Abstract
Miniature Schnauzer dogs are predisposed to idiopathic hypertriglyerceridemia, which increases risk for diseases such as pancreatitis and gallbladder mucocele. Recently, elevated triglyceride concentrations have been associated with proteinuria in this breed, although it is difficult to determine which abnormality is primary. Retrospective review of renal tissue from 27 proteinuric Miniature Schnauzers revealed that 20 dogs had ultrastructural evidence of osmophilic globules consistent with lipid in glomerular tufts. Seven of these dogs had lipid thromboemboli in glomerular capillary loops that distorted their shape and compressed circulating erythrocytes. Triglyceride concentrations were reported in 6 of these 7 dogs, and all were hypertriglyceridemic. In addition, glomerular lipidosis (defined as accumulation of foam cells within peripheral capillary loops) was identified in a single dog. The remaining 12 dogs had smaller amounts of lipid that could only be identified ultrastructurally. Neither signalment data nor clinicopathologic parameters (serum albumin, serum creatinine, urine protein-to-creatinine ratio, and blood pressure) differed among the various types of lipid lesions. During the time course of this study, all dogs diagnosed with glomerular lipid thromboemboli were Miniature Schnauzers, underscoring the importance of recognizing these clear spaces within capillary loops as lipid.
Keywords: dogs, glomerulonephropathy, hyperlipidemia, hypertriglyceridemia, proteinuria
Miniature Schnauzer dogs are predisposed to idiopathic hypertriglyceridemia. In this breed, elevated triglyceride levels have been associated with pancreatitis,23,26 gallbladder mucocele,11 insulin resistance,22 and elevated liver enzymes in serum.25 A recent study documented a strong association between hypertriglyceridemia and proteinuria in Miniature Schnauzers, but the temporal relationship between the 2 abnormalities could be not be determined.5
Hyperlipidemia is a well-established component of nephrotic syndrome in dogs and humans. Experimental data suggest that increases in cholesterol and triglycerides result from decreased lipoprotein lipase activity, impairment of lipoprotein lipase binding to very low-density lipoproteins, inhibition of lipid degradation pathways, and upregulation of lipid biosynthesis pathways.4,12,15,17,28 Importantly, the converse relationship also exists between these 2 conditions wherein lipids induce renal disease, a phenomenon known as lipid nephrotoxicity.6 Experimental data in multiple studies of hyperlipidemic rats demonstrated progressive glomerular injury.9,10,18 Focal segmental glomerulosclerosis (FSGS) is the typical lesion in these hyperlipidemic rat models, and lipid deposition in glomeruli is common. Hyperlipidemia and glomerular disease are also observed together in a rare genetic disorder in people, lipoprotein glomerulopathy.7,20 The hallmark of this disease in humans is aneurysmally dilated glomerular capillary loops due to the presence of “lipid thrombi.”14
The goal of this study was to characterize the glomerular lesions of Miniature Schnauzer dogs that have undergone renal biopsy for the clinical evaluation of proteinuric kidney disease. Given the frequency of hyperlipidemia in Miniature Schnauzer dogs and its recent link with proteinuria, we were especially interested in the prevalence and characteristics of glomerular lipid deposition in renal biopsy samples from this breed. During the course of this study, we observed an unusual lesion, glomerular lipid thromboemboli, which was only identified in Miniature Schnauzer dogs. The description of the histologic and ultrastructural characteristics of these lipid thromboemboli was a second goal of this study.
Materials and Methods
Case Selection
Retrospective evaluation of all samples from Miniature Schnauzer dogs submitted to the International Veterinary Renal Pathology Service (IVRPS) from January 1, 2007, to July 31, 2014, was performed. Inclusion criteria were presence of proteinuria (urine protein-to-creatinine ratio [UPC] ≥ 0.5) and adequate samples such that intact, nonsclerotic glomeruli were available for histologic and ultrastructural evaluation.
Specimen Evaluation
Specimens had been routinely processed, serially sectioned at 3 μm thickness, and stained with hematoxylin and eosin (HE), periodic acid–Schiff reagent (PAS), Masson’s trichrome (TRI), and Jones’s methenamine silver (JMS). Congo Red staining was performed on 8-μm-thick samples. Oil Red O method was performed on samples in which lipid was suspected and intact glomeruli were present in the frozen (unfixed/unprocessed) tissue. Transmission electron microscopic (TEM) specimens were processed as described previously.3 Briefly, glutaraldehyde-fixed samples were embedded, sectioned, and stained by routine methods, and samples were examined by pathologists at Texas Heart Institute, University of Georgia, or the Ohio State University. Immunofluorescence (IF) was performed on fresh tissue after embedding in Optimal Cutting Temperature (OCT) medium and freezing. Samples were sectioned at 5 μm thickness and stained with fluorescein isothiocyanate (FITC)–conjugated polyclonal goat anti–canine IgG, IgM, IgA, and C3 (Bethyl Labs, Montgomery, TX) and FITC-conjugated polyclonal rabbit anti–human C1q, λ light chain and κ light chains antibodies (Dako North America, Carpinteria, CA), as previously described.2 Samples were examined with an epifluorescence microscope using appropriate filters at the time of the original diagnosis, and glomeruli that had positive staining patterns were digitally photographed. All relevant case materials (clinicopathologic data, histopathology slides, TEM images, IF images if available, and associated scores) were reviewed.
Statistical Analysis
Descriptive statistics evaluated patient characteristics (age and sex) and renal lesions. Distribution of clinical data was visually assessed with Q-Q plots. Normally distributed data are reported as mean ± standard deviation. Serum creatinine concentration was not normally distributed and required logarithmic transformation for regression analysis; this variable is reported as median (range). Univariate analysis of variance (ANOVA) was performed to determine if UPC, serum creatinine concentration, serum albumin concentration, or systolic blood pressure differed by category of renal lesion. Categories of renal lesions included glomerular thromboemboli, other lipid deposits, or no lipid deposits; glomerular lipidosis was not included as a category in the analyses due to insufficient numbers (n = 1). A multivariate analysis of variance (MANOVA) was also performed for those individuals with complete data. The statistical analyses were performed using the R software for statistical computing (R Development Core Team, Vienna Austria), and a P value of <.05 was considered significant.
Results
Samples from Miniature Schnauzers represented 31 of 960 (3.2%) of all canine submissions to the IVRPS during the study period. One dog was biopsied twice within 1 month because the first sample did not obtain enough glomeruli for a complete evaluation; this dog was only counted once in the descriptions below. One case was excluded because the dog had nonproteinuric (UPC = 0.1) acute kidney injury (serum creatinine = 3.1 mg/dl). A second case was excluded because it was not evaluated comprehensively (TEM and IF were not performed), and a third case was excluded because it had a limited biopsy specimen such that a definitive diagnosis could not be rendered. This left 27 patients for evaluation. One sample was an autopsy submission, whereas the other cases were biopsies. Signalment data are presented in Table 1 and Supplemental Table S1, separated according to lesion type. Overall, there were 14 males and 13 females, and the average age was 6.7 years.
Table 1.
Patient Signalment and Laboratory Values for 27 Miniature Schnauzer Dogs With Glomerular Disease.a
| Lipid Lesion | Sex | Age, y | UPC | Creatinine, mg/dl | Albumin, g/dl | SBP, mm Hg |
|---|---|---|---|---|---|---|
| Glomerular lipid thromboemboli (n = 7) | 3M, 4F | 9.3 ± 2.5 | 7.7 ± 5.7 | 0.8 (0.6–2.9) | 3.1 ± 0.3 | 137 ± 8 |
| Glomerular lipidosis (n = 1) | 1M | 1.7 | 5.3 | 1.1 | 3.8 | 194 |
| Other lipid deposits (n = 12) | 6M, 6F | 6.8 ± 3.7 | 6.2 ± 3.3 | 2.0 (0.6–2.7) | 3.2 ± 0.6 | 150 ± 27 |
| No lipid deposits (n = 7) | 4M, 3F | 5.5 ± 2.8 | 8.7 ± 6.6 | 1.1 (0.6–1.6) | 2.6 ± 0.9 | 148 ± 24 |
SBP, systolic blood pressure; UPC, urine protein-to-creatinine ratio.
Dogs are grouped based on type of lipid lesion observed in the biopsy evaluation. Mean ± standard deviation or median (range) is reported, depending on whether the data followed a normal distribution.
None of the laboratory variables differed by category of renal lipid lesion in univariate or multivariate analyses (ANOVA and MANOVA p>0.05).
Pathology Findings
Six dogs had definitive evidence of immune complex–mediated glomerulonephritis (ICGN) with or without secondary sclerosis. One additional case had positive IF staining without supporting ultrastructural evidence of electron-dense deposits. The remaining 20 cases were non–immune complex–mediated glomerulonephropathies. Two of the 7 cases with confirmed or suspected immune complex–mediated disease and 18 of the 20 non–immune complex–mediated cases had ultrastructural and occasionally histologic evidence of lipid accumulation within the glomerular tufts. The remaining 7 dogs did not have lipid observed histologically or ultrastructurally, of which 5 were diagnosed with ICGN.
The lipid-associated lesions were divided into categories as follows: glomerular lipid thromboemboli, glomerular lipidosis, and lipid material within the glomerular basement membranes (GBMs) or mesangium.
Glomerular Lipid Thromboemboli
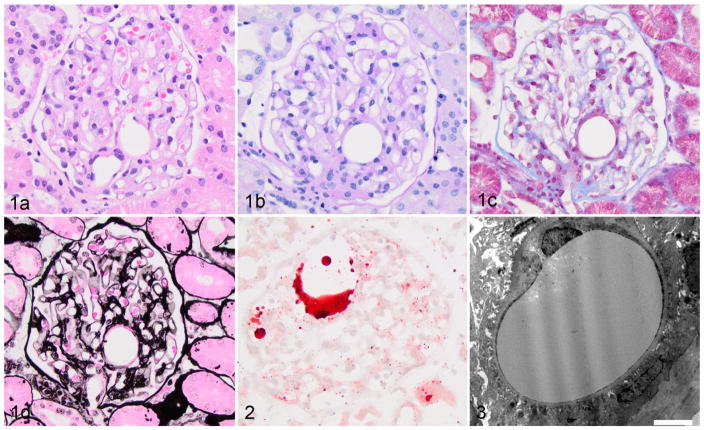
Seven cases had nonstaining circular clear spaces within capillary lumena, which often resulted in significant distortion in the glomerular architecture (Figs. 1A–D), consistent with lipid thromboemboli. Some of these thromboemboli were up to 50 μm in diameter. Glomerular lipid thromboemboli were present in 4% to 91% (median, 26%) of sampled glomeruli in these 7 cases. The presence of lipid was confirmed by Oil Red O staining in most cases (Fig. 2). Notably, this lesion was not observed in any breed other than Miniature Schnauzers during the study period. Ultrastructural evaluation revealed moderately osmophilic material within capillary lumena, which compressed circulating erythrocytes (Fig. 3). These thromboemboli did not demonstrate positivity with any of the IF stains. In addition to the lipid thromboemboli, 5 of these 7 dogs had FSGS, 1 had glomerular synechiae without FSGS, and 1 had arterionephrosclerosis.
Figures 1–3.
Lipid thromboembolus, kidney glomerulus, dog. Figure 1. Glomerulus with an intracapillary nonstaining circular structure, which is a lipid thromboembolus. (a) Hematoxylin and eosin. (b) Periodic acid–Schiff. (c) Masson’s trichrome. (d) Jones’s methenamine silver. Figure 2. Lipid droplets inside glomerular capillary lumens. Oil red O. Figure 3. Glomerular capillary loop with an intraluminal osmophilic structure, consistent with lipid. Bar = 5 μm. Transmission electron microscopy.
Glomerular Lipidosis
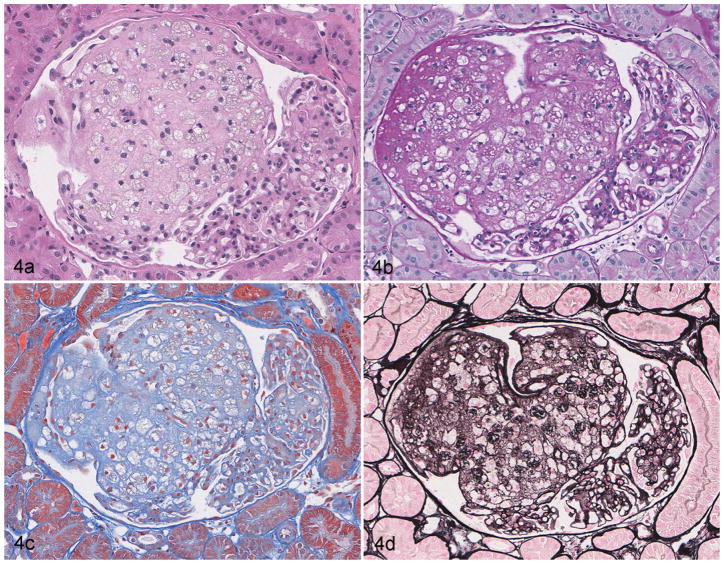
Large aggregates of foam cells were present in the glomeruli of 1 dog. These foam cells distorted the glomerular capillaries, sometimes reaching diameters of 100 μm (Fig. 4). This process involved 30% of sampled glomeruli. In addition, 30% of glomeruli had immature morphology. Specifically, the glomerular tufts were small, contained few capillaries, and had a prominent rim of podocyte precursors. Ultrastructural evaluation verified the accumulation of cells with abundant lipid in cytoplasm of cells, many of which had characteristics of mesangial cells. Evaluation with IF did not identify definitive evidence of immune complex deposition in glomeruli.
Figure 4.
Glomerular lipidosis, dog. Approximately two-thirds of the glomerular tuft is effaced by the presence of numerous foam cells containing small clear vacuoles consistent with lipid. The remaining portion of the glomerulus is compressed against Bowman’s capsule but is neither hypercellular nor sclerotic. (a) Hematoxylin and eosin. (b) Periodic acid–Schiff. (c) Masson’s trichrome. (d) Jones’s methenamine silver.
Other Forms of Glomerular Lipid
Ultrastructural evidence of glomerular lipid deposition in the mesangium and/or in the GBM was found in 6 of the 7 cases with glomerular lipid thromboemboli, the dog with glomerular lipidosis, and 12 additional dogs. The histopathologic diagnoses rendered in these cases ranged from FSGS to diffuse segmental and global glomerulosclerosis. Four dogs had an additional histopathologic lesion of markedly dilated Bowman’s capsules and compressed glomerular tufts (glomerulocystic atrophy). All dogs had small (<1 μm) osmophilic globules distributed along the GBM of capillary walls, within the mesangial matrix, and/or inside the mesangial cell cytoplasm. One case also had abundant lipid in the podocyte cytoplasm.
Clinical Data and Relationship to Pathology
Signalment (age and sex) and laboratory data (UPC, serum albumin and creatinine concentrations, and systolic blood pressure) for dogs with each category of lipid lesions are presented in Table 1. Dogs with more than 1 type of lipid lesion were grouped based on the predominant lesion. None of the laboratory variables differed significantly between categories of lipid lesion in univariate analyses (ANOVA, P = .15–.48) or in a multivariate analysis of the subset of 20 dogs with complete data (MANOVA, P = .36). Serum triglyceride concentrations were available for 6 of 7 dogs with lipid thromboemboli, and all were hypertriglyceridemic (median, 572 mg/dl; range, 380–2481 mg/dl; the upper limit of laboratory reference ranges varied from 70–108 mg/dl). Serum triglyceride concentrations were also available for 2 other dogs: a dog with immune complex–mediated glomerulonephritis (without lipid) with a triglyceride concentration of 115 mg/dl and a dog with glomerulosclerosis with lipid deposition in the capillary walls with a triglyceride concentration of 51 mg/dl. Regarding comorbidities, one of the dogs with lipid thromboemboli had hyperadrenocorticism that was reported to be well controlled with trilostane, and another dog with non–immune complex–mediated disease and lipid deposition in the capillary walls and mesangium had untreated hyperadrenocorticism. None of the dogs were reported to have diabetes mellitus or hypothyroidism.
Discussion
Idiopathic hypertriglyceridemia is a well-known clinicopathologic finding in Miniature Schnauzer dogs. The condition is believed to be genetic, but the age of onset and features of the disease can vary,24 suggesting the pathogenesis might be polygenic or multifactorial in nature. There is a significant association between hypertriglyceridemia and proteinuria in the breed.8 The results of the current study demonstrate that glomerular lipid deposits are present in most renal biopsy samples examined from proteinuric Miniature Schnauzers, and 7 of 26 (27%) of the samples had glomerular lipid thromboemboli. To our knowledge, glomerular lipid thromboemboli have previously only been reported in a dog with diabetes mellitus; in that case report, the term glomerular emboli was used.1 At the IVRPS, glomerular lipid thromboemboli were only observed in Miniature Schnauzers during the study period. During the course of the preparation of this article, this lesion was identified in a single Shetland sheepdog with mild proteinuria and elevated liver enzymes. Therefore, it appears that this lesion is closely breed associated.
In humans, lipoprotein glomerulopathy is a disease in which glomerular lipid thrombi are identified. Of note, there is a difference in terminology wherein the term thromboemboli is used in veterinary medicine because the manner in which lipid accumulates in glomerular capillary loops is unknown. Lipoprotein glomerulopathy is a rare genetic disease caused by autosomal dominant, incompletely penetrant mutations in the apolipoprotein E (APOE) gene that raise the serum level of the protein.20 The mutations responsible for lipoprotein glomerulopathy are theorized to impair binding and uptake of APOE by peripheral tissues and/or increase the tendency toward aggregation of the protein.20 Renal involvement is always present, but hyperlipidemia can range from absent to severe. This disease is distinct from APOE deficiency, a more common autosomal recessive, or occasionally dominant, genetic disease that causes Type III hyperlipoproteinemia (increased intermediate density lipoproteins) with only rare renal manifestations.13 Lipoprotein glomerulopathy can progress to nephrotic syndrome,7 and segmental sclerosis and periglomerular fibrosis are observed in advanced cases.14 Treatment with fibrates was shown to improve lipid profiles, decrease proteinuria, increase serum albumin, and improve survival in a population of patients affected by lipoprotein glomerulopathy.7 Although the ultrastructural features of the current cases are very similar to human lipoprotein glomerulopathy, they are not identical. Comparison of the ultrastructural evaluations by one of the authors (R.E.C.) reveals a difference in the osmophilia of the droplets, but the significance of this difference is unclear.
The clinical consequences of glomerular lipid thromboemboli in Miniature Schnauzers are unknown. One of the dogs with lipid thromboemboli was reported to develop hypoalbuminemia, and a different dog was azotemic at the time of the biopsy, but for the other dogs, proteinuria was the sole sign of renal disease. No difference in laboratory data was found between dogs with glomerular lipid thromboemboli and those with other types of glomerular lesions, but the sample size for each category was small. Future studies are needed to determine the progression and severity of the disease and whether dietary fat restriction and fibrate therapy reduce proteinuria. Importantly, recognition that these clear spaces are indicative of lipid droplets in capillary lumens and not merely an artifact of processing is needed to identify more cases.
Glomerular lipidosis is distinct from glomerular lipid thromboemboli. The former lesion is an aneurysmally dilated capillary loop that contains aggregates of lipid-laden foam cells, whereas the latter is a dilated capillary lumen with a nonstaining structure. Of note, these 2 lesions were not identified in the same case, which suggests that the lesions are not merely different stages of the same lesion. Glomerular lipidosis has been previously described in 33 Beagles from a large colony of research dogs.27 The lesions were noted incidentally when kidneys were evaluated for drug safety studies. The dogs were reported to have normal plasma lipid levels, and there was no clinical evidence of renal dysfunction. The authors concluded that glomerular lipidosis does not cause functional renal impairment. Only 1 dog in the current study was found to have glomerular lipidosis. This dog was hypertensive but had normal serum albumin and creatinine concentrations. Serum triglyceride concentration was not measured in the dog; thus, it is possible that the pathology was unrelated to familial hyperlipidemia in Miniature Schnauzer dogs.
Varying degrees of lipid deposition were also noted in glomerular capillary walls and the mesangium in many of the biopsy specimens from Miniature Schnauzer dogs. One of these dogs had a normal serum triglyceride concentration, but serum triglyceride measurements were not reported for the other dogs. Lipid deposits in the mesangium have been reported in clinically healthy Beagle dogs with and without proteinuria.19 The significance of these findings and relationship to hyperlipidemia have not been determined. Of note, the Beagle is another breed where familial hyperlipidemia has been reported.21
Interestingly, of the 27 Miniature Schnauzer dogs in this study, only 6 (22%) had a definitive diagnosis of immune complex–mediated glomerulonephritis. This is appreciably lower than the general proportion of proteinuric dogs with immune complex–mediated glomerulonephritis recently reported by our group. In that study, 48% of dogs that underwent renal biopsy for the clinical indication of proteinuria had definitive evidence of immune complex deposition.16 This could be due to a decreased incidence of immune complex glomerular disease in the breed or an increased risk for other glomerulopathies such as FSGS, the most common lesion association with hyper-triglyceridemia in rodent models.
A selection bias is expected to exist in the samples submitted to the IVRPS. Epidemiologic analysis of breed over-representation and comparison of the prevalence of various lesions using the IVRPS database is difficult since some breeds are more or less likely to be biopsied if a heritable condition is known. Because renal biopsy is invasive and expensive, multiple factors are involved in the decision to perform the procedure. For instance, some practitioners may attribute the clinical signs to a “breed nephropathy” and forego biopsy, whereas others may decide to biopsy the patient to prove or disprove that the specific lineage carries the trait.
Renal specimens from this cohort of proteinuric Miniature Schnauzer dogs demonstrated the presence of lipid in glomeruli of most dogs. Interestingly, glomerular lipid thromboemboli appear to be a fairly breed-specific lesion and should not be overlooked in autopsy or biopsy specimens. Glomerular lipidosis can be observed in this breed; however, more Miniature Schnauzer dogs might have mesangial lipid, which is only detectable with ultrastructural evaluation. The results from this study underscore the necessity of a comprehensive evaluation of renal tissue by thin sections, special stains, and TEM examination. Furthermore, the diagnoses need to be interpreted together with the sufficient clinicopathologic data.
Supplementary Material
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
We thank Alan Flechtner and Anne Saulsbery from the Comparative Mouse Phenotyping Shared Resource (Cancer Center Support Grant P30 CA016058) for histopathology and electron microscopy specimen preparation. In addition, we thank Mary Sanders (Department of Small Animal Clinical Sciences, Texas A&M University, College Station, TX) for her expert technical assistance.
Footnotes
Supplemental material for this article is available on the Veterinary Pathology website at http://journals.sagepub.com/doi/suppl/10.1177/0300985816681412.
Reprints and permission: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Brown TP, Fitzpatrick RK. Glomerular lipid emboli in a diabetic dog. Vet Pathol. 1986;23(2):209–211. doi: 10.1177/030098588602300219. [DOI] [PubMed] [Google Scholar]
- 2.Cianciolo RE, Brown CA, Mohr FC, et al. Pathologic evaluation of canine renal biopsies: methods for identifying features that differentiate immune-mediated glomerulonephritides from other categories of glomerular diseases. J Vet Intern Med. 2013;27:S10–S18. doi: 10.1111/jvim.12226. [DOI] [PubMed] [Google Scholar]
- 3.Cianciolo RE, Mohr FC, Aresu L, et al. World Small Animal Veterinary Association renal pathology initiative: classification of glomerular diseases in dogs. Vet Pathol. 2016;53(1):113–135. doi: 10.1177/0300985815579996. [DOI] [PubMed] [Google Scholar]
- 4.Clement LC, Mace C, Avila-Casado C, et al. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20(1):37–46. doi: 10.1038/nm.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furrow E, Jaeger JQ, Parker VJ, et al. Proteinuria and lipoprotein lipase activity in Miniature Schnauzer dogs with and without hypertriglyceridemia. Vet J. 2016;212:83–89. doi: 10.1016/j.tvjl.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyebi L, Soltani Z, Reisin E. Lipid nephrotoxicity: new concept for an old disease. Curr Hypertens Rep. 2012;14(2):177–181. doi: 10.1007/s11906-012-0250-2. [DOI] [PubMed] [Google Scholar]
- 7.Hu Z, Huang S, Wu Y, et al. Hereditary features, treatment, and prognosis of the lipoprotein glomerulopathy in patients with the APOE Kyoto mutation. Kidney Int. 2014;85(2):416–424. doi: 10.1038/ki.2013.335. [DOI] [PubMed] [Google Scholar]
- 8.Jaeger JQ, Johnson S, Hinchcliff KW. Characterization of biochemical abnormalities in idiopathic hyperlipidemia of Miniature Schnauzer dogs. J Vet Intern Med. 2003;17:394. [Google Scholar]
- 9.Kasiske BL, Cleary MP, O’Donnell MP, et al. Effects of genetic obesity on renal structure and function in the Zucker rat. J Lab Clin Med. 1985;106(5):598–604. [PubMed] [Google Scholar]
- 10.Kondo S, Yoshizawa N, Wakabayashi K. Natural history of renal lesions in spontaneously hypercholesterolemic (SHC) male rats. Nihon Jinzo Gakkai Shi. 1995;37(2):91–99. [PubMed] [Google Scholar]
- 11.Kutsunai M, Kanemoto H, Fukushima K, et al. The association between gall bladder mucoceles and hyperlipidaemia in dogs: a retrospective case control study. Vet J. 2014;199(1):76–79. doi: 10.1016/j.tvjl.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Mace C, Chugh S, Clement L, et al. The EU and glomerular diseases. Nephrol Dial Transplant. 2012;27(suppl 2):ii32–ii33. [Google Scholar]
- 13.Matsunaga A, Saito T. Apolipoprotein E mutations: a comparison between lipoprotein glomerulopathy and type III hyperlipoproteinemia. Clin Exp Nephrol. 2014;18(2):220–224. doi: 10.1007/s10157-013-0918-1. [DOI] [PubMed] [Google Scholar]
- 14.Saito T, Oikawa S, Sato H, et al. Lipoprotein glomerulopathy: renal lipidosis induced by novel apolipoprotein E variants. Nephron. 1999;83(3):193–201. doi: 10.1159/000045511. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, Liang K, Vaziri ND. Down-regulation of lipoprotein lipase and VLDL receptor in rats with focal glomerulosclerosis. Kidney Int. 2002;61(1):157–162. doi: 10.1046/j.1523-1755.2002.00104.x. [DOI] [PubMed] [Google Scholar]
- 16.Schneider SM, Cianciolo RE, Nabity MB, et al. Prevalence of immune-complex glomerulonephritides in dogs biopsied for suspected glomerular disease: 501 cases (2007–2012) J Vet Intern Med. 2013;27(suppl 1):S67–S75. doi: 10.1111/jvim.12247. [DOI] [PubMed] [Google Scholar]
- 17.Shearer GC, Stevenson FT, Atkinson DN, et al. Hypoalbuminemia and proteinuria contribute separately to reduced lipoprotein catabolism in the nephrotic syndrome. Kidney Int. 2001;59(1):179–189. doi: 10.1046/j.1523-1755.2001.00478.x. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson FT, Wheeldon CM, Gades MD, et al. Estrogen worsens incipient hypertriglyceridemic glomerular injury in the obese Zucker rat. Kidney Int. 2000;57(5):1927–1935. doi: 10.1046/j.1523-1755.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- 19.Stuart BP, Phemister RD, Thomassen RW. Glomerular lesions associated with proteinuria in clinically healthy dogs. Vet Pathol. 1975;12(2):125–144. doi: 10.1177/030098587501200205. [DOI] [PubMed] [Google Scholar]
- 20.Tsimihodimos V, Elisaf M. Lipoprotein glomerulopathy. Curr Opin Lipidol. 2011;22(4):262–269. doi: 10.1097/MOL.0b013e328345ebb0. [DOI] [PubMed] [Google Scholar]
- 21.Wada M, Minamisono T, Ehrhart LA, et al. Familial hyperlipoproteinemia in beagles. Life Sci. 1977;20(6):999–1008. doi: 10.1016/0024-3205(77)90287-9. [DOI] [PubMed] [Google Scholar]
- 22.Xenoulis PG, Levinski MD, Suchodolski JS, et al. Association of hypertrigly-ceridemia with insulin resistance in healthy Miniature Schnauzers. J Am Vet Med Assoc. 2011;238(8):1011–1016. doi: 10.2460/javma.238.8.1011. [DOI] [PubMed] [Google Scholar]
- 23.Xenoulis PG, Levinski MD, Suchodolski JS, et al. Serum triglyceride concentrations in Miniature Schnauzers with and without a history of probable pancreatitis. J Vet Intern Med. 2011;25(1):20–25. doi: 10.1111/j.1939-1676.2010.0644.x. [DOI] [PubMed] [Google Scholar]
- 24.Xenoulis PG, Suchodolski JS, Levinski MD, et al. Investigation of hypertrigly-ceridemia in healthy Miniature Schnauzers. J Vet Intern Med. 2007;21(6):1224–1230. doi: 10.1892/07-051.1. [DOI] [PubMed] [Google Scholar]
- 25.Xenoulis PG, Suchodolski JS, Levinski MD, et al. Serum liver enzyme activities in healthy Miniature Schnauzers with and without hypertriglyceridemia. J Am Vet Med Assoc. 2008;232(1):63–67. doi: 10.2460/javma.232.1.63. [DOI] [PubMed] [Google Scholar]
- 26.Xenoulis PG, Suchodolski JS, Ruaux CG, et al. Association between serum triglyceride and canine pancreatic lipase immunoreactivity concentrations in Miniature Schnauzers. J Am Anim Hosp Assoc. 2010;46(4):229–234. doi: 10.5326/0460229. [DOI] [PubMed] [Google Scholar]
- 27.Zayed I, Gopinath C, Hornstra HW, et al. A light and electron microscopical study of glomerular lipoidosis in beagle dogs. J Comp Pathol. 1976;86(4):509–517. doi: 10.1016/0021-9975(76)90060-8. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Zhang X, Chen L, et al. Expression profiling of hepatic genes associated with lipid metabolism in nephrotic rats. Am J Physiol Renal Physiol. 2008;295(3):F662–F671. doi: 10.1152/ajprenal.00046.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.