Abstract
Purpose
Based on the international reports, consumption of opioid analgesics in Poland is relatively low. There is limited information on possible impediments to optimal opioid use. This study was aimed to identify possible barriers to access to opioid analgesics and causes of failure to comply with current clinical guidelines.
Methods
Consumption data per capita in 2000–2015 were analyzed in terms of oral morphine equivalents in total, per prescription type, per reimbursement status, to identify the impact of regulations specific for Poland.
Results
The consumption of opioid analgesics has been consistently growing from 36.0 in 2000 to 103.4 mg oral morphine equivalents (OME) per capita in 2015, mainly thanks to strong opioid consumption growth. Tramadol is the most commonly used opioid in Poland. Fentanyl and buprenorphine transdermal formulations are the most frequently used strong opioid analgesics in terms of OME. The vast majority (92.8 %) of opioids were distributed upon for outpatient use in 2015, with a almost fourfold growth of consumption of strong opioids and almost threefold of weak opioids between 2000 and 2015. Strong opioids were 41 % of OME used upon prescription in 2015. Acceleration of consumption growth has been observed since 2013.
Conclusions
The prescription pattern does not abide by the current clinical guidelines for pain treatment, and the most often used opioids in Poland are tramadol, buprenorphine, and fentanyl. The use of opioids in Poland grows fast, with acceleration since 2013. The most important legal impediments of optimal opioid analgesics use have been lack of reimbursement, special prescription forms, and complicated prescribing rules.
Keywords: Opioids, Pain, Guidelines
Introduction
Pain relief is one of the fundamental human rights. According to a UN Convention, certain medicines are indispensable for the relief of pain and suffering so that their availability needs to be ensured by local and regional authorities [1]. Opioid analgesics have played a key role in pain management in patients with cancer or other chronic incurable diseases since WHO published the concept of the analgesic ladder in 1996 [2]. Consecutively released clinical guidelines by the acknowledged international organizations, e.g., the European Association for Palliative Care (EAPC), the European Society of Medical Oncology (ESMO), the National Comprehensive Cancer Network (NCCN) and others, were aimed to improve clinical practice of optimal pain treatment [3–5].
Although supported by international agreements, such as the Universal Declaration of Human Rights, which includes the right to medical care and encompasses palliative care, more than 5 billion people worldwide had little or no access to essential analgesics, such as codeine or morphine, in 2013, while more than 90 % of global use of opioid analgesics occurred in the USA, Canada, Australia, New Zealand, and several European countries. This inequity has been reported in several studies [6, 7].
In 2012–2014, Poland was ranked 40 globally and 28 in Europe in opioid consumption that reached 1773 defined daily doses for statistical purposes per million inhabitants per day (excluding buprenorphine). In the same time in Germany, the leading country in the region, the opioid analgesics consumption was 15 times higher excluding buprenorphine, and buprenorphine use was fivefold higher [8]. The use of morphine, fentanyl, and oxycodone per capita is greater than global mean value yet below the European average. However, it does not differ from other Central European countries (Hungary, Czech Republic, and Slovakia) much and correlates with general socio-economic indices (GDP—gross domestic product, HDI—human development index) [9].
The officially reported data have some limitations and may be misleading. They usually do not include buprenorphine or methadone in the statistics. The defined daily doses (DDD) do not represent clinical use. It is not a recommended prescription dose but only provides a rough measure to rank opioid use of countries [10]. Tramadol is not included in any reports, as it is not regarded as “narcotic drugs” [8]. The information on opioid use in non-cancer pain is unavailable. In spite of thorough effort to create regional reports, they may not reflect factors unique to particular countries when searching common, rather than country-specific phenomena [11].
This study was aimed to analyze the opioid consumption pattern and dynamics over the period of 2000–2014 to identify possible barriers to access to opioid analgesics and causes of failure to comply with current clinical guidelines. Information about impediments and mechanisms affecting optimal opioid analgesics use may be helpful in specifying of policies to improve the accessibility of opioid medicines.
Methods
National unit sales data 2000–2015 collected by and yielded by courtesy of IMS Health (http://www.imshealth.com/) were analyzed. This database presents real numbers of units sold from wholesalers to pharmacies on the territory of Poland. In Poland, pharmacies do not keep opioid products in stock but order them from wholesalers once the prescription is placed. So, although these data represent wholesaler sales to drugstores, they quite precisely equal real consumption without effects of time lag nor stock.
To determine the extent of use, we assessed the data in terms of oral morphine equivalents (OME), which are accepted by the current clinical guidelines, as presented in Table 1. In our opinion, such approach better represents “the amount of pain treated” and better recognizes real clinical practice in the moment of therapeutic choice, rather than defined daily doses for statistical purposes (S-DDD) that might be more suitable for comparisons between countries.
Table 1.
Oral morphine equivalents (OME)
| Opioid | OME [mg] |
|---|---|
| Fentanyl | 100 |
| Buprenorphine | 75 |
| Methadone | 4 |
| Oxycodone | 1.5 |
| Morphine | 1 |
| Tramadol | 0.15 |
| Dihydrocodeine | 0.25 |
| Codeine | 0.25 |
| Pentazocine | 0.3 |
| Pethidine | 0.125 |
Pentazocine and pethidine are not recommended for chronic pain treatment
The consumption values were divided by the population in millions during the year for the correctness of the calculation; however, the population of Poland in the analyzed period was stable and varied from 0.999 to 1008 of the value in 2000 (2000 = and could be skipped). Data were obtained from government statistical office for 2000–2014 with official prognosis for 2015 [12].
We included all weak and strong opioid medicines accessible and indicated for pain treatment in Poland in the analyzed period: buprenorphine, codeine, dihydrocodeine, fentanyl, morphine, oxycodone, pethidine, pentazocine, and tramadol. We included also methadone, but only its formulation reimbursed for cancer pain treatment. Other formulations of methadone may also legally be prescribed for cancer pain treatment and cannot be distinguished in the data from their predominant use in the treatment of dependence on illicit opioids; however, due to high prices, their use as a pain extremely sparse so negligible for the statistics.
To identify the impact of regulations specific for Poland, the consumption was analyzed in total, per prescription type, per reimbursement status. Essential information on regulatory changes was also added to allow correct interpretation of the data.
This analysis was taken as the basis for further in-depth qualitative and questionnaire-based surveys.
Results
Overall consumption
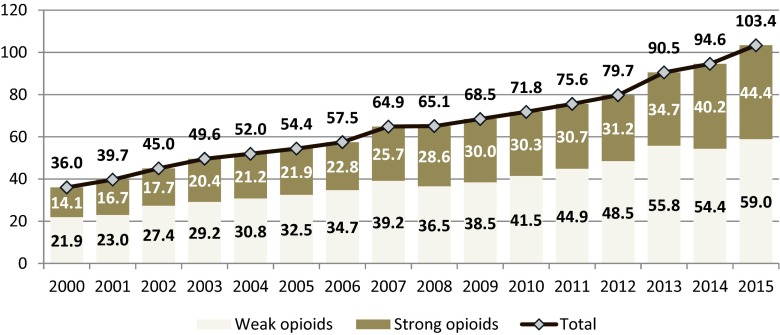
The overall consumption of opioid analgesics has been consistently growing since from 36.0 in 2000 to 103.4 mg OME per capita in 2015 (Fig. 1). This almost threefold growth was generated both by weak and strong opioids, with rather a stable ratio strong to weak opioids of 38–44 %. However, strong opioid consumption grew faster than weak opioids and reached 44.4 mg OME per capita.
Fig. 1.
. Consumption of opioid analgesics in Poland in 2000–2015 (mg of oral morphine equivalents per capita)
Strong opioids
There has been a stable use of morphine products observed during all period 2000–2016, with minor changes. In 2000, morphine was the main opioid analgesic with 8.9 mg OME per capita that stood for almost 3/4 of all OME of strong opioids used then. In 2015, its consumption equaled 10.0 mg OME per capita, which ranked morphine on the third position among strong opioids.
Fentanyl was the second most widely used strong opioid in 2000, responsible for about 1/4 of their use (3.1 mg OME per capita). Its consumption grew fast till 2003 and then stayed roughly stable up to 2015 with the use of 13.0 mg OME per capita. Since 2003, fentanyl transdermal formulations are the most frequently used opioid analgesics in terms of OME.
Consumption of buprenorphine was insignificant in 2000–2006, mostly generated by sublingual tablets. In 2003, patches were launched and since obtaining full reimbursement in pain treatment in 2007, its consumption has been growing very fast, making this molecule the leading opioid analgesic in 2015 (13.1 mg OME per capita). Buprenorphine has preferential prescription status, as it is the only strong opioid for which regular prescription is used.
Oxycodone was available in pharmacies from 2009; however, its consumption was negligible till 2011 when it gained reimbursement status by the National Health Fund (NHF). Since then, its use grew to 6.3 mg OME per capita in 2015, which ranked it on the fourth position.
Methadone was available during the whole period; however, it began to be used in cancer pain treatment in 2011, after including one of its formulations on the NHF reimbursement list of medicines used in cancer. In 2015, its consumption in cancer treatment reached 2.0 mg OME per capita and stands for only 10 % of its overall sales in Poland.
Pethidine was ranked the third strong opioid used in Poland in 2000 (1.2 mg OME per capita). Its consumption has been consistently decreasing to marginal values in 2005.
The use of pentazocine has been insignificant over the analyzed period.
Weak opioids
There have been three weak opioids available in Poland in 2000–2015, with different prescription requirements.
Codeine is available in composite products with paracetamol or ibuprofen as the only self-medication opioid painkiller (available without a prescription). Its consumption was the highest in 2002 (6.3 mg OME per capita) and then after has been slowly decreasing to 3.0 mg OME per capita.
Dihydrocodeine was launched in 2008, as the only weak opioid has been prescribed on the special prescription form (the same as used for most of the strong opioids) till 2010. After change of prescription regulations to regular prescription forms, its consumption grew to stable values of 0.7–0.8 mg OME per capita.
Tramadol is the most commonly used opioid in Poland. Its consumption equals 55.3 mg OME per capita in 2015, which is over a half of OME prescribed in Poland now. This analgesic has been prescribed on regular prescription forms. Composite formulations with paracetamol are mainly responsible for the continuous fast growth of consumption since they appeared in 2006 and made for almost half of the overall tramadol consumption in 2015.
Inpatient consumption
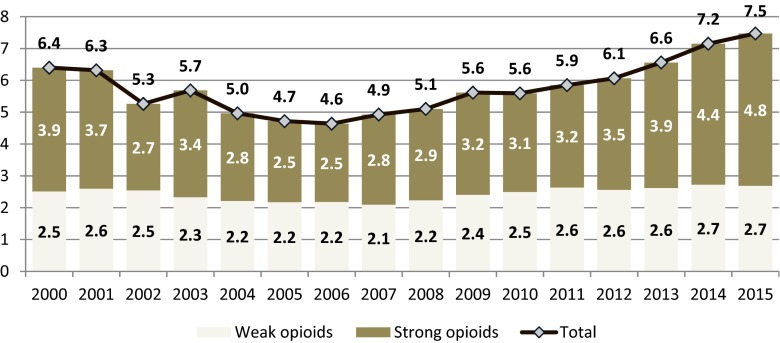
7.2 % of all opioids calculated as OME were consumed in inpatient health centers in 2015 (10.8 % of all strong opioids and 4.6 % of all weak opioids). The amount of strong opioids consumed in inpatient centers is almost twice higher than of weak opioids and is growing faster, while the use of weak opioids remains stable over years (Fig. 2).
Fig. 2.
Inpatient consumption of opioid analgesics in Poland in 2000–2015 (mg of oral morphine equivalents per capita)
Outpatient consumption
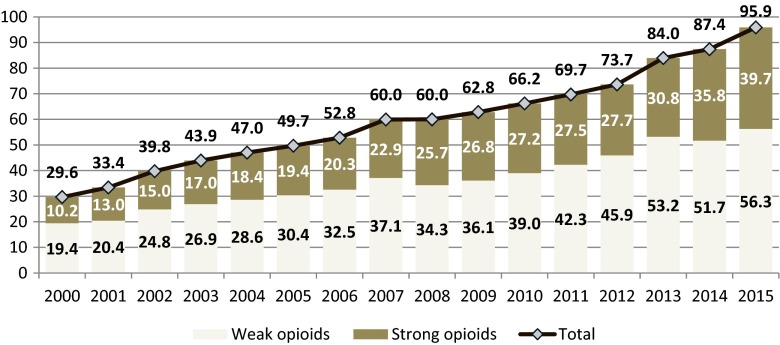
The vast majority (92.8 %) of opioids were distributed upon prescription for outpatient use in 2015, with a fast growth of consumption (almost fourfold of strong opioids and almost threefold of weak opioids) between 2000 and 2015 (Fig. 3). Strong opioids were made for 34 % of OME used upon prescription in 2000 and for 41 % in 2015. Acceleration of consumption growth is observed in the last 3 years (2013–2015).
Fig. 3.
Outpatient consumption of opioid analgesics in Poland in 2000–2015 (mg of oral morphine equivalents per capita)
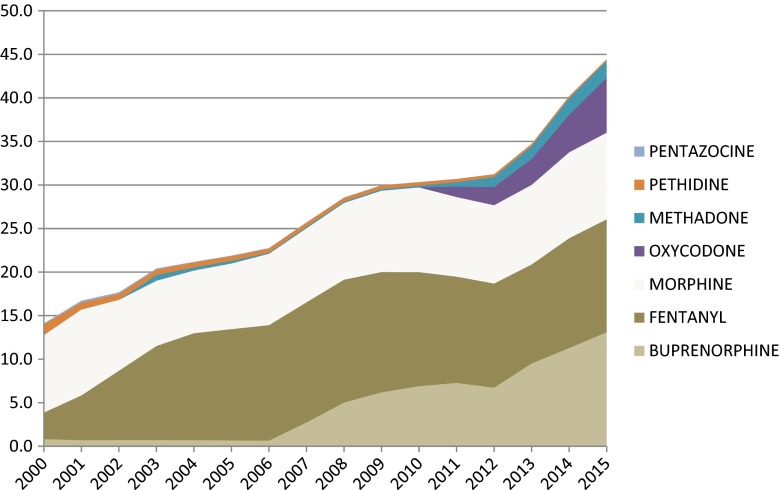
Not only the consistent fast growth of strong opioids is observed, but also a variety of opioid products is available in pharmacies now. There was only morphine (oral and injectable) and one fentanyl (transdermal therapeutic system) product in 2000 available. In 2015, the outpatient practitioner might choose from 15 formulations of morphine, oxycodone, fentanyl, buprenorphine, and methadone (Fig. 4) of different routes of administration (injectable, spinal, oral, transdermal, transmucosal).
Fig. 4.
Strong opioids use dynamics in Poland 2000–2015 (OME mg per capita; pethidine and pentazocine excluded due to insignificant values)
Discussion
Consumption of opioid analgesics in Poland grew 3 times over the 15-years period. The mean annual growth of 8 % makes this group of medicines one of the fastest growing ones. Not only the amount of opioids used increased but also the variety of molecules, competitive products, and different formulations. Except for hydromorphone, there have been available all medicines recommended in current guidelines for cancer pain treatment since 2007 [3]. The total increase of opioid consumption can only be partly explained by the growth of incidence of neoplasms by 36.5 % (2015 vs. 2000) that has been the leading indication for opioid analgesics [13]. The primary driver of their consumption growth was an increase of their use in cancer and non-cancer pain treatment. However, we do not know what is an amount of opioids that are used in numerous non-cancer indications, such as post-operative pain, low back pain, and osteoarthrosis. The common observation is that non-cancer pain is rarely managed with strong opioids and physicians are reluctant to prescribe strong opioids for non-cancer patients. This thesis needs further investigation.
Morphine was the most often used strong opioid in 2000. Its consumption has not significantly changed over the next 15 years, while all other opioids grew. This is usually expounded by the phenomenon of morphinofobia, apart from the phenomenon of opioidophobia, which seems not to be the same. In practice, patients accept treatment with fentanyl or oxycodone easier, than with morphine, which is probably more often associated with connotations and prejudices.
The most widely used opioid formulations are fentanyl and buprenorphine patches. Strong marketing activities of pharmaceutical industry and favorable reimbursement status resulted in fast growth of fentanyl transdermal therapeutic system (fentanyl TTS) consumption in 2000–2003. In 2007, buprenorphine obtained the status of reimbursed medicine. Since then, its consumption has been growing consistently and in 2015, use of buprenorphine patches reached values of fentanyl use. The crucial driver of buprenorphine growth was, however, the exceptional status of this medicine, as it was the only strong opioid prescribed on regular prescription forms. This seems to be one of the most important enabler for opioids prescription, besides patient effective price. Regular prescription forms are in the use of all practitioners and do not require any effort to be obtained from them. There was also an easier way of prescribing of buprenorphine required by the regulations. This made buprenorphine technically much more accessible than other opioids. It is likely that buprenorphine was also perceived as “safer” drug than others, as regular prescription forms were required.
The similar impact of special prescription forms on drug consumption may be observed in dynamics of the use of tramadol versus codeine and dihydrocodeine.
Special prescription forms, mandatory to prescribe “narcotic drugs,” have been accessible for every practitioner without any charges; however, this required additional effort to obtain them and was subject to scrutiny by the National Health Fund controllers. In our opinion, they seem to be one of the most important impediment to optimal opioids use. This thesis might be supported by the buprenorphine and tramadol cases. Although no special permit or license was required to obtain the special prescription forms, few practitioners ordered such forms. Currently, all prescriptions in Poland have a unique code and are registered electronically, so there is no need to keep special prescription forms to track the prescription.
Since 2012, the national multi-channel campaign against untreated pain has been carried on. The Polish Society of Palliative Medicine (PTMP) and several non-government organizations supported by pharmaceutical industry launched different programs to raise public awareness of the need for treatment of chronic pain and insist on authorities to take the legislative initiative. The Polish Ministry of Health (MoH) founded an expert group for palliative and hospice care in 2011/12, consisting of several palliative care leaders, which worked out the recommendations to improve the accessibility of palliative care and pain treatment [14]. Although not included in the summary document, some of those proposals were reported to the MoH and consecutively implemented. In 2014, special pink-colored two-copy forms were changed, upon the MoH regulation, to one-copy blanks that did not differ much from the regular ones. The change of the color of the script eliminated stigmatization of patients taking opioid drugs. The total abolition of special prescription has been one of the postulates of the expert group; however, it requires major legislative effort at the Parliament of Poland. By the same MoH regulation, the amount of controlled medicine to be prescribed in written is still obligatory, however, in a simplified way. Dozens of local and national conferences, NGO-driven mass media campaigns, and involvement of authorities resulted in speeding up the growth of opioid consumption in Poland.
The second largest impediment to opioid use seems patient effective price that depends on obtaining the status of reimbursed medicine. Oxycodone, oxycodone/naloxone, buprenorphine, and methadone were almost not prescribed until getting reimbursement status. The unquestioned observation from this study is that only reimbursed drugs are accessible to patients.
The impact of clinical guidelines of the acknowledged national and international medical societies on the prescribing pattern was difficult to assess. The EAPC published its recommendations in 1996, 2001, and 2002 and was dedicated mainly to palliative care professionals [15, 16, 3]. The ESMO guidelines, updated almost every year in 2005–2012, should theoretically be observed by oncologists [17, 18]. According to the EAPC (2012), transdermal formulations of fentanyl and buprenorphine should be alternative to oral morphine and oxycodone. Buprenorphine was not recommended in a document from 2001. Both transdermal opioids have been the leading opioid analgesics. Fentanyl case indicates that rather industry-driven information was more effective than official clinical guidelines. However, there might have been observed the impact of the EAPC guidelines since 2012, as oral morphine and oxycodone formulations grew faster than transdermal opioids, on a stipulation that buprenorphine is becoming more and more popular in non-cancer patients. The authors of this article published a 12-point simple summary of the basic rules of chronic pain treatment in 2014 [19]. The aim of this paper was to popularize the EAPC and ESMO guidelines, as well as the newest evidence-based medicine updates, in native language among Polish physicians. In our opinion, it might be an important element of education of Polish practitioners, as well as a learning point that any guidelines should be ensured in local languages if they have to be respected.
The most frequently used opioid is tramadol. It is available on regular prescription forms and has been widely accepted in cancer and non-cancer pain. Its use grows very fast consistently. However, there is a threat that tramadol may be overused or overdosed, in particular by the physicians with prejudices to strong opioids.
Acceleration of growth of strong opioid consumption in the last 3 years (2013–2015), both in inpatient and outpatient practices, can be explained only by the impact of public campaign against untreated pain, resulting in raising awareness, facilitation of prescribing, imposing pain diagnosis as mandatory in hospitals, and increasing working knowledge of healthcare professionals.
Based on the official international reports, consumption of opioid analgesics in Poland is still relatively low. This implies that the use of opioid analgesics may be suboptimal, although there is no agreement on indices of optimal opioid use per capita. Our study does not make a distinction between opioid use in cancer and non-cancer patients; however, due to strong attachment of opioids as the medicines dedicated for cancer patients, non-cancer pain cases may remain undertreated. This thesis needs to be addressed in a further investigation as well as educational programs.
This study cannot answer the questions on the internal impediments of physicians to prescribe opioid analgesic. Questionnaire-based study needs to be performed then.
Conclusions
The use of opioids grows fast, with acceleration since 2013.
The most often used opioids in Poland are tramadol, buprenorphine, and fentanyl. The prescription pattern does not reflect the current clinical guidelines for pain treatment.
The important legal impediments of optimal opioid analgesics use have been price (lack of reimbursement), special prescription forms, and complicated prescribing rules.
The further questionnaire-based study is necessary to investigate internal barriers to opioid use.
Compliance with ethical standards
Conflict of interest
This research received no specific grant from any funding agency, public or commercial, or from non-profit sectors, except data yielded by courtesy of IMS Health. We declare no financial relationship with the organization that delivered the database (IMS Health) nor with any organizations.
We agree to allow the journal to review the primary data for transparency needs if requested.
References
- 1.UN Office on Drugs and Crime (2009) The international drug control conventions. New York: United Nations
- 2.World Health Organization (1995) Cancer pain relief, 2nd edn. WHO, Geneva
- 3.Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, Haugen DF, Juhl G, King S, Klepstad P, Laugsand EA, Maltoni M, Mercadante S, Nabal M, Pigni A, Radbruch L, Reid C, Sjogren P, PC S, Tassinari D, Zeppetella G. For the European Palliative Care Research Collaborative (EPCRC) on behalf of the European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;Volume 13(Issue 2):e58–e68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 4.Ripamonti C, Santini D, Maranzano E, Berti M, Roila F. On behalf of the ESMO guidelines working group management of cancer pain: ESMO clinical practice guidelines. Ann of Oncol. 2012;23(Supplement 7):vii139–vii154. doi: 10.1093/annonc/mds233. [DOI] [PubMed] [Google Scholar]
- 5.NCCN clinical practice guidelines in oncology, adult cancer pain version 2.2016, https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf
- 6.UN general assembly. UNIVERSAL declaration of human rights. New York: United Nations (1948) http://www.ohchr.org/EN/UDHR/Documents/UDHR_Translations/eng.pdf. Accessed 26 Jan 2016
- 7.International Narcotics Control Board 2011 Availability of internationally controlled drugs: ensuring adequate access for medical and scientifi c purposes. New York, United Nations
- 8.International Narcotics Control Board (2016) Narcotic drugs 2015. United Nations, New York
- 9.University of Wisconsin/WHO Collaborating Center (2015) http://www.painpolicy.wisc.edu/who-regional-office-europe-euro
- 10.Berterame S, Erthal J, Thomas J, Fellner S, Vosse B, Clare P, Hao W, Johnson DT, Mohar A, Pavadia J, Samak AK, Sipp W, Sumyai V, Suryawati S, Toufiq J, Yans R, Mattick RP Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. Lancet 387(10028):1644–1656
- 11.Vranken MJ, Lisman JA, Mantel-Teeuwisse AK, Jünger S, Scholten W, Radbruch L, Payne S, Schutjens MH. Barriers to access to opioid medicines: a review of national legislation and regulations of 11 central and eastern European countries. Lancet Oncol. 2016;17(1):e13–e22. doi: 10.1016/S1470-2045(15)00365-4. [DOI] [PubMed] [Google Scholar]
- 12.Central Statistical Office of Poland, Information Portal, http://stat.gov.pl/en/topics/population/
- 13.Didkowska J, Wojciechowska U (2015) Cancer in Poland in 2013. Polish National Cancer Registry Department of Epidemiology, Warsaw. http://onkologia.org.pl/wp-content/uploads/BIUL2013.pdf
- 14.Ciałkowska-Rysz A, Dzierżanowski T, Łuczak J, et al. Podsumowanie pracy Zespołu do spraw opieki paliatywnej i hospicyjnej (sierpień 2011 r. – czerwiec 2012 r.) Medycyna Paliatywna. 2014;6(4):177–189. [Google Scholar]
- 15.(1996) Morphine in cancer pain: modes of administration. Expert Working Group of the European Association for Palliative Care. Br Med J 312(7034):823–826 [PMC free article] [PubMed]
- 16.Hanks GW, Conno F, Cherny N, Hanna M, Kalso E, McQuay HJ, Mercadante S, Meynadier J, Poulain P, Ripamonti C, Radbruch L, Casas JR, Sawe J, Twycross RG, Ventafridda V. Expert working group of the Research Network of the European Association for Palliative Care. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer. 2001;84(5):587–593. doi: 10.1054/bjoc.2001.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jost EN1; ESMO Guidelines Task Force ESMO minimum clinical recommendations for the management of cancer pain. Ann Oncol. 2005;16(Suppl 1):i83–i85. doi: 10.1093/annonc/mdi833. [DOI] [PubMed] [Google Scholar]
- 18.Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F. ESMO guidelines working group.Management of cancer pain: ESMO clinical practice guidelines. Ann Oncol. 2012;23(Suppl 7):vii139–vii154. doi: 10.1093/annonc/mds233. [DOI] [PubMed] [Google Scholar]
- 19.Ciałkowska-Rysz A, Dzierżanowski T. Podstawowe zasady farmakoterapii bólu u chorych na nowotwory i inne przewlekłe, postępujące, zagrażające życiu choroby. Medycyna Paliatywna. 2014;6(1):1–6. [Google Scholar]