Abstract
The aim of this immunohistochmical study was to evaluate the distribution of kisspeptin neurons in the pre-optic area (POA) of gonadally intact adult male and female rhesus monkeys, and to determine whether imposition of an estradiol (E2) positive feedback signal in the castrate male increased kisspeptin in the POA. Additionally, kisspeptin in POA of the intact female was examined during an LH surge induced prematurely by E2 administered in the early follicular phase. The number of kisspeptin neurons in POA of males and females was similar. Immunoactive kisspeptin perikarya were not observed in the POA of castrate adult males but such neurons in these animals were present within 12h of imposing an increment in circulating E2 concentrations, that in a screening study conducted 4-6 weeks earlier elicited an LH surge. As expected, premature induction of an LH surge by E2 early in the follicular phase was associated with up-regulation of kisspeptin in POA. These results represent the first description of immunoreactive kisspeptin cell bodies in POA of the macaque brain and provide further support for the view that 1) kisspeptin neurons in POA of the female monkey are a target for the positive feedback action of E2 and 2) the hypothalamic mechanism, which mediates this action of E2 in primates, is not subjected to perinatal programming by testicular testosterone. Moreover, our findings indicate that maintenance of kisspeptin content in POA of intact male monkeys requires the action of E2, presumably generated by aromatization of testicular testosterone at the hypothalamic level.
Keywords: Kisspeptin, pre-optic area, LH surge, sexual dimorphism
Introduction
Classical studies of rodents have demonstrated that, in these species, the pre-ovulatory LH surge is generated by a neural signal that originates in the rostral hypothalamus, specifically in the POA and is transmitted to the pituitary via the hypophysial portal circulation by a large discharge or surge of GnRH (1). The initiation of this GnRH surge is triggered by the interaction of a circadian signal (2) originating from the suprachiasmatic nucleus, and a positive feedback action of pre-ovulatory levels of E2 in the POA (1). The mandatory role of the POA for the LH surge in rodents was elegantly demonstrated in the rat by Halasz and Gorski (3), who showed that after surgical isolation of the POA from the mediobasal hypothalamus (MBH), females became acyclic and failed to ovulate. Similar studies of female rhesus monkeys were performed by Knobil's group (4) but, in contrast to rodents, surgical isolation of the MBH in these animals did not consistently interrupt menstrual cyclicity and ovulation, suggesting that the POA is not essential for the positive feedback action of E2 that generates the pre-ovulatory LH surge in highly evolved primates.
Kisspeptin neurons in the anteroventral periventricular area (AVPV) and the periventricular nucleus of the POA are generally recognized as a major target for the positive feedback action of E2 that underlies the GnRH surge in rodents (5,6). Interestingly, KISS1 mRNA is also found in the POA of the female monkey (7,8) and human (9), and elevated KISS1 expression in this hypothalamic area has been described in the female rhesus monkey during the late follicular phase of the menstrual cycle at the time the pre-ovulatory LH surge was to be anticipated (8). Together these findings suggest that the POA of the primate hypothalamus may play a role, albeit a redundant one, in mediating the positive feedback action of E2 to elicit the pre-ovulatory surge of LH.
In rodents, the POA mechanism that triggers the pre-ovulatory LH surge in adult females is decommissioned in males by an action of testicular testosterone secreted during the early postnatal period of development (10). As may have been predicted from the established role of POA kisspeptin neurons in triggering the LH surge during the estrous cycle, kisspeptin expression in the POA of the mouse and rat is sexually dimorphic showing a dramatic female-dominant sex difference in the number of these peptidergic neurons (11,12). Not surprisingly this sexual dimorphism is also determined by testicular testosterone secretion during early postnatal development, as demonstrated by the finding that in female rats a single injection of testosterone propionate on the day of birth leads to a characteristic male-like pattern of kisspeptin neurons in the adult POA (13).
In contrast to the rodent, the pre-ovulatory gonadotropin surge in primates is not “masculinized” by testicular androgen exposure during perinatal development. Adult male monkeys, castrated as adults, are able to generate LH surges in response to exogenous E2 (14), and when such animals are exposed for 30-60 days to low follicular phase levels of E2 before elevating levels of this steroid to produce a positive feedback signal, the induced LH surge is markedly amplified (15). A sensational demonstration of the ability of the hypothalamic-pituitary unit of this male primate to respond to the positive feedback action of E2, was provided by the finding that an ovarian fragment transplanted subcutaneously in a castrated adult male rhesus monkey exhibited repetitive ovulatory cycles with approximately normal periodicity (16). Similarly, intact and gonadectomized adult men also respond to the positive feedback action of E2, as demonstrated by a LH surge elicited after 5-9 days of exposure to exogenous E2 (17,18).
Given the lack of sexual dimorphism in the neuroendocrine mechanism that generates the pre-ovulatory LH surge in highly evolved primates, Watanabe et al conducted a study to determine if POA kisspeptin neurons are activated by E2 in male as well as in female monkeys (19). To test this idea, castrate adult male Japanese macaques were implanted with E2-containing capsules to produce circulating levels of the steroid typical of those observed in the early follicular phase of the menstrual cycle. After two weeks of exposure to these relatively low levels of E2, the animals received an intramuscular injection of E2 benzoate at a dose previously established to induce an LH surge in this model; KISS1 mRNA levels in POA kisspeptin neurons together with c-Fos expression, a marker of neuronal activation, were up-regulated in association with the estrogen-induced LH surge. While this observation suggests that E2 activates POA kisspeptin neurons when a LH surge is elicited in male monkeys, it should be noted, that the study design did not allow for the positive feedback action of E2 (generated by the E2 benzoate injection) to be separated from the effects of the 2 weeks of low dose E2 exposure.
The present study was conducted to extend the foregoing findings on the regulation by E2 of kisspeptin neurons in the POA of the rhesus monkey. Specifically, we attempted to 1) compare the distribution of immunoreactive kisspeptin in the POA of intact adult male and follicular phase female monkeys, 2) examine whether the premature induction of an LH surge by exogenous E2 administration early in the follicular phase of the menstrual cycle evokes increased kisspeptin expression in the POA, and 3) determine whether imposition of a positive E2 feedback signal in the castrate adult male monkey is associated with an increase in kisspeptin immunoactive elements in the POA. The use of immunohistochemistry, rather than in situ hybridization as previously employed, to monitor the distribution and activity of KISS1 neurons in the POA provides the first description of the location of the peptide encoded by the gene in this region of the monkey hypothalamus.
Materials and Methods
Animals and hypothalamic tissue
Hypothalamic tissue for this study was obtained from 3 different sources:
-
1)
Four adult female rhesus monkeys (Macaca mulatta) ranging from 5-8 years of age and weighing 5-9 kg, and 6 castrate adult males (8-11 years of age, 8-12 kg body weight) were used. The females had menstruated spontaneously for 2-4 months with cycles ranging from 29 to 40 days before the study. The males had been gonadectomized 3-7 months earlier for other purposes. All monkeys were maintained under controlled photoperiod (lights on between 0700–1900 h) and temperature (approximately 21°C) in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. At the completion of each experimental protocol (see Experimental Design), animals were first sedated with ketamine hydrochloride (approximately 20 mg/kg body weight; Ketaject, Phoenix Scientific Inc., St Joseph, MO, USA) and deeply anesthetized with sodium pentobarbital (approximately 30 mg/kg body weight, iv; Nembutal sodium solution, Abbott Laboratories, North Chicago, IL, USA). The thoracic cavity was exposed, the dorsal aorta clamped and the head perfused transcardially at a flow rate of approximately 40 ml/min initially with approximately 1 L of physiological saline containing 2% sodium nitrite, and 5,000 U of heparin/L and immediately followed with 2-3 L of 4% paraformaldehyde (PFA) in 0.1M phosphate buffered saline (PBS, pH 7.2). Following perfusion, the brain was removed from the cranium and the hypothalamus isolated. A parasagittal cut was made at approximately 4 mm on either side of midline and a horizontal cut was made immediately dorsal to the anterior commissure. The hypothalamic block was immersed in fixative for 1-2h and was then transferred to a 30% sucrose solution (in 0.1 m PBS, pH 7.2) for at least 24h at 4 C. Serial sections throughout the entire hypothalamic block were cut on a freezing microtome every 25 μm. The sections were placed sequentially into a train of 10 wells, with each well containing a sequence of sections collected at 250 μm intervals from the region of the organum vasculosum of the lamina terminalis (OVLT) to the mammillary bodies. Sections were stored in an antifreeze cryoprotectant solution at −20C (20). The foregoing experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
-
2)
Hypothalami from 3 hysterectomized rhesus monkeys in the follicular phase of the menstrual cycle ranging in age from 8 to 15 years of age (9-11 kg body weight) were obtained post mortem after the animals had been venepunctured, euthanized with Beuthanasia-D (Henry Schein Animal Health, Dublin, OH, USA) and their brains immediately perfused with approximately 1 L of saline containing 2% sodium nitrite and 5,000 U heparin/L followed by approximately 2 L of 4% PFA in 0.1M PBS (pH 7.2). Following removal of the brain from the cranium the hypothalamus was post-fixed in 4% PFA at 4C overnight prior to immersion in 30% sucrose. Serial 25 μm sections throughout the entire hypothalamus were cut and collected as described above.
-
3)
Twenty five μm sections from 3 intact adult males (6-10 years old and 8-10 kg body weight) and 3 adult castrate males (4-14 kg body weight and gonadectomized at age 3-10 years old) were obtained from our tissue bank. The animals had been previously used for studies of testicular function, and castration had been performed 1-5 months before perfusion and post fixation, which were conducted exactly as described for the animals obtained from the first source. The hypothalamic sections had also been cryopreserved in wells containing a sequence of sections collected at 250 μm from the OVLT to the mammillary bodies.
Experimental design
Experiment 1: Patterns of kisspeptin immunoreactivity in POA perikarya and the distribution of these neurons in intact males and females
Hypothalamic tissue obtained from 3 hysterectomized adult females and 3 intact adult males was used. It was determined that the females were in the follicular phase of the menstrual cycle at the time of perfusion based on the following hormonal data obtained on the morning of perfusion: circulating LH levels ranged from 0.01-1.1 ng/ml, E2 from 22.9-63.9 pg/ml, and progesterone from 0-0.3 ng/ml. Sequential series of sections collected every 250 μm through the POA from a male-female pair were processed together to minimize immunohistochemical variation.
Experiment 2: Immunoreactive kisspeptin expression in the POA associated with a E2-induced LH surge prematurely elicited in the early follicular phase
Four intact females on day 3 of the menstrual cycle were implanted under sedation with ketamine hydrochloride with either blank Silastic capsules or E2-containing Silastic capsules to produce circulating E2 levels of 200-300 pg/ml (N=2/group). The ability of this estrogen regimen to induce a premature LH surge in a control cycle was established for all 4 monkeys prior to the experimental cycle. The interval between control and experimental cycles ranged from 4 to 11 weeks. Blood samples were collected by femoral venipuncture under sedation with ketamine hydrochloride every 12h for the duration of capsule implantation during both control and experimental cycles. Hypothalami were collected during the experimental cycle after 36h of exposure to E2. A sequential series of sections collected every 250 μm through the POA of each of the 4 monkeys were processed in a single immunohistochemical run.
Experiment 3: A comparison of immunoreactive kisspeptin neurons in the POA of intact and castrate adult male monkeys and the effect of E2 treatment on the castrate
Six castrate males were implanted with E2-containing Silastic capsules for 12h (N=3) or 48h (N=3) prior to perfusion with the aim of producing circulating E2 levels of 200-400 pg/ml. Before the experiment, the ability of this E2 treatment to induce a LH surge was established in all 6 monkeys. The time between the control and experimental E2 challenges ranged from 4 and 6 weeks. Blood samples were collected by femoral venipuncture under sedation with ketamine hydrochloride every 12h for the duration of capsule implantation during both control and experimental E2 challenges. A sequential series of sections collected every 250 μm through the POA from a 12h and 48h E2 treated castrate were processed together with a comparable series of sections from an intact and untreated castrate adult male in a single immunohistochemical run.
Antibodies and blocking peptides
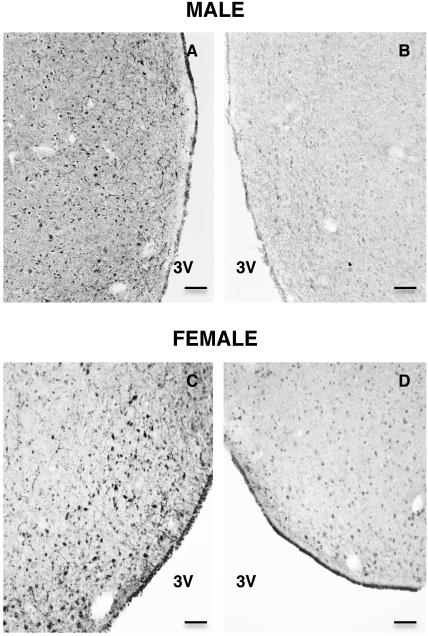
The kisspeptin antibody used was a sheep anti-human kisspeptin-54 antibody (GQ2), kindly provided by Dr. Stephen Bloom (Imperial College London, Hammersmith Hospital, London, UK). The specificity of GQ2 has been previously validated for immunohistochemical studies of monkey hypothalamus (21). Biotinylated rabbit antisheep IgG (Vector Laboratories, Burlingame, CA, USA) was used as the secondary antibody to detect GQ2. Kisspetin-54 (Phoenix Pharmaceuticals Inc., Burlingame, CA, USA) was used to preabsorb GQ2 for control purposes as described previously (21) (Fig. 1).
Figure 1.
Photomicrographs (x10) of coronal hemisections of POA taken at the level of the AVPe from the same section of a male (A,B) and a female (C,D) monkey, and immunostained for kisspeptin using the primary antibody, GQ2. A,C - without preabsorbtion of GQ2; B,D -preabsorbed with human kisspeptin-54. 3V, Third ventricle. Scale bar, 100 μm.
E2-containing Silastic capsules
Crystalline E2 (Steraloids Inc., Newport, RI, USA) was used to prepare E2-filled Silastic brand capsules (4 cm long; Dow Corning Corp., Midland, MI, USA) as described previously (22). Blank Silastic capsules of the same dimensions were also prepared for control experiments.
Nickel-intensified diaminobenzidine (Ni-DAB) immunohistochemistry
Sections were rinsed at room temperature in 50 mM potassium phosphate buffered saline (KPBS) (pH 7.3, 6×10 min), incubated for 30 min in 3% hydrogen peroxide (Fisher Scientific, Fair Lawn, NJ, USA), and rinsed again in 50 mM KPBS (4×5 min). They were then incubated in blocking serum buffer (5% normal rabbit serum + 0.005% albumin from bovine serum [Sigma-Aldrich, St. Louis, MO, USA] + 0.05% Triton-X [Sigma-Aldrich, St. Louis, MO, USA]) for 1h to reduce non-specific binding and then incubated in GQ2 (1:60,000) for 1h at room temperature followed by 48h of incubation at 4°C on a shaker. After 10×6 min rinses in 50 mM KPBS, sections were incubated in biotinylated rabbit anti sheep IgG (1:600 in blocking serum) for 1h. Sections were rinsed again in 50 mM KPBS (5×10 min) and incubated in Vectasin Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) for 1h. This was followed by rinses in 50 mM KPBS and 0.175M sodium acetate (3×5 min each). The tissue was then incubated in Ni-DAB solution (Nickel(II) sulfate hexahydrate [Sigma-Aldrich, St. Louis, MO, USA] and 3,3′-diaminobenzidine tetrahydrochloride hydrate [Sigma-Aldrich, St. Louis, MO, USA]). The duration of incubation in the Ni-DAB, generally 3-8 min, was adjusted for optimal staining. To stop the reaction, sections were rinsed with 0.175M sodium acetate (3×5 min) followed by 3×5 min rinses in 50 mM KPBS. Sections were mounted on slides (Fisherbrand Superfrost Plus, Fisher Scientific, Pittsburgh, PA, USA) treated with subbing solution (0.1% gelatin and 0.01% chromium potassium sulfate). Mounted sections were placed in distilled water × 2 min and then dehydrated in grades of ethyl alcohol (50%, 75%, and 100% twice × 4 min each). Before coverslipping with Permount (Fisher Scientific, Fair Lawn, NJ, USA), the slides were immersed in Histo-Clear (National Diagnostics, Atlanta, GA, USA) × 4 min. Slides were stored at room temperature.
Cell location and cell counting
The atlas of the monkey brain by Paxinos et al (23) was used as a reference. Here, it should be noted that while this atlas identifies a specific anteroventricular periventricular nucleus AVPe in the POA of the monkey brain, this is not the case for the atlas of Bleier (24). The location of the AVPe in the POA of the monkey corresponds closely to that of the AVPV in rodents and sheep. For enumeration of kisspeptin cell body number, a series of coronal sections from individual monkeys taken every 250 μm from the level of the OVLT and continuing posteriorly to the caudal aspect of the optic chiasm were examined at 20x, and neuron number per section was determined by blinded observers. The section containing the highest number of kisspeptin neurons for a given monkey was designated section 0, and the overall distribution of immunoreactive kisspeptin neurons for a given group of animals was derived by aligning the counts with respect to section 0, as previously described (25).
Hormone assays
Plasma LH levels were measured as previously described using a homologous macaque radioimmunoassay (RIA) employing recombinant cynomolgus monkey LH (rcLH; AFP6936A) as standard (26). The sensitivity of the assay ranged from 0.09-0.42 ng/ml and the inter- and intra-assay coefficients of variation were less than 5% and 13%, respectively.
Plasma E2 levels were measured using a double antibody RIA kit (Diagnostics Products Corp., CA, USA) as described previously (22). The sensitivity of the assay ranged from 3-5 pg/ml and the intra- and inter-assay coefficients of variation were less than 7% and 11%, respectively.
Plasma progesterone concentrations were measured a RIA previously described (27). The sensitivity of the assay ranged from 0.02-0.03 ng/ml and the intra- and inter-assay coefficients of variation were less than 13% and 24%, respectively.
Statistical Analyses
The significance of differences in circulating E2 and LH levels and in mean number of kisspeptin neurons per section were assessed using one-way ANOVA (Prism 5, GraphPad Software, Inc., La Jolla, CA, USA). Significance was accepted at P≤0.05.
Results
Experiment 1: Patterns of kisspeptin immunoreactivity in POA perikarya and the distribution of these neurons in intact males and females
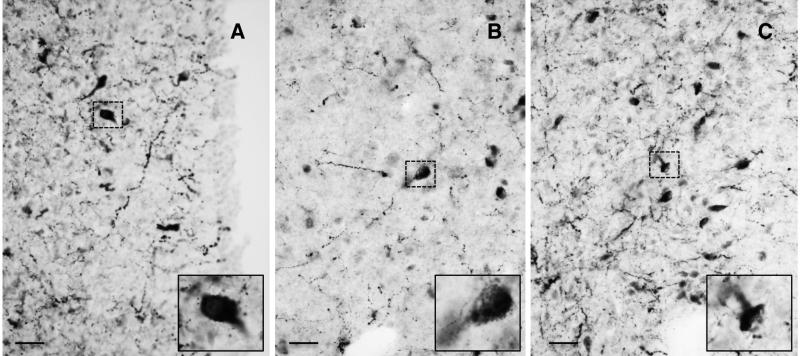
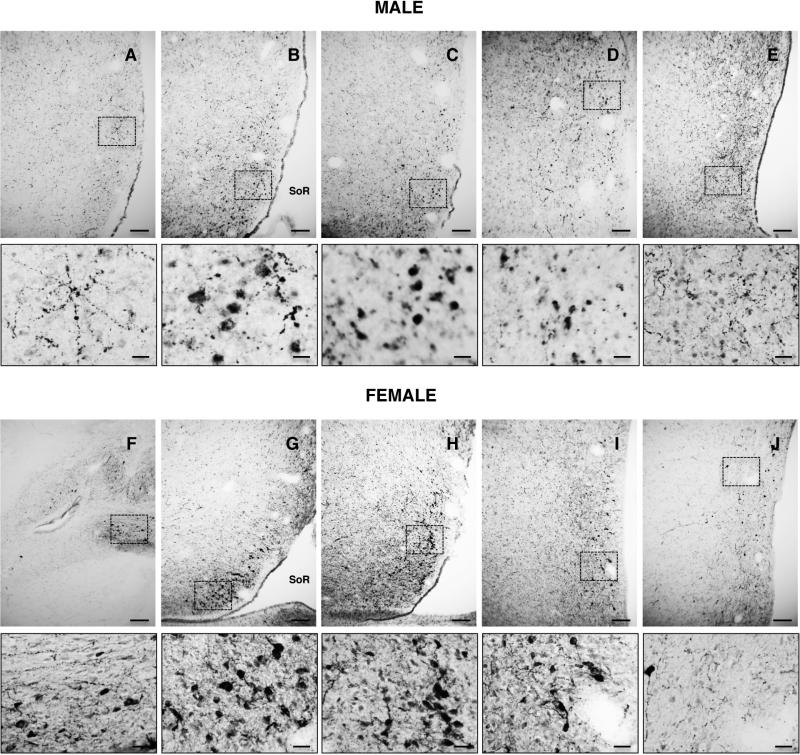
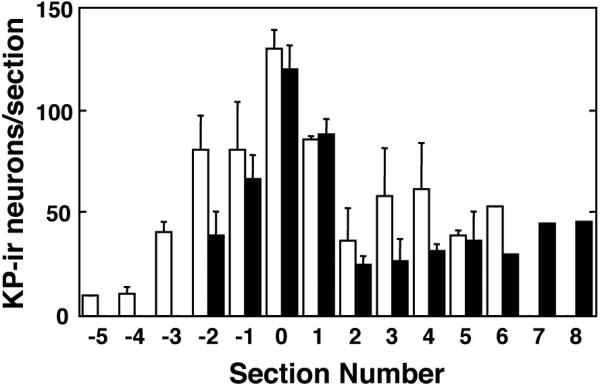
Kisspeptin cell bodies in the POA of both male and female rhesus monkeys exhibited three distinct patterns of cytoplasmic kisspeptin immunoreactivity that gave the cell body the appearance of either 1) a homogenously filled “pear” generally with a pale central nucleus, 2) a granular and irregularly filled “balloon”, or 3) a homogeneous “cap” typically located in the region of a dendrite (Fig. 2). In the female, the predominant pattern was that of the homogenous “pear”, while in the male granular and irregular cytoplasmic staining prevailed. Kisspeptin perikarya in the POA of both male and females were distributed in an antero-posterior continuum spanning up to 2,750 μm (Figs. 3 and 4). For quantification, the 3 types of immunostained kisspetin neurons were counted. Based on the topology of the base of the third ventricle, the greatest number of kisspeptin perikarya were found at an anteroposterior level corresponding to the region of the (AVPe) (23). The number of immunoreactive kisspeptin cell bodies recorded in the analyzed sequence of sections through the POA of males and females was 591 + 91 and 477 + 79 (Mean + SEM), respectively. The difference was not significant. The distribution and intensity of immunoreactive kisspeptin fibers, within the region of the AVPe and beyond, was also similar in males and females (Figs. 3 and 4).
Figure 2.
Photomicrographs (x40) of hemi-sections of POA from monkey showing neurons with 3 distinct patterns of kisspeptin immunostaining giving the perikaryon a pear-like (A), granular (B) or cap-like (C) appearance. Insets show neurons demarcated by dashed boxes at higher magnification. Scale bar, 20 μm.
Figure 3.
Photomicrographs (x10) of sequential hemi-sections taken at 250 μm intervals through the POA of an intact adult male (upper panels) and a female in the follicular phase of the menstrual cycle (lower panels) stained for kisspeptin immunoreactivity. Sections are shown in order from anterior to posterior (A-E, male and F-J, female). Areas demarcated by dashed boxes in the low magnification panels are shown underneath at higher magnification (x40). SoR, supra-optic recess of third ventricle. Scale bars, 100 μm (A-J) and 20 μm (smaller panels below A-J).
Figure 4.
Mean number (±SEM) of immunoreactive kisspeptin neurons in the POA of intact males (open bars, N=3) and females in the follicular phase (black bars, N=3) enumerated in a series of sections taken at 250 μm intervals through the POA. For each animal, the section containing the highest number of kisspeptin neurons was assigned section 0. Each data bar is comprised of 1-3 observations.
Experiment 2: Immunoreactive kisspeptin expression in the POA associated with an E2 induced LH surge prematurely elicited in the early follicular phase
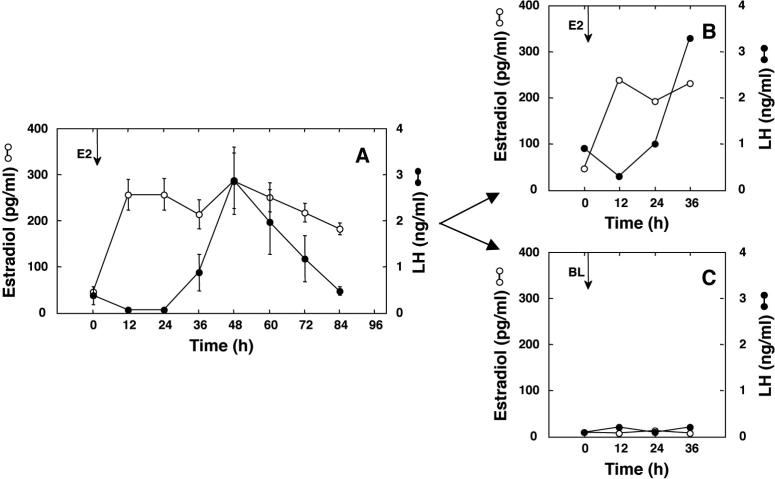
Implantation of E2-containing Silastic capsules on day 3 of the control menstrual cycle in the 4 monkeys resulted in an increase in circulating E2 concentrations from a mean of 46 pg/ml to a mean of 232 pg/ml (Fig. 5A). Between 24h to 36h after implantation of E2, an LH surge was elicited in all 4 monkeys with peak LH concentrations being observed at 48h (Fig. 5A). In the 2 monkeys which subsequently received E2 during the experimental cycle, the increment in circulating E2 was similar to that in the control cycle and an LH surge was initiated at 24h (Fig. 5C). By the time of perfusion at 36h, LH values were comparable to peak concentrations of this gonadotropin during the control cycle. As anticipated, implantation of blank Silastic capsules during the experimental cycle in the remaining 2 monkeys was not associated with an LH surge (Fig. 5B).
Figure 5.
Panel A. Mean (±SEM) concentrations of circulating E2 (open circles) and LH (closed circles) in females during a control menstrual cycle to demonstrate the ability of an E2 challenge early in the follicular phase (day 3) to elicit a premature LH surge (N=4). Panels B and C. Mean E2 and LH levels in the 2 monkeys that were subsequently implanted with either E2-containing capsules (N=2; B) or blank capsules (N=2; C). The arrows denote the time of implantation with either E2-containing (E2) or blank (BL) capsules.
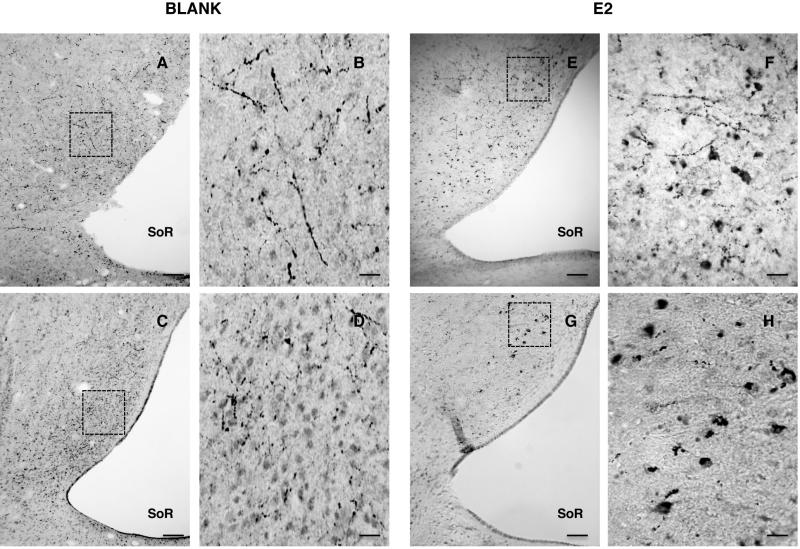
Kisspeptin immunoreactivity in cell bodies in the region of the AVPe was markedly upregulated in the E2 treated females when compared to the animals that received empty implants (Fig. 6). Because there were only two animals in each group kisspeptin cell number in this experiment was not enumerated.
Figure 6.
Photomicrographs (x10) of hemi-sections of POA at the level of the AVPe showing kisspeptin immunoreactive neurons in intact females implanted on day 3 of the follicular phase with either blank (A-D) or E2-containing (E-H) Silastic capsules to induce a premature LH surge. The areas demarcated by the dotted boxes in panels A, C, E and G are shown at higher magnification (x40) in B, D, F and H. SoR, supra-optic recess of the third ventricle. Scale bars, 100 μm (A,C,E,G) and 50 μm (B,D,F,H).
Experiment 3: A comparison of immunoreactive kisspeptin neurons in the POA of intact and castrate adult male monkeys and the effect of E2 treatment on the castrate
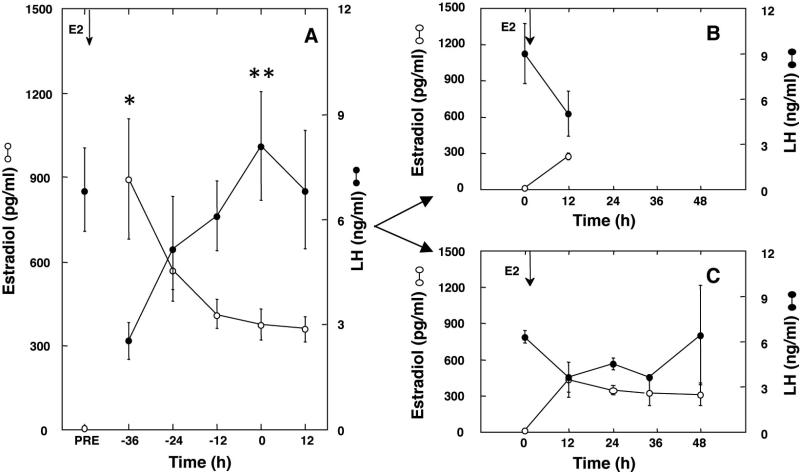
In this experiment, kisspeptin immunoreactive perikarya and fibers in the POA were examined in four groups of adult male monkeys, namely intact; castrate; and two cohorts of castrates, one exposed to approximately 300 pg/ml estradiol for 12h and the other for 48h. Prior to conducting this study the LH response to the E2 signal was first characterized in all 6 E2-treated castrate monkeys (Fig. 7A, note data in this panel are plotted with respect to the time of the LH peak: ie time 0). Implantation of E2-containing Silastic capsules resulted in a marked increase in circulating E2 from low castrate levels of <10 pg/ml to a mean concentration of approximately 1000 pg/ml 12h after implantation. Subsequently E2 concentrations declined progressively although they remained above 300 pg/ml for the duration of the time course (Fig. 7A). Circulating LH concentrations declined from a mean of 6.9 + 1.2 ng/ml before implantation of E2 (Fig. 7A) to 3 + 1.1 ng/ml 12h later. Subsequently LH concentration rose in all 6 animals reaching peak levels of approximately 8 ng/ml, 48h-72h after implantation of the E2-containing capsules (Fig. 7A). During the subsequent 12h and 48h E2 challenges, conducted 4-6 weeks after removing the capsules initially implanted for characterizing the LH response, circulating levels of this steroid were again increased from low castrate values to approximately 300 pg/ml, and, in both E2 groups, circulating LH concentrations were reduced by approximately 50% after 12h of steroid exposure (Fig. 7B and C). In the 3 monkeys in which E2 administration continued for 48h, an unambiguous LH surge was observed in one animal. In this monkey, LH concentrations increased 400% between 36 and 48h.
Figure 7.
Panel A. Mean (±SEM) circulating concentrations of E2 (open circle) and LH (closed circle) in castrate adult male monkeys (N=6) implanted with E2-containing capsules for 96h during a control experiment to confirm the action of E2 to induce a LH surge. Panels B and C. Mean E2 and LH levels during a subsequent implantation of the same monkeys with E2-containing capsules for either 12h (N=3; B) or 48h (N=3; C). Arrows denote the time of implantation with E2-containing capsules (E2); Time 0 represents the time of the LH peak (panel A) or the time of E2 implantation (panels B and C); PRE, prior to E2 implantation. *,P≤0.05 from PRE for E2 and **, P≤0.05 from −36h for LH .
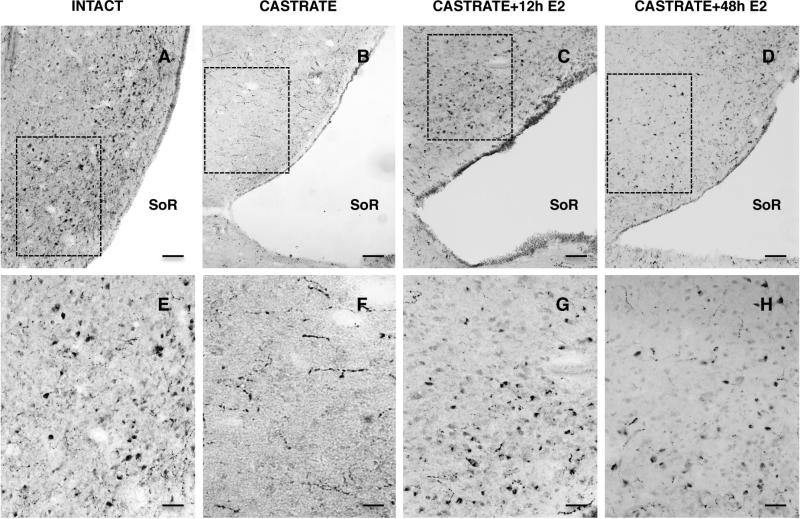
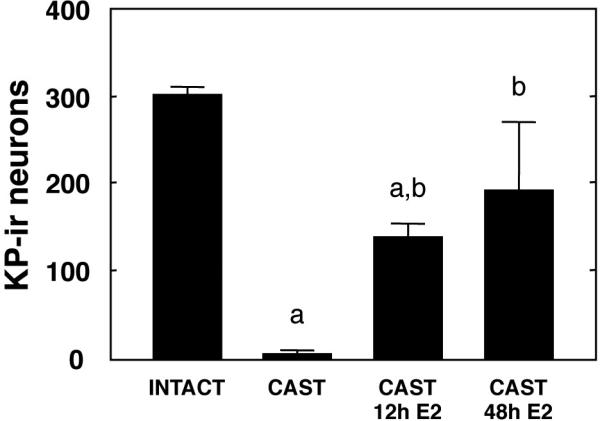
In striking contrast to the situation reported for intact males in Experiment 1, immunoreactive kisspeptin perikarya were very rarely seen in the POA of castrate adult male monkeys although fiber staining in this region of the brain continued to be observed (Fig. 8). In castrates treated with E2 for either 12h or 48h, however, the pattern and intensity of immunoreactive kisspeptin in perikarya in the POA closely resembled that of intact animals (Fig. 8). In the E2 treated castrates, kisspeptin cell bodies were observed along an anteroposterior continuum of 1,500 μm in the region of the AVPe. The mean number of immunoreactive kisspeptin cell bodies in each of the 4 groups are shown in Fig. 9. E2 treated castrates had a significantly greater number of kisspeptin neurons in the POA than that in the castrate group, although kisspeptin neuron number was unrelated to duration of E2 exposure.
Figure 8.
Representative photomicrographs of hemi-sections of POA at the level of the AVPe stained for kisspeptin immunoreactivity from intact (A,E), castrate (B,F), 12h E2-treated castrate (C,G), and 48h E2-treated castrate (D,H) adult male monkeys (left to right, respectively). High power (x20) images below (E,F,G,H) show areas demarcated by the dotted boxes in the top panels (A,B,C,D x10). SoR, supra-optic recess of third ventricle. Scale bars, 100 μm (top panels) and 50 μm (bottom panels).
Figure 9.
Number (mean±SEM) of immunoreactive kisspeptin neurons in a sequential series of sections (8-11) collected at 250 μm intervals throughout the POA of intact males, castrate males, and castrate males treated with E2 for 12h or 48h. a,b, P≤0.05 from intact and castrate group, respectively.
Discussion
Although KISS1 expressing neurons have been previously described in the POA of both male and female monkeys (7,8,19), the present study is the first to confirm the expectation that, in these primates, KISS1 mRNA in the POA is translated, and that kisspeptin may be readily detected in cell bodies and fibers in this hypothalamic area of both sexes by standard immunohistochemical procedures. In addition, and more importantly, the present study provides evidence to indicate that the maintenance of kisspeptin content in the POA in male primates is dependent upon the testis and that this action of the male gonad may be accounted for by E2 receptor (ER) signaling.
As discussed in the Introduction, the POA in highly evolved primates is not essential for generating an LH surge and triggering ovulation (28). Interestingly, however, Smith et al (8) found that KISS1 expression in the POA of the rhesus monkey is up-regulated late in the follicular phase in association with an elevation in circulating E2 concentrations, that in primates, as in rodents, constitutes the ovarian signal that triggers the pre-ovulatory LH surge (1,29). In the present study, imposition of an increment in circulating E2 concentrations of approximately 300 pg/ml early in the follicular phase in 2 monkeys resulted in an unequivocal increase in the number of immunoreactive kisspeptin neurons in the region of the AVPe in association with the initiation of a premature LH surge. This result, albeit achieved with a limited number of animals, supports the earlier finding of Smith et al (8) and reinforces the view that the population of kisspeptin neurons in the POA of the primate hypothalamus plays a role in mediating the positive feedback action of E2 that elicits the pre-ovulatory surge of LH in the normal menstrual cycle. The latter idea, however, must be tempered with the view, derived from the classical studies by Knobil's laboratory, that participation of the POA is not obligatory for eliciting an LH surge and triggering ovulation in the monkey (reviewed in 28). In order to fully appreciate the relative physiological importance of the populations of kisspeptin neurons of the POA and MBH in mediating the positive feedback action of E2 on LH secretion in the monkey, selective ablation of ER signaling in these two populations of kisspeptin neurons will be required.
Since the neural mechanisms underlying the initiation of the pre-ovulatory LH surge in the rhesus monkey are not de-commissioned by the testis during perinatal development (14,16), the finding that the number and distribution of kisspeptin neurons in the POA of testis-intact male monkeys was similar to that of female monkeys in the follicular phase of the menstrual cycle is perhaps not surprising. In both sexes, kisspeptin perikarya were observed in the region of the AVPe extending periventricularly into the medial POA, the same general area previously reported to contain KISS1 expressing neurons in the monkey (8) and other species (6). There were however, differences between the male and female in the distribution of immunoreactive kisspeptin within the perikarya of these neurons. In the male, there was a much larger proportion of neurons with cell bodies that appeared to be incompletely filled with immunoreactive kisspeptin. The reason for this subtle sex difference is unknown, but is likely to be related to the respective hormonal milieu generated by the testis or ovary. It should also be recalled that the method of fixation of the male (perfusion) and female (immersion) brain was different, although it is to be expected that perfusion rather than immersion would guarantee better preservation of immunoreactivity.
In striking contrast to intact males, immunoreactive kisspeptin perikarya were very rarely seen in the POA of adult males that had been castrated postpubertally. Implantation of castrate males for either 12h or 48h with E2 containing capsules that produced a quasi-square wave increment in circulating concentrations of the steroid of 300 pg/ml or greater resulted in marked up-regulation of kisspeptin immunoreactivity; an effect that was sufficiently robust to restore the number of immunoreactive kisspeptin neurons to values that were not significantly different from those in the POA of intact males. Here, it should be noted that the castrate group did not receive blank Silastic capsules nor were they sedated and venipunctured at 12h intervals before perfusion, as were the E2 treated castrates. This minor flaw in experimental design is considered to be of little importance because the number of kisspeptin neurons and immunoreactive kisspeptin fibers in the groups of animals that were sedated and venipunctured (E2-treated) was dramatically greater than that in the untreated castrate group. Intuitively, it would be anticipated that the effect, if any, of the additional experimental manipulations in the estrogen treated groups would be to exert a suppressive influence on the kisspeptin neuronal network underlying gonadotropin secretion.
The action of E2 treatment to regulate kisspeptin immunoreactivity in the POA of the castrate male monkey appeared to be unrelated to the action of the steroid to regulate LH secretion. This is because kisspeptin immunoreactivity was dramatically upregulated at both 12h when LH secretion was suppressed due to the negative feedback action of E2 and at 48h when the positive feedback action of the steroid was anticipated to be operative. The similarity in the kisspeptin response at the two phases of E2 feedback in the present model is consistent with the generally accepted notion that the site of action of E2 negative feedback in primate and non-primate species is within the MBH-pituitary unit (6,29). Indeed, E2 replacement to the ovariectomized monkey results in the down regulation of both KISS1 mRNA and kisspeptin in neurons of the arcuate nucleus in the MBH (30). Thus, it is reasonable to propose that kisspeptin content of kisspeptin neurons in the POA of the male monkey is maintained by an action of the testis and that this action may be mimicked by high circulating concentrations of E2 presumably via increasing signaling by ER alpha (ERα) expressed by the kisspeptin neurons (31,32). The pharmacological levels of circulating E2 achieved in the present study should not necessarily be used as an argument against the view that ERα signaling is the physiological pathway. This is because in the testis-intact situation, E2 in the POA of the male will be generated primarily by the local aromatization of circulating testosterone (33,34), which in the intact adult monkey ranges in concentrations from 2,000-20,000 pg/ml (35). Thus, the concentration of E2 in the POA of intact males may be greater than those in the E2 treated castrates examined in the present study.
In order for E2 to exert its positive feedback action on LH secretion in the follicular phase of the menstrual cycle, elevated concentrations of the circulating steroid must be maintained for approximately 36h (36). If the strength-duration characteristics of the E2 positive feedback signal in the castrate male are similar to those in the intact female, the question must be asked - what relevance, if any, does the upregulation of POA kisspeptin, observed in the present study after a 12h exposure to E2, have to do with the positive feedback action of the steroid and surge generation that is not anticipated until 24h later? Clearly, to answer this question, there will be a need for a more discerning paradigm of E2 replacement, such as that originally developed by Karsch et al (15), wherein a “pseudofollicular phase” is first induced in a castrate male by chronically increasing circulating E2 to concentrations normally observed early in the follicular phase (ie 50 pg/ml) before imposing an additional elevation in E2 to elicit positive feedback.
Two other issues need to be noted. First, kisspeptin staining in untreated monkeys in the follicular phase of the menstrual cycle studied in experiments 1 and 2 appeared to be related to whether or not the animals had been implanted with blank Silastic capsules. In the implanted animals (Experiment 2), the number of kisspeptin neurons was noticeable less than that in follicular phase females without implants (Experiment 1). A possible explanation for this discrepancy is that the monkeys which received Silastic capsules were sedated every 12h; first to implant the capsules and then to collect bi-daily blood samples. Thus, the added stress imposed by the protocol employed in Experiment 2 may have been responsible for the relatively low levels of kisspeptin immunoreactivity in this group of follicular phase females.
Second, unambiguous evidence that the positive feedback action of E2 had been initiated in the 3 castrate males that were exposed to E2 for 48h prior to perfusion was forthcoming in only 1 of the 3 monkeys. Although circulating E2 levels during the E2 challenge at the time of perfusion were lower than those during the earlier pre-experimental challenge, when an LH surge was induced in all 3 of these monkeys, levels of the steroid were nevertheless maintained above 300 pg/ml. Therefore an inadequate positive E2 feedback signal is unlikely to account for variability of the LH response. A more likely explanation is that the LH surge had not yet been initiated in 2 animals at 48h; a view consistent with the finding that during the pre-experimental E2 challenge the peak of the LH surge in the 3 monkeys was not observed until 60 or 72h after implantation of the E2 capsules.
In summary, the present study provides the first description of immunoreactive kisspeptin in the POA of the adult male and female macaque and suggests that the number and distribution of these peptidergic neurons is similar in the two sexes. In castrate adult males, however, kisspeptin neurons were rarely observed in the POA, although kisspeptin immunoreactivity in this region of the brain was readily restored in male castrates following exposure to E2 for as little as 12h. The later finding suggests that maintenance of kisspeptin content in the POA of the intact male requires local synthesis of E2 achieved via intra-hypothalamic aromatization of circulating testicular testosterone. In addition, our results further support the view that kisspeptin neurons in the POA of the female monkey are a target for the positive feedback action of E2 that underlies initiation of the pre-ovulatory LH surge, although this action of E2 in highly evolved primates appears to be a redundant one and not obligatory for ovulation (4). Finally, the presence of a robust kisspeptin neuronal network in the POA of the adult male monkey raises the question of what is the function of this peptidergic system in the brain of male primates?
Acknowledgements
We thank Dr. Pam Maoli (University of Pittsburgh School of Medicine, Pittsburgh, PA, USA) for allowing us post mortem access to the brain of the follicular phase females, Dr. Gloria E Hoffman (Morgan State University, Baltimore, MD, USA) for valuable guidance in the immunohistochemical technique, Dr. Stephen R. Bloom (Imperial College, London, UK) for the kisspeptin antibody, and Ms. Carolyn Phalin for conducting the radioimmunoassays. This study was supported by NIH grants R01 HD13254, R01 HD072189 and U54 HD08610 (T.M.P) and 5T32DK007729-19 (M.V.T)., and the São Paulo Research Foundation (2013/03596-1) (B.K.).
References
- 1.Levine JE. Neuroendocrine control of the ovarian cycle of the rat. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction. 4th edition Vol. 2. Elsevier; 2015. pp. 1199–1257. [Google Scholar]
- 2.Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96:57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- 3.Halasz B, Gorski RA. Gonadotrophic hormone secretion in female rats after partial or total interruption of neural afferents to the medial basal hypothalamus. Endocrinology. 1967;80:608–622. doi: 10.1210/endo-80-4-608. [DOI] [PubMed] [Google Scholar]
- 4.Krey LC, Butler WR, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology. 1975;96:1073–1087. doi: 10.1210/endo-96-5-1073. [DOI] [PubMed] [Google Scholar]
- 5.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbison AE. Physiology of the adult gonadotropin-releasing hormone neuronal network. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction. 4th edition Vol. 1. Elsevier; 2015. pp. 399–467. [Google Scholar]
- 7.Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in Kiss-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides. 2009;30:103–110. doi: 10.1016/j.peptides.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. Hypothalamic expression of Kiss-1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod. 2010;83:568–577. doi: 10.1095/biolreprod.110.085407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- 10.Feder HH. Hormonal actions on the sexual differentiation of the genitalia and the gonadotropin-regulating systems. In: Adler NT, editor. Neuroendocrinology of Reproduction. Vol. 2. Plenum Press; 1981. pp. 89–118. [Google Scholar]
- 11.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kauffman AS. Sexual differentiation and the Kiss1 system: hormonal and developmental considerations. Peptides. 2009;30:83–93. doi: 10.1016/j.peptides.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 14.Yamaji T, Dierschke DJ, Hotchkiss J, Bhattacharya AN, Surve AH, Knobil E. Estrogen induction of LH release in the rhesus monkey. Endocrinology. 1971;89:1034–1041. doi: 10.1210/endo-89-4-1034. [DOI] [PubMed] [Google Scholar]
- 15.Karsch FJ, Dierschke DJ, Knobil E. Sexual differentiation of pituitary function: apparent difference between primates and rodents. Science. 1973;179:484–486. doi: 10.1126/science.179.4072.484. [DOI] [PubMed] [Google Scholar]
- 16.Norman RL, Spies HG. Cyclic ovarian function in a male macaque: additional evidence for a lack of sexual differentiation in the physiological mechanisms that regulate the cyclic release of gonadotropins in primates. Endocrinology. 1986;118:2608–2610. doi: 10.1210/endo-118-6-2608. [DOI] [PubMed] [Google Scholar]
- 17.Barbarino A, De Marinis L. Estrogen induction of luteinizing hormone release in castrated adult human males. J Clin Endocrinol Metab. 1980;51:280–286. doi: 10.1210/jcem-51-2-280. [DOI] [PubMed] [Google Scholar]
- 18.Kulin HE, Reiter EO. Gonadotropin and testosterone measurements after estrogen administration to adult men, prepubertal and pubertal boys, and men with hypogonadotropism: evidence for maturation of positive feedback in the male. Pediatr Res. 1976;10:46–51. doi: 10.1203/00006450-197601000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe Y, Uenoyama Y, Suzuki J, Takase K, Suetomi Y, Ohkura S, Inoue N, Maeda KI, Tsukamura H. Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J Neuroendocrinol. 2014;26:909–917. doi: 10.1111/jne.12227. [DOI] [PubMed] [Google Scholar]
- 20.Watson RE, Jr., Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 21.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramaswamy S. Pubertal augmentation in juvenile rhesus monkey testosterone production induced by invariant gonadotropin stimulation is inhibited by estrogen. J Clin Endocrinol Metab. 2005;90:5866–5875. doi: 10.1210/jc.2005-0092. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Huang X-F, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2000. [Google Scholar]
- 24.Bleier R. A Cytoarchitectonic Atlas. The University of Wisconsin Press Ltd; Wisconsin: 1984. The Hypothalamus of the Rhesus Monkey. [Google Scholar]
- 25.Kalil B, Ramaswamy S, Plant TM. The distribution of substance P and kisspeptin in the mediobasal hypothalamus of the male rhesus monkey and a comparison of intravenous administration of these peptides to release GnRH as reflected by LH secretion. Neuroendocrinology. 2015 doi: 10.1159/000442420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Majdoubi M, Ramaswamy S, Sahu A, Plant TM. Effects of orchidectomy on levels of the mRNAs encoding gonadotropin-releasing hormone and other hypothalamic peptides in the adult male rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2000;12:167–176. doi: 10.1046/j.1365-2826.2000.00433.x. [DOI] [PubMed] [Google Scholar]
- 27.Williams NI, Caston-Balderrama AL, Helmreich DL, Parfitt DB, Nosbisch C, Cameron JL. Longitudinal changes in reproductive hormones and menstrual cyclicity in cynomolgus monkeys during strenuous exercise training: abrupt transition to exercise-induced amenorrhea. Endocrinology. 2001;142:2381–2389. doi: 10.1210/endo.142.6.8113. [DOI] [PubMed] [Google Scholar]
- 28.Plant TM. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front Neuroendocrinol. 2012;33:160–168. doi: 10.1016/j.yfrne.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Zeleznik AJ, Plant TM. Control of the menstrual cycle. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction. 4th edition Vol. 2. Elsevier; 2015. pp. 1307–1361. [Google Scholar]
- 30.Alcin E, Sahu A, Ramaswamy S, Hutz ED, Keen KL, Terasawa E, Bethea CL, Plant TM. Ovarian regulation of kisspeptin neurones in the arcuate nucleus of the rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2013;25:488–496. doi: 10.1111/jne.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 32.Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roselli CE, Resko JA. Cytochrome p450 aromatase (CYP19) in the non-human primate brain: distribution, regulation, and functional significance. J Steroid Biochem Mol Biol. 2001;79:247–253. doi: 10.1016/s0960-0760(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 34.Michael RP, Bonsall RW, Rees HD. Sites at which testosterone may act as an estrogen in the brain of the male primate. Neuroendocrinology. 1987;46:511–521. doi: 10.1159/000124874. [DOI] [PubMed] [Google Scholar]
- 35.Plant TM. Time courses of concentrations of circulating gonadotropin, prolactin, testosterone, and cortisol in adult male rhesus monkeys (Macaca mulatta) throughout the 24 h light-dark cycle. Biol Reprod. 1981;25:244–252. doi: 10.1095/biolreprod25.2.244. [DOI] [PubMed] [Google Scholar]
- 36.Karsch FJ, Weick RF, Butler WR, Dierschke DJ, Krey LC, Weiss G, Hotchkiss J, Yamaji T, Knobil E. Induced LH surges in the rhesus monkey: strength-duration characteristics of the estrogen stimulus. Endocrinology. 1973;92:1740–1747. doi: 10.1210/endo-92-6-1740. [DOI] [PubMed] [Google Scholar]