Summary
Purpose
Clinicians commonly teach patients alternative clearing behaviors to reduce coughing and hard throat clearing with the assumption that these behaviors clear mucus from the vocal folds. Yet there is limited evidence of the effectiveness of these alternative behaviors at clearing mucus. This study’s purpose was to evaluate the efficacy of reducing laryngeal mucus aggregation using alternative approaches in comparison with hard coughing and hard throat clearing in people with and without voice disorders.
Method
Mucus aggregation of 46 participants, 22 with and 24 without voice disorders, was evaluated from stroboscopy recordings taken before and after each of six clearing behaviors: hard coughing, hard throat clearing, silent coughing, soft throat clearing, dry swallowing, and swallowing with a fluid bolus. Each participant performed each clearing behavior twice. Two trained raters evaluated mucus aggregation for type, thickness, and pooling.
Results
Of the six clearing behaviors studied, only hard throat clearing changed vocal fold mucus aggregation. The features of mucus aggregation that were changed by hard throat clearing were the severity of mucus thickness and the presence of type 3 mucus.
Conclusions
Despite the widespread clinical use of alternative clearing behaviors, the results of this study indicate that hard throat clearing is the only clearing behavior to have a significant impact on removing mucus aggregation from the vocal folds. This finding should be further investigated in a larger scale study. If the results of this study are replicated, clinicians should consider changing their use and description of alternative clearing behaviors in clinical practice.
Keywords: voice, larynx, mucus, throat clear, cough
INTRODUCTION
A thin layer of laryngeal mucus is considered necessary to maintain healthy vocal fold tissue.1 This thin, clear mucus is in contrast to mucus aggregation commonly seen in patients with voice disorders, which is typically opaque, thicker, and more abundant.2 Mucus aggregation can occur as a protective reaction and is believed to be part of the healing process. This increased laryngeal mucus can cause patients to cough and/or clear their throat to clear the mucus. It is believed that this can progress into a harmful cycle of habitual coughing and throat clearing that can cause vocal fold edema through tissue shearing, friction, and contact forces, and perpetuate or cause a voice disorder.
Patients with voice disorders frequently complain about laryngeal mucus and associated chronic, habitual coughing and throat clearing during their evaluation. More than 4 million patients with voice disorders present with habitual coughing and throat clearing.3–6 Mucus aggregation in these patients is often visible during laryngeal endoscopy with or without stroboscopy. Patients with voice disorders have been found to have larger amounts of mucus that is thicker in comparison with those of healthy controls.2 Speech-language pathologists (SLPs) and laryngologists attempt to reduce laryngeal mucus complaints by advocating for increased hydration and discussing the importance of extinguishing the habitual clearing behaviors. Often alternative behaviors, believed to be less harmful, are promoted to provide the patient with a replacement for the behaviors believed to be harmful.
Silent coughing, soft throat clearing, dry swallowing, and swallowing a fluid bolus of water are the four most common alternative clearing behaviors used. Despite their common use in the voice clinic, there is no evidence of their ability to clear mucus from the vocal folds. The purpose of this study was to evaluate the efficacy of reducing laryngeal mucus aggregation by these alternative approaches in comparison with hard coughing and hard throat clearing in people with and without voice disorders.
METHOD
Participants
Forty-six people, 22 with and 24 without voice disorders, participated in this study. There were 15 women without voice disorders, 9 men without voice disorders, 13 women with voice disorders, and 9 men with voice disorders who participated. The average age for the participants were 40.5 years for vocally normal women, 37.9 years for vocally normal men, 40.3 years for women with voice disorders, and 40.7 years for men with voice disorders. The age ranges were 27–59 years for vocally normal women, 30–59 years for vocally normal men, 24–60 years for women with a voice disorder, and 27–59 years for men with a voice disorder. People were categorized as being with or without a voice disorder based on voice quality of life survey,7 perceptual judgment of voice quality, participant interview, self-categorization, and endoscopy with stroboscopy completed by an SLP who specializes in voice disorders. The diagnoses of people with voice disorders and their voice-related quality of life scores are displayed in Table 1. Participation in the study was accepted through an informed consent form. The clinical data for this study were collected at the Charlotte Eye Ear Nose and Throat Associates specialized voice center in Charlotte, North Carolina. The SLPs involved with data collection were specifically trained in voice. The data collection, storage, and use were in accordance with human subjects regulations.
TABLE 1.
Type of Voice Disorder, V-RQOL Score, and Number of Participants With Each Disorder for All Persons Included in This Study
| Patient ID | V-RQOL Score | Type of Disorder |
|---|---|---|
| 1 | 16 | Right polyp, left reactive nodule, left bowing |
| 2 | 24 | Muscle tension dysphonia, left bowing |
| 3 | 29 | Left polyp, right reactive nodule, bilateral generalized edema |
| 4 | 25 | Left recurrent paralysis, bilateral generalized edema |
| 5 | 13 | Bilateral muscle tension dysphonia, erythema, and edema |
| 6 | 24 | Nodules, erythema, prominent vascularization |
| 7 | 15 | Left bowing, laryngopharyngeal reflux |
| 8 | 15 | Glottal insufficiency, left bowing |
| 9 | 36 | Bilateral diffuse edema and varices, anterior glottal gap |
| 10 | 30 | Bilateral edema medially |
| 11 | 23 | Bilateral polypoid degeneration |
| 12 | 28 | Right bowing, mild tremor, intermittent medial glottal gap |
| 13 | 14 | Left pseudosulcus, mild glottal insufficiency |
| 14 | 10* | Bilateral edema, erythema, and muscle tension dysphonia, pseudosulcus |
| 15 | 19 | Right cyst, left reactive nodule, bilateral prominent vascularization, anterior glottal gap |
| 16 | 15 | Mild bilateral edema and erythema |
| 17 | 12 | Left hemorrhage, cyst with a polyp underneath, bilateral glottal gap |
| 18 | 23 | Postop nodule removal, bilateral irregular leading edges, glottal gap, adynamic segments |
| 19 | 18 | Postop right polyp removal, anterior erythema |
| 20 | 45 | Bilateral polypoid lesions |
| 21 | 12 | Pedunculated ventricular cyst extending to impede vocal fold contact |
| 22 | 11 | Bilateral bowing, anterior gap, muscle tension dysphonia |
Patient’s complaints focused on singing voice.
Abbreviation: V-RQOL, voice-related quality of life.
Instrumentation and procedures
We used a digital Rhino-Laryngeal Stroboscopic System Model 9100B (KayPentax, Lincoln Park, NJ, USA) with a 70-degree rigid endoscope (KayPentax, Model 9106) to visualize the vocal folds. We used stroboscopy, instead of continuous light endoscopy, as it has been shown to be superior for the visualization of mucus.8 Two sustained phonations of/i/for each participant for each clearing task were recorded. One recording was conducted before the task and the other immediately after. Participants were instructed to phonate at habitual pitch and loudness levels. The duration of the sample was dependent on the ability of the participant to sustain phonation.
Participants underwent a series of 12 clearing behavior trials with each behavior assessed twice. The clearing behaviors were elicited in a standardized order with the first behavior counterbalanced, using the Latin square approach, across groups (people with and without voice disorders). This allowed for all of the tasks to be elicited in the first position. After the first tasks, tasks were elicited in the a priori determined standardized order: hard coughing, hard throat clearing, silent coughing, soft throat clearing, swallowing, and swallowing with a fluid bolus of water. This allowed for all tasks to be elicited in all positions. This standardized order was chosen to prevent differences in participant response due to an anticipated position effect. That is, this method allows for the evaluation of all participants having undergone the same clearing task before the one elicited so that there is not a differing response across participants due to the prior task. The same clinician provided verbal instructions and an example of each task for all of the participants. For example, the clinician described a silent cough and then modeled one. There was no required training for the participant. However, if the participant did not accurately produce the instructed task, that trial was discarded and the task was elicited again until the participant accurately produced the correct task. Instruction for the swallowing with a fluid bolus of water was “take a sip of water from the water bottle.” A self-selected bolus size was used and, therefore, the amount of water was not standardized across participants.
Mucus ratings
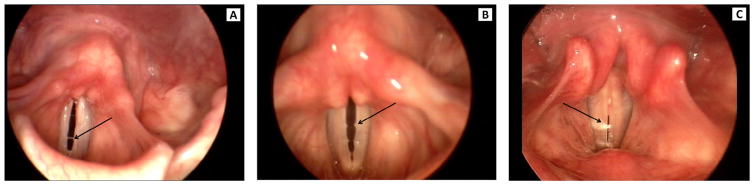
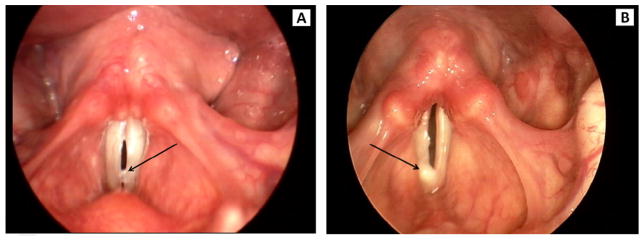
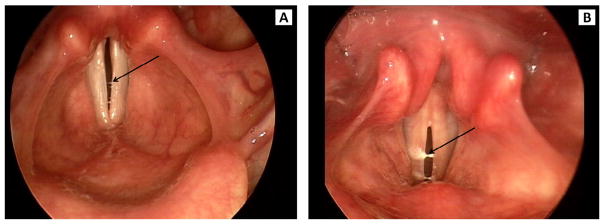
Visual judgments from stroboscopy of mucus aggregation type, pooling, and thickness were made using Alvin software.10 Mucus aggregation was classified, as in Hsiao, Liu, and Lin,10 into three types (Figure 1). Type 1 was defined by a rough surface of the vocal fold and by mucus threads between the vocal folds noted during open phase. Type 2 was defined as mucus bubbles visible on phonation and resembling vocal fold nodules. Type 3 was defined as mucus lumps visible either before or during phonation. Mucus pooling and thickness were classified as not apparent, mild, or severe (Figures 2 and 3, respectively). Two raters independently scored the three features of mucus aggregation. Rater reliability was assessed as percent exact agreement. Inter-rater reliability was 92%. Intra-rater reliability was 91% for rater 1 and 88% for rater 2. Discrepancies between raters were resolved by the raters reviewing the scores and stroboscopic recordings together and reaching a consensus score.
FIGURE 1.
Examples of type 1 (A), type 2 (B), and type 3 (C) mucus aggregation from stroboscopy.
FIGURE 2.
Examples of mild pooling (A) and severe pooling (B) of mucus from stroboscopy.
FIGURE 3.
Examples of mildly thick (A) and severely thick (B) mucus aggregation from stroboscopy.
Statistical analysis
Generalized linear mixed models (GLMMs) were used to compare changes in study outcomes (presence of mucus, severe pooling, and severe thickness) among the various trial types. GLMMs are ideal for modeling measurements obtained on repeated occasions within subjects.7 Comparing changes, and not absolute values, further reduced the possibility of an order effect influencing the results. Separate models were constructed for each study outcome of interest, which served as the dependent variables in the models. The models assumed a compound symmetry covariance structure, and all models included covariate adjustment for diagnosis group (people with and without voice disorders) and repetition order (first or second). P values were obtained from the models, and P values <0.05 were considered statistically significant. All analyses were conducted using SAS v9.4 (Cary, NC, USA).
RESULTS
Table 2 lists the occurrence frequency of each mucus type before and after each clearing behavior. Although each subject performed each trial type twice, results are presented as averages across repetitions. Mucus was present before clearing in 99.26% of trials. Results of the GLMMs indicated that in general, pre/post changes in the presence of type 1 and type 2 mucus were minimal, and none were statistically significant. The presence of type 3 mucus declined as a result of several different clearing behaviors; however, only hard throat clearing resulted in a decline in type 3 mucus that was statistically significant (from 68.0% down to 28.7%, P < 0.05).
TABLE 2.
Frequency of Each Mucus Type Before and After the Clearing Behavior Among Study Subjects (n = 46)
| Trial Type | Mucus Type 1 | Difference | |
|---|---|---|---|
|
| |||
| Pretrial (% Present) | Posttrial (% Present) | ||
| Hard coughing | 100.0% | 96.7% | −3.3% |
| Hard throat clearing | 98.9% | 98.9% | 0.0% |
| Silent coughing | 100.0% | 98.9% | −1.1% |
| Soft throat clearing | 97.8% | 98.9% | 1.1% |
| Swallowing | 100.0% | 98.9% | −1.1% |
| Swallowing with bolus | 98.9% | 100.0% | 1.1% |
|
| |||
| Mucus Type 2 | Difference | ||
|
| |||
| Pretrial (% Present) | Posttrial (% Present) | ||
|
| |||
| Hard coughing | 93.6% | 94.7% | 1.1% |
| Hard throat clearing | 94.7% | 89.4% | −5.3% |
| Silent coughing | 94.8% | 93.7% | −1.1% |
| Soft throat clearing | 96.8% | 97.9% | 1.1% |
| Swallowing | 94.7% | 95.8% | 1.1% |
| Swallowing with bolus | 90.4% | 92.6% | 2.2% |
|
| |||
| Mucus Type 3 | Difference | ||
|
| |||
| Pretrial (% Present) | Posttrial (% Present) | ||
|
| |||
| Hard coughing | 52.4% | 44.1% | −8.2% |
| Hard throat clearing | 68.0% | 28.7% | −39.2%*** |
| Silent coughing | 64.8% | 61.4% | −3.4% |
| Soft throat clearing | 51.2% | 45.4% | −5.9% |
| Swallowing | 61.0% | 64.5% | 3.5% |
| Swallowing with bolus | 52.2% | 66.2% | 14.1% |
P < 0.05.
Tables 3 and 4 list the frequency of severe pooling and thickness before and after the clearing behaviors. Results of the GLMMs indicated that although the presence of severe pooling declined after some trials, none of the declines in the frequency of severe pooling were statistically significant. The presence of severe thickness declined after some trials, and again only hard throat clearing resulted in a decline that was statistically significant (from 88.0% down to 59.0%, P < 0.05). Because we included diagnosis group and repetition order into the models as covariates, we were also able to assess the association between these variables and the mucus aggregation measures. Neither repetition order of the clearing tasks nor diagnosis group normal/pathological voice status had a statistically significant effect on any of the mucus aggregation measures.
TABLE 3.
Frequency of Severe Pooling Before and After the Clearing Behaviors Among Study Subjects (n = 46)
| Trial Type | Pooling | Difference | |
|---|---|---|---|
|
| |||
| Pretrial (% Severe) | Posttrial (% Severe) | ||
| Hard coughing | 73.9% | 71.6% | −2.3% |
| Hard throat clearing | 76.6% | 66.6% | −10.1% |
| Silent coughing | 79.0% | 71.3% | −7.7% |
| Soft throat clearing | 74.0% | 63.7% | −10.3% |
| Swallowing | 66.5% | 77.7% | 11.2% |
| Swallowing with bolus | 72.6% | 79.3% | 6.8% |
TABLE 4.
Frequency of Severe Thickness Before and After the Clearing Behaviors Among Study Subjects (n = 46)
| Thickness | Difference | ||
|---|---|---|---|
|
| |||
| Pretrial (% Severe) | Posttrial (% Severe) | ||
| Hard coughing | 82.2% | 77.8% | −4.5% |
| Hard throat clearing | 88.0% | 59.0% | −28.9%*** |
| Silent coughing | 84.9% | 82.8% | −2.1% |
| Soft throat clearing | 79.1% | 72.3% | −6.8% |
| Swallowing | 82.6% | 86.9% | 4.3% |
| Swallowing with bolus | 85.4% | 79.9% | −5.5% |
P < 0.05.
DISCUSSION
SLPs and ENTs who treat patients with voice disorders commonly describe the harmful effects of coughing and clearing and advocate for less harmful clearing behaviors including soft throat clearing, silent coughing, dry swallowing, and swallowing a fluid bolus. Although these behaviors are clearly less harmful from a biomechanical perspective, the effectiveness of these clearing behaviors had not been tested. The results of this study suggest that although these behaviors are “less harmful” to vocal fold tissue, they are not effective at removing mucus aggregation. This may be an important finding because these alternative clearing behaviors are commonly used in current clinical practice. However, given the relatively small sample size, it is premature to change clinical practice at this point. Instead, a larger scale study is needed to verify the results and provide substantial evidence to motivate a change in clinical practice.
Interestingly, the results of the study indicate that hard throat clearing is more effective at clearing laryngeal mucus aggregation than coughing. Coughing and throat clearing are both innate clearing behaviors and have been presumed to both be effective at clearing mucus aggregation from the vocal folds. Coughing is typically thought to be more effective and more harmful to the vocal folds than throat clearing. Coughing is often described to patients with voice disorders as their vocal folds slamming together, like hands clapping vigorously. Given the stronger action of coughing, it makes sense to hypothesize that coughing is more effective than hard throat clearing at removing mucus aggregation; however, the results of this study suggest the opposite. It is likely, although not formally investigated in this study, that the reason that coughing was not found to be effective at clearing mucus aggregation is that coughing has a larger respiratory component than hard throat clearing and, thus, may have caused new mucus to aggregate on the vocal folds. This may be a positive finding for clinicians treating patients with voice disorders, if indeed habitual coughing is more deleterious than hard throat clearing, because it provides evidence to support the clinical practice of inhibiting coughing. If the results of this study are replicated, it would provide additional evidence for supporting clinical practice patterns that promote the concept that coughing is not helpful at removing mucus aggregation.
In a prior study, hard throat clearing was found to be the most effective clearing technique for removing laryngeal sensation in persons without voice disorders and the second most effective, after swallowing with a fluid bolus, in persons with voice disorders.12 This indicates that patient report of laryngeal sensation after clearing may accurately reflect laryngeal mucus aggregation. The present study investigating the actual clearing of laryngeal mucus did not find a difference between persons with and persons without voice disorders. This may be a reflection of the relatively small sample size or the counseling that patients with voice disorders in the laryngeal sensation study received on the beneficial effects of swallowing a fluid bolus compared with the harmful effects of hard throat clearing. Future studies should investigate the correspondence between laryngeal mucus aggregation and patient report of laryngeal sensation due to mucus to determine the accuracy of self-reported laryngeal mucus.
Limitations
There are three main limitations of this study. First, the study asked the participants to perform and raters to judge unfamiliar and somewhat odd tasks. This is especially true of the vocally normal participants who are novices at silent coughs. This is also true of raters who are not used to judging types of mucus. Although all tasks were verified for accuracy and the raters had high reliability, this is an emerging type of investigation, and more elegant methods to investigate the topic may be available in the future. Second, this study had a relatively small sample size, which precludes generalizations about the population at large. Third, although vocally normal participants were screened for voice disorders, the main criterion for inclusion in the study was self-associating with either a voice disordered or a non–voice disordered group. In the absence of visible laryngeal pathology and a perceptible auditory voice disorder, people who self-identified as vocally normal were considered vocally normal in this study. Future studies should consider a thorough medical work-up of people self-identifying as vocally normal.
CONCLUSIONS
Despite the widespread clinical use of alternative clearing behaviors, the results of this study indicate that only hard throat clearing has a significant impact on removing mucus aggregation from the vocal folds. This finding should be further investigated in a larger scale study. If the results of this study are replicated, clinicians should consider modifying their use of alternative clearing behaviors in clinical practice.
Acknowledgments
This project was supported by an R03 grant (R03 DC00843) funded by the National Center for Deafness and Other Communication Disorders at the National Institutes of Health. This publication was supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCRR Grant number UL1 TR000062.
References
- 1.Levendoski EE, Leydon C, Thibeault SL. Vocal fold epithelial barrier in health and injury: a research review. J Speech Lang Hear Res. 2014;57:1679–1691. doi: 10.1044/2014_JSLHR-S-13-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonilha HS, White L, Kuckhahn K, et al. Mucus aggregation in persons with voice disorders. J Commun Disord. 2012;45:304–311. doi: 10.1016/j.jcomdis.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colton RH, Casper JK. Understanding Voice Problems: A Physiological Perspective for Diagnosis and Treatment. Baltimore, MD: Williams and Wilkins Press; 1996. [Google Scholar]
- 4.Boone DR, McFarlane SC, Von Berg SL. The Voice and Voice Therapy. 7. Boston, MA: Pearson Education Inc; 2005. [Google Scholar]
- 5.Brodnitz F. Vocal Rehabilitation. Rochester, MN: American Academy of Ophthalmology and Otolaryngology; 1971. [Google Scholar]
- 6.Stemple JC, Glaze LE, Klaben BG. Clinical Voice Pathology: Theory and Management. 3. San Diego, CA: Singular; 2000. [Google Scholar]
- 7.Hogikyan ND, Sethuraman G. Validation of an instrument to measure voice-related quality of life (V-RQOL) J Voice. 1999;13:557–569. doi: 10.1016/s0892-1997(99)80010-1. [DOI] [PubMed] [Google Scholar]
- 8.Bonilha H, Aikman A, Hines K, et al. Vocal fold mucus aggregation in vocally normal speakers. Logoped Phoniatr Vocol. 2008;33:136–142. doi: 10.1080/14015430701875588. [DOI] [PubMed] [Google Scholar]
- 9.Hillenbrand J, Gayvert R. Open source software for experiment design and control. J Speech Lang Hear Res. 2005;48:45–60. doi: 10.1044/1092-4388(2005/005). [DOI] [PubMed] [Google Scholar]
- 10.Hsiao T, Liu C, Lin K. Videostrobolaryngoscopy of mucus layer during vocal fold vibration in patients with laryngeal tension-fatigue syndrome. Ann Otol Rhinol Laryngol. 2002;111:537–541. doi: 10.1177/000348940211100610. [DOI] [PubMed] [Google Scholar]
- 11.McCulloch C, Searle SR. Generalized, Linear, and Mixed Models. NewYork: John Wiley & Sons, Inc; 2001. [Google Scholar]
- 12.Bonilha HS, Gerlach TT, Sutton LE, et al. Laryngeal sensation before and after clearing behaviors. J Voice. 2012;26:674. doi: 10.1016/j.jvoice.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]