Summary
Introduction
The association between obesity and asthma control/quality of life commonly relies on body mass index (BMI) as the anthropomorphic measure. Due to limitations of BMI and the existence of alternative measures, such as neck circumference (NC), we examined the association between NC and asthma control/quality of life, with particular attention to male–female differences
Materials and Methods
The AsthMaP-2 Project is an observational study of youth with physician–diagnosed asthma. NC was stratified according to age- and sex-specific cutoffs and associated with asthma control (via Asthma Control Test [ACT]) and quality of life (via Integrated Therapeutics Group [ITG]—Asthma Short Form)
Results
The mean±SD age was 11.9±3.6 years, and 53% were male (N=116). The mean BMI percentile was at the 71±28 percentile. Thirty-one participants (27%) met criteria for high NC. Males with high NC had significantly worse asthma control (P =0.02) and lower quality of life than those with low NC. No similar association was found for females and the proportion of variability in ACTand ITG was best explained by BMI percentile. Conversely, for males, the proportion of variability in these scores explained by NC was larger than BMI percentile alone (Cohen’s f2 =0.04–0.09, a small to medium effect size)
Discussion
Among male youth with asthma, combined use of NC and BMI percentile explained asthma control and quality of life better than BMI alone. Future studies of asthma should include measurement of NC and other anthropogenic measures of regional adiposity to clarify sex differences in asthma.
Keywords: adiposity, body mass index, pediatric obesity, pediatrics
INTRODUCTION
Approximately, 17% of youth in the United States are considered obese.1 Obesity is associated with an increased prevalence of asthma, increased severity, decreased control, and poor response to therapy.2,3 However, the pathophysiological link between the obesity and asthma has yet to be fully explained. This is further complicated by emerging male–female differences with regard to patterns of adiposity4 and accompanying risk of co-morbid diseases,5 such as asthma.6
Studies evaluating the relationship between obesity and asthma have most commonly utilized body mass index (BMI) as the anthropomorphic measure of adiposity.7 BMI is defined as the ratio of body weight (kg) to the square of height (cm). In youth, BMI is compared to reference ranges to determine a percentile. Recently, it has become clear that the use of BMI has limitations, including but not limited to its inadequacy in describing regional adiposity and inability to differentiate between lean body mass and fat mass.3,8 Other anthropomorphic measurements for obesity include percent body fat, waist-to-hip circumference ratio, and waist circumference. Of note, these measures are all associated with increased exercise-induced asthma symptoms while BMI is not.9
Neck circumference (NC, measured at the neck midpoint) is an additional anthropomorphic measure of obesity. It is associated with cardiovascular disease risk,10 another disease for which the predictive value of BMI could be improved upon.11 Further, NC has been shown to better correlate with visceral adiposity than BMI does in multiple populations.12–16 The objective of this study was to examine the association between NC and asthma control/quality of life in youth with asthma, with particular attention to male–female differences. We hypothesized that higher NC would be associated with worse asthma control/quality of life in both sexes. Some of the results of these studies have been previously reported in the form of an abstract.17
MATERIALS AND METHODS
Study Cohort
The Asthma Severity Modifying Polymorphisms (AsthMaP)-2 Project is a single-center longitudinal observational study of asthma. AsthMaP-2 follows a convenience sample of youth between the ages of 6 and 20 years. Participants must have physician–diagnosed asthma for at least 1 year prior to the time of recruitment from the emergency department, inpatient units and outpatient clinics of an urban pediatric medical center. Individuals who report a medical history of chronic or complex cardiorespiratory disease are ineligible. Specific methodology from the prior AsthMaP cohort has been published elsewhere.18–21 This study was approved by our Institutional Review Board and consent/assent were given by parents and participants. Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at Children’s National Medical Center.
Anthropomorphic Measurements
All anthropomorphic measurements were taken using standard techniques as follows: (i) Weight (average of two measurements) to the nearest 0.1 kg by digital scale (ScaleTronix, White Plains, NY); (ii) height (average of three measurements) to the nearest 0.1 cm by stadiometer (Holtain Limited, Crymych, Pembrokeshire, UK); and (iii) neck circumference (NC) (average of three measurements) measured at mid-neck height, between mid-cervical spine and below the cricothyroid cartilage, with the subject standing upright and facing the investigator, having their shoulders relaxed10 to within 1 mm with plastic tapes. BMI was calculated from average weight and height values collected from each participant and plotted on CDC growth charts according to age and sex to determine BMI percentile (http://www.cdc.gov/healthyweight/assessing/bmi/childrens_BMI/tool_for_schools.html). NC was stratified according to a previously defined set of age- and sex-specific NC measurement cutoffs shown by Nafiu et al.22 A NC equal to or greater than the age- and sex-specific cutoff was considered “high” and a NC less than the cutoff was considered “low.” For example, a 6-year-old male with a NC =30.0 cm would be classified as “high” since the NC cutoff for 6-year-old males is 28.8 cm.
Outcome Measures
The Asthma Control Test (ACT®) and Childhood Asthma Control Test (C-ACT®) were used to assess asthma control.23,24 Scores on the ACT and C-ACT range from 0 to 27 and 5 to 25, respectively. Scores of 23 and 22 or higher, respectively, on the ACT and C-ACT indicate adequate asthma control.25 As the minimally-important difference for the ACT has been established to be around two points,25 scores below 20 are commonly considered indicative of inadequate asthma control.
The Integrated Therapeutics Group—Asthma Short Form (ITG) was used to assess quality of life.26 Domains include self-reported functional limitation and symptoms. The ITG is scored between 0 and 100 where low scores indicate lower asthma-related quality of life.27
Statistical Analysis
The statistical analyses focused on two predictors (BMI percentile and NC) and five outcomes (ACT, ITG, and its three subscales). Normality of continuous quantitative outcomes was assessed using the Shapiro–Wilk normality test and evaluation of histograms. ACT score was found to be non-normally distributed; therefore, square-transformations were applied for analyses.
Analyses were performed on data collected at study enrollment. Initial analyses compared asthma outcomes between NC groups (meets/exceeds cutoff =high vs. does not meet cutoff =low) using analysis of covariance with adjustment for sex and age. A P-value ≤0.05 was considered evidence of some association with sex. Further analyses were stratified by sex and adjusted for age. Mean ITG and ACT scores were compared between NC groups and between BMI percentile groups (<85th vs. ≥85th percentile) using Student’s t tests. Frequencies of NAEPP chronic severity classification groups were compared between NC groups using an exact χ2 test. Assessment of the contribution of NC in addition to BMI percentile was tested using hierarchical linear regression models. A full model including BMI percentile and NC as predictors was compared to a constrained model including only BMI percentile or NC. The difference in r2 values for the models described the proportion of variability in outcome that could be attributed to NC. A likelihood-ratio test assessed whether the proportion of variability attributable to NC was significantly different from zero. For each pair of hierarchical models, Cohen’s f2 was calculated to measure the effect size (i.e., the contribution) of NC to the outcome. All analyses were performed using Stata V13 (College Station, TX).
RESULTS
The AsthMaP-2 cohort included 116 youths at the time of these analyses. The mean ± SD age was 11.9 ± 3.6 years, and 53% were male. The mean BMI ± SD percentile for age and sex was 71 ± 28%. Fifty-two youths (45%) exceeded a BMI percentile of 85 and therefore were classifiable as overweight/obese. NC met or exceeded high criteria (i.e., above age- and sex-specific cutoffs22) in 31 (27%) of the participants. Of the 114 cases with information available, 105 (92%) had persistent asthma as defined by NAEPP criteria.28 Participant demographics and clinical characteristics are shown in Table 1.
TABLE 1.
Participant Demographics and Clinical Characteristics
| Total cohort | Low NC | High NC | P-value (High vs. Low NC) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| N (out of 116) | N (%) or Mean ± SD | N (out of 85) | N (%) or Mean ± SD | N (out of 31) | N (%) or Mean ± SD | ||
| Age, years | 116 | 11.9 ± 3.6 | 85 | 11.4 ± 3.8 | 31 | 11.7 ± 3.1 | 0.63 |
| Sex, % male | 116 | 61 (53%) | 85 | 48 (56%) | 31 | 13 (42%) | 0.16 |
| Race4 | |||||||
| American Indian/Alaskan Native | 116 | 10 (8.6%) | 85 | 9 (11%) | 31 | 1 (4.9%) | 0.21 |
| Asian | 116 | 0 (0.0%) | 85 | 0 (0.0%) | 31 | 0 (0.0%) | N/A |
| Black/African-American | 116 | 107 (92.2%) | 85 | 79 (93%) | 31 | 28 (96.7%) | 0.64 |
| Native Hawaiian/other pacific islander | 116 | 0 (0.0%) | 85 | 0 (0.0%) | 31 | 0 (0.0%) | N/A |
| White | 116 | 12 (10.3%) | 55 | 8 (9%) | 31 | 4 (13%) | 0.58 |
| Ethnicity, % hispanic | 116 | 7 (6.0%) | 84 | 7 (8%) | 31 | 2 (6%) | 0.74 |
| Public health insurance | 116 | 94 (81%1) | 85 | 66 (78%) | 31 | 27 (87%) | 0.26 |
| BMI, percentile | 116 | 71 ± 28 | 85 | 62 ± 28 | 31 | 96 ± 6 | <0.001 |
| BMI | 116 | 22 ± 7 | 85 | 20 ± 4 | 31 | 30 ± 6 | <0.001 |
| NC, % high | 116 | 31 (27%) | 85 | 0 (0%) | 31 | 31 (100%) | N/A |
| Age of asthma onset, years | 114 | 5 ± 4 | 84 | 4 ± 4 | 31 | 5 ± 4 | 0.59 |
| Premature birth | 115 | 17 (15%2) | 85 | 9 (11%) | 31 | 8 (26%) | 0.04 |
| Allergic rhinitis | 115 | 54 (47%2) | 85 | 39 (46%) | 31 | 15 (48%) | 0.81 |
| Atopic dermatitis | 115 | 68 (59%) | 85 | 54 (64%) | 31 | 14 (45%) | 0.08 |
| Parent with asthma | 115 | 68 (59%) | 85 | 47 (55%) | 31 | 21 (68%) | 0.23 |
| Sibling with asthma | 115 | 70 (61%3) | 85 | 54 (64%) | 31 | 17 (55%) | 0.40 |
| FEV1 (% predicted) change with bronchodilator | 100 | 10 ± 18 | 74 | 11 ± 20 | 28 | 8 ± 9 | 0.40 |
| Post bronchodilator FEV1 (% predicted) | 104 | 100 ± 87 | 74 | 100 ± 101 | 28 | 101 ± 25 | 0.95 |
| FEV1/FVC (% predicted) change with bronchodilator | 99 | 7 ± 10 | 74 | 7 ± 11 | 28 | 6 ± 7 | 0.54 |
| Post bronchodilator FEV1/FVC (%) | 109 | 81 ± 10 | 74 | 86 ± 9 | 28 | 84 ± 7 | 0.24 |
| FEF25–75 (% predicted) change with bronchodilator | 108 | 71 ± 28 | 74 | 50 ± 93 | 28 | 37 ± 44 | 0.32 |
| Post bronchodilator FEF25–75 (% predicted) | 102 | 90 ± 27 | 74 | 89 ± 25 | 28 | 93 ± 32 | 0.55 |
| Serum IgE, IU/mL | 111 | 553 ± 728 | 51 | 560 ± 681 | 60 | 531 ± 855 | 0.87 |
| Blood eosinophils, % | 111 | 6 ± 5 | 84 | 6 ± 5 | 31 | 5 ± 4 | 0.50 |
| ACT | 115 | 20 ± 4 | 54 | 21 ± 4 | 61 | 18 ± 4 | <0.01 |
| ITG composite | 110 | 66 ± 21 | 85 | 70 ± 20 | 31 | 58 ± 20 | 0.01 |
| ITG functional | 111 | 70 ± 22 | 85 | 73 ± 22 | 31 | 61 ± 22 | 0.02 |
| ITG daytime | 113 | 63 ± 24 | 85 | 68 ± 23 | 31 | 54 ± 22 | 0.01 |
| ITG nighttime | 112 | 64 ± 27 | 85 | 67 ± 26 | 31 | 58 ± 29 | 0.13 |
NC, neck circumference; IgE, immunoglobulin E; BMI, body mass index; ACT, asthma control test; ITG, integrated therapeutics group’s child asthma short form.
2 unsure and 2 uninsured.
2 unsure.
10 with no sibling.
Individuals could select all race options that applied.
At the time of their assessments, those participants with a high NC had significantly poorer asthma control as indicated by lower ACTand a lower quality of life via ITG scores (Table 2). The mean ACT score for the high NC group was 17 ± 4 compared with the low NC group whose mean ACT was 19 ± 5 (P =0.004). ITG composite, functional, and daytime scores were all significantly lower in the high NC group. The mean ITG composite for high NC individuals was 55 ± 21 compared to 66 ± 28 for those with a low NC (P =0.02). The mean ITG functional score for high NC participants was 60 ± 24 compared to 71 ± 31 (P =0.02) for those with a low NC. For ITG daytime score, those with a high NC had a mean score of 51 ± 24 versus 62 ± 31 for those with low NC values (P =0.02). There was no significant association between NC and ITG nighttime or NAEPP chronic severity classification.
TABLE 2.
Comparison of Asthma Control Test and Integrated Therapeutics Group’s Child Asthma Short Form Scores Between High and Low Neck Circumference Groups
| Dependent variable | Low NC | High NC | P-value | ||
|---|---|---|---|---|---|
|
|
|
||||
| N | Mean ± SD | N | Mean ± SD | ||
| ACT1,2 | 84 | 19 ± 5 | 31 | 17 ± 4 | 0.004 |
| ITG composite1 | 82 | 66 ± 28 | 28 | 55 ± 21 | 0.02 |
| ITG functional1 | 83 | 71 ± 31 | 28 | 60 ± 24 | 0.02 |
| ITG daytime1 | 85 | 62 ± 31 | 28 | 51 ± 24 | 0.02 |
| ITG nighttime | 84 | 64 ± 37 | 28 | 55 ± 29 | 0.15 |
ACT, asthma control test; ITG, integrated therapeutics group’s child asthma short form.
Showed evidence of a sex effect (P ≤ 0.05).
Raw values presented, but analyses were sex- and age-adjusted using square-transformed data.
We saw male–female differences for group effects for the associations between NC and several asthma outcomes (P ≤ 0.05 for ACT score and for ITG composite, functional, and daytime scores). In sex-stratified age-adjusted analyses, male participants with a high NC had significantly worse asthma control and lower quality of life than males with a low NC as indicated by lower ACT and ITG scores (Table 3). The mean ± SD ACT score for high NC males was 19 ± 4 compared with low NC males whose mean ACT was 22 ± 3 (P =0.02). The mean ITG composite score for high NC males (59 ± 20) was significantly lower than that for low NC males (73 ± 19; P =0.04). The mean ITG functional score for high NC males (60 ± 25) was also significantly lower than in low NC males (76 ± 21; P =0.03). Although mean ITG daytime and nighttime scores were lower in the high NC versus low NC males, the difference was not statistically significant (P =0.10 and 0.10, respectively). Females showed a similar pattern with those in the high NC category having lower mean ACT scores than the low NC females (18 ± 5 vs. 20 ± 3; P =0.07), but this and ITG scores were not significantly different among females.
TABLE 3.
Sex-Stratified Comparison of Asthma Control Test and Integrated Therapeutics Group’s Child Asthma Short Form Scores Between High and Low Neck Circumference Groups
| Dependent variable | Females | Males | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Does not exceed NC cutoff | Exceeds NC cutoff | P-value | Does not exceed NC cutoff | Exceeds NC cutoff | P-value | |||||
|
|
|
|
|
|||||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | |||
| ACT1 | 36 | 20 ± 3 | 18 | 18 ± 5 | 0.07 | 48 | 22 ± 3 | 13 | 19 ± 4 | 0.02 |
| ITG composite | 35 | 66 ± 21 | 17 | 60 ± 21 | 0.18 | 47 | 73 ± 19 | 11 | 59 ± 20 | 0.04 |
| ITG functional | 35 | 70 ± 23 | 17 | 62 ± 22 | 0.22 | 48 | 76 ± 21 | 11 | 60 ± 25 | 0.03 |
| ITG daytime | 37 | 62 ± 25 | 17 | 51 ± 22 | 0.11 | 48 | 72 ± 21 | 11 | 60 ± 20 | 0.10 |
| ITG nighttime | 37 | 63 ± 27 | 17 | 58 ± 32 | 0.60 | 47 | 71 ± 25 | 11 | 57 ± 23 | 0.10 |
ACT, asthma control test; ITG, integrated therapeutics group’s child asthma short form.
Raw values presented, but analyses performed on square-transformed data.
When participants were categorized by BMI percentile (<85th vs. ≥85th percentile), comparisons of mean asthma control and quality of life measures showed no significant differences in either males or females (Table 4).
TABLE 4.
Sex-Stratified Comparison of Asthma Control Test and Integrated Therapeutics Group’s Child Asthma Short Form Scores between Body Mass Index Percentile Groups
| Dependent variable | Females | Males | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| BMI < 85th percentile | BMI ≥85th percentile | P-value | BMI < 85th percentile | BMI ≥ 85th percentile | P-value | |||||
|
|
|
|
|
|||||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | |||
| ACT1 | 27 | 20 ± 3 | 25 | 18 ± 4 | 0.07 | 33 | 22 ± 3 | 27 | 21 ± 4 | 0.29 |
| ITG composite | 26 | 68 ± 18 | 24 | 58 ± 21 | 0.06 | 33 | 72 ± 20 | 25 | 69 ± 20 | 0.57 |
| ITG functional | 26 | 74 ± 20 | 24 | 63 ± 24 | 0.09 | 33 | 76 ± 22 | 25 | 69 ± 22 | 0.25 |
| ITG daytime | 28 | 65 ± 23 | 24 | 54 ± 24 | 0.09 | 33 | 71 ± 22 | 25 | 69 ± 21 | 0.64 |
| ITG nighttime | 28 | 66 ± 25 | 24 | 57 ± 29 | 0.24 | 32 | 68 ± 25 | 25 | 69 ± 26 | 0.98 |
BMI, body mass index; NC, neck circumference; ACT, asthma control test; ITG, integrated therapeutics group’s child asthma short form.
Unadjusted raw values presented, but analysis performed on square-transformed data. Results reflective of only those subjects with a NC measurement.
These findings were furthered using hierarchical regression analyses (adjusted for age) to determine whether the addition of NC significantly added to the ability of BMI percentile to explain the variability seen in asthma control and quality of life (Table 5). Even though BMI percentile and NC are closely related to each other (r2=0.32; P <0.001), the addition of NC affected models differently by sex. For males only, BMI and NC percentile in combination explain a statistically significantly greater portion of the variability in ACT and ITG composite and nighttime scores than does BMI percentile alone. The Cohen’s f2 effect sizes in males ranged from 0.04 to 0.09, using conventional standards, a small to medium effect size. This was not the case in females where the addition of NC to the model did not significantly contribute to describing the outcomes.
TABLE 5.
Sex-Stratified Contribution of Neck Circumference to the Relationship Between Body Mass Index Percentile and Outcomes
| Sex | Asthma outcome | Model with NC alone (r2) | Model with BMI percentile alone (r2) | Model with BMI percentile +NC (r2) | % variability in outcome attributable to NC (P-value)1 | Cohen’s f2 effect size |
|---|---|---|---|---|---|---|
| Female | ACT2 | 0.081 | 0.142 | 0.147 | 0.5% (0.60) | <0.01 |
| ITG composite | 0.056 | 0.096 | 0.099 | 0.3% (0.68) | <0.01 | |
| ITG functional | 0.046 | 0.093 | 0.094 | 0.1% (0.80) | <0.01 | |
| ITG daytime | 0.068 | 0.039 | 0.070 | 3.2% (0.19) | 0.033 | |
| ITG nighttime | 0.008 | 0.073 | 0.082 | 0.9% (0.49) | 0.01 | |
| Male | ACT2 | 0.096 | 0.021 | 0.096 | 7.5% (0.029) | 0.083 |
| ITG composite | 0.081 | 0.004 | 0.085 | 8.1% (0.028) | 0.093 | |
| ITG functional | 0.081 | 0.017 | 0.081 | 6.4% (0.049) | 0.073 | |
| ITG daytime | 0.050 | 0.011 | 0.050 | 3.9% (0.13) | 0.043 | |
| ITG nighttime | 0.052 | 0.002 | 0.075 | 7.3% (0.037) | 0.083 |
BMI, body mass index; NC, neck circumference; ACT, asthma control test; ITG, integrated therapeutics group’s child asthma short form.
Likelihood-ratio P-value shown.
Analyses performed on square-transformed data.
By convention, a Cohen’s f2 effect size of 0.02, 0.15, and 0.35 are considered small, medium and large, respectively.
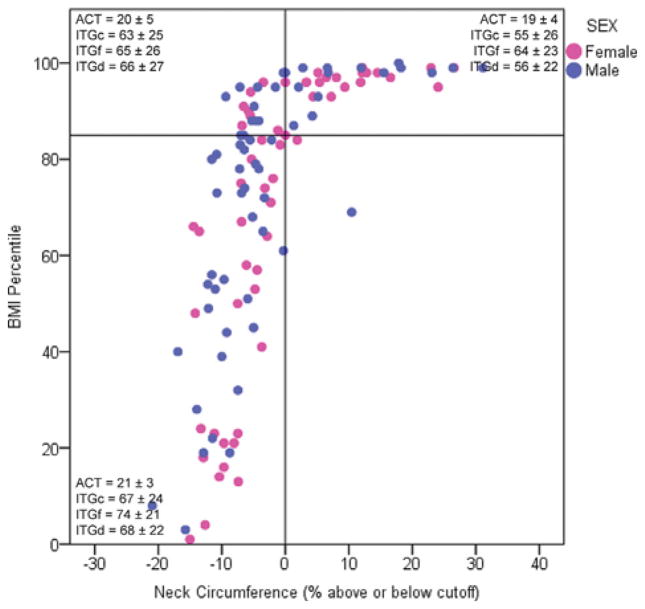
To visualize the relationship between BMI and NC, we plotted BMI percentiles against the percent difference between the actual NC and the NC cutoff for each participant. The resulting scatterplot is shown in Figure 1 and is divided into four quadrants using lines at the [NCactual−NCcutoff)]/NCcutoff = 0 and at a BMI percentile of 85, the value which the CDC considers overweight. Mean ACT and ITG composite and daytime scores are overlaid for each quadrant according to sex. The plot is notable for the worst control and quality of life (i.e., lowest ACT and ITG scores) in the upper right quadrant, where both BMI percentile and NC are above their respective cutoffs.
Fig. 1.
Scatterplot of BMI percentile and percent difference between actual NC and NC cutoff. Mean ± SD ACT and ITG composite, functional, and daytime scores are shown for all four quadrants. The plot is notable for the worst control and quality of life (i.e., lowest ACT and ITG scores) in the upper right quadrant, where both BMI percentile and NC are above their respective cutoffs. BMI, body mass index; NC, neck circumference; ACT, asthma control test; ITG, integrated therapeutics group’s child asthma short form; ITGc, ITG composite; ITGf, ITG functional; ITGd, ITG daytime.
DISCUSSION
In our investigation, we studied the relationship between asthma and obesity using neck circumference (NC) as an alternative measure to BMI. Notably, we found ACT and ITG scores to be significantly associated overall with NC among youth with asthma, indicating inadequate asthma control, and poor quality of life, but these associations varied by sex. Among the males, the lower ACT scores associated with high NC exceeded the minimal clinically important difference (i.e., 2 points25). Additionally, among males only, NC significantly added to the ability of BMI percentile to explain variability in asthma control and quality of life. Although there were trends toward worse asthma control among females with high NC, we did not detect significant associations for quality of life.
During the pre-pubertal period of low sex steroid hormones (i.e., estrogen, progesterone, testosterone), males predominate with regard to prevalence and severity of asthma. Following onset of puberty, asthma has a female predominance in both prevalence and severity.29 As they mature through childhood and puberty, females with asthma exhibit increased bronchial responsiveness compared to males based on methacholine sensitivity.30,31 In fact, post-pubertal women with asthma are more likely to be categorized as severe32 and more likely to be hospitalized for acute asthma than men.33 Although this sex disparity in asthma is clear, the underlying etiology is unknown.
Obesity is a state of chronic inflammation34 associated with an increased prevalence of asthma, decreased symptom control, and poor response to therapy.2,3 The study of this link requires accurate measures of obesity. However, the optimal measure of adiposity for defining obesity is unclear. Unfortunately, many of the most accurate measures (e.g., underwater weighing, dual-energy x-ray absorptiometry (DXA), and isotope dilution) are labor intensive and expensive, preventing their use in large cohort studies. Therefore, BMI (and BMI percentile in children) has become the most commonly used surrogate measure of body type and adiposity.
Increasingly, there are concerns regarding the relevance of BMI with regard to regional adiposity and its inability to differentiate between lean body mass and fat mass.3,8 This is important in asthma research as there appears to be a disparity between the two sexes with adiposity playing a role.3,19,29 Importantly, fat distribution differs between the sexes with women generally accumulating subcutaneous fat around their hips and buttocks while men accumulate visceral fat in their abdomen (i.e., gynecoid vs. android patterns).35 In addition, sex differences exist in cardiometabolic disease where NC is more strongly associated with increased risk in females.36
We and others showed previously that obese individuals with asthma tend to display non-eosinophilic airway inflammation.19,37 There are at least two features of asthma that are compounded by obesity: (i) physical forces and (ii) inflammation. Regarding physical forces, Al-Alwan et al. showed increased lung compliance in obese individuals with asthma as opposed to obese controls.38 On the other hand, there are studies showing normal or decreased lung compliance in obese individuals with asthma.39 This suggests that the physical forces of excess weight are not solely responsible for the altered lung volumes in these patients. Relevant to asthmatic inflammation specifically, leptin is an adipocyte-derived mediator of obstructive lung disease in mice.40
Our analyses have several potential limitations. First, the data used in our analyses are cross-sectional data at enrollment from a population composed largely of African American youth living in the Washington, DC area. As such, the range of phenotypes represented by the study population may not fully represent the full range of clinical phenotypes and may be affected by the mix of pre-pubertal and pubertal youth. The lack of pubertal measurement and sleep apnea symptoms are limitations. Further, our analyses, particularly those examining sex-differences were derived post-hoc using a limited sample size. In particular, the power in many of our stratified analyses is likely too low to truly rule out associations. Additionally, while ACT and ITG are validated measures, both are self-reported surveys and as such carry a degree of subjectivity. The NC cutoffs were determined in the original publication using ROC analyses22 and we did not assess inter-rater reliability for measurement of NC in our population. These potential sources of random misclassification would tend to obscure associations but there is no reason to suspect any misclassification would be more common in females than males. Finally, because this is an observational study, some of the subjects were not on controller medications while others were, but with variable compliance.
In conclusion, in a sample of primarily urban and minority youth with asthma, increased NC was associated with worse asthma control and decreased asthma-related quality of life, and these associations varied by sex. For males, lower ACT and ITG scores were associated with high NC and NC significantly added to the ability of BMI percentile to explain variability in asthma control and quality of life. Although there were trends toward worse asthma control among females with a high NC, we did not detect significant associations for quality of life. Use of BMI and NC percentile together explain differences in asthma control and quality of life better than BMI alone, but only in males. NC and other anthropomorphic measures of regional adiposity may be useful in the clinical evaluation of pediatric patients with asthma. Future clinical studies of asthma should consider the inclusion of NC measurement to clarify its role in males and females.
Acknowledgments
Funding source: NIH, Numbers: R01MD007075, UL1TR000075; Clark Charitable Foundation.
Footnotes
Conflict of interest: None.
Disclaimer: The views expressed in this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Clinicaltrials.gov Identifier: NCT01647399
AUTHORS’ CONTRIBUTIONS
LM and RJF conceptualized and designed the project. LM, SB, NC, RM, FN, GDP, RL, and YT recruited the subjects and acquired the data. HGD and RJF performed data analysis. LM, EM, and RJF interpreted data and prepared the manuscript. DKP, SJT, CAC, MH, DMM, and RJF drafted or revised the manuscript for important intellectual content.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 3.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, Kercsmar CM, Visness CM, Matsui EC, Steinbach SF, Szefler SJ, Sorkness CA, Morgan WJ, Teach SJ, Gan VN. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125:584–592. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medrikova D, Jilkova ZM, Bardova K, Janovska P, Rossmeisl M, Kopecky J. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obes (Lond) 2012;36:262–272. doi: 10.1038/ijo.2011.87. [DOI] [PubMed] [Google Scholar]
- 5.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues—the biology of pear shape. Biol Sex Differ. 2012;3:13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang JE, Holbrook JT, Wise RA, Dixon AE, Teague WG, Wei CY, Irvin CG, Shade D, Lima JJ. Obesity in children with poorly controlled asthma: sex differences. Pediatr Pulmonol. 2013;48:847–856. doi: 10.1002/ppul.22707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visness CM, London SJ, Daniels JL, Kaufman JS, Yeatts KB, Siega-Riz AM, Calatroni A, Zeldin DC. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. J Asthma. 2010;47:822–829. doi: 10.3109/02770903.2010.489388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 9.Forno E, Acosta-Perez E, Brehm JM, Han YY, Alvarez M, Colon-Semidey A, Canino G, Celedon JC. Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J Allergy Clin Immunol. 2014;133:1308–1314. 1314 e1301–1305. doi: 10.1016/j.jaci.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Noun LL, Laor A. Relationship between changes in neck circumference and cardiovascular risk factors. Exp Clin Cardiol. 2006;11:14–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Chrysant SG, Chrysant GS. New insights into the true nature of the obesity paradox and the lower cardiovascular risk. J Am Soc Hypertens. 2013;7:85–94. doi: 10.1016/j.jash.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Li HX, Zhang F, Zhao D, Xin Z, Guo SQ, Wang SM, Zhang JJ, Wang J, Li Y, Yang GR. Neck circumference as a measure of neck fat and abdominal visceral fat in Chinese adults. BMC Public Health. 2014;14:311. doi: 10.1186/1471-2458-14-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Samarasinghe Y, Kane P, Amiel S, Aylwin S. Visceral adiposity is closely correlated with neck circumference and represents a significant indicator of insulin resistance in WHO grade III obesity. Clin Endocrinol (Oxf) 2010;73:197–200. doi: 10.1111/j.1365-2265.2009.03772.x. [DOI] [PubMed] [Google Scholar]
- 14.Onat A, Hergenç G, Yüksel H, Can G, Ayhan E, Kaya Z, Dursunoğlu D. Neck circumference as a measure of central obesity: associations with metabolic syndrome and obstructive sleep apnea syndrome beyond waist circumference. Clin Nutr. 2009;28:46–51. doi: 10.1016/j.clnu.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Laakso M, Matilainen V, Keinanen-Kiukaanniemi S. Association of neck circumference with insulin resistance-related factors. Int J Obes Relat Metab Disord. 2002;26:873–875. doi: 10.1038/sj.ijo.0802002. [DOI] [PubMed] [Google Scholar]
- 16.Hatipoglu N, Mazicioglu MM, Kurtoglu S, Kendirci M. Neck circumference: an additional tool of screening overweight and obesity in childhood. Eur J Pediatr. 2010;169:733–739. doi: 10.1007/s00431-009-1104-z. [DOI] [PubMed] [Google Scholar]
- 17.Behniwal S, Gordish-Dressman H, Marwah R, Certner N, Mansell D, Pillai D, Lazaroff R, Tsegaye Y, Maltz L, Freishtat R. Larger neck circumference is associated with poor asthma control in obese youth. Am J Respir Crit Care Med. 2014;189:A6318. [Google Scholar]
- 18.Benton AS, Kumar N, Lerner J, Wiles AA, Foerster M, Teach SJ, Freishtat RJ. Airway platelet activation is associated with airway eosinophilic inflammation in asthma. J Investig Med. 2010;58:987–990. doi: 10.231/JIM.0b013e3181fa02f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benton AS, Wang Z, Lerner J, Foerster M, Teach SJ, Freishtat RJ. Overcoming heterogeneity in pediatric asthma: tobacco smoke and asthma characteristics within phenotypic clusters in an African American cohort. J Asthma. 2010;47:728–734. doi: 10.3109/02770903.2010.491142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freishtat RJ, Iqbal SF, Pillai DK, Klein CJ, Ryan LM, Benton AS, Teach SJ. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr. 2010;156:948–952. doi: 10.1016/j.jpeds.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stemmy EJ, Benton AS, Lerner J, Alcala S, Constant SL, Freishtat RJ. Extracellular cyclophilin levels associate with parameters of asthma in phenotypic clusters. J Asthma. 2011;48:986–993. doi: 10.3109/02770903.2011.623334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nafiu OO, Burke C, Lee J, Voepel-Lewis T, Malviya S, Tremper KK. Neck circumference as a screening measure for identifying children with high body mass index. Pediatrics. 2010;126:e306–310. doi: 10.1542/peds.2010-0242. [DOI] [PubMed] [Google Scholar]
- 23.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Lieu TA, Quesenberry CP, Sorel ME, Mendoza GR, Leong AB. Computer-based models to identify high-risk children with asthma. Am J Respir Crit Care Med. 1998;157:1173–1180. doi: 10.1164/ajrccm.157.4.9708124. [DOI] [PubMed] [Google Scholar]
- 25.Voorend-van Bergen S, Vaessen-Verberne AA, Landstra AM, Brackel HJ, van den Berg NJ, Caudri D, de Jongste JC, Merkus PJ, Pijnenburg MW. Monitoring childhood asthma: web-based diaries and the asthma control test. J Allergy Clin Immunol. 2014;133:1599–1605. e1592. doi: 10.1016/j.jaci.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Bukstein DA, McGrath MM, Buchner DA, Landgraf J, Goss TF. Evaluation of a short form for measuring health-related quality of life among pediatric asthma patients. J Allergy Clin Immunol. 2000;105:245–251. doi: 10.1016/s0091-6749(00)90072-1. [DOI] [PubMed] [Google Scholar]
- 27.Gorelick MH, Brousseau DC, Stevens MW. Validity and responsiveness of a brief, asthma-specific quality-of-life instrument in children with acute asthma. Ann Allergy Asthma Immunol. 2004;92:47–51. doi: 10.1016/S1081-1206(10)61709-7. [DOI] [PubMed] [Google Scholar]
- 28.Burns JS, Dockery DW, Neas LM, Schwartz J, Coull BA, Raizenne M, Speizer FE. Low dietary nutrient intakes and respiratory health in adolescents. Chest. 2007;132:238–245. doi: 10.1378/chest.07-0038. [DOI] [PubMed] [Google Scholar]
- 29.Fu L, Freishtat RJ, Gordish-Dressman H, Teach SJ, Resca L, Hoffman EP, Wang Z. Natural progression of childhood asthma symptoms and strong influence of sex and puberty. Ann Am Thorac Soc. 2014;11:939–944. doi: 10.1513/AnnalsATS.201402-084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tantisira KG, Colvin R, Tonascia J, Strunk RC, Weiss ST, Fuhlbrigge AL. Airway responsiveness in mild to moderate childhood asthma: sex influences on the natural history. Am J Respir Crit Care Med. 2008;178:325–331. doi: 10.1164/rccm.200708-1174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forastiere F, Corbo G, Dell’Orco V, Pistelli R, Agabiti N, Kriebel D. A longitudinal evaluation of bronchial responsiveness to methacholine in children: role of baseline lung function, gender, and change in atopic status. Am J Respir Crit Care Med. 1996;153:1098–1104. doi: 10.1164/ajrccm.153.3.8630551. [DOI] [PubMed] [Google Scholar]
- 32.The ENFUMOSA Study Group. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J. 2003;22:470–477. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Stewart P, Johansen H, McRae L, Taylor G. Sex difference in hospitalization due to asthma in relation to age. J Clin Epidemiol. 2003;56:180–187. doi: 10.1016/s0895-4356(02)00593-0. [DOI] [PubMed] [Google Scholar]
- 34.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sood A. Sex differences: implications for the obesity-asthma association. Exerc Sport Sci Rev. 2011;39:48–56. doi: 10.1097/JES.0b013e318201f0c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preis SR, Massaro JM, Hoffmann U, D’Agostino RB, Sr, Levy D, Robins SJ, Meigs JB, Vasan RS, O’Donnell CJ, Fox CS. Neck circumference as a novel measure of cardiometabolic risk: the Framingham Heart study. J Clin Endocrinol Metab. 2010;95:3701–3710. doi: 10.1210/jc.2009-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. Eur Respir J. 2013;42:1012–1019. doi: 10.1183/09031936.00124912. [DOI] [PubMed] [Google Scholar]
- 38.Al-Alwan A, Bates JH, Chapman DG, Kaminsky DA, DeSarno MJ, Irvin CG, Dixon AE. The nonallergic asthma of obesity. A matter of distal lung compliance. Am J Respir Crit Care Med. 2014;189:1494–1502. doi: 10.1164/rccm.201401-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 2010;108:206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 40.Vernooy JH, Ubags ND, Brusselle GG, Tavernier J, Suratt BT, Joos GF, Wouters EF, Bracke KR. Leptin as regulator of pulmonary immune responses: involvement in respiratory diseases. Pulm Pharmacol Ther. 2013;26:464–472. doi: 10.1016/j.pupt.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]