Abstract
Background
Autoantibodies (AAbs) against islet autoantigens (AAgs) are used for type 1 diabetes (T1D) diagnosis and prediction. Islet-specific AAbs usually appear early in life and may fluctuate in terms of number and titer sometimes for over 20 years before T1D develops. Whereas their predictive power is high for pediatric subjects with high genetic risk who rapidly progress to multiple AAb positivity, they are less reliable for children with low genetic risk, single AAb positivity and slow disease progression.
Objective
It is unknown how AAbs develop and whether they are involved in T1D pathogenesis. So far an increase in AAb number seems to only indicate AAg spreading and progression towards clinical T1D. The goal of this review is to shed light into the possible involvement of AAbs in T1D development.
Method
We thoroughly review the current literature and discuss possible mechanisms of AAb development and roles they may play in disease pathogenesis.
Results
Genetic and environmental factors instigate changes at the molecular and cellular levels that promote AAb development. Although direct involvement of AAbs in T1D is less clear, autoreactive B cells are clearly involved in various immune and autoimmune responses via antigen presentation, immunoregulation and cytokine production.
Conclusion
Our analysis suggests that understanding the mechanisms that lead to islet-specific AAb development and the diabetogenic processes that autoreactive B cells promote may uncover additional biomarkers and therapeutic targets.
Conclusion
Our analysis suggests that delineating the mechanisms that lead to islet-specific AAb development and the diabetogenic processes that autoreactive B cells promote may lead in the discovery of additional biomarkers and therapeutic targets.
Keywords: autoantibodies, type 1 diabetes, prediction, prevention, biomarker, diabetogenesis
1. Introduction
Type 1 diabetes (T1D) is one of the most common autoimmune disorders in the industrially-developed countries that mostly manifests in children and young adults [1]. The incidence of T1D has considerably increased worldwide and significant effort is placed for its prediction, prevention and cure [2, 3]. T1D is considered a chronic immune-mediated disease directed against the insulin-producing beta cells of the pancreatic islets in genetically-susceptible individuals [4–6]. Recent reports on pancreas histology however, have challenged this “dogma” after demonstrating that lymphocytes infiltrate the exocrine pancreas as well suggesting that T1D is a pancreas rather than a beta-cell-specific autoimmune disease [7, 8]. T1D has a similar disease pattern worldwide: quite rare before the first year of age, a peak between 5 and 15 years of age and a decline in incidence thereafter, with a constant low rate manifestation in adults [9]. One of the most intriguing features of T1D is the sometimes long, subclinical period that precedes T1D onset characterized by the presence of autoantibodies (AAbs) targeted against islet-specific autoantigens (AAgs) [10]. From three major prediction and prevention studies (DIPP, BabyDiab and DAISY), which followed large cohorts of individuals with risk for developing T1D (mostly first degree relatives-FDRs of patients with T1D) we learned that seroconversion occurs very early (less than 3 years of age), epitope spreading indicates the advancement of the disease, 84% is the cumulative risk for developing T1D in 15 yrs after seroconversion in multiple AAb-positive (AAb+) subjects, and the median time from sercoconversion to T1D onset is 3.5 years [11, 12]. These studies also showed that the most important factors contributing to AAb development are genetic, although their development can be also influenced by environmental triggers [13, 14].
Despite the progress made in understanding the natural history of T1D, several pivotal questions remain regarding the mechanisms that lead to loss of B cell tolerance and the development of islet-specific AAbs. To name some: is AAb development genetically determined? What influences AAg spreading? Is AAg spreading also genetically determined? Why do certain AAb+ donors never progress to T1D? At which stage do environmental factors impact the diabetogenic process? In this review, we address the factors that determine AAb development and lead to the clinical onset of T1D and discuss the possible mechanisms that induce AAg spreading and contribute to disease progression. We argue that AAbs despite their prediction value tell us little about the mechanism that lead to T1D and that additional biomarkers linked to their development and overall to disease activity need to be discovered as they could serve as predictive biomarkers and therapeutic targets.
1.1 Levels of tolerance bypassed during progression to T1D
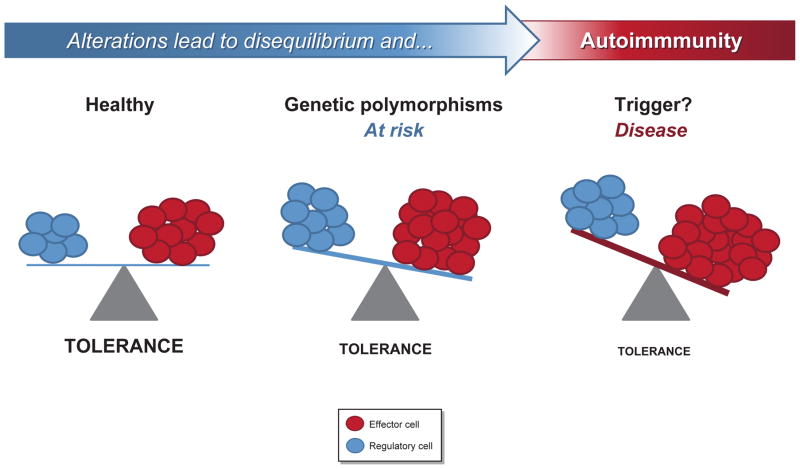
With regards to T1D several levels of tolerance are thought to be bypassed; at the level of the thymus and bone marrow (BM), and at the level of the periphery i.e. in secondary lymphoid organs, especially the spleen and pancreatic draining lymph nodes, including the target tissue itself, i.e. the pancreas [15]. T cell tolerance is essential to maintain the immune system in equilibrium in the steady-state and during pathogen recognition. Most autoimmune diseases such as T1D are associated with genetic determinants that introduce qualitative and quantitative defects in effector T cells (Teffs) and regulatory T cells (Tregs), altering their balance and increasing the risk of disease development (Figure 1). For the disease to manifest however, additional events in the form of environmental triggers instigate further alterations in the Teff/Treg equilibrium that ultimately result in progressive beta killing and T1D (Figure 1).
Figure 1. Genetic determinants and environmental triggers alter the Teff/Treg equilibrium and lead to T1D.
Genetic determinants that introduce qualitative and quantitative defects in effector T cells (Teffs) and regulatory T cells (Tregs), alter their balance and increase the risk of disease development. For the disease to manifest however, additional events in the form of environmental triggers instigate further alterations in the Teff/Treg equilibrium that ultimately result in progressive beta killing and T1D.
1.1.1 Thymus
Most of our knowledge with regards to the role of thymic central tolerance in T1D has derived from studies in non-obese diabetic (NOD) mice. In the thymus of NOD mice two primary checkpoints are thought to be dysfunctional: clonal deletion and generation of Tregs [16, 17]. Consequently, autoreactive T cells specific for a large number of islet AAgs escape into the periphery that given the reduced Treg number and function, predispose to T1D [18]. The escape of islet-specific T cells from the thymus results from defects in apoptosis [19–21], and lack of islet-specific AAg presentation by thymic stromal cells [22]. Certain major histocompatibility complex (MHC) class II haplotypes are linked to either susceptibility or resistance to T1D via mechanisms that at least in humans remain undefined [23]. In mice, resistance MHC class II haplotypes seem to operate via clonal deletion [24], Treg induction [25] or positive selection of T cells expressing additional T cell receptor (TCR) specificities in the thymus [26]. The release of pathogenic T cells in the periphery plays an important role in the instruction of B cell autoreactive responses, as we describe below.
In addition to MHC genes, polymorphisms in the insulin gene (INS-VNTR [variable number of tandem repeats]) promoter strongly associate with T1D predisposition [27]. The INS-VNTR locus affects expression of the insulin gene in the thymus, and consequently plays important role in thymic selection of preproinsulin (PPI)-specific T cells [28, 29]. The polymorphisms found in the INS-VNTR-locus of patients with T1D are thought to decrease expression of PPI by about three-fold, leading to defective negative selection and the development of autoreactive T cells [30, 31]. The importance of insulin expression in the thymus was demonstrated in NOD mice ablated of the insulin gene: Ins2−/− NOD mice gene developed accelerated T1D and with 100% penetrance [32, 33]. The importance of insulin as a driver of AAg in T1D was illustrated by the elegant studies of Nakayama M et al. In these studies, Ins1/Ins2 double-deficient NOD mice expressing a proinsulin transgene with a mutation at position B16 (Y→A), that alters antigen reactivity, were rescued from T1D development [34]. By using T-cell receptor (TCR) transgenic (Tg) mice specific for insulin beta chain 9-23 (insB:9-23), we and others subsequently showed that, insulin expression in the thymus of NOD mice is involved in clonal deletion rather than thymic induction of Tregs [35, 36]. In humans, T cell responses against PPI/insulin, including the insB:9-23 epitope have been identified [37–40], which may drive the development of insulin-specific B cells. Of interest, high insulin AAbs (IAA) levels are predictors of rapid disease onset in mice and pediatric subjects [41, 42]. Thus, it is possible that polymorphisms in the insulin gene promoter result in defective central tolerance in the thymus and the escape of insulin-reactive T cells that instruct the development of autoreactive B cells.
1.1.2 Bone marrow
Bone marrow is the site where autoreactive B cells are deleted during development. Not till recently was it revealed that central B cell tolerance is defective in patients with T1D resulting in the accumulation of self-reactive mature naïve B cells [43]. The increased production of autoreactive B cells is thought to play key role in the development of islet-specific autoimmunity by promoting the presentation of beta-cell antigens to autoreactive T cells, which in turn by providing B-cell help signals, promote the production of AAbs [44]. Therefore, defects in central tolerance in the thymus and BM produce autoreactive T and B cells that create a pathogenic loop contributing to T1D development.
1.1.3 Periphery
It has been puzzling how exactly naïve islet-reactive T and B cells that usually have low affinity for their cognate antigen become activated and escape the control mechanisms of peripheral tolerance [45]. One of the most intriguing mechanisms proposed lately involves the generation of highly immunogenic AAgs via post-translational modification (PTM). PTM within the endoplasmic reticulum (ER) of “stressed” beta-cells were recently described to trigger AAg T cell activation [46–49]. During ER-stress, proteins are misfolded or modified in a way that alters their immunogenicity. In T1D susceptible individuals, this was shown to generate new AAgs (neoAgs), which activate autoreactive T cells and possibly B cells. Interestingly, aberrant proteins produced by the stressed beta-cells were shown to promote their self-destruction (‘beta-cell suicide’) [50]. This finding generated a novel therapeutic paradigm for T1D, where administration of a chemical ER stress mitigator, resulted in a marked protection from T1D in mice [51]. Thus the targeted beta cells are not innocent players in the destruction process. It is still unclear whether genetic or environmental factors trigger the differential processing of insulin and other islet-AAgs. These PMT AAgs are probably taken up by phagocytosis by antigen presenting cells (dendritic cells, macrophages, neutrophils) for eventual processing and presentation to self-reactive T cells either locally i.e. in ectopic lymphoid pancreatic structures, or in the pancreatic lymph nodes. It will be interesting to know whether B cells make part of this autoreactive process, i.e by presenting PTM AAgs to autoreactive T cells.
An enormous amount of data generated in mouse models and from analysis of human samples has identified Tregs and type 1 regulatory (Tr1) cells as key components of peripheral immune tolerance. Tregs and Tr1 cells play major role in controlling effector and autoreactive T cell responses [52, 53]. Alterations in Treg, Tr1 or Teff quantity and quality contribute to most autoimmune diseases, including T1D [54, 55]. Thus, several therapeutic strategies aim to invigorate Treg/Tr1 function and number [56]. Among them, two therapeutic strategies are particularly appealing: the first strategy aims at expanding endogenous Tregs via low dose interleukin-2 (IL-2) administration, whereas the second via administration of autologous ex vivo expanded Tregs or Tr1 cells. Recently, a clinical study with low dose IL-2 showed good safety profile and Tregs expanded in patients with T1D [57, 58]. A phase I trial was also recently conducted where the safety of ex vivo expanded autologous polyclonal Tregs was tested in patients with T1D [59]. The results of this trial are very promising as no adverse events were seen and the Tregs remained stable during the observation period.
Recent findings showed that Tregs are essential in controlling autoreactive B cell development and function. Tregs and a particular subset that resides in B cell follicles known as follicular Treg (Tfr), restrain the B cell helper activity of T follicular helper (Tfh) cells and are considered critical for optimal generation, function and termination of germinal center responses, which could otherwise lead to autoimmunity [60, 61]. Notably, the B cell peripheral tolerance checkpoint is also dysfunctional in patients with T1D [43], suggesting that Tfh and/or Tfr cells might be responsible for this defect. Interestingly, patients with T1D have an expanded frequency of Tfh cells in circulation [62, 63], suggesting that possibly the proportion of Tfh to Tfr cells is altered and involved in AAb pathogenesis. Thus, while the results on the efficacy of Treg-enhancing treatments in a larger cohort of patients are much awaited, studies on AAb number, titer and autoreactive B cell counts could tell us how Treg-targeted therapies affect autoreactive humoral immunity.
Increasing evidence point to a pivotal role of regulatory B cells (Breg) in pathogenesis of autoimmune diseases, mainly through production of anti-inflammatory cytokines [64]. In the absence of a specific surface phenotype, Bregs are often identified based on their production of IL-10, hence they are referred to as “B10 cells” by some investigators [65]. In autoimmune diabetes, NOD mice are deficient in Breg cells [66] and recent evidence showed that there is deficiency of Breg cells in T1D patients [67, 68]. While the exact reasons of Breg deficiency in T1D is not fully understood, there is evidence of their negative regulation by Fas-mediated apoptosis in NOD mice as those carrying loss-of-function mutation in FasL or treated with FasL-neutralizing mAb have increased frequency of Bregs and protected from overt disease [66]. Subversion of IL-10 tolerogenic role through Fas-mediated deletion of IL-10-producing B cells could be one of the reasons that deletion of IL-10-encoding gene has a negligible effect on disease pathogenesis in NOD mice [69, 70].
Taken together, published data show that B cells play an essential role in immunity and tolerance by participating in various immune and autoimmune responses, ie in antigen presentation, production of AAbs, immunoregulation, cytokine production. Thus, despite being less recognized, B cells and their products are important players in T1D pathogenesis and understanding the mechanisms by which they are generated and their positive and negative effects on T cell tolerance may lead to the discovery of new biomarkers and possibly targets to prevent further disease progression.
1.2 Triggers of islet-specific AAbs: genetic determinants and environmental factors
Human leukocyte antigen (HLA) complex is the strongest genetic determinant of T1D as it confers 40 to 60% of the inheritable risk [71]. The risk is specifically associated with HLA-DR3/DR4 and DQ8/DQ2, which are in tight linkage disequilibrium. On the other hand, HLA-DR15 and DQ6 are protective as they are negatively associated with the disease. There is also evidence of association of certain MHC alleles with specific AAbs. For example DR3 and/or DQB1*0201 are associated with GAD65 AAb and DQ8/DR4 with IA-2 AAb [72, 73]. On the other hand, IAA AAbs are associated with DR3 and DQ8 [72]. Conversely, DR3/DQB*0201 are reported to be negatively associated with IA-2 AAbs [74, 75]. Based on these pattern, a combination of DR4/IA-2 is considered more specific for T1D [76], whereas that of DR3/GAD65 AAbs is considered a general marker of autoimmunity. Mechanistically, there is no known association of specific MHC alleles with certain AAgs. Nonetheless, it is not difficult to envisage that such mechanistic relationship exist given that MHC class II present peptides including AAgs to CD4 T cells, which in turn promote autoreactive B cell activation and AAb production.
Besides HLA, a specific allele of PTPN22 (protein tyrosine phosphatase type 22) has been associated with T1D [77]. The largest fold increase in disease risk among GAD65 AAb+ subjects who carry low risk HLA-DQ alleles has been linked to the PTPN22 R620W risk allele [78]. In addition, Steck et al associated the R620W allele with the progression from preclinical to clinical diabetes in ICA–positive individuals [79]. However, it remains unclear how the R620W allele, a gain-of-function variant that reduces T cell activation, increases disease susceptibility. One hypothesis is that impairment of T cell activation carrying this allele shifts thymic selection thereby allowing autoreactive thymocytes to bypass negative selection and enter the peripheral repertoire [80]. In addition, while the R620W allele is tightly associated with GAD65 AAbs, the mechanism underlying this relationship is poorly understood [81]. We speculate that this may also be related to the role of MHC haplotype given that the tyrosine kinase lck, a main target of the PTPN22 encoded lymphoid specific phosphatase (LYP), is the kinase that transduces CD4 co-receptor signaling upon its binding to MHC class II. Besides PTPN22, the IL2RA is another non-HLA risk gene that is associated with IAA-positive but not IAA-negative patients [78]. Thus, while MHC polymorphisms play a major role in dictating the type of islet AAbs, the overall composition of AAbs in high risk individuals is shaped by multiple risk alleles.
The most convincing evidence that non-genetic factors play a major role in causing T1D comes from the study of monozygotic (MZ) twins [82]. Twin siblings often remain AAb+ for years and never progress to T1D. Although nongermline genetic events such as somatic mutations could be responsible for the observed discordance, environmental factors, either viruses, vaccines, toxins or dietary factors (e.g. breast feeding vs. cow’s milk) are thought to modify susceptibility by affecting the epigenome [83]. Also in the general population a great proportion of single-AAb+ subjects never progress to T1D. It is believed that these subjects bear some autoimmune-resistant alleles, or, as in the case of MZ twins, environmental factors have modified disease progression by acting on the epigenome.
Parent-related events also alter the risk for T1D in the offspring. Children born to diabetic fathers have higher risk to develop T1D compared to those born to diabetic mothers [84]. It remains unclear how the disease is “transmitted” from diabetic fathers to their children and why diabetic mothers “transmit” the disease less frequently to the offspring. Some speculations have been generated in this regard, and among them, transplacental passage of maternal islet AAbs has been suspected. At birth the child of a diabetic mother often has islet-specific AAbs, which gradually disappear [85, 86]. Thus, there are thoughts that perhaps mother transmitted islet-specific AAbs protect from T1D.
NOD mice are different from humans in this respect. Genetic abrogation of maternal AAb transmission in NOD mice protects the offspring from T1D development, suggesting that the maternal transmission of AAbs is a critical environmental component for T1D in mice [87–89]. Interestingly, antigen-specific therapy with PPI-fused with the immunoglobulin (Ig)G Fc fragment (PPI-Fc) in pregnant diabetogenic T-cell receptor-transgenic mice efficiently accumulated in fetuses through the placental FcRn and protected them from subsequent diabetes development [90]. Taken together, these observations show that the islet-specific autoimmune process becomes activated during infancy and environmental events can deviate, prolong, inhibit, trigger or accelerate its progression.
Infections with bacteria and enteroviruses are among the most suspected triggers of T1D. Evidence of coxsackie virus infection in patients with T1D and AAb+ subjects [91, 92] and the recent discovery of IFH1, an innate receptor specific for enteroviruses, as a risk gene of T1D, have fortified this notion (reviewed in [93]). Triggering of innate inflammatory responses from these pathogens is thought to affect the entire pancreas, despite the fact that the clinical manifestation of T1D is due to the loss of insulin-producing beta cells. Molecular mimicry is one of the postulated mechanisms by which enterovirus or bacterial infections are thought to promote AAb development and T1D [94, 95]. The infection is considered to cause antibodies that are cross-reactive to islet AAgs [96, 97]. But other than hypothesis and association studies, so far there is no clear evidence how bacterial and enteroviral infections promote AAb development and the loss of beta cells.
1.3 Role of islet-specific autoantibodies in T1D development
While the value of islet AAbs as biomarkers of disease risk and progression has grown exponentially since their discovery in 1970s [98], their exact role in the disease process remains poorly understood with no clearly defined pathogenic role in either experimental animals or T1D patients. In addition, the case of a child with genetic B cell deficiency who had developed overt T1D is often cited as evidence that neither AAbs nor B cells are essential instigators of the disease [99]. Nevertheless, the selective association of certain AAbs with specific genetic risk alleles, as described above, is consistent with an involvement of B cells and AAbs in T1D pathogenesis and raises the question of why these AAbs are produced. Furthermore, the levels and presence of IAA AAbs are dramatically age-dependent as more than 90% of children who progress to overt disease before the of age 5 years are positive for IAA and have high titer levels, while less than half of individuals developing the disease after age of 15 year are IAA positive [100]. Another indirect evidence of pathogenic role for AAbs is the tight correlation between the number of AAbs and progression to overt diabetes. Although still unproven, it is conceivable that by binding to more AAgs, these AAbs can influence AAg uptake and presentation by B cells and follicular dendritic cells. The uptake can be facilitated by Ag-specific B cell receptors (BCR) or occurs non-specifically via FC receptors, which can subsequently increase Ag presentation to autoreactive T cells leading to the initiation and perpetuation of autoimmune responses or both [101]. In addition, Silva D et al implicated secretion of islet AAbs in the expansion of autoreactive CD4 T cells via Fcγ-R-mediated mechanism [102]. On the other hand, there is evidence that islet AAbs are largely innocuous and perhaps protective. As we mentioned before, the presence of anti-islet AAbs at birth in offspring of mothers with T1D was correlated with a decrease of developing anti-islet AAbs. In addition, there is no evidence of beta cell damage via trans-placental transmission of anti-islet AAbs (GAD65 and IAA) that are readily detected in the infants [89]. Thus, beyond their use as biomarkers, the role of islet AAbs in disease pathogenesis remains poorly understood.
1.4 Islet-specific AAbs for T1D staging, prediction and prevention
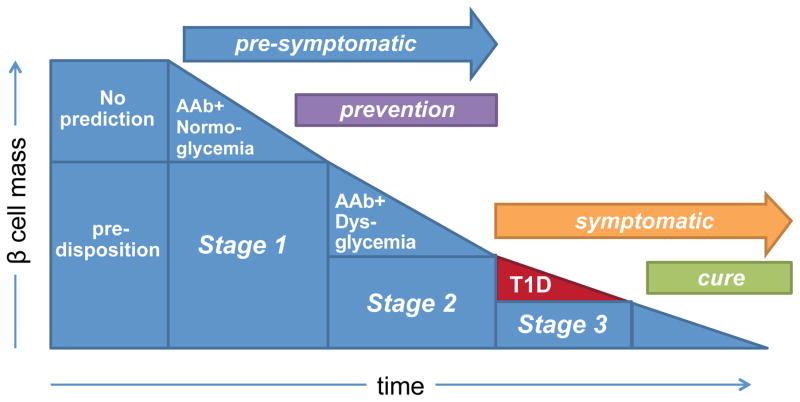
Recently, a new staging classification for T1D was made by JDRF, the Endocrine Society and the American Diabetes Association. Stage 1 is defined by the presence of two or more islet AAbs with lack of metabolic defects (normoglycemia), stage 2 is determined by the presence of two or more islet AAbs with dysglycemia, and stage 3 is the time when T1D appears (Figure 2). Both stage 1 and 2 are considered pre-symptomatic, whereas stage 3 is symptomatic [103]. This staging classification system is thought to improve the design of clinical trials, where risk profiles will be calculated, patients will be stratified and treated according to their disease stage, and stage-specific clinical endpoints will be used. For example, the clinical endpoint in prevention studies for patients with stage 1 T1D will be progression to stage 2 and not necessarily development of T1D.
Figure 2. New T1D staging according to JDRF, the Endocrine Society and the American Diabetes Association.
Presymptomatic T1D is defined by two stages, stage 1, with evident autoimmunity (AAb+) and lack of metabolic defects (normoglycemia), and stage 2, with autoimmunity (≥2AAb+) and dysglycemia. T1D new-onset is defined as stage 3. Interventions taking place at stage 1 or 2 are considered preventive and their clinical endpoint could be different from those in stage 3, where interventions are considered curative.
There are several beneficial outcomes for staging and predicting T1D. Our experience so far has shown that FDRs followed by Trialnet or subjects participated in natural history studies such as the DAISY and TEDDY are less likely to experience some of the severe T1D symptoms, ie diabetic ketoacidosis (KDA) and end up hospitalized [104–106]. Also, children diagnosed early with T1D, are more likely to go through the “honeymoon phase”, their insulin needs are reduced, have slower beta-cell loss, better metabolic control and reduced secondary complications i.e. atherosclerosis, neuropathy, nephropathy [107, 108].
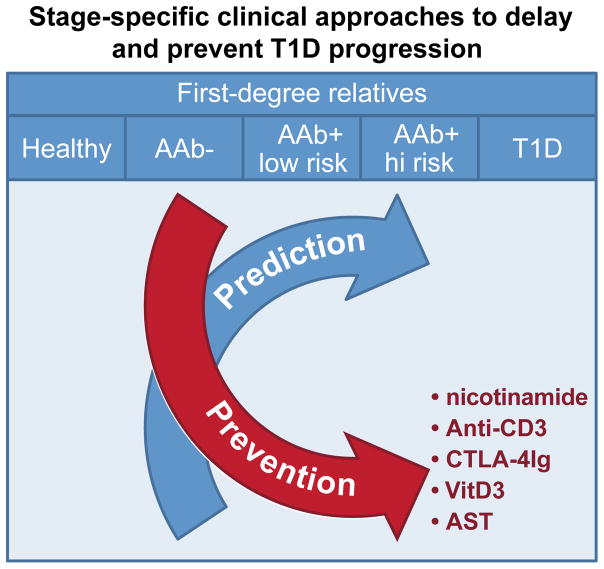
The power to relatively accurately predict T1D in high-risk individuals (more than 2AAb+) seems to have revolutionized the field of T1D prevention, where shorter, smaller and perhaps less expensive clinical trials are being designed. However, whereas our predictive ability is high for stage 2 FDRs, we are less able to make predictions in single AAb+ subjects where prevention therapies we hope will have better outcome (Figure 3). Today, several new clinical trials have are testing nicotinamide, anti-CD3, CTLA-4Ig, VitD3 combined with antigen-specific therapy (AST) in AAb+ subjects at stage 1 (clinicaltrials.gov). In these studies, transition to stage 2 will be the trials’ primary outcome.
Figure 3. Stage-specific clinical approaches to delay and prevent T1D progression.
The further the disease progresses the better we can make predictions of T1D development. However, preventive studies such antigen-specific therapies (AST) are more efficacious at earlier stages of T1D. Today, several clinical approaches already tested at T1D onset i.e. anti-CD3, are tested for prevention. Some of them are indicated in the figure.
2. Conclusion
Our recent advancements in understanding T1D pathogenesis have allowed us to predict the disease development with rather high efficacy based on the measurement of AAbs and other risk factors (i.e. HLA, glucose tolerance etc). Today, combining genetic, immunologic, and metabolic strategies, we can predict T1D progression with high degree of accuracy within 10 yrs of follow-up [109]. There is growing advocacy for screening of the general population but this would not gain momentum until intervention that can lead to their protection are developed. The recent T1D classification system is expected to help with the design of interventions in the early stages of T1D to prevent symptomatic disease and assessment of benefit/risk ratio. In line with this notion, rituximab (anti-CD20) therapy, which deletes CD19+ B cells, have described to suppress IAAs, but had a minor effect on anti-GAD65, IA2, and ZnT8 AAbs [110]. In addition, higher levels of IAAs were associated with better therapeutic response to anti-CD3 in recent-onset T1D patients [111]. Thus, it appears that the value of islet AAbs as biomarkers could be extended not only to design better clinical trials, but also to evaluate their outcomes. However, we need to understand how AAbs develop as this knowledge will lead in the development of additional biomarkers and perhaps therapeutic targets.
Acknowledgments
This work was funded by the NIH R01AI99027 to RAH and GR-2011-02348732 (Italian Ministry of Health) to GF.
References
- 1.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51:3353–3361. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- 2.Levy-Marchal C, Patterson CC, Green A. Geographical variation of presentation at diagnosis of type I diabetes in children: the EURODIAB study. European and Dibetes. Diabetologia. 2001;44(Suppl 3):B75–80. doi: 10.1007/pl00002958. [DOI] [PubMed] [Google Scholar]
- 3.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 6.Castano L, Eisenbarth GS. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63:3880–3890. doi: 10.2337/db14-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkinson MA. Losing a grip on the notion of beta-cell specificity for immune responses in type 1 diabetes: can we handle the truth? Diabetes. 2014;63:3572–3574. doi: 10.2337/db14-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 10.Nokoff N, Rewers M. Pathogenesis of type 1 diabetes: lessons from natural history studies of high-risk individuals. Ann N Y Acad Sci. 2013;1281:1–15. doi: 10.1111/nyas.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmons KM, Michels AW. Type 1 diabetes: A predictable disease. World J Diabetes. 2015;6:380–390. doi: 10.4239/wjd.v6.i3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillespie KM, Gale EA, Bingley PJ. High familial risk and genetic susceptibility in early onset childhood diabetes. Diabetes. 2002;51:210–214. doi: 10.2337/diabetes.51.1.210. [DOI] [PubMed] [Google Scholar]
- 14.Knip M, Veijola R, Virtanen SM, Hyoty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S125–136. doi: 10.2337/diabetes.54.suppl_2.s125. [DOI] [PubMed] [Google Scholar]
- 15.Rossini AA. Autoimmune diabetes and the circle of tolerance. Diabetes. 2004;53:267–275. doi: 10.2337/diabetes.53.2.267. [DOI] [PubMed] [Google Scholar]
- 16.Pearson JA, Wong FS, Wen L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun. 2016;66:76–88. doi: 10.1016/j.jaut.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zucchelli S, Holler P, Yamagata T, Roy M, Benoist C, Mathis D. Defective central tolerance induction in NOD mice: genomics and genetics. Immunity. 2005;22:385–396. doi: 10.1016/j.immuni.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman SM, DiLorenzo TP. A comprehensive guide to antibody and T-cell responses in type 1 diabetes. Tissue Antigens. 2003;62:359–377. doi: 10.1034/j.1399-0039.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto H, Sprent J. A defect in central tolerance in NOD mice. Nat Immunol. 2001;2:1025–1031. doi: 10.1038/ni726. [DOI] [PubMed] [Google Scholar]
- 20.Lesage S, Hartley SB, Akkaraju S, Wilson J, Townsend M, Goodnow CC. Failure to censor forbidden clones of CD4 T cells in autoimmune diabetes. J Exp Med. 2002;196:1175–1188. doi: 10.1084/jem.20020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liston A, Lesage S, Gray DH, et al. Generalized resistance to thymic deletion in the NOD mouse; a polygenic trait characterized by defective induction of Bim. Immunity. 2004;21:817–830. doi: 10.1016/j.immuni.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Viret C, Leung-Theung-Long S, Serre L, et al. Thymus-specific serine protease controls autoreactive CD4 T cell development and autoimmune diabetes in mice. J Clin Invest. 2011;121:1810–1821. doi: 10.1172/JCI43314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wicker LS. Major histocompatibility complex-linked control of autoimmunity. J Exp Med. 1997;186:973–975. doi: 10.1084/jem.186.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt D, Verdaguer J, Averill N, Santamaria P. A mechanism for the major histocompatibility complex-linked resistance to autoimmunity. J Exp Med. 1997;186:1059–1075. doi: 10.1084/jem.186.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai S, Serra P, Clemente-Casares X, et al. Antidiabetogenic MHC class II promotes the differentiation of MHC-promiscuous autoreactive T cells into FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2013;110:3471–3476. doi: 10.1073/pnas.1211391110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luhder F, Katz J, Benoist C, Mathis D. Major histocompatibility complex class II molecules can protect from diabetes by positively selecting T cells with additional specificities. J Exp Med. 1998;187:379–387. doi: 10.1084/jem.187.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barratt BJ, Payne F, Lowe CE, et al. Remapping the insulin gene/IDDM2 locus in type 1 diabetes. Diabetes. 2004;53:1884–1889. doi: 10.2337/diabetes.53.7.1884. [DOI] [PubMed] [Google Scholar]
- 28.Pugliese A, Zeller M, Fernandez A, Jr, et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 29.Vafiadis P, Bennett ST, Todd JA, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 30.Chentoufi AA, Polychronakos C. Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: the mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes. 2002;51:1383–1390. doi: 10.2337/diabetes.51.5.1383. [DOI] [PubMed] [Google Scholar]
- 31.Chentoufi AA, Palumbo M, Polychronakos C. Proinsulin expression by Hassall’s corpuscles in the mouse thymus. Diabetes. 2004;53:354–359. doi: 10.2337/diabetes.53.2.354. [DOI] [PubMed] [Google Scholar]
- 32.Thebault-Baumont K, Dubois-Laforgue D, Krief P, et al. Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest. 2003;111:851–857. doi: 10.1172/JCI16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan Y, Rudert WA, Grupillo M, He J, Sisino G, Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 2009;28:2812–2824. doi: 10.1038/emboj.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fousteri G, Jasinski J, Dave A, et al. Following the fate of one insulin-reactive CD4 T cell: conversion into Teffs and Tregs in the periphery controls diabetes in NOD mice. Diabetes. 2012;61:1169–1179. doi: 10.2337/db11-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jasinski JM, Yu L, Nakayama M, et al. Transgenic insulin (B:9-23) T-cell receptor mice develop autoimmune diabetes dependent upon RAG genotype, H-2g7 homozygosity, and insulin 2 gene knockout. Diabetes. 2006;55:1978–1984. doi: 10.2337/db06-0058. [DOI] [PubMed] [Google Scholar]
- 37.Alleva DG, Crowe PD, Jin L, et al. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest. 2001;107:173–180. doi: 10.1172/JCI8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arif S, Tree TI, Astill TP, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113:451–463. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kent SC, Chen Y, Bregoli L, et al. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435:224–228. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- 40.Pathiraja V, Kuehlich JP, Campbell PD, et al. Proinsulin-specific, HLA-DQ8, and HLA-DQ8-transdimer-restricted CD4+ T cells infiltrate islets in type 1 diabetes. Diabetes. 2015;64:172–182. doi: 10.2337/db14-0858. [DOI] [PubMed] [Google Scholar]
- 41.Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A. 2000;97:1701–1706. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steck AK, Johnson K, Barriga KJ, et al. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care. 2011;34:1397–1399. doi: 10.2337/dc10-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menard L, Saadoun D, Isnardi I, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121:3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mallone R, Brezar V. To B or not to B: (anti)bodies of evidence on the crime scene of type 1 diabetes? Diabetes. 2011;60:2020–2022. doi: 10.2337/db11-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeker LT, Bour-Jordan H, Bluestone JA. Breakdown in peripheral tolerance in type 1 diabetes in mice and humans. Cold Spring Harb Perspect Med. 2012;2:a007807. doi: 10.1101/cshperspect.a007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marre ML, James EA, Piganelli JD. beta cell ER stress and the implications for immunogenicity in type 1 diabetes. Front Cell Dev Biol. 2015;3:67. doi: 10.3389/fcell.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGinty JW, Chow IT, Greenbaum C, Odegard J, Kwok WW, James EA. Recognition of posttranslationally modified GAD65 epitopes in subjects with type 1 diabetes. Diabetes. 2014;63:3033–3040. doi: 10.2337/db13-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Lummel M, Duinkerken G, van Veelen PA, et al. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes. 2014;63:237–247. doi: 10.2337/db12-1214. [DOI] [PubMed] [Google Scholar]
- 49.Marrack P, Kappler JW. Do MHCII-presented neoantigens drive type 1 diabetes and other autoimmune diseases? Cold Spring Harb Perspect Med. 2012;2:a007765. doi: 10.1101/cshperspect.a007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atkinson MA, Bluestone JA, Eisenbarth GS, et al. How does type 1 diabetes develop?: the notion of homicide or beta-cell suicide revisited. Diabetes. 2011;60:1370–1379. doi: 10.2337/db10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engin F, Yermalovich A, Nguyen T, et al. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci Transl Med. 2013;5:211ra156. doi: 10.1126/scitranslmed.3006534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yadav M, Stephan S, Bluestone JA. Peripherally induced tregs - role in immune homeostasis and autoimmunity. Front Immunol. 2013;4:232. doi: 10.3389/fimmu.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 54.Pot C, Apetoh L, Kuchroo VK. Type 1 regulatory T cells (Tr1) in autoimmunity. Semin Immunol. 2011;23:202–208. doi: 10.1016/j.smim.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geiger TL, Tauro S. Nature and nurture in Foxp3(+) regulatory T cell development, stability, and function. Hum Immunol. 2012;73:232–239. doi: 10.1016/j.humimm.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roncarolo MG, Gregori S, Bacchetta R, Battaglia M. Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr Top Microbiol Immunol. 2014;380:39–68. doi: 10.1007/978-3-662-43492-5_3. [DOI] [PubMed] [Google Scholar]
- 57.Pham MN, von Herrath MG, Vela JL. Antigen-Specific Regulatory T Cells and Low Dose of IL-2 in Treatment of Type 1 Diabetes. Front Immunol. 2015;6:651. doi: 10.3389/fimmu.2015.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenzwajg M, Churlaud G, Mallone R, et al. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J Autoimmun. 2015;58:48–58. doi: 10.1016/j.jaut.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bluestone JA, Buckner JH, Fitch M, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanderleyden I, Linterman MA, Smith KG. Regulatory T cells and control of the germinal centre response. Arthritis Res Ther. 2014;16:471. doi: 10.1186/s13075-014-0471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sage PT, Sharpe AH. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 2015;36:410–418. doi: 10.1016/j.it.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferreira RC, Simons HZ, Thompson WS, et al. IL-21 production by CD4+ effector T cells and frequency of circulating follicular helper T cells are increased in type 1 diabetes patients. Diabetologia. 2015;58:781–790. doi: 10.1007/s00125-015-3509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kenefeck R, Wang CJ, Kapadi T, et al. Follicular helper T cell signature in type 1 diabetes. J Clin Invest. 2015;125:292–303. doi: 10.1172/JCI76238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunol. 2015;194:1395–1401. doi: 10.4049/jimmunol.1401329. [DOI] [PubMed] [Google Scholar]
- 66.Xiao Z, Mohamood AS, Uddin S, et al. Inhibition of Fas ligand in NOD mice unmasks a protective role for IL-10 against insulitis development. Am J Pathol. 2011;179:725–732. doi: 10.1016/j.ajpath.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kleffel S, Vergani A, Tezza S, et al. Interleukin-10+ regulatory B cells arise within antigen-experienced CD40+ B cells to maintain tolerance to islet autoantigens. Diabetes. 2015;64:158–171. doi: 10.2337/db13-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deng C, Xiang Y, Tan T, et al. Altered Peripheral B-Lymphocyte Subsets in Type 1 Diabetes and Latent Autoimmune Diabetes in Adults. Diabetes Care. 2016;39:434–440. doi: 10.2337/dc15-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serreze DV, Chapman HD, Post CM, Johnson EA, Suarez-Pinzon WL, Rabinovitch A. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J Immunol. 2001;166:1352–1359. doi: 10.4049/jimmunol.166.2.1352. [DOI] [PubMed] [Google Scholar]
- 70.Saxena A, Khosraviani S, Noel S, Mohan D, Donner T, Hamad AR. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2015;74:27–34. doi: 10.1016/j.cyto.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baschal EE, Eisenbarth GS. Extreme genetic risk for type 1A diabetes in the post-genome era. J Autoimmun. 2008;31:1–6. doi: 10.1016/j.jaut.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Graham J, Hagopian WA, Kockum I, et al. Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51:1346–1355. doi: 10.2337/diabetes.51.5.1346. [DOI] [PubMed] [Google Scholar]
- 73.Hagopian WA, Sanjeevi CB, Kockum I, et al. Glutamate decarboxylase-, insulin-, and islet cell-antibodies and HLA typing to detect diabetes in a general population-based study of Swedish children. J Clin Invest. 1995;95:1505–1511. doi: 10.1172/JCI117822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knip M, Kukko M, Kulmala P, et al. Humoral beta-cell autoimmunity in relation to HLA-defined disease susceptibility in preclinical and clinical type 1 diabetes. Am J Med Genet. 2002;115:48–54. doi: 10.1002/ajmg.10343. [DOI] [PubMed] [Google Scholar]
- 75.Savola K, Sabbah E, Kulmala P, Vahasalo P, Ilonen J, Knip M. Autoantibodies associated with Type I diabetes mellitus persist after diagnosis in children. Diabetologia. 1998;41:1293–1297. doi: 10.1007/s001250051067. [DOI] [PubMed] [Google Scholar]
- 76.Savola K, Bonifacio E, Sabbah E, et al. IA-2 antibodies--a sensitive marker of IDDM with clinical onset in childhood and adolescence. Childhood Diabetes in Finland Study Group. Diabetologia. 1998;41:424–429. doi: 10.1007/s001250050925. [DOI] [PubMed] [Google Scholar]
- 77.Fousteri G, Liossis SN, Battaglia M. Roles of the protein tyrosine phosphatase PTPN22 in immunity and autoimmunity. Clin Immunol. 2013;149:556–565. doi: 10.1016/j.clim.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 78.Maziarz M, Janer M, Roach JC, et al. The association between the PTPN22 1858C>T variant and type 1 diabetes depends on HLA risk and GAD65 autoantibodies. Genes Immun. 2010;11:406–415. doi: 10.1038/gene.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steck AK, Zhang W, Bugawan TL, et al. Do non-HLA genes influence development of persistent islet autoimmunity and type 1 diabetes in children with high-risk HLA-DR,DQ genotypes? Diabetes. 2009;58:1028–1033. doi: 10.2337/db08-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vang T, Miletic AV, Bottini N, Mustelin T. Protein tyrosine phosphatase PTPN22 in human autoimmunity. Autoimmunity. 2007;40:453–461. doi: 10.1080/08916930701464897. [DOI] [PubMed] [Google Scholar]
- 81.Maziarz M, Hagopian W, Palmer JP, et al. Non-HLA type 1 diabetes genes modulate disease risk together with HLA-DQ and islet autoantibodies. Genes Immun. 2015;16:541–551. doi: 10.1038/gene.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salvetti M, Ristori G, Bomprezzi R, Pozzilli P, Leslie RD. Twins: mirrors of the immune system. Immunol Today. 2000;21:342–347. doi: 10.1016/s0167-5699(00)01658-3. [DOI] [PubMed] [Google Scholar]
- 83.Elboudwarej E, Cole M, Briggs FB, et al. Hypomethylation within gene promoter regions and type 1 diabetes in discordant monozygotic twins. J Autoimmun. 2016 doi: 10.1016/j.jaut.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Warram JH, Krolewski AS, Gottlieb MS, Kahn CR. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311:149–152. doi: 10.1056/NEJM198407193110304. [DOI] [PubMed] [Google Scholar]
- 85.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48:460–468. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 86.Hamalainen AM, Savola K, Kulmala PK, Koskela P, Akerblom HK, Knip M. Disease-associated autoantibodies during pregnancy and at birth in families affected by type 1 diabetes. Clin Exp Immunol. 2001;126:230–235. doi: 10.1046/j.1365-2249.2001.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greeley SA, Katsumata M, Yu L, et al. Elimination of maternally transmitted autoantibodies prevents diabetes in nonobese diabetic mice. Nat Med. 2002;8:399–402. doi: 10.1038/nm0402-399. [DOI] [PubMed] [Google Scholar]
- 88.Washburn LR, Dang H, Tian J, Kaufman DL. The postnatal maternal environment influences diabetes development in nonobese diabetic mice. J Autoimmun. 2007;28:19–23. doi: 10.1016/j.jaut.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koczwara K, Ziegler AG, Bonifacio E. Maternal immunity to insulin does not affect diabetes risk in progeny of non obese diabetic mice. Clin Exp Immunol. 2004;136:56–59. doi: 10.1111/j.1365-2249.2004.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Culina S, Gupta N, Boisgard R, et al. Materno-Fetal Transfer of Preproinsulin Through the Neonatal Fc Receptor Prevents Autoimmune Diabetes. Diabetes. 2015;64:3532–3542. doi: 10.2337/db15-0024. [DOI] [PubMed] [Google Scholar]
- 91.Simonen-Tikka ML, Pflueger M, Klemola P, et al. Human enterovirus infections in children at increased risk for type 1 diabetes: the Babydiet study. Diabetologia. 2011;54:2995–3002. doi: 10.1007/s00125-011-2305-3. [DOI] [PubMed] [Google Scholar]
- 92.Tapia G, Cinek O, Rasmussen T, et al. Human enterovirus RNA in monthly fecal samples and islet autoimmunity in Norwegian children with high genetic risk for type 1 diabetes: the MIDIA study. Diabetes Care. 2011;34:151–155. doi: 10.2337/dc10-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tauriainen S, Oikarinen S, Oikarinen M, Hyoty H. Enteroviruses in the pathogenesis of type 1 diabetes. Semin Immunopathol. 2011;33:45–55. doi: 10.1007/s00281-010-0207-y. [DOI] [PubMed] [Google Scholar]
- 94.Christen U, Bender C, von Herrath MG. Infection as a cause of type 1 diabetes? Curr Opin Rheumatol. 2012;24:417–423. doi: 10.1097/BOR.0b013e3283533719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Afonso G, Mallone R. Infectious triggers in type 1 diabetes: is there a case for epitope mimicry? Diabetes Obes Metab. 2013;15(Suppl 3):82–88. doi: 10.1111/dom.12166. [DOI] [PubMed] [Google Scholar]
- 96.Masala S, Cossu D, Piccinini S, et al. MAP3865c homologous epitopes are a target of antibody response in new-onset type 1 diabetes children from continental Italy. Pediatr Diabetes. 16:189–195. doi: 10.1111/pedi.12269. [DOI] [PubMed] [Google Scholar]
- 97.Hiemstra HS, Schloot NC, van Veelen PA, et al. Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 2001;98:3988–3991. doi: 10.1073/pnas.071050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2:1279–1283. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 99.Martin S, Wolf-Eichbaum D, Duinkerken G, et al. Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. N Engl J Med. 2001;345:1036–1040. doi: 10.1056/NEJMoa010465. [DOI] [PubMed] [Google Scholar]
- 100.Vardi P, Ziegler AG, Mathews JH, et al. Concentration of insulin autoantibodies at onset of type I diabetes. Inverse log-linear correlation with age. Diabetes Care. 1988;11:736–739. doi: 10.2337/diacare.11.9.736. [DOI] [PubMed] [Google Scholar]
- 101.Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54(Suppl 2):S52–61. doi: 10.2337/diabetes.54.suppl_2.s52. [DOI] [PubMed] [Google Scholar]
- 102.Silva DG, Daley SR, Hogan J, et al. Anti-islet autoantibodies trigger autoimmune diabetes in the presence of an increased frequency of islet-reactive CD4 T cells. Diabetes. 2011;60:2102–2111. doi: 10.2337/db10-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964–1974. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barker JM, Goehrig SH, Barriga K, et al. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27:1399–1404. doi: 10.2337/diacare.27.6.1399. [DOI] [PubMed] [Google Scholar]
- 105.Elding Larsson H, Vehik K, Bell R, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34:2347–2352. doi: 10.2337/dc11-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes. 2012;13:308–313. doi: 10.1111/j.1399-5448.2011.00829.x. [DOI] [PubMed] [Google Scholar]
- 107.Fernandez Castaner M, Montana E, Camps I, et al. Ketoacidosis at diagnosis is predictive of lower residual beta-cell function and poor metabolic control in type 1 diabetes. Diabetes Metab. 1996;22:349–355. [PubMed] [Google Scholar]
- 108.Bowden SA, Duck MM, Hoffman RP. Young children (<5 yr) and adolescents (>12 yr) with type 1 diabetes mellitus have low rate of partial remission: diabetic ketoacidosis is an important risk factor. Pediatr Diabetes. 2008;9:197–201. doi: 10.1111/j.1399-5448.2008.00376.x. [DOI] [PubMed] [Google Scholar]
- 109.Xu P, Wu Y, Zhu Y, et al. Prognostic performance of metabolic indexes in predicting onset of type 1 diabetes. Diabetes Care. 2010;33:2508–2513. doi: 10.2337/dc10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu L, Herold K, Krause-Steinrauf H, et al. Rituximab selectively suppresses specific islet antibodies. Diabetes. 2011;60:2560–2565. doi: 10.2337/db11-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Demeester S, Keymeulen B, Kaufman L, et al. Preexisting insulin autoantibodies predict efficacy of otelixizumab in preserving residual beta-cell function in recent-onset type 1 diabetes. Diabetes Care. 2015;38:644–651. doi: 10.2337/dc14-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]