquisition.
Footnotes
Conflicts: The authors have no conflicts of interest to disclose.
References
- 1.Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72:1850–1857. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibbs RB. Estrogen Therapy and Cognition: A Review of the Cholinergic Hypothesis. Endocr Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuh JL, Wang SJ, Lu SR, Juang KD, Lee SJ. Alterations in cognitive function during the menopausal transition. J Am Geriatr Soc. 2003;51:431–432. doi: 10.1046/j.1532-5415.2003.51124.x. [DOI] [PubMed] [Google Scholar]
- 4.Greendale GA, Wight RG, Huang MH, Avis N, Gold EB, Joffe H, Seeman T, Vuge M, Karlamangla AS. Menopause-associated symptoms and cognitive performance: results from the study of women's health across the nation. Am J Epidemiol. 2010;171:1214–1224. doi: 10.1093/aje/kwq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab. 2013;98:3829–3838. doi: 10.1210/jc.2013-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson VW, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Estrogen exposures and memory at midlife: A population-based study of women. Neurology. 2003;60:1369–1371. doi: 10.1212/01.wnl.0000059413.75888.be. [DOI] [PubMed] [Google Scholar]
- 7.Kok HS, Kuh D, Cooper R, van der Schouw YT, Grobbee DE, Wadsworth ME, Richards M. Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause. 2006;13:19–27. doi: 10.1097/01.gme.0000196592.36711.a0. [DOI] [PubMed] [Google Scholar]
- 8.Luetters C, Huang MH, Seeman T, Buckwalter G, Meyer PM, Avis NE, Sternfeld B, Johnston JM, Greendale GA. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the study of women's health across the nation (SWAN) J Womens Health (Larchmt) 2007;16:331–344. doi: 10.1089/jwh.2006.0057. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell ES, Woods NF. Cognitive symptoms during the menopausal transition and early postmenopause. Climacteric. 2001;14:252–261. doi: 10.3109/13697137.2010.516848. [DOI] [PubMed] [Google Scholar]
- 10.Hara Y, Waters EM, McEwen BS, Morrison JH. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiological reviews. 2015;95:785–807. doi: 10.1152/physrev.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kritzer M, Kohama S. Ovarian hormones differentially influence immunoreactivity for dopamine beta-hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkey. Journal of Comparative Neurology. 1999;409:438–451. doi: 10.1002/(sici)1096-9861(19990705)409:3<438::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Dumas JA, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estrogen interacts with the cholinergic system to affect the verbal memory in postmenopausal women: evidence for the critical period hypothesis. Hormones and Behavior. 2008;53:159–169. doi: 10.1016/j.yhbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen's effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35:847–865. doi: 10.1002/hbm.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baddeley AD. Working Memory. Oxford: Clarendon Press; 1986. [Google Scholar]
- 15.Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Hormones & Behavior. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- 16.Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen's affect on cognition. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- 17.Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosceince and Biobehavioral Reviews. 2002;26:809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 19.Wong DF, Wagner HN, Jr, Dannals RF, Links JM, Frost JJ, Ravert HT, Wilson AA, Rosenbaum AE, Gjedde A, Douglass KH, et al. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science. 1984;226:1393–1396. doi: 10.1126/science.6334363. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, Gur RC, Wang G, Fowler JS, Moberg PJ, Ding Y, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- 21.Kaasinen V, Nagren K, Hietala J, Farde L, Rinne JO. Sex differences in extrastriatal dopamine d(2)-like receptors in the human brain. Am J Psychiatry. 2001;158:308–311. doi: 10.1176/appi.ajp.158.2.308. [DOI] [PubMed] [Google Scholar]
- 22.Craig MC, Cutter WJ, Wickham H, van Amelsvoort TA, Rymer J, Whitehead M, Murphy DG. Effect of long-term estrogen therapy on dopaminergic responsivity in post-menopausal women--a preliminary study. Psychoneuroendocrinology. 2004;29:1309–1316. doi: 10.1016/j.psyneuen.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Gardiner SA, Morrison MF, Mozley PD, Mozley LH, Brensinger C, Bilker W, Newberg A, Battistini M. Pilot Study on the Effect of Estrogen Replacement Therapy on Brain Dopamine Transporter Availability on Healthy, Postmenopausal Women. American Journal of Geriatric Psychiatry. 2004;12:621–630. doi: 10.1176/appi.ajgp.12.6.621. [DOI] [PubMed] [Google Scholar]
- 24.Epperson CN, Pittman B, Czarkowski KA, Bradley J, Quinlan DM, Brown TE. Impact of atomoxetine on subjective attention and memory difficulties in perimenopausal and postmenopausal women. Menopause. 2011;18:542–548. doi: 10.1097/gme.0b013e3181fcafd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;6625:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 26.Comasco E, Frokjaer VG, Sundstrom-Poromaa I. Functional and molecular neuroimaging of menopause and hormone replacement therapy. Front Neurosci. 2014;8:388. doi: 10.3389/fnins.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibbs SE, D'Esposito M. A functional MRI study of the effects of bromocriptine, a dopamine receptor agonist, on component processes of working memory. Psychopharmacology (Berl) 2005;180:644–653. doi: 10.1007/s00213-005-0077-5. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs E, D'Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women's health. J Neurosci. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Estradiol treatment altered anticholinergic-related brain activity in postmenopausal women. Neuroimage. 2012;60:1394–1403. doi: 10.1016/j.neuroimage.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Reisberg B, Ferris SH. Brief cognitive rating scale (BCRS) Psychopharmacol Bull. 1988;24:629–635. [PubMed] [Google Scholar]
- 33.Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2. Lutz, FL: Psychological Assessment Resources Inc.; 2001. [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JBW. In: Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. SCID-I/P, editor. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 35.Beck AT, Steer RA, Brown GT. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 36.Luciana M, Collins PF, Depue RA. Opposing roles for dopamine and serotonin in the modulation of human spatial working memory functions. Cereb Cortex. 1998;8:218–226. doi: 10.1093/cercor/8.3.218. [DOI] [PubMed] [Google Scholar]
- 37.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dumas JA, Kutz AM, McDonald BC, Naylor MR, Pfaff AC, Saykin AJ, Newhouse PA. Increased working memory-related brain activity in middle-aged women with cognitive complaints. Neurobiology of Aging. 2013;34:1145–1147. doi: 10.1016/j.neurobiolaging.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNair DM, Lorr M, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 40.Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- 41.Hoddes ElZ V, Smythe H, Phillips R, Dement W. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 42.Newhouse PA, Potter A, Corwin J, Lenox R. Age-related effects of the nicotinic antagonist mecamylamine on cognition and behavior. Neuropsychopharmacology. 1994;10:93–107. doi: 10.1038/npp.1994.11. [DOI] [PubMed] [Google Scholar]
- 43.Overall J, Gorham D. The brief psychiatric rating scale. Psychological Report. 1993;10:799–812. [Google Scholar]
- 44.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 45.Snodgrass J, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. Journal of Experimental Psychology. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 46.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A Parametric Study of Prefrontal Cortex Involvement in Human Working Memory. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 47.Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–607. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 48.Kimberg DY, Aguirre GK, Lease J, D'Esposito M. Cortical effects of bromocriptine, a D-2 dopamine receptor agonist, in human subjects, revealed by fMRI. Hum Brain Mapp. 2001;12:246–257. doi: 10.1002/1097-0193(200104)12:4<246::AID-HBM1019>3.0.CO;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE, Katz L, Shankweiler DP, Fletcher JM, Lacadie C, Keltz M, Gore JC. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- 50.Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- 51.Epperson CN, Shanmugan S, Kim DR, Mathews S, Czarkowski KA, Bradley J, Appleby DH, Iannelli C, Sammel MD, Brown TE. New onset executive function difficulties at menopause: a possible role for lisdexamfetamine. Psychopharmacology (Berl) 2015;232:3091–3100. doi: 10.1007/s00213-015-3953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morcom AM, Bullmore ET, Huppert FA, Lennox B, Praseedom A, Linnington H, Fletcher PC. Memory encoding and dopamine in the aging brain: a psychopharmacological neuroimaging study. Cereb Cortex. 2010;20:743–757. doi: 10.1093/cercor/bhp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honey G, Bullmore E. Human pharmacological MRI. Trends in Pharmacological Sciences. 2004;25:366–374. doi: 10.1016/j.tips.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Backman L, Nyberg L, Lindenberger U, Li S, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neuroscience and Biobehavioral Reviews. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]