Abstract
Twin studies have estimated the relative contribution of genes and the environment to variance in exercise behavior and it is known that parental education positively affects exercise levels. This study investigates the role of parental education as a potential modifier of variance in exercise behavior from age 7 to 18 years. The study is based on large datasets from the Netherlands Twin Register (NTR: N= 24,874 twins; surveys around the ages of 7, 10, 12, 14, 16 and 18 years) and two Finnish twin cohorts (FinnTwin12: N= 4,399; 12, 14 and 17 years; FinnTwin16: N=4,648; 16, 17 and 18 years). Regular participation in moderate-to-vigorous exercise activities during leisure time was assessed by survey. Parental education was dichotomized (“both parents with a low education” versus “at least one parent with a high education”). The mean in exercise behavior tended to be higher and the variance tended to be lower in children of high educated parents. Evidence for gene-by-environment interaction was weak. To develop successful interventions that specifically target children of low educated parents, the mechanisms causing the mean and variance differences between the two groups should be better understood.
Keywords: gene-environment interaction, family environment, genetics, physical activity, sport, health, adolescence
INTRODUCTION
A wealth of literature supports the notion that regular physical exercise conveys strong health benefits, such as a lower risk of cardiovascular disease, diabetes and cancer, and improved cardiorespiratory, musculoskeletal and neuromotor fitness (Janssen & Leblanc, 2010; Warburton et al., 2010; Garber et al., 2011). In view of these positive effects that are well-advertised by public health organizations, it is surprising that only a modest proportion of the population engages in regular voluntary exercise. This suggests that we are currently far from understanding the determinants of this important lifestyle behavior. Twin studies can provide a valuable contribution to this understanding as they allow the disentanglement of genetic and environmental influences on behavior. Twin studies have investigated the genetic architecture of exercise behavior across the lifespan (Huppertz et al., 2012; de Geus et al., 2014). For younger children, environmental factors shared by co-twins explain most of the variance in exercise behavior (Huppertz et al., 2012). For adolescents, genetic factors have shown to be a major source of individual differences with heritability estimates between 50% and 85% and with environmental factors specific to each twin individual explaining the remaining variance (van der Aa et al., 2010). In adults, about 40% of the variance is explained by genetic factors and 60% is explained by unique environmental factors (Stubbe et al., 2006; Vink et al., 2011).
An important limitation of these twin studies is that they have ignored the possibility of interaction between genes and the environment. The expression of genes and thus also the genetic variance, however, may depend on environmental circumstances (Purcell, 2002). A more facilitating environment might increase genetic variance, whereas a more restrictive environment might suppress genetic effects. Parental education constitutes an environmental factor that can be relevant to exercise behavior as both knowledge of health behaviors and the economic position, which is positively correlated with parental education (Harden et al., 2007), might facilitate the pursuit of a healthy lifestyle. Parental education has indeed found to be positively associated with physical activity in youth (Ferreira et al., 2006; Hanson & Chen, 2007; Singh et al., 2008). Parental education - as a single measure or combined with occupational status and income - has also been shown to modify the heritability of a whole range of phenotypes in young individuals, including intelligence (Turkheimer et al., 2003), problem behavior (Rosenberg et al., 2012) and body-mass index (Lajunen et al., 2012). Its effect on exercise behavior has not been modelled in previous studies. If there is an effect of parental education on exercise behavior, it may work in two directions: Genetic variance might be lower in children of high educated parents as their parents might be more inclined to support their children in pursuing physical exercise, thereby leaving less choice to the children of whether or not to participate in this behavior. Or genetic variance might be higher in children of high educated parents as more resources, including more alternative hobbies and interests, might be provided, meaning that the children can freely express their genetic preferences. Although these mechanisms might not be mutually exclusive and could cancel each other out, differences in the magnitude of effects would make it possible to detect interactions.
A requirement to detect GxE interaction are very large datasets of twins. Although there are a few large twin registers in the world, not many of them have collected data on exercise behavior in children and adolescents as well as on parental education. The Netherlands Twin Register and two Finnish twin cohorts are exceptions. The Netherlands Twin Register (NTR) was founded in 1987 at the Vrije Universiteit Amsterdam, the Netherlands, to study individual differences in health and behavior (Boomsma et al., 2002; van Beijsterveldt et al., 2013; Willemsen et al., 2013). It has since grown to be one of the largest twin registers in the world with around 85,000 twins and their family members registered to take part in research. Exercise data in young twins have been collected for more than 10 years and now provide a rich resource for research. The University of Helsinki, Finland, hosts two twin cohorts that include data on twins between age 12 and 18 years (Kaprio et al., 2002; Kaprio, 2013). In 1991, data collection of the FinnTwin16 cohort was initiated, when twins born in 1975 were 16 years old and it eventually targeted all Finnish twins born in 1975-79, with follow-up measures later on. Data collection of the FinnTwin12 cohort started in 1994 when the twins born in 1983 were 11-12 years old and eventually targeted all Finnish twins born in 1983-87 that were, again, followed over time. We are fortunate enough to be able to base our study on these impressive resources. Replication of any findings in independent samples is essential and even though some differences in assessments attenuate comparability, the datasets that will be used still provide the best possible approximation of the ideal data that should be used for this study, even on a world-wide scale. The datasets will be analyzed separately to account for any differences between countries and cohorts.
We aim to investigate the role of parental education as a potential modifier of exercise behavior from the ages 7 to 18 years. Genetic models will be fitted conditional on parental education. It is hypothesized that higher parental education will be associated with higher means in offspring's exercise behavior and that there will be (genetic) variance differences between the two groups.
MATERIALS AND METHODS
Participants
The data were derived from the Netherlands Twin Register, the FinnTwin12 cohort and the FinnTwin16 cohort.
The Netherlands Twin Register
In the NTR, parents of twins fill out surveys when their children are born and at the ages of 2 (“survey 2”), 3 (“survey 3”), 5 (“survey 5”), 7 (“survey 7”), 9/10 (“survey 10”), and 12 (“survey 12”) years. From 13 years onwards, the twins are asked to self-report on their health and behavior every two to three years. If individuals decide not to participate in one survey, they will still be approached for subsequent surveys. Participants are mainly Caucasian and live in all regions of the Netherlands.
For this particular study, twins were selected with data on exercise behavior for the surveys around 7, 10, 12, 14, 16 and/or 18 years of age (with a maximum age range of +−2 years) and data on education of at least one parent. Exclusion criteria were a serious illness or disability (e.g., hemiplegia or heart disease, N= 354 individuals) and unknown zygosity (N= 1 pair). If participants reported an injury that interferes with physical activity on surveys 14, 16 and/or 18 years of age, data were excluded for that specific survey (N= 403 individuals for survey 14, 439 for survey 16 and 65 for survey 18). The sample thus consisted of 24,874 twins born between 1986 and 2005 (49% males). Zygosity classification of same-sex twin pairs was based on blood group or DNA typing for 20% of the pairs and it was survey-based for 80%. Classification based on questions on physical similarities and mistaking one twin for another by relatives and strangers has previously shown 93%-97% agreement with DNA polymorphisms in the NTR (Rietveld et al., 2000; Willemsen et al., 2005). Participants consented to take part in research of the NTR and the data collection protocol was approved by the Medical Research Ethics Committee of the VU University Medical Center
The Finnish twin cohort
The Finnish twin data were collected in two young cohorts (FinnTwin12 and FinnTwin16). At the baseline assessment of the FinnTwin12 cohort, a survey was mailed to all Finnish twins born between 1983-87, when they were approximately 12 years old (N= 5,184 twins, response rate: 94%) and their parents (response rates >86%). The twins received follow-up surveys around the ages of 14 and 17 years.
At the baseline assessment of the FinnTwin16 cohort, surveys were sent out to all Finnish twins born between 1975-79, when they were approximately 16 years old. The surveys were sent out to the twins (N= 4,940 twins, response rate: 88%) and to their parents (response rates >79%). The twins were approached with follow-up surveys around the ages of 17 and 18 years. All participating families provided written informed consent and the data collection protocol was approved by the ethics committee of the Department of Public Health, University of Helsinki, and the Institutional Review Board (IRB), Indiana University.
For FinnTwin12, individuals were selected with data on education of at least one parent and on exercise behavior around the ages 12, 14 and/or 17 years. After exclusion of twins with unknown zygosity (N= 134 pairs), the sample consisted of 4,399 individuals (51% males). Zygosity classification for 72% of the same-sex twin pairs was based on survey items on physical similarity at school age, supplemented with additional information such as photographs if classification was unclear. Zygosity classification based on survey items has shown 97% correspondence with classification based on DNA polymorphisms in 395 same-sex twin pairs from the FinnTwin12 study (Jelenkovic et al., 2011). For the remaining pairs, zygosity classification was based on DNA typing.
For FinnTwin16, data were selected of individuals with information on parental education and on exercise behavior around the ages 16, 17 and/or 18 years. Exercise measurements were changed to missing when a serious illness or disability was consistently reported over time (N= 33 individuals). Furthermore, 103 pairs were excluded due to missing information on zygosity. The sample thus consisted of 4,648 individuals of which 48% were males. For 75% of the same-sex twin pairs, zygosity classification was based on validated survey items (Sarna et al., 1978) and for 25%, it was based on DNA typing.
Measures
Parental education
Within the NTR, both mothers and fathers were asked to indicate their level of education shortly after their twins were born and when the twins were 3, 7 and 10 years old. This information was used to classify both mothers and fathers into two levels (more recent surveys were preferred): 1) low education (66% of the mothers, 69% of the fathers) and 2) high education (34%, 31%). “High education” corresponds to a university degree or a university of applied sciences degree. In 313 families, one parent was low educated and the other had not provided any information on education. These families were excluded as they could not be clearly assigned to one of the two groups. Next, the parental data were combined into two groups of parental education: families where at least one parent was high educated (the other parent could be low educated, high educated or missing; 43%), and families where both parents were low educated (57%).
Within the FinnTwin cohorts, both mothers and fathers indicated their level of education at the baseline assessment when their twins were 12 (FinnTwin12) or 16 (FinnTwin16) years old. In these cohorts, “high education” corresponds to a high school degree that allows entry to further training at a university. Again, both mothers and fathers were grouped into two levels of education (mothers of the FinnTwin12 cohort: 62% low, 38% high; fathers of the FinnTwin12 cohort: 76% low, 24% high; mothers of the FinnTwin16 cohort: 73% low, 27% high; fathers of the FinnTwin16 cohort: 80% low, 20% high). Families where one parent was low educated and the other had not provided any information on education were excluded (N= 260 families for FinnTwin12 and N= 369 families for FinnTwin16). Next, parental data were combined into two groups: for 44% of the families in the FinnTwin12 cohort, at least one of the parents was high educated, whereas for 56%, both were low educated. For the FinnTwin16 cohort, these figures were 34% and 66%, respectively. Table 1 depicts the number of twins and complete twin pairs for each survey of the NTR, the FinnTwin12 cohort and the FinnTwin16 cohort that were included in this study, split by the two levels of parental education and sex x zygosity group.
TABLE 1.
Number of twins (complete pairs), split by parental education and zygosity
| A. Low parental
education | |||||
|---|---|---|---|---|---|
| Survey | MZM | DZM | MZF | DZF | DOS |
|
Netherlands Twin Register
| |||||
| 7 | 542 (269) | 647 (321) | 623 (310) | 549 (273) | 1,165 (580) |
| 10 | 677 (335) | 701 (345) | 760 (378) | 684 (339) | 1,433 (708) |
| 12 | 1,344 (664) | 1,260 (611) | 1,588 (785) | 1,281 (630) | 2,551 (1,250) |
| 14 | 687 (302) | 619 (245) | 1,046 (466) | 801 (342) | 1,370 (555) |
| 16 | 472 (193) | 370 (133) | 753 (316) | 601 (234) | 867 (302) |
| 18 | 185 (74) | 155 (53) | 423 (175) | 314 (118) | 390 (122) |
|
FinnTwin12 | |||||
| 12 | 394 (195) | 413 (204) | 426 (212) | 377 (187) | 811 (401) |
| 14 | 364 (180) | 371 (182) | 398 (196) | 336 (164) | 726 (347) |
| 17 | 323 (157) | 335 (161) | 375 (183) | 308 (149) | 645 (308) |
|
FinnTwin16 | |||||
| 16 | 397 (196) | 520 (254) | 549 (274) | 476 (236) | 1,122 (556) |
| 17 | 364 (175) | 489 (236) | 536 (265) | 456 (224) | 1,066 (513) |
| 18 | 353 (169) | 485 (234) | 527 (260) | 465 (227) | 1,053 (499) |
| B. High parental
education | |||||
|---|---|---|---|---|---|
| Survey | MZM | DZM | MZF | DZF | DOS |
|
Netherlands Twin Register
| |||||
| 7 | 603 (301) | 580 (289) | 643 (320) | 535 (266) | 1,091 (541) |
| 10 | 644 (321) | 644 (318) | 645 (321) | 502 (248) | 1,160 (575) |
| 12 | 954 (474) | 944 (466) | 1,128 (560) | 813 (401) | 1,954 (962) |
| 14 | 554 (239) | 525 (229) | 781 (348) | 586 (261) | 1,193 (493) |
| 16 | 386 (166) | 361 (135) | 553 (231) | 358 (130) | 783 (285) |
| 18 | 140 (59) | 141 (51) | 271 (111) | 187 (78) | 320 (111) |
|
FinnTwin12 | |||||
| 12 | 318 (158) | 347 (171) | 322 (160) | 301 (147) | 629 (308) |
| 14 | 284 (138) | 317 (155) | 298 (148) | 286 (140) | 597 (289) |
| 17 | 256 (126) | 281 (136) | 291 (143) | 275 (137) | 542 (262) |
|
FinnTwin16 | |||||
| 16 | 236 (116) | 248 (121) | 312 (154) | 253 (123) | 493 (245) |
| 17 | 221 (108) | 239 (116) | 306 (151) | 252 (122) | 472 (229) |
| 18 | 220 (106) | 238 (114) | 308 (152) | 247 (118) | 478 (233) |
MZM=monozygotic male, DZM=dizygotic male, MZF=monozygotic female, DZF=dizygotic female, DOS=dizygotic of opposite-sex.
Exercise behavior
Within the NTR, exercise behavior was quantified as weekly metabolic equivalents of task (MET) hours spent on regular exercise behavior during leisure time. Exercise behavior was assessed through parental reports in the surveys around 7, 10 and 12 years of age and by self-reports in the surveys around 14, 16 and 18 years of age. Participants were asked to indicate what kind of activities their children (parental report) or they (self-report) participated in and - if any - 1) for how many years, 2) for how many months a year, 3) how many times a week and 4) how many minutes each time. Activities that were done for less than half a year or less than three months a year were excluded (e.g., skiing, sailing camps), as well as activities that merely increase energy expenditure (e.g., playing chess), mandatory physical education at school and activities that are related to transportation (e.g., walking, cycling). If exercise frequency or duration were missing while the other one was indicated, the missing value was replaced with the median of that specific activity within the respective survey (see (Huppertz et al., 2012)). All reported activities were assigned a MET score based on Ridley et al.'s compendium of energy expenditures for youth (Ridley et al., 2008). The respective values represent the energy that is expended during the activity relative to energy expenditure at rest (which would be 1 MET). Weekly MET hours were calculated by summing the product of frequency, duration and the MET score over all activities that an individual took part in. Good test-retest reliability of this measure has been established in previous studies (Stubbe et al., 2007; de Moor et al., 2008). For age 7, data were provided by both parents for 62% of the children, by mothers only for 35% of the children and by fathers only for 3% of the children. For age 10, these were 24%, 75% and 1%, respectively, and for age 12, these were 44%, 55% and 1%. As mothers’ and fathers’ ratings correlated high at all ages (0.73 for age 7, 0.88 for age 10 and 0.89 for age 12), their ratings were averaged when both parents had reported on the same child.
Within the FinnTwin cohorts, the twins self-reported on their exercise behavior across all ages. They were asked how often they engage in moderate-to-vigorous exercise or sport activities during their leisure time, with the following answer options: 1) not at all, 2) less than once a month, 3) one or two times a month, 4) about once a week, 5) two or three times a week, 6) four or five times a week and 7) just about every day (or more). For the baseline assessment at age 12 in the FinnTwin12 cohort, the answer options were different, namely: 1) daily, 2) a few times a week, 3) a few times a month, 4) a few times in 6 months and 5) never. They were reversely scored for the analyses so that a higher score corresponds to a higher exercise level, with “1= never”, in order to better match the other assessments. The response categories were treated as continuous scores in all analyses.
Data analysis
The analyses were done for each dataset and age group separately (six age groups in the NTR, three age groups in FinnTwin12 and three age groups in FinnTwin16). Twin data allow the phenotypic variance to be decomposed into variance that is due to 1) additive genetic factors (“A”), 2) shared environmental factors (“C” for “common”) and 3) unique environmental factors (“E”; which includes measurement error). Shared environmental factors are common to both individuals of a twin pair (e.g., growing up in the same family or attending the same school), whereas unique environmental factors are unique to each child (e.g., having different friends or non-shared illnesses).
To get a first indication of the relative contribution of A, C and E to exercise behavior within the two groups of parental education, twin correlations were estimated for each of the five sex x zygosity groups, for children of low versus high educated parents separately. Monozygotic (MZ) twins are genetically virtually identical at the sequence level, whereas dizygotic (DZ) twins share, on average, 50% of their segregating genes. As environmental influences are assumed to be the same for MZ and DZ twins, a higher MZ twin resemblance (rMZ > rDZ) indicates genetic influences. A DZ twin correlation that is larger than half of the MZ twin correlation indicates environmental influences shared by co-twins that make DZ twins more similar to each other than what would be expected based on their genetic similarity alone. An MZ twin correlation that is not unity (rMZ < 1) points towards environmental influences that the two twins of a pair do not share and that therefore make them more different from each other. This includes measurement error.
Twin correlations may also indicate quantitative and/or qualitative sex differences. The former denotes that the same genetic and/or environmental factors operate to different degrees in males and females, which is reflected in different twin correlations for males and females. Qualitative sex differences, in contrast, are present if different genetic and/or shared environmental factors operate in males and females. This is reflected in correlations of dizygotic twins of opposite-sex (DOS) that cannot be predicted based on the dizygotic male (DZM) and the dizygotic female (DZF) correlations (Falconer & Mackay, 1996). Qualitative genetic and shared environmental sex differences cannot be modelled at the same time. In our previous work, we found the shared environmental differences to be more relevant in this respect (Stubbe et al., 2005; Huppertz, 2012).
The twin correlations were derived from saturated models with separate means and variances for the first-born and the second-born twin and for each sex x zygosity x parental education group. Next, just one mean and one variance were estimated across twin order and zygosity, for both sexes and parental education groups separately (e.g., one mean for sons of low educated parents, one mean for sons of high educated parents, one mean for daughters of low educated parents and one mean for daughters of high educated parents). One-by-one, it was tested whether constraining the 1) means of males, 2) means of females, 3) variances of males or 4) variances of females to be equal across parental education led to a significant deterioration of the model fit. This was done to identify any differences in means or variances between children of low versus high educated parents. A stringent alpha level of 0.01 was chosen to account for the large number of tests in this study.
Next, a series of genetic models were fitted to the data. First, an ACE model was fitted allowing for quantitative and qualitative sex differences in the variance components and sex differences in the means. In order to control for gene-environment correlation, separate means were estimated for children of low and high educated parents (Purcell, 2002). For children of low versus high educated parents separately, the phenotypic variance of exercise behavior was decomposed into additive genetic variance, shared environmental variance and unique environmental variance. The latent A-components were constrained to correlate 1 for MZ twins (100% shared genes) and 0.5 for DZ twins (50% shared genes). The latent C-components were constrained to correlate 1 for both types of (same-sex) twins and the E-components were, by definition, not allowed to correlate.
Separate parameters were estimated for males and females to allow for quantitative sex differences and the correlations between the C-components were initially estimated freely for DOS twins to allow for qualitative sex differences at the outset, whereas the correlations between the A-components were not allowed to vary, in accordance with our previous work (Stubbe et al., 2005; Huppertz et al, 2012). Next, it was tested whether the correlations between the C-components of DOS twins could be constrained to 1 without a significant deterioration of the model fit (α= 0.01). Only if this did not change the model fit significantly, subsequent tests for differences in the variance components between children of low and high educated parents were performed on the ACE models without qualitative sex differences.
In order to identify differences in the variance decomposition between children of low versus high educated parents, various constraints were subsequently imposed on the unstandardized variance components. For males and females separately, it was tested whether equating the A-, C- and E-components for children of low and high educated parents, simultaneously and one-by-one, led to a significant deterioration of the model fit (Purcell, 2002). For the simultaneous test, again a stringent alpha level of 0.01 was chosen to account for the large number of tests, and for the separate tests, a Bonferroni correction was applied (α= 0.01/3). For all analyses, the raw-data maximum likelihood procedure was used to estimate the parameters. Nested submodels were compared with hierarchic χ2-tests. The −2 log-likelihood (LL) of the constrained model was subtracted from the −2LL of the less constrained model, and significance was tested based on the χ2-distribution and given the difference in degrees of freedom between the respective models. All analyses were run with the software package OpenMx 2.0.1 in R 3.1.2 (Boker et al., 2011).
RESULTS
Table 2 contains the means and variances of exercise behavior for each survey, stratified by sex and parental education, as well as the mean ages with standard deviations. In the NTR, means and variances of exercise behavior tended to increase with age. Mean exercise behavior was lower for children of parents with a low education in 7-, 10-, 12-, 14- and 16-year-old females and for 12-year-old males. Means were also lower at other ages, but this did not reach statistical significance. The variance in exercise behavior tended to be larger in children of parents with a low education - the effect was significant for 5 of the 12 comparisons. For 18-year-old females, an effect in the opposite direction was found, with a significantly lower variance in the group of low educated parents. In the Finnish twin cohorts, means and variances were relatively stable across ages. The means do not represent “mean frequency”, but rather the mean of the response categories that were assessed. It is important to note that the means at age 12 reflect a 5-point scale, whereas at the other ages, they reflect a 7-point scale, which explains the much lower means for age 12. Again, means were consistently lower for children of parents with a low education, whereas the variances were consistently larger for this group, with the exception of 18-year-old females in the FinnTwin16 cohort. However, as indicated by the p-values, the differences in means and variances were partly significant in the FinnTwin12 cohort, but not in the FinnTwin16 cohort.
TABLE 2.
Means and variances of weekly MET hours (NTR) and items on frequency of exercise behavior (FinnTwin), split by sex and parental education, and p-values that result from equating means or variances to be equal for children of low and high educated parents
| A. Males | |||||
|---|---|---|---|---|---|
| Survey | Mean age (SD) | Low parental education | High parental education | p-value meana | p-value variancea |
|
Netherlands Twin Register
| |||||
| 7 | 7.53 (.34) | 14.31 (151.00), 1772 | 15.27 (127.40), 1727 | .04 | 2.6e-3 |
| 10 | 9.83 (.44) | 22.36 (388.51), 2092 | 22.88 (363.64), 1867 | .46 | .20 |
| 12 | 12.24 (.39) | 24.54 (437.36), 3872 | 27.33 (454.48), 2872 | 3.3e-6 | .33 |
| 14 | 14.63 (.59) | 29.05 (909.67), 1939 | 31.18 (734.93), 1648 | .04 | 2.1e-5 |
| 16 | 16.87 (.44) | 30.76 (1175.00), 1212 | 32.67 (944.54), 1114 | .19 | 4.2e-4 |
| 18 | 18.76 (.53) | 24.43 (800.51), 491 | 23.78 (731.59), 410 | .75 | .37 |
|
FinnTwin12 | |||||
| 12 | 11.42 (.30) | 3.39 (2.00), 1215 | 3.65 (1.55), 980 | 7.9e-5 | 1.3e-4 |
| 14 | 14.05 (.09) | 5.05 (2.42), 1088 | 5.13 (2.10), 896 | .25 | .04 |
| 17 | 17.62 (.22) | 4.79 (2.97), 970 | 5.02 (2.56), 804 | 9.7e-3 | .04 |
|
FinnTwin16 | |||||
| 16 | 16.17 (.13) | 4.69 (2.82), 1475 | 4.81 (2.76), 730 | .14 | .76 |
| 17 | 17.14 (.08) | 4.78 (2.82), 1372 | 4.99 (2.71), 693 | .02 | .57 |
| 18 | 18.61 (.17) | 4.69 (2.71), 1344 | 4.78 (2.52), 694 | .25 | .31 |
| B. Females | |||||
|---|---|---|---|---|---|
| Survey | Mean age (SD) | Low parental education | High parental education | p-value meana | p-value variancea |
|
Netherlands Twin Register
| |||||
| 7 | 7.51 (.34) | 9.47 (92.24), 1754 | 10.63 (85.97), 1725 | 1.9e-3 | .21 |
| 10 | 9.85 (.43) | 14.58 (244.58), 2163 | 16.61 (229.69), 1728 | 4.3e-4 | .24 |
| 12 | 12.24 (.39) | 16.53 (317.13), 4152 | 19.32 (303.72), 2921 | 1.9e-8 | .28 |
| 14 | 14.63 (.61) | 19.58 (625.80), 2584 | 23.32 (519.88), 1991 | 2.6e-6 | 5.2e-5 |
| 16 | 16.89 (.46) | 18.16 (567.56), 1851 | 22.34 (585.12), 1327 | 1.3e-5 | .58 |
| 18 | 18.76 (.49) | 14.68 (434.10), 976 | 17.77 (528.98), 649 | .01 n.s. | 8.7e-3 |
|
FinnTwin12 | |||||
| 12 | 11.41 (.30) | 2.85 (2.11), 1206 | 3.04 (2.08), 937 | 9.3e-3 | .80 |
| 14 | 14.04 (.08) | 4.90 (2.41), 1107 | 5.08 (1.97), 886 | .02 | 3.5e-3 |
| 17 | 17.61 (.23) | 4.80 (2.62), 1016 | 5.00 (2.19), 841 | 1.3e-2 | 1.2e-2 |
|
FinnTwin16 | |||||
| 16 | 16.15 (.13) | 4.67 (2.46), 1589 | 4.68 (2.13), 812 | .90 | .03 |
| 17 | 17.13 (.07) | 4.74 (2.27), 1539 | 4.84 (1.97), 797 | .16 | .04 |
| 18 | 18.59 (.16) | 4.72 (2.13), 1539 | 4.70 (2.20), 797 | .74 | .64 |
Compared to the model where means and variances are equal across twin order and zygosity status, but not across sex and parental education.
Table 3 depicts the twin correlations and their 99% confidence intervals (CIs). In the NTR, MZ correlations were consistently higher than DZ twin correlations, with the exception of 16-year-old daughters of high educated parents, and the same-sex DZ twin correlations were larger than half of the MZ twin correlations in all but a few cases, implying that both genetic effects and shared environmental effects contribute to the variance in children's and adolescents’ exercise behavior. DZ correlations tended to be higher for females than for males, implying a larger influence of shared environmental factors in females. Especially for the younger ages, the DOS correlations were lower than what would be expected based on the same-sex DZ correlations, suggesting qualitative sex differences. In the Finnish twin cohorts, the MZ twin correlations were - with the exception of 14-year-old daughters of high educated parents - consistently higher than the DZ correlations and the same-sex DZ correlations were higher than half of the MZ correlations, with three exceptions. The twin correlations of males and females were fairly similar, but the DOS correlations were consistently lower than the same-sex DZ correlations.
TABLE 3.
Twin correlations, split by zygosity and parental education (99% CIs)
| A. Netherlands Twin
Register | |||||||
|---|---|---|---|---|---|---|---|
| Zygosity | Education | Survey 7 | Survey 10 | Survey 12 | Survey 14 | Survey 16 | Survey 18 |
| MZM | Low | .90(.86;.92) | .90(.87;.92) | .87(.85;.90) | .57(.46;.66) | .60(.46;.70) | .64(43;.78) |
| High | .91(.88;.93) | .89(.86;.92) | .88(.85;.90) | .53(.40;.63) | .43(.25;.57) | .47(.18;.68) | |
| DZM | Low | .80(.74;.84) | .57(.47;.66) | .61(.54;.67) | .38(.24;.51) | .41(.19;.58) | .45(.17;.66) |
| High | .80(.74;.85) | .70(.62;.77) | .62(.54;.68) | .31(.15;.46) | .23(−.01;.44) | .33(−.01;.59) | |
| MZF | Low | .89(.85;.92) | .91(.88;.93) | .92(.91;.93) | .74(.68;.79) | .64(.55;.72) | .58(.45;.69) |
| High | .86(.81;.89) | .85(.81;.89) | .86(.83;.88) | .51(.40;.60) | .61(.50;.70) | .74(.59;.83) | |
| DZF | Low | .82(.76;.87) | .67(.58;.74) | .69(.64;.74) | .45(.32;.56) | .26(.11;.40) | .15(−.09;.37) |
| High | .81(.75;.86) | .80(.74;.85) | .76(.70;.81) | .39(.24;.52) | .67(.49;.78) | .16(−.15;.44) | |
| DOS | Low | .40(.30;.48) | .38(.29;.46) | .40(.34;.46) | .13(.03;.24) | .20(.04;.34) | .35(.15;.51) |
| High | .50(.41;.58) | .45(.36;.53) | .44(.37;.51) | .28(.16;.39) | .21(.05;.35) | .20(−.05;.42) | |
| B. The Finnish twin
cohorts | |||||||
|---|---|---|---|---|---|---|---|
| FinnTwin12 | FinnTwin16 | ||||||
| Zygosity | Education | Survey 12 | Survey 14 | Survey 17 | Survey 16 | Survey 17 | Survey 18 |
| MZM | Low | .70(.59;.78) | .66(.54;.76) | .71(.59;.79) | .64(.52;.74) | .59(.45;.70) | .63(.50;.74) |
| High | .59(.44;.71) | .62(.46;.73) | .77(.65;.85) | .75(.63;.84) | .75(.62;.84) | .72(.58;.82) | |
| DZM | Low | .50(.35;.62) | .43(.26;.57) | .41(.22;.57) | .41(.26;.53) | .37(.21;.51) | .38(.22;.52) |
| High | .46(.29;.60) | .31(.11;.48) | .49(.29;.64) | .49(.29;.65) | .44(.22;.61) | .38(.16;.57) | |
| MZF | Low | .70(.60;.78) | .66(.55;.75) | .64(.52;.74) | .71(.62;.78) | .70(.61;.77) | .66(.56;.74) |
| High | .70(.59;.79) | .49(.31;.63) | .65(.51;.76) | .68(.55;.78) | .79(.70;.86) | .74(.63;.82) | |
| DZF | Low | .61(.47;.71) | .41(.23;.57) | .32(.12;.50) | .44(.29;.56) | .50(.36;.62) | .30(.13;.45) |
| High | .66(.52;.76) | .51(.33;.65) | .43(.23;.59) | .51(.31;.66) | .43(.22;.60) | .31(.09;.51) | |
| DOS | Low | .28(.15;.39) | .28(.15;.40) | .22(.08,.35) | .19(.08;.29) | .20(.09;.31) | .20(.09;.31) |
| High | .34(.20;.46) | .08(−.07;.23) | .15(−.01;.30) | .20(.04;.35) | .24(.08;.39) | .25(.09;.40) | |
Based on the fully saturated model; MZM=monozygotic male, DZM=dizygotic male, MZF=monozygotic female, DZF=dizygotic female, DOS=dizygotic of opposite-sex.
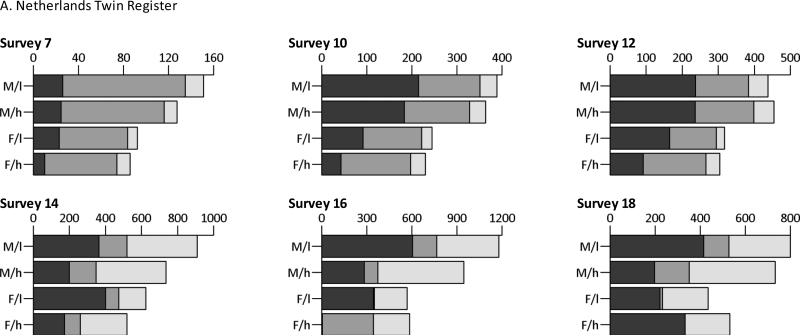
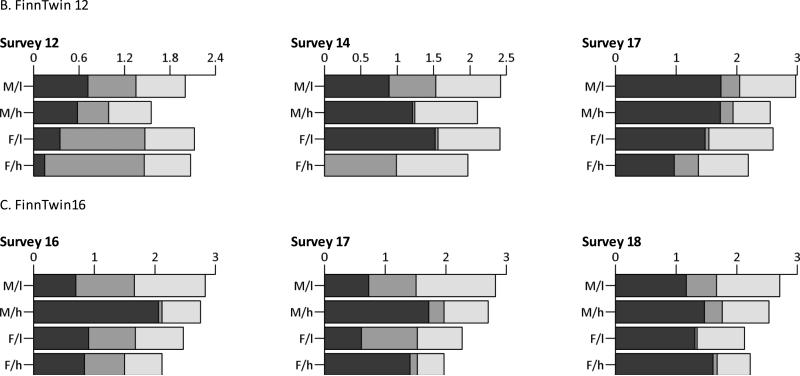
Figure 1, Table 4 and Figure 2 contain the results of the genetic model fitting. The full ACE models were compared to models that did not allow for qualitative sex differences. In the NTR, qualitative sex differences were present for the ages 7, 10 and 12 years in both groups of parental education, but not for the ages 14, 16 and 18 years. The figures thus depict the A, C and E estimates of the models with a freely estimated correlation between the C-components for DOS twins for the first three age groups and with a correlation that was constrained to 1 for the last three age groups. In the Finnish twin cohorts, the genetic models revealed that there were no sex-specific shared environmental effects and thus qualitative sex differences were not taken into account in the ACE models. Figure 1 depicts the unstandardized variance components (A, C, E), for children of low versus high educated parents separately. Supplementary table 1 contains the exact numbers with 99% CIs.
Fig. 1.
Unstandardized additive genetic (dark gray), shared environmental (gray) and non-shared environmental (light gray) variance components, split by sex and parental education (M/l= males, low educated parents; M/h= males, high educated parents; F/l= females, low educated parents; F/h= females, high educated parents)
TABLE 4.
Model fit indices for constraining the unstandardized additive genetic (A), shared environmental (C) and non-shared environmental (E) variance components to be equal for children of low and high educated parents, split by sex
| Males | Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Survey | −2LL | χ 2 | Δdf | p-value | −2LL | χ 2 | Δdf | p-value | |
|
Netherlands Twin Register
| |||||||||
| 7 | Full modela | 49321.1 | - | - | - | 49321.1 | - | - | - |
| ACE equal | 49342.3 | 21.2023 | 3 | 9.6e-05 | 49332.95 | 11.8514 | 3 | .0079 | |
| A equal | 49321.14 | .0431 | 1 | .8355 | 49327.63 | 6.5305 | 1 | .0106 | |
| C equal | 49324.09 | 2.9934 | 1 | .0836 | 49321.33 | .2325 | 1 | .6297 | |
| E equal | 49330.68 | 9.5878 | 1 | .0020 | 49329.52 | 8.4230 | 1 | .0037 | |
| 10 | Full model | 63814.18 | - | - | - | 63814.18 | - | - | - |
| ACE equal | 63817.19 | 3.0086 | 3 | .3903 | 63830.40 | 16.2249 | 3 | .0010 | |
| A equal | 63815.25 | 1.0721 | 1 | .3005 | 63823.99 | 9.8151 | 1 | .0017 | |
| C equal | 63814.25 | .0701 | 1 | .7912 | 63815.93 | 1.7513 | 1 | .1857 | |
| E equal | 63814.44 | .2628 | 1 | .6082 | 63824.77 | 10.5922 | 1 | .0011 | |
| 12 | Full model | 115286.6 | - | - | - | 115286.6 | - | - | - |
| ACE equal | 115287.7 | 1.1094 | 3 | .7748 | 115340.6 | 53.9805 | 3 | 1.1e-11 | |
| A equal | 115286.6 | .0004 | 1 | .9835 | 115304.1 | 17.5573 | 1 | 2.8e-05 | |
| C equal | 115286.8 | .2302 | 1 | .6314 | 115291.9 | 5.2743 | 1 | .0216 | |
| E equal | 115286.8 | .1943 | 1 | .6594 | 115333.1 | 46.4837 | 1 | 9.2e-12 | |
| 14 | Full model | 75413.25 | - | - | - | 75413.25 | - | - | - |
| ACE equal | 75432.01 | 18.7622 | 3 | .0003 | 75462.5 | 49.2505 | 3 | 1.2e-10 | |
| A equal | 75414.54 | 1.2905 | 1 | .2560 | 75422.62 | 9.3752 | 1 | .0022 | |
| C equal | 75413.25 | .0024 | 1 | .9607 | 75413.29 | .0452 | 1 | .8317 | |
| E equal | 75413.25 | .0011 | 1 | .9739 | 75442.35 | 29.1015 | 1 | 6.9e-08 | |
| 16 | Full model | 51489.31 | - | - | - | 51489.31 | - | - | - |
| ACE equal | 51509.80 | 20.4875 | 3 | .0001 | 51502.88 | 13.5651 | 3 | .0036 | |
| A equal | 51492.59 | 3.2733 | 1 | .0704 | 51501.79 | 12.4781 | 1 | .0004 | |
| C equal | 51489.47 | .1611 | 1 | .6882 | 51502.76 | 13.4432 | 1 | .0002 | |
| E equal | 51494.41 | 5.1001 | 1 | .0239 | 51490.03 | .7140 | 1 | .3981 | |
| 18 | Full model | 22933.47 | - | - | - | 22933.47 | - | - | - |
| ACE equal | 22936.82 | 3.3488 | 3 | .3409 | 22940.87 | 7.4019 | 3 | .0601 | |
| A equal | 22934.24 | .7744 | 1 | .3789 | 22935.60 | 2.1312 | 1 | .1443 | |
| C equal | 22933.50 | .0339 | 1 | .8540 | 22933.68 | .2127 | 1 | .6446 | |
| E equal | 22935.61 | 2.1406 | 1 | .1434 | 22933.48 | .0129 | 1 | .9097 | |
|
FinnTwin12 | |||||||||
| 12 | Full model | 14383.79 | - | - | - | 14383.79 | - | - | - |
| ACE equal | 14399.29 | 15.5043 | 3 | .0014 | 14385.59 | 1.8028 | 3 | .6143 | |
| A equal | 14384.19 | .4042 | 1 | .5249 | 14384.44 | .6510 | 1 | .4198 | |
| C equal | 14385.07 | 1.2857 | 1 | .2568 | 14384.46 | .6710 | 1 | .4127 | |
| E equal | 14384.87 | 1.0828 | 1 | .2981 | 14383.99 | .2017 | 1 | .6534 | |
| 14 | Full model | 14024.63 | - | - | - | 14024.63 | - | - | - |
| ACE equal | 14030.19 | 5.5637 | 3 | .1349 | 14038.75 | 14.1226 | 3 | .0027 | |
| A equal | 14025.31 | .6893 | 1 | .4064 | 14030.48 | 5.8522 | 1 | .0156 | |
| C equal | 14026.08 | 1.4513 | 1 | .2283 | 14026.77 | 2.1491 | 1 | .1427 | |
| E equal | 14024.69 | .0625 | 1 | .8026 | 14025.96 | 1.3333 | 1 | .2482 | |
| 17 | Full model | 13267.41 | - | - | - | 13267.41 | - | - | - |
| ACE equal | 13277.62 | 10.2019 | 3 | .0169 | 13277.72 | 10.3034 | 3 | .0162 | |
| A equal | 13267.41 | 1.47e-06 | 1 | .9990 | 13268.36 | .9438 | 1 | .3313 | |
| C equal | 13267.45 | .0344 | 1 | .8528 | 13267.96 | .5421 | 1 | .4616 | |
| E equal | 13273.88 | 6.4670 | 1 | .0110 | 13270.71 | 3.2928 | 1 | .0696 | |
|
FinnTwin16 | |||||||||
| 16 | Full model | 16713.28 | - | - | - | 16713.28 | - | - | - |
| ACE equal | 16725.04 | 11.7615 | 3 | .0082 | 16720.99 | 7.7081 | 3 | .0524 | |
| A equal | 16716.38 | 3.0935 | 1 | .0786 | 16713.31 | .0282 | 1 | .8665 | |
| C equal | 16714.57 | 1.2825 | 1 | .2574 | 16713.39 | .1037 | 1 | .7474 | |
| E equal | 16723.59 | 10.3076 | 1 | .0013 | 16715.94 | 2.6574 | 1 | .1031 | |
| 17 | Full model | 15760.69 | - | - | - | 15760.69 | - | - | - |
| ACE equal | 15773.01 | 12.3249 | 3 | .0063 | 15778.14 | 17.4459 | 3 | .0006 | |
| A equal | 15764.20 | 3.5088 | 1 | .0610 | 15766.28 | 5.5907 | 1 | .0181 | |
| C equal | 15762.25 | 1.5564 | 1 | .2122 | 15766.74 | 6.0525 | 1 | .0139 | |
| E equal | 15773.00 | 12.3126 | 1 | .0004 | 15774.21 | 13.5205 | 1 | .0002 | |
| 18 | Full model | 15638.47 | - | - | - | 15638.47 | - | - | - |
| ACE equal | 15642.24 | 3.7706 | 3 | .2873 | 15645.87 | 7.4005 | 3 | .0602 | |
| A equal | 15638.87 | .4028 | 1 | .5256 | 15640.06 | 1.5935 | 1 | .2068 | |
| C equal | 15638.74 | .2720 | 1 | .6020 | 15638.52 | .0501 | 1 | .8229 | |
| E equal | 15641.59 | 3.1229 | 1 | .0772 | 15645.26 | 6.7912 | 1 | .0092 | |
The unstandardized variance components were equated simultaneously (ACE) and separately (A, C, E).
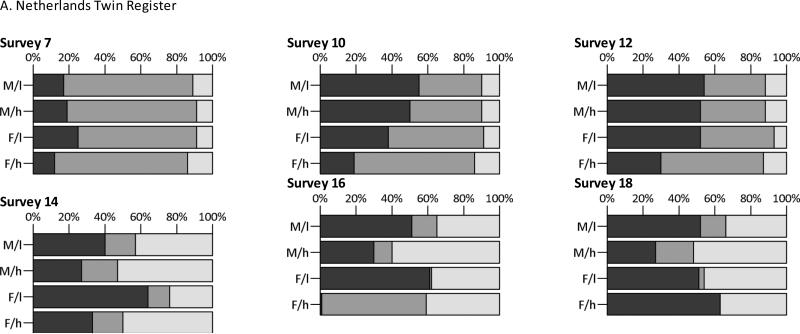
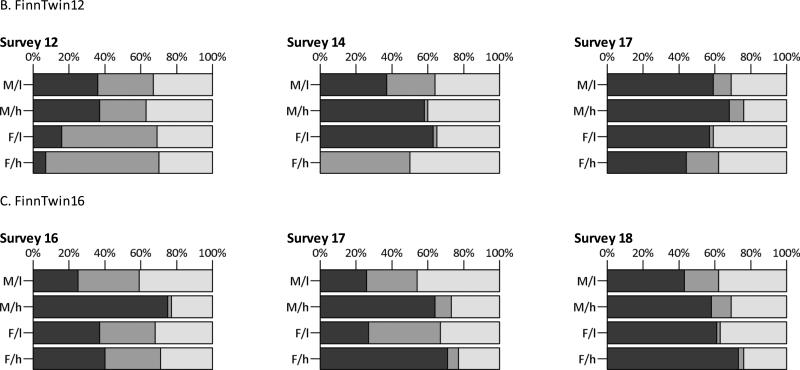
Fig. 2.
Standardized additive genetic (dark gray), shared environmental (gray), and non-shared environmental (light gray) variance components, split by sex and parental education (M/l= males, low educated parents; M/h= males, high educated parents; F/l= females, low educated parents; F/h= females, high educated parents)
In the NTR, the variance of the unstandardized A-components tended to be attenuated in children of high educated parents compared to children of low educated parents. The C-components tended to be smaller in daughters of low educated parents with a large and statistically significant effect in 16-year-olds. In the Finnish twin cohorts, no consistent differences in the unstandardized A-components according to parental education could be observed. The genetic variance tended to be lower in children of parents with a high education in FinnTwin12 but higher in FinnTwin16. The shared environmental variance in males of the FinnTwin12 cohort and in both males and females of the FinnTwin16 cohort tended to be lower in high educated parents with the exception of 18-year-old females in the FinnTwin16 cohort. The E-component was consistently lower in children of high educated parents, with the exception of 14-year-old females of the FinnTwin12 cohort.
Table 4 depicts the model fitting indices of the models 1) simultaneously constraining the unstandardized A, C and E to be equal across groups of parental education, 2) only constraining the unstandardized A to be equal, 3) only constraining the unstandardized C to be equal and 4) only constraining the unstandardized E to be equal across the two groups. In the NTR, comparing the models that estimated A, C and E freely for the two groups and the models that equated the three components at the same time led to significant p-values in the ages 7, 14 and 16 for males and in all but the last age group for females. Subsequently constraining each variance component separately indicated significant differences after Bonferroni correction - namely in males, a lower E-component with high educated parents for 7-year-olds, and in females, lower A-components with high educated parents for the ages 10, 12, 14 and 16 years, a higher C-component for age 16 and higher E-components for the ages 10, 12 and 14. In the Finnish twin cohorts, few of the observed differences were significant. For the FinnTwin12 cohort, constraining A, C and E simultaneously led to a significant deterioration of the model fit for 12-year-old males and for 14-year-old females. For the FinnTwin16 cohort, significant differences were apparent for 16-year-old males and both males and females aged 17 years old. Post-hoc tests consistently revealed significant differences in the E-components, with a smaller unique environmental variances in children of higher educated parents.
Figure 2 depicts the variance components relative to the total variance (e.g., for A: A/V) as percentages. The exact numbers with 99% CIs can be found in supplementary table 2. In the NTR, the relative contribution of genetic effects to the total variance tended to be lower in children of high educated parents compared to children of low educated parents, whereas the relative influence of shared and non-shared environmental effects was comparable between the two groups, in accordance with the findings for the unstandardized variance components. In the Finnish twin cohorts, patterns were again less consistent.
DISCUSSION
This study aimed to investigate the role of parental education as a potential modifier of genetic and environmental effects on exercise behavior in children and adolescents based on data of the Netherlands Twin Register and two Finnish twin cohorts. To this end, means, variances and genetic and environmental variance components were compared between children with two low educated parents and children with at least one high educated parent. Based on twin data, it was tested whether 1) means and variances were different for the two groups and 2) whether the contribution of genetic, shared environmental and unique environmental factors to the variance in exercise behavior differed. It was hypothesized that higher parental education would be associated with a higher mean in offspring's exercise behavior which was largely confirmed in both the Dutch and the Finnish data. Total variances tended to be lower in children of high educated parents. Evidence for gene-by-environment interaction was weak. Data in Dutch females partly supported the hypothesis of a reduction in genetic variance in children of high educated parents, but data of males did not. The Finnish data provided no support at all.
Based on a large number of previous studies, we expected that children of high educated parents would exercise more than children of low educated parents (Ferreira et al., 2006; Hanson & Chen, 2007; Singh et al., 2008). This trend was indeed seen both in the Dutch and the Finnish data, but significant differences were mainly seen in Dutch females which is in accordance with previous studies suggesting a stronger association between socioeconomic status (Hanson & Chen, 2007), as well as parental education in particular (Drewnowski et al., 1994), and exercise behavior in females than in males. Hanson and Chen argued that males might be physically more active whilst interacting with their peers, whereas for females, exercise levels might be more dependent on structured activities that in turn are more likely to involve parental influence. As an addition to our analyses, we calculated the percentage of non-exercisers for both parental education groups separately and found that this percentage was consistently – and for a large part significantly – lower in children of high educated parents (supplementary table 3).
It is not known why children of high educated parents exercise more than children of low educated parents, but several possible mechanisms have been proposed. Most obviously, high educated parents might be more aware of the benefits of regular exercise behavior and might therefore be more inclined to promote this behavior in their children. In addition, these parents are likely to have better financial resources to promote healthy behavior. It has also been shown that high educated parents spent more time with their children and that they are better at effectively tailoring their own behavior to their children's specific needs. In addition to a more sensitive and responsive handling of their children, these parents are also thought to be more effective at managing their children's lives, including recreational activities, and at promoting talent and skill development (Kalil et al., 2012). It is reasonable to assume that these more optimal parenting behaviors ultimately lead to healthier behavior in the children.
This direct effect of parenting might mainly apply to younger children that are more dependent on their parents when it comes to exercise activities as opposed to older children (Huppertz, 2012). Adolescents, in contrast, spend less time at home and the direct influence of parents might be outweighed by the influence of peers and the school environment (West, 1997). In this age group, the influence of parents may take a more indirect path. High educated parents tend to have high educated children that in turn might pursue health behaviors to take care of themselves, although their priorities might lie elsewhere. It is important to shed further light on the possible mechanisms causing children of low educated parents to exercise less in order to develop effective interventions.
Interestingly, we also found a consistent trend for a lower variance in children of high educated parents, both in the Dutch and the Finnish data, although only a few differences were significant. Variance differences are hardly even mentioned in studies that assess differences in health behavior by education level. There is no reason to assume that parental education only affects mean levels of exercise behavior and not the variance, however.
Voluntary exercise behavior has been hypothesized to be influenced by genetic effects on the general drive to be physically active, on exercise ability and on the balance of the appetitive and aversive effects in the psychological response during and shortly after exercise (de Geus & de Moor, 2008). We expected these genetic effects to be affected by parental education. There was a tendency for the unstandardized genetic components to be attenuated in daughters of high educated parents in the Dutch data. Combined with the fact that exercise behavior is higher in this group, these children or their parents may be more capable to suppress an unfavorable genetic predisposition that would prevent engagement in regular voluntary exercise behavior, such as a low innate drive, ability and/or enjoyment. Low educated parents may leave the choice to exercise much more to the children themselves, thereby increasing genetic variance. However, neither in the Dutch data of males nor in the two large Finnish twin cohorts, a clear pattern in the variance decomposition emerged. One possible explanation could be that for part of the sample, the genetic variance was actually larger in high educated parents, as outlined in the introduction, which might have attenuated the effect of a lower genetic variance in the remainder of the sample. Additional covariates such as parenting style and (financial) resources should be assessed and taken into account in the future in order to differentiate between possibly diverse groups in this regard. The most consistent finding in the Finnish data was that the unique environmental variance tended to be higher in children of low educated parents. Possible reasons for this could be earlier individuation of children in low educated families and/or more twin-specific peer influences in this group, or simply more measurement error.
When interpreting our results, one should bear in mind some fundamental differences between the Dutch and the Finnish data. First of all, the definition of what constitutes a “high education” was largely different in the two datasets. In the Dutch dataset, a high education corresponded to a university degree or a university of applied sciences degree. In the Finnish dataset, this was a high school degree which is a requirement, but no guarantee, for university education. Although the distribution of low versus high educated individuals turned out to be comparable (about 40% high and 60% low), a relatively high educational level in the “low education” group of the Dutch dataset and a relatively low education level in the “high education” group of the Finnish dataset may have occurred. Second, exercise behavior was quantified as weekly MET hours in the Dutch twins and as frequency of moderate-to-vigorous activity in the Finnish twins. The former takes duration and intensity of the activity into account, the latter does not. A person that exercises twice a week, for instance, might have a weekly MET hours score that, depending on the activity and the duration, could vary between 2 (2×15 minutes at an intensity of 4 MET) and 20 (2×60 minutes at an intensity of 10 MET) or more. The partly differential findings in the Dutch and Finnish datasets may thus reflect differences in assessments and should not be interpreted as genuine differences between the two countries based on cultural or even genetic effects.
Gene-by-environment (GxE) interaction has been investigated several times with physical activity as a potential modifier of, for instance, body size and/or body composition, with promising results. Both, effects of physical activity on the heritability of body size/composition (Mustelin et al., 2009) and effects of physical activity on expression of specific genes related to body size/composition have been reported (Vimaleswaran et al., 2009). Taken the substantial genetic contribution to physical activity (den Hoed et al., 2013) and the genetic correlations between exercise, fitness and body composition (Mustelin et al., 2011), these studies might qualify as tests of gene-by-gene (GxG) interaction, which may apply to the present study as well. The exposure to certain environments (such as parental education) is partly under genetic control (Kendler & Eaves, 1986), which adds complexity to the interpretation of the results. Moreover, there might be gene frequency differences present in the two parental education groups. If the genes in question then also affect children's exercise behavior, this gene-environment correlation (rGE) might lead to results that mimic GxE interaction in absence of any true interaction. One way to control for rGE is estimating separate means for low and high educated parents, which has been done in the present study (Purcell, 2002).
Notwithstanding its limitations, this study constitutes a relevant addition to the literature as it is the first to investigate GxE interaction with exercise behavior as the outcome variable. Identifying GxE interaction is of importance for at least two reasons. From a public health perspective, identifying modifiers of genetic effects on exercise behavior can improve intervention strategies. From a scientific perspective, the identification of GxE interaction might improve gene-hunting studies as these could add an interaction term (Sung et al., 2014). This would not only increase the probability to find significant associations, but it may also lead to the identification of loci or genes that are selectively expressed under certain circumstances.
PERSPECTIVE
Our study found only weak evidence for GxE interaction effects of parental education on children's and adolescents’ exercise behavior. Nonetheless, the fairly consistent trends in the expected directions for the means and the total variances of exercise behavior in both datasets do point to a role for parental education in offspring exercise behavior. The lower genetic variances in exercise behavior in Dutch daughters of high educated parents suggest that these trends could in part be due to a suppression of genetic effects in children of high educated parents that would act against exercise behavior. That significant effects were found in females only fits the stronger association between socioeconomic status, as well as parental education, and physical activity in females that has been reported in previous studies (Drewnowski et al., 1994; Hanson & Chen, 2007). A better understanding of the parental behaviors that lead to the observed patterns is needed to develop appropriate interventions that increase exercise behavior in children of low educated parents. In addition, it is of importance that further GxE interaction studies with exercise behavior as the outcome variable are performed to shed more light on potential modifiers of this behavior and for comparability purposes.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the twin families that contributed to this study for their support of scientific research. Data collection and analyses in the Netherlands Twin Register (NTR) were supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (RO1DK092127), the National Institute of Mental Health (RO1MH58799-03), the European Research Council (230374), and the Netherlands Organisation for Scientific Research (480-04-004, SPI-56-464, 463-06-001, VENI 451-04-034). Data collection and analyses in the Finnish twin cohorts were supported by the National Institute of Alcohol Abuse and Alcoholism (AA 12502, AA 00145, and AA 09203 to R J Rose) and the Academy of Finland (100499, 205585, 118555, 141054, 213506, 129680, 265240, 263278, and 264146 to J Kaprio). CH's visit to the University of Helsinki was funded by an EMGO+ Travel Grant. CH is currently funded by a grant of the ReVanche Program EMGO+. KS was supported by the Academy of Finland (266592) and the Finnish Ministry of Education and Culture.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Jaakko Kaprio has consulted for Pfizer on nicotine dependence in 2012-2014. The authors declare that they have no further conflict of interest.
REFERENCES
- Boker S, Neale MC, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma DI, Vink JM, van Beijsterveldt CEM, de Geus EJC, Beem AL, Mulder EJ, Derks EM, Riese H, Willemsen GA, Bartels M, van den Berg M, Kupper NH, Polderman TJ, Posthuma D, Rietveld MJ, Stubbe JH, Knol LI, Stroet T, van Baal GC. Netherlands Twin Register: a focus on longitudinal research. Twin research : the official journal of the International Society for Twin Studies. 2002;5:401–406. doi: 10.1375/136905202320906174. [DOI] [PubMed] [Google Scholar]
- de Geus EJC, Bartels M, Kaprio J, Lightfoot JT, Thomis M. Genetics of regular exercise and sedentary behaviors. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2014;17:262–271. doi: 10.1017/thg.2014.42. [DOI] [PubMed] [Google Scholar]
- de Geus EJC, de Moor MHM. A genetic perspective on the association between exercise and mental health. Ment Health Phys Act. 2008;1:53–61. [Google Scholar]
- de Moor MHM, Boomsma DI, Stubbe JH, Willemsen G, de Geus EJC. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Archives of general psychiatry. 2008;65:897–905. doi: 10.1001/archpsyc.65.8.897. [DOI] [PubMed] [Google Scholar]
- den Hoed M, Brage S, Zhao JH, Westgate K, Nessa A, Ekelund U, Spector TD, Wareham NJ, Loos RJ. Heritability of objectively assessed daily physical activity and sedentary behavior. The American journal of clinical nutrition. 2013;98:1317–1325. doi: 10.3945/ajcn.113.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Kurth CL, Krahn DD. Body weight and dieting in adolescence: impact of socioeconomic status. Int J Eat Disord. 1994;16:61–65. doi: 10.1002/1098-108x(199407)16:1<61::aid-eat2260160106>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TF. Introduction to quantitative genetics. 4th ed. Vol. 280. Pearson Education; Essex (UK): 1996. [Google Scholar]
- Ferreira I, van der Horst K, Wendel-Vos W, Kremers S, van Lenthe FJ, Brug J. Environmental correlates of physical activity in youth - a review and update. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2006;8:129–154. doi: 10.1111/j.1467-789X.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports Medicine. American College of Sports Medicine position stand Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine and science in sports and exercise. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Hanson MD, Chen E. Socioeconomic status and health behaviors in adolescence: a review of the literature. Journal of behavioral medicine. 2007;30:263–285. doi: 10.1007/s10865-007-9098-3. [DOI] [PubMed] [Google Scholar]
- Harden KP, Turkheimer E, Loehlin JC. Genotype by environment interaction in adolescents' cognitive aptitude. Behavior genetics. 2007;37:273–283. doi: 10.1007/s10519-006-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz C, Bartels M, van Beijsterveldt CEM, Boomsma DI, Hudziak JJ, de Geus EJC. Effect of shared environmental factors on exercise behavior from age 7 to 12 years. Medicine and science in sports and exercise. 2012;44:2025–2032. doi: 10.1249/MSS.0b013e31825d358e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Leblanc AG. Systematic review of the health benefits of physical activity and fitness in school- aged children and youth. The international journal of behavioral nutrition and physical activity. 2010;7:40. doi: 10.1186/1479-5868-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenkovic A, Ortega-Alonso A, Rose RJ, Kaprio J, Rebato E, Silventoinen K. Genetic and environmental influences on growth from late childhood to adulthood: a longitudinal study of two Finnish twin cohorts. American journal of human biology : the official journal of the Human Biology Council. 2011;23:764–773. doi: 10.1002/ajhb.21208. [DOI] [PubMed] [Google Scholar]
- Kalil A, Ryan R, Corey M. Diverging destinies: maternal education and the developmental gradient in time with children. Demography. 2012;49:1361–1383. doi: 10.1007/s13524-012-0129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprio J. The Finnish twin cohort study: an update. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2013;16:157–162. doi: 10.1017/thg.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin research : the official journal of the International Society for Twin Studies. 2002;5:366–371. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Eaves LJ. Models for the joint effect of genotype and environment on liability to psychiatric illness. Am J Psychiatry. 1986;143:279–289. doi: 10.1176/ajp.143.3.279. [DOI] [PubMed] [Google Scholar]
- Lajunen HR, Kaprio J, Rose RJ, Pulkkinen L, Silventoinen K. Genetic and environmental influences on BMI from late childhood to adolescence are modified by parental education. Obesity. 2012;20:583–589. doi: 10.1038/oby.2011.304. [DOI] [PubMed] [Google Scholar]
- Mustelin L, Latvala A, Pietilainen KH, Piirila P, Sovijarvi AR, Kujala UM, Rissanen A, Kaprio J. Associations between sports participation, cardiorespiratory fitness, and adiposity in young adult twins. Journal of applied physiology (Bethesda, Md : 1985) 2011;110:681–686. doi: 10.1152/japplphysiol.00753.2010. [DOI] [PubMed] [Google Scholar]
- Mustelin L, Silventoinen K, Pietilainen K, Rissanen A, Kaprio J. Physical activity reduces the influence of genetic effects on BMI and waist circumference: a study in young adult twins. International journal of obesity. 2009;33:29–36. doi: 10.1038/ijo.2008.258. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin research : the official journal of the International Society for Twin Studies. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Ridley K, Ainsworth BE, Olds TS. Development of a compendium of energy expenditures for youth. The international journal of behavioral nutrition and physical activity. 2008 doi: 10.1186/1479-5868-5-45. 10.1186/1479-5868-1185-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld MJ, van der Valk JC, Bongers IL, Stroet TM, Slagboom PE, Boomsma DI. Zygosity diagnosis in young twins by parental report. Twin research : the official journal of the International Society for Twin Studies. 2000;3:134–141. doi: 10.1375/136905200320565409. [DOI] [PubMed] [Google Scholar]
- Rosenberg J, Pennington BF, Willcutt EG, Olson RK. Gene by environment interactions influencing reading disability and the inattentive symptom dimension of attention deficit/hyperactivity disorder. Journal of child psychology and psychiatry, and allied disciplines. 2012;53:243–251. doi: 10.1111/j.1469-7610.2011.02452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna S, Kaprio J, Sistonen P, Koskenvuo M. Diagnosis of twin zygosity by mailed questionnaire. Hum Hered. 1978;28:241–254. doi: 10.1159/000152964. [DOI] [PubMed] [Google Scholar]
- Singh GK, Kogan MD, Siahpush M, van Dyck PC. Independent and joint effects of socioeconomic, behavioral, and neighborhood characteristics on physical inactivity and activity levels among US children and adolescents. Journal of community health. 2008;33:206–216. doi: 10.1007/s10900-008-9094-8. [DOI] [PubMed] [Google Scholar]
- Stubbe JH, Boomsma DI, de Geus EJC. Sports participation during adolescence: a shift from environmental to genetic factors. Medicine and science in sports and exercise. 2005;37:563–570. doi: 10.1249/01.mss.0000158181.75442.8b. [DOI] [PubMed] [Google Scholar]
- Stubbe JH, Boomsma DI, Vink JM, Cornes BK, Martin NG, Skytthe A, Kyvik KO, Rose RJ, Kujala UM, Kaprio J, Harris JR, Pedersen NL, Hunkin J, Spector TD, de Geus EJC. Genetic influences on exercise participation in 37,051 twin pairs from seven countries. PloS one. 2006;1:e22. doi: 10.1371/journal.pone.0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe JH, de Moor MHM, Boomsma DI, de Geus EJC. The association between exercise participation and well-being: a co-twin study. Preventive medicine. 2007;44:148–152. doi: 10.1016/j.ypmed.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Sung YJ, Schwander K, Arnett DK, Kardia SL, Rankinen T, Bouchard C, Boerwinkle E, Hunt SC, Rao DC. An empirical comparison of meta-analysis and mega-analysis of individual participant data for identifying gene-environment interactions. Genetic epidemiology. 2014;38:369–378. doi: 10.1002/gepi.21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D'Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychological science. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CEM, Groen-Blokhuis M, Hottenga JJ, Franic S, Hudziak JJ, Lamb D, Huppertz C, de Zeeuw E, Nivard M, Schutte N, Swagerman S, Glasner T, van Fulpen M, Brouwer C, Stroet T, Nowotny D, Ehli EA, Davies GE, Scheet P, Orlebeke JF, Kan KJ, Smit D, Dolan CV, Middeldorp CM, de Geus EJC, Bartels M, Boomsma DI. The Young Netherlands Twin Register (YNTR): longitudinal twin and family studies in over 70,000 children. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2013;16:252–267. doi: 10.1017/thg.2012.118. [DOI] [PubMed] [Google Scholar]
- van der Aa N, de Geus EJC, van Beijsterveldt CEM, Boomsma DI, Bartels M. Genetic Influences on Individual Differences in Exercise Behavior during Adolescence. International journal of pediatrics. 2010 doi: 10.1155/2010/138345. 10.1155/2010/138345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimaleswaran KS, Li S, Zhao JH, Luan J, Bingham SA, Khaw KT, Ekelund U, Wareham NJ, Loos RJ. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. The American journal of clinical nutrition. 2009;90:425–428. doi: 10.3945/ajcn.2009.27652. [DOI] [PubMed] [Google Scholar]
- Vink JM, Boomsma DI, Medland SE, de Moor MHM, Stubbe JH, Cornes BK, Martin NG, Skytthea A, Kyvik KO, Rose RJ, Kujala UM, Kaprio J, Harris JR, Pedersen NL, Cherkas L, Spector TD, de Geus EJC. Variance components models for physical activity with age as modifier: a comparative twin study in seven countries. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2011;14:25–34. doi: 10.1375/twin.14.1.25. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Charlesworth S, Ivey A, Nettlefold L, Bredin SS. A systematic review of the evidence for Canada's Physical Activity Guidelines for Adults. The international journal of behavioral nutrition and physical activity. 2010;7:39. doi: 10.1186/1479-5868-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West P. Health inequalities in the early years: is there equalisation in youth? Soc Sci Med. 1997;44:833–858. doi: 10.1016/s0277-9536(96)00188-8. [DOI] [PubMed] [Google Scholar]
- Willemsen G, Posthuma D, Boomsma DI. Environmental factors determine where the Dutch live: results from the Netherlands Twin Register. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2005;8:312–317. doi: 10.1375/1832427054936655. [DOI] [PubMed] [Google Scholar]
- Willemsen G, Vink JM, Abdellaoui A, den Braber A, van Beek JH, Draisma HH, van Dongen J, van 't Ent D, Geels LM, van Lien R, Ligthart L, Kattenberg M, Mbarek H, de Moor MHM, Neijts M, Pool R, Stroo N, Kluft C, Suchiman HE, Slagboom PE, de Geus EJC, Boomsma DI. The Adult Netherlands Twin Register: twenty-five years of survey and biological data collection. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2013;16:271–281. doi: 10.1017/thg.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.