Abstract
Treatment of large peripheral nerve damages ranges from the use of an autologous nerve graft to a synthetic nerve growth conduit. Biological grafts, in spite of many merits, show several limitations in terms of availability and donor site morbidity, and outcomes are suboptimal due to fascicle mismatch, scarring, and fibrosis. Tissue engineered nerve graft substitutes utilize polymeric conduits in conjunction with cues both chemical and physical, cells alone and or in combination. The chemical and physical cues delivered through polymeric conduits play an important role and drive tissue regeneration. Electrical stimulation (ES) has been applied toward the repair and regeneration of various tissues such as muscle, tendon, nerve, and articular tissue both in laboratory and clinical settings. The underlying mechanisms that regulate cellular activities such as cell adhesion, proliferation, cell migration, protein production, and tissue regeneration following ES is not fully understood. Polymeric constructs that can carry the electrical stimulation along the length of the scaffold have been developed and characterized for possible nerve regeneration applications. We discuss the use of electrically conductive polymers and associated cell interaction, biocompatibility, tissue regeneration, and recent basic research for nerve regeneration. In conclusion, a multifunctional combinatorial device comprised of biomaterial, structural, functional, cellular, and molecular aspects may be the best way forward for effective peripheral nerve regeneration.
Keywords: peripheral nerve regeneration, electrically conducting polymers, electrical stimulation, nerve growth conduit, tissue engineering
I. INTRODUCTION
Peripheral nerve damage is a common injury affecting as many as 1.8% of trauma patients1 and an estimated 20 million Americans,2 many of who sustain associated lifelong disabilities. Nerve injuries are a significant burden on the health-care system, resulting in $150 billion of annual health-care dollars spent in the United States alone.2 Although certain nerve gaps can spontaneously repair through the body’s natural repair mechanisms, many injuries must be repaired surgically in order to reconstruct a damaged nerve. If unrepaired within a certain time, these injuries can block communication via the sensory and motor nerves of the peripheral nervous system (PNS) to the brain and spinal cord. As such, the proximal stump forms a neuroma and the muscle that had been previously innervated by the nerve becomes atrophic.3 Furthermore, failure of these nerves to regenerate can cause painful neuropathies, which affect a patient’s daily activities.3 Thus, proper and full regenerative efforts must be sought to avoid these morbidities. The present manuscript provides an in-depth, state-of-the-art review of past, present, and avenues for future research associated with peripheral nerve regeneration using various tissue engineering strategies including advanced nerve growth conduits (NGCs) using traditional electrically stimulating polymers and ideas for the use of ionically conducting polymers for incorporation into NGCs.
To better understand the repair of the PNS, it is necessary to explain the neurophysiology of the PNS. The PNS lies outside of the central nervous system (brain and spinal cord). It is made up of bundles of axons that transmit information to and from the central nervous system via action potentials, which are electrical impulses.4 Each axon is a part of a neuron, which is made up of dendrites, a cell body, and an axon. Dendrites are small spines on the cell body that receive electrical input from other neurons. The cell body contains the nucleus in which the proteins, hormones, and neurotransmitters produced by the neuron are produced. The axon is the portion of the neuron that communicates with other neurons and is often surrounded by a protective layer called myelin. The initial segment of the axon is the axon hillock, at which both inhibitory and excitatory input to the neuron are summed up and the decision on whether or not to send an action potential through the axon is made. Once the decision to send an action potential is made, the electrical impulse is transmitted down the axon to the synapse. Here, there is a cascade of signal events that release neurotransmitters into the synapse, the space between two communicating neurons, and the neuron is thus able to communicate with surrounding neurons or target organs to convey a signal. When a peripheral nerve is injured, it is the bundle of axons that is damaged and thus the communication between target organs and the nervous system is lost at that injury site. While the PNS has the capacity to regenerate injured nerves, there are multiple factors (i.e., size and location of the defect and age of the patient) that determine the quality of functional regeneration.5 When the size of the injury is too large for tension-free, end-to-end anastomosis, there are various treatment options that can be considered.
Currently, the gold standard for peripheral nerve reconstruction is direct end-to-end repair or interposition of an autologous nerve graft (autograft), if there is excessive tension.2 However, use of such autografts presents several drawbacks such as sensory loss at the donor site as well as risk for neuroma.2 Furthermore, at the repair site, there is often fascicle mismatch, scarring, and fibrosis, which limit the regenerative benefits of biological grafting procedure.2 Limitations of current treatments in the clinical setting call for a need for novel methods of repairing injured nerves. One alternative is the development of NGCs that serve to guide the regenerating axon to the distal stump.6 Initial studies focused on the use of biostable and bioinert materials such as silicone to overcome the mechanical property requirements associated with autografts.7 These growth conduits were used to house only the cells and tissue that were native to the regenerating peripheral nerve.7 Initially, the use of silicone and other synthetic materials was attractive for developing NGCs because of the relative ease of manipulating their physical and chemical properties through various chemical means.8 Many studies reported the possibility of nerve regeneration through synthetic nerve growth tubes.9, 10 As these silicone growth conduits were improved on, conduits made of silicone and poly(tetrafluoroethylene) (PTFE) became frequently used polymers for use in NGCs.6 These polymers, however, are non-resorbable, which led to drawbacks, such as chronic foreign body reaction, resulting in excessive scarring and ultimately limiting a full recovery of the nerve function.6, 7, 11, 12 The next generation of nerve growth tubes used biodegradable polymers. The early set of polymers adopted for these applications included the use of polyesters such as polyglycolic acid (PGA), polylactic acid (PLA), and their copolymer poly(lactic-co-glycolic acid) (PLGA) for NGCs, and the concept of tissue engineering gained momentum in the field of neural tissue engineering.8 Over the past two decades, tissue engineering has emerged as an innovative method to assist in peripheral nerve regeneration using synthetic biomaterials, and continued research may overcome many of the drawbacks associated with biological grafts.13–21
Tissue engineering combines cells, scaffolds, and signals (growth factors and/or chemotactic factors and external stimulus in the form of physical forces) to repair or replace a damaged or missing tissue. The overall goal of tissue engineering is to use the combination of these three factors to make use of the cellular capacity to generate new functional tissue.22, 23 In peripheral nerve regeneration, various cell types have been utilized including skeletal muscle-derived multipotent stem cells24 and Schwann cells.25 Additional efforts take advantage of cell signaling to stimulate nerve growth such as nerve growth factor (NGF),26–28 topographical cues,29 and electrical stimulation (ES),30–33 all of which may serve to enhance the rate of nerve regeneration. Biodegradable scaffolds derived from the materials of both natural and synthetic origin including PLA, PGA, PLGA, polycaprolactone (PCL), collagen, chitosan, and different material compositions have been used for the fabrication of nerve conduits and as well as other tissue regenerative techniques.23, 34, 35 These nerve conduits were designed to provide optimal mechanical strength, degradation properties, and porosity to support regeneration. In the tissue engineering approach, efforts are also made to incorporate and release biological factors from scaffolds alone or in combination with cells to regain some degree of functional recovery.36 Growth factors, such as NGF, brain-derived neurotrophic factor (BDNF), and Neurotrophin-3 (NT-3), have also been used to stimulate nerve growth.5, 26–28 These bioactive factors were physically incorporated into scaffolds or chemically conjugated.5, 26–28 These factors were able to stimulate proliferation and differentiation of various cell types that aid in nerve regeneration.5 However, growth factors can be challenging to work with due to their relatively short half-life, poor stability, and potential for spreading to other areas of the body. The latter part of this review discusses some of these growth factor strategies in detail.
More recent efforts take advantage of external stimuli such as electrical, magnetic, and mechanical loading to enhance the rate of nerve regeneration.30–33 The benefits of ES for the regeneration of bone, cartilage, skin, spinal nerves, and peripheral nerves have been widely documented and demonstrated in the literature.37 Electrical stimulation is a commonly used therapy to promote functional recovery of muscle and nerve tissue following injury that can result in enhanced tissue regeneration.38, 39 In general, ES has been shown to enhance cell multiplication in connective tissue and formation of new collagen in injured tendons.40–45 These studies suggest that increased collagen biosynthesis is due to an increased number of collagen producing cells at the injured site. ES also results in accelerated healing of ligament and tendon injuries, with increased rat tendon healing by >250% histologically.46 When severed dog tendons were treated with implantable electrodes (20 µA direct current), the tendons showed a 92% return to normal strength in eight weeks, compared to 50% in control animals.46, 47 These studies also demonstrated an increase in capillary and fibroblast number at the wound site that preceded collagen synthesis. Electrical stimulation and exercise has been shown to improve blood vessel growth via expression of vascular endothelial growth factor (VEGF).48–50 For instance, expression of VEGF protein was observed in rabbit skeletal muscles after three days and up to eight weeks of ES.51 Exercise and ES increases blood flow, which has been suggested to release nitric oxide-like humoral agents that are critical regulatory molecules for angiogenesis.52–54 In studies where the right tibialis anterior (TA) and extensor digitorum longus (EDL) muscles of Sprague-Dawley rats were stimulated for 8 h/day for seven days, chronic ES induced vessel proliferation (increased vessel density) and increased expression of VEGF protein in the stimulated skeletal muscle.48 Many recent publications report the use of electrically conductive polymer derived conduits to enable ES locally, at the repair site, to promote tissue regeneration.13–21, 55–66 Polymers such as polyaniline (PANI), poly(3,4-ethylenedioxythiophene) (PEDOT), poly(pyrrole) (PPY), and their copolymers were presented in the forms of sheets, fibers, hydrogels, and 3D porous matrices as scaffolds.67, 68 Application of electrical stimulation to scaffolds derived from these materials alters certain of their properties in terms of volume or wettability in the biological environment, enabling them to conduct electrical charges along the length of a scaffold.69, 70
II. CHARACTERISTICS OF A GOOD NERVE GROWTH CONDUIT
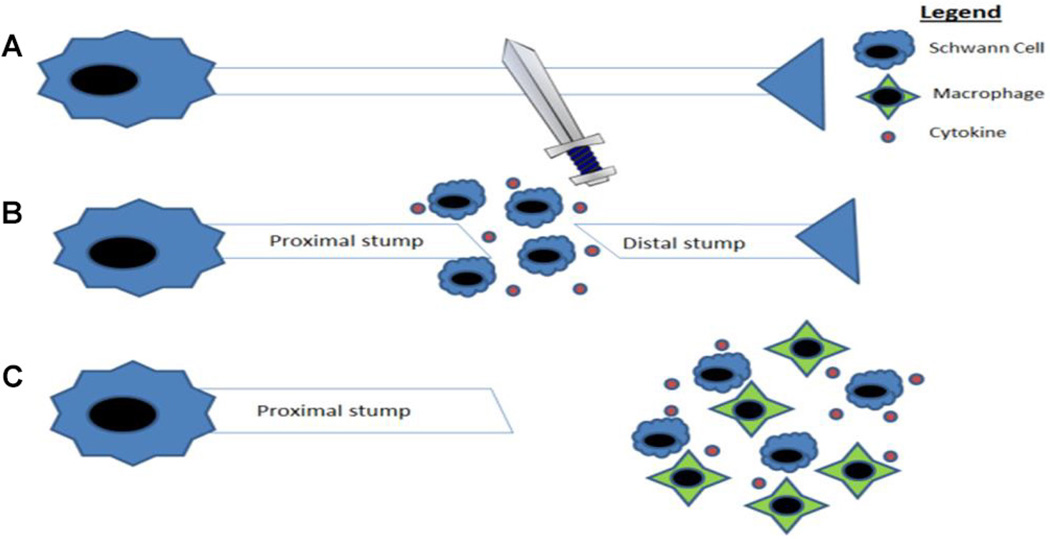
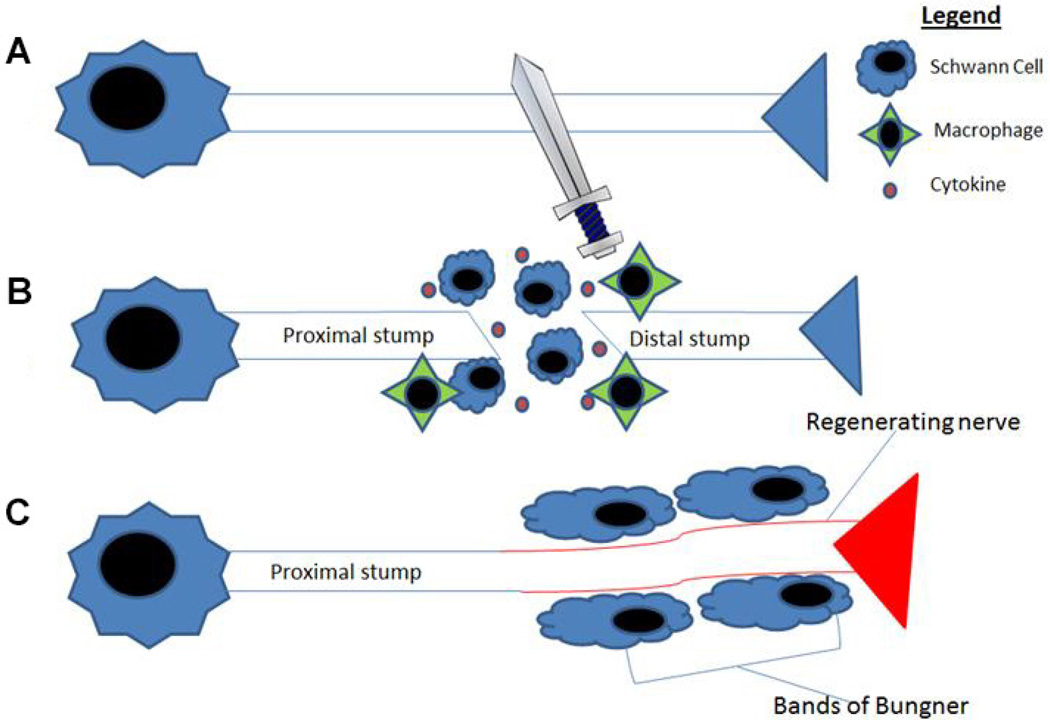
A nerve growth conduit (NGC), also referred to as a nerve guidance conduit or nerve graft substitute, is an artificial means to guide the regeneration and regrowth of nerve axons to facilitate more complete nerve regeneration. When a nerve is severed in the PNS, the distal portion of the nerve begins to degenerate, the cytoskeleton breaks down, and there is dissolution of the cell membrane.71 Next, the Schwann cells and macrophages begin to clear myelin and axonal debris.71 Eventually, Schwann cells and macrophages release cytokines, which lead to enhancement of axonal growth beginning at the proximal end (the end closer to the body) and continues toward the distal stump,71 a process known as Wallerian degeneration (Fig. 1). The goal of NGCs is to improve on the regeneration process, thus promoting better recovery from injury. As seen in Fig. 2, the NGC is a hollow tube that connects the proximal nerve stump to the distal nerve stump, designed to bridge the gap between the two ends. Other functions of the conduit include: reducing infiltration of fibrous tissue, presenting a barrier for selective diffusion of macromolecules and neurotrophic factors between the device and the surroundings, and increasing the concentration of endogenous proteins/neurotrophic factors inside the conduit.6, 72 Furthermore, these channels prevent neuroma formation and excessive branching. While these are the primary functions of the NGC, there are many other factors that go into optimizing its function that allow it to play a role in the stimulation of nerve growth (Fig. 3).
FIG. 1.
(A) Injury of the nerve axon. (B) Schwann cell proliferation and release of cytokines. (C) The cytokines activate macrophages to clear myelin and axon debris from the distal nerve, which allows for the production of an environment that supports axonal regrowth. (Adapted from Gaudet et al.)157
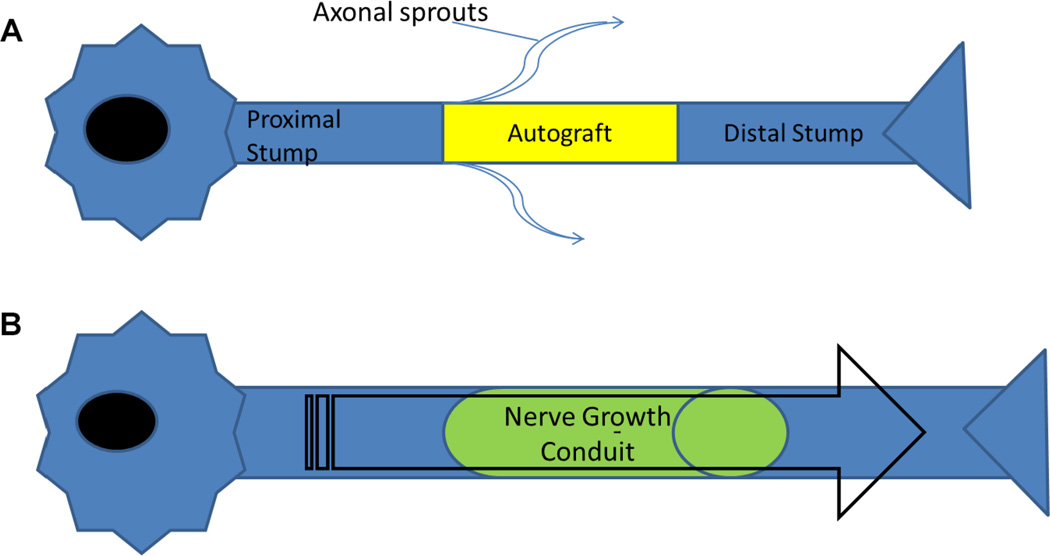
FIG. 2.
(A) An example of a nerve defected repaired with biological graft (autograft). Autografts used to repair the defect results in axonal sprouts and regeneration often includes scarring. (B) Nerve defect treated with a polymeric nerve growth conduit helps to prevent axonal sprouting and localize the growth factors and cells that help promote nerve regeneration.
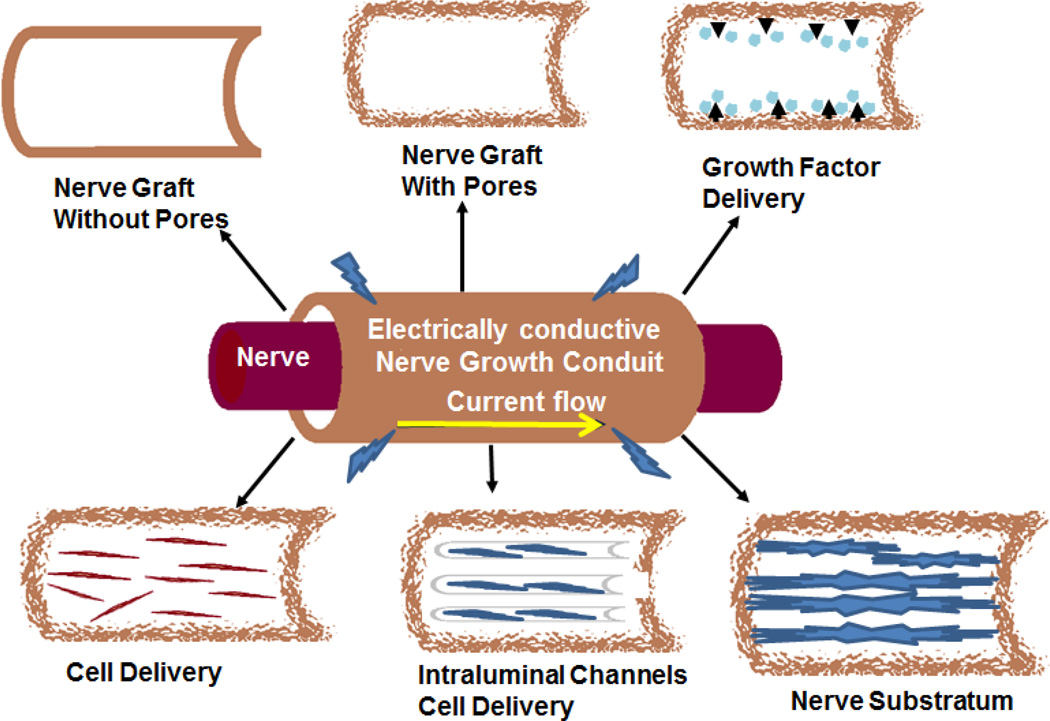
FIG. 3.
Examples of peripheral nerve conduits that present various aspects of scaffold properties to promote guided nerve regeneration. There are multiple modifications to the classic peripheral nerve conduit that have been used to help stimulate nerve growth. (Adapted from Hudson et al.)158
Besides being biodegradable and biocompatible, it is also essential that NGCs have other qualities, such as (i) permeability, (ii) flexibility, and (iii) minimal swelling, to be effective.73 Permeability allows nutrients and oxygen exchange that can properly diffuse on both sides through the tube before it becomes vascularized, which is needed for the beginning of the regeneration process.73 Vascularization of NGCs also improves the Wallerian degeneration process, which leads to greater axonal growth with more rapid and complete myelination.11 Making the conduit porous can also encourage quick and abundant revascularization.11 Flexibility becomes increasingly important as the size of the nerve gap increases, which is most relevant in areas of extensive movement, such as joints. Increasing flexibility of the nerve conduit will allow the conduit to comply with critical tensile and bending stress, thus ensuring the stability of the conduit and the regenerating nerve.73, 74 Flexibility, however, must be balanced with the ability to withstand pulling forces that will be exerted by sutures inserted into the tube during surgery.74 Additionally, the structural mechanics of the NGC should enable it to remain intact during the regenerative process with minimal swelling, so as not to block the progression of the regenerative process.73 Figure 3 summarizes various aspects of NGCs and their benefits.
Another aspect that should be considered in the design of a NGC is the form of internal structures. These include features such as filaments, collagen sponges, and multichannel nerve tubes, which may enhance regeneration by stabilizing the fibrin matrix to more precisely guide the regenerating nerve.73 Examples of intrinsic frameworks previously investigated include incorporation of filaments, collagen sponges, and multiple channels.73 Intrinsic structures can be advantageous through providing increased surface area for cell attachment, better stabilization of fibrin matrix formed inside the nerve tube, and better contact guidance of the regenerating nerve.73 The hydrophilic nature of the polymeric NGC results in swelling and dimensional changes following its implantation at the defect site due to absorption of biological fluids.11 The internal diameter and wall thickness of the NGC are also important design parameters; the conduit should have an internal diameter that is large enough to easily place the nerve stumps inside the lumen of the growth conduit, and the conduit wall should be thin enough to account for swelling that will lead to compression of the regenerating nerve.11
Careful design of biodegradable scaffolds is necessary in order to produce a scaffold that will provide an optimal regeneration environment.6 A major parameter that must be taken into consideration with biodegradable scaffolds is determination of the timing of degradation such that the scaffolds degrade at the same rate as nerve growth. If the scaffold degrades too fast, the guidance properties of the scaffold are lost and nerve regrowth is impaired. If, however, the nerve conduit does not degrade fast enough, there is compression of the regrowing nerve and nerve regeneration is again impaired. Currently marketed NGCs include Neurotube (Synovis Surgical Innovations, Deerfield, Illinois), Neurolac (Ascension Orthopedics, Plainsboro, New Jersey), and NeuraGen (Integra LifeSciences, Plainsboro, New Jersey).6 These products are used only for treating short nerve defects that are greater than or equal to 8 mm, but less than or equal to 30 mm, and thus serve a limited patient population.
Various studies have incorporated growth factors into their scaffold design to promote nerve growth.5, 26–28, 75–77 Growth factors can be incorporated in solution inside of the lumen12 or entrapped into a matrix that is loaded into the lumen,78–80 or they can be embedded in the conduit wall using microspheres.81 Some of the drawbacks of using growth factors include: short half-life, diffusion of the growth factor out of the intended site, and variable release rates of the growth factor.64, 82, 83 Thus, while using growth factors can be helpful, there are aspects of their use that need to be addressed in order to best take advantage of their potential.
Another modification to the single-lumen NGC that has been explored is the use of Schwann cells.73 It is thought that because Schwann cells are involved in the natural regenerative process in the PNS, they will encourage regeneration over longer nerve gaps,73 as evidenced through several studies that show that Schwann cells enhance neurite (axons and dendrites) growth.84, 85 Clinically, however, it is difficult to translate the benefits of Schwann cells to meaningful gains due to limited tissue availability, long cell culture times, and donor site morbidity.86 Other cell types that have been used include bone marrow stromal cells,87 adipose tissue-derived stem cells,88, 89 and human mesenchymal stem cells (hMSCs).90 Working with these cells is challenging for the aforementioned reasons. A clinically relevant cell population, seeding density, and cell culture design need to be optimized to translate to better clinical relevance. Another conduit modification that has emerged over the past few decades is ES enabled through the incorporation of electrically conducting polymers into NGCs.73 Because the nervous system is highly influenced by the electrical stimuli,31 it is understandable that NGC technology has become invested in incorporating and optimizing such a stimulus. The optimization of electrical conductivity in NGCs will be further discussed throughout this review. The various methods that have been used to optimize NGC function are reviewed in Table 1 and Fig. 3.
TABLE 1.
Literature Summary—Ideal Scaffold Properties for Nerve Regeneration
| Nerve growth conduit property | Findings |
|---|---|
| Porous | |
| Growth factor incorporation | |
| Incorporation of support cells |
|
| Intraluminal channels | |
| Electrical activity |
|
III. EFFECT OF ELECTRICAL STIMULATION ON NEURONS
The nervous system (both central and peripheral) is highly influenced by electrical stimuli that serve as the primary means of communication. The goal of ES is to depolarize the membrane resulting in an action potential.31 Electrical stimulation methods seek to take advantage of the electrical properties that are inherent within the nervous system to better promote cell differentiation and axonal outgrowth. While the mechanism of ES-promoted neuronal growth is not fully understood, several different theories exist. Electrical stimulation has been shown to regulate cellular activities such as cell adhesion,91, 92 proliferation,93 cell migration,76, 77, 94 and protein production.75, 76 ES might augment natural neuroregeneration through the promotion of neural stem cell migration. Studies of the central nervous system (CNS) have shown neurite extension to be essential in the process of neuron regeneration.64 Research suggests that electric fields redistribute receptors on cell surfaces, leading to downstream signaling in cellular pathways that are linked to the cytoskeleton and various organelles;95 signaling through these pathways encourages directional sensing and motility, causing cells to migrate toward the source of electrical current.95 It has been hypothesized that ES is guiding the regenerating proximal nerve stump by stimulating various cellular pathways that lead to cytoskeletal changes and redistribution of cellular organelles.
Electrical stimulation has been shown to aid in the process of neurite extension, and, when compared to topographical cues, neurite length measurements were larger in the electrically stimulated group.64 Yan et al. have carried out a series of experiments to elucidate the direct effect of ES on neurite outgrowth.95 From these studies it is evident that ES induces neurite outgrowth through calcium pathway signaling. For these studies, dorsal root ganglion neurons (DRGNs) were grown on electrically conductive glass. These cells were then electrically stimulated for 30 min intervals under a 10Hz/5V electric field, which led to a 1.7-fold increase in neurite outgrowth as compared to controls without ES. The authors hypothesized the role of voltage-dependent calcium channels in causing the enhanced neurite outgrowth. Through various drug interventions, it was shown that depletion of both intracellular and extracellular calcium eliminated the benefits of ES on neurite outgrowth, indicating that ES may work via voltage-dependent calcium channels.95 Additionally, it was determined that the voltage and frequency of ES was important to the mechanism of electrically stimulated neuron differentiation. Duration and frequency of ES is also a factor that can have dramatic effects on peripheral nerve regeneration. A study by Chen et al. showed accelerated maturation of regenerated nerves following ES.21 Application of ES resulted in a larger mean number of axons, endoneurial area, and total nerve area, and increased blood vessel number and increased blood vessel area over controls. These findings were in contrast to an earlier study by the same group that showed that ES significantly suppressed the formation of nerve cables across a nerve.21 These two studies were performed using different electrical signals and timings for application of ES. To unravel the role of ES and ideal timing of ES, a series of studies was performed.21 It was concluded that increasing the intensity of the electrical signal led to decreased function of the regenerated nerve.96 Previous studies reported that the immediate application of ES following injury led to better nerve regeneration.97 The intensity of applied ES, frequency, duration, and timing following nerve injury plays an important role in determining tissue healing. It was discovered that introducing a seven-day incubation (delayed ES) period following cell seeding led to an increased rate of regeneration and resulted in enhanced maturity of the neural components within the growth conduit.98 The number of myelinated axons that successfully grew across the 10 mm nerve gap was twofold greater in the delayed ES group than in the immediate ES group.98 The same study also showed that the nerves were larger and the amount of blood vessels and revascularization was greater in the delayed ES group than in the immediate ES group.98 These accounts highlight the importance of temporal relationships in ES experimental design in the optimization of NGCs.
The effect of ES on growth factors is another means by which ES has been shown to enhance neurite outgrowth. Application of ES through polypyrrole NGCs increased fibronectin adsorption on immediate stimulation, leading to enhanced neurite outgrowth,17 Huang et al. examined the impact of BDNF on the ES pathway leading to increased nerve regeneration and functional recovery.99 Stimulation using 3V, 20 Hz for 20 min led to improved motor functional recovery and reduced muscle atrophy.99 In a delayed sciatic nerve injury rat model, a defect was left untreated for a period ranging from two to 24 weeks with or without ES stimulation.99 The electrically stimulated group had better axonal recovery (increased diameter of myelinated axons, increased number of myelinated axons, and increased thickness of myelin sheath) and better functional outcomes (increased wet weight of gastrocnemius muscle and increased area of muscle fiber).99 Decreasing concentrations of BDNF in the healing nerves were observed through immunohistochemistry over time that suggested an inverse relationship between age of nerve injury and the effect of ES on factor release.99 Furthermore, an upregulation of BDNF was observed in the anterior horn of the spinal cord when proximal nerve stumps in the delayed nerve lesions were electrically stimulated. The findings of these studies are highlighted in Fig. 4. By creating NGCs that are able to conduct electricity, the benefits of ES can be utilized. Many electrically conducting polymers alone or in combination with other degradable polymers as well as different anionic dopants have been fabricated into tubular conduits for nerve regeneration applications. The present review aims to highlight the application of electrically conductive polymers for nerve growth and innovative ways to improve their performance in conjunction with ES.
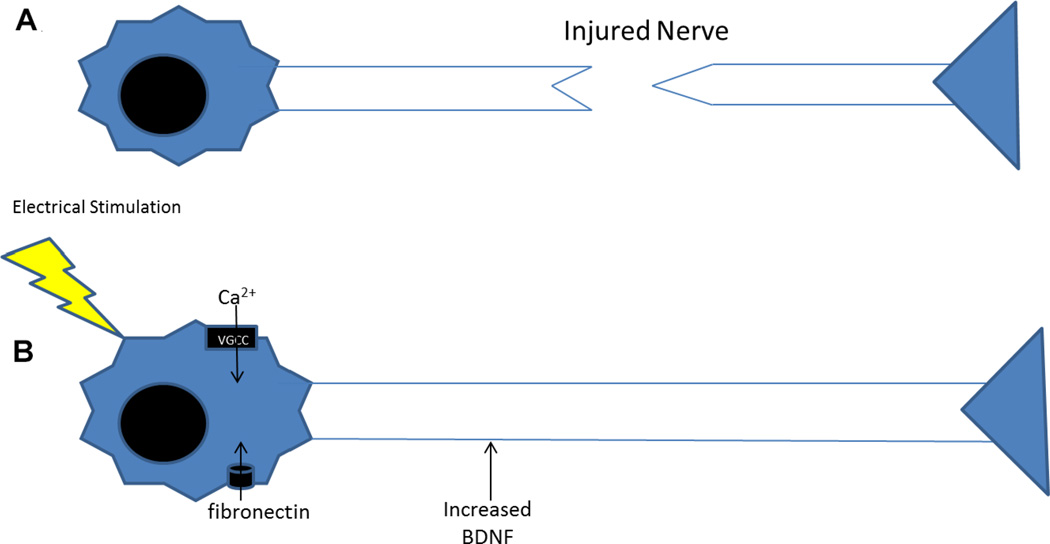
FIG. 4.
(A) Injured nerve. (B) Electrical stimulation leads to increased BDNF and fibronectin adsorption, which lead to increased neurite outgrowth. One pathway that is thought to be related to increased neurite outgrowth is the calcium signaling pathway through voltage-gated calcium channels (VGCC).
IV. CONDUCTIVE POLYMERS FOR NERVE GROWTH CONDUITS
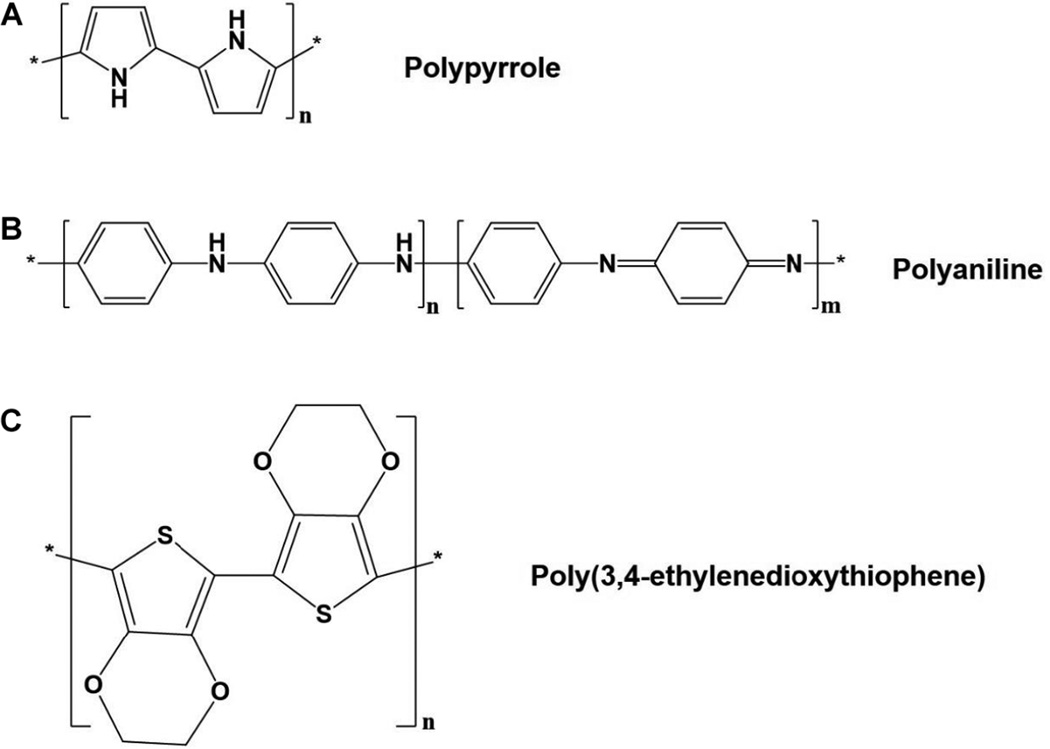
Conductive polymers have been studied for many years. The first major breakthrough came in 1978 when it was shown that polyacetylene exhibits a dramatic increase in electrical conductivity when it is oxidized (loses electrons) or reduced (accepts electrons).100, 101 Many other electrically conductive polymers such as polypyrrole, polyaniline, and poly(3,4-ethylenedioxythiophene) (Fig. 5) were discovered.101 In certain polymer chains, molecular distortion is energetically favored over molecular ionization in the presence of a dopant, leading to distortion of the polymer lattice and charge localization.101 In such a scenario, there is a localized distortion of the molecule lattice structure and an accompanying radical ion, or polaron, which comes from localization of the charge.101 When there is an electrical stimulus applied to the conductive polymer, dopants are able to move throughout the structure, creating polarons and allowing charge to flow through.68 Schmidt et al. were the first to show an increase in neurite outgrowth on the cells cultured on PPY scaffold following ES that triggered immense interest in using conductive polymers to promote peripheral nerve regeneration.102 These conductive polymers, along with various innovative scaffolds, and methodologies have been adopted to promote neural tissue regeneration. These studies are focused on identifying specific polymer compositions, scaffold design, and identifying appropriate ES parameters to promote nerve regeneration. The following sections summarize these efforts and provide an overview of the research in the laboratory setting.
FIG. 5.
Chemical structures of conducting polymers for nerve-growth conduits. (A) Polypyrrole (PPY). (B) Polyaniline (PANI). (C) Poly(3,4-ethylenedioxythiphene) (PEDOT).
A. Polypyrrole
Polypyrrole (PPY) is the most frequently studied electrically conducting polymer for tissue engineering. Apart from its conducting properties, it also has antioxidant properties.103 It can be easily synthesized and it readily accommodates anionic biomolecules within its structure, enhancing its biocompatibility.104 As a biomaterial, PPY presents several advantages including biocompatibility, chemical stability in air and water, and reasonably high electrical conductivity under physiological conditions.68 Polypyrrole has been utilized in a diverse array of technology development such as high-voltage batteries,105 supercapacitor electrodes,106–109 and biosensors,110, 111 and in drug delivery systems.112 The use of PPY derived scaffold materials in neural tissue engineering is extensive.13–19, 68, 113–115 In addition, PPY scaffolds have been also used for bone,116, 117 liver,118 and cardiac tissue engineering.119
PPY is electrically conductive through the conjugation in its backbone. When the PPY molecule is oxidized, pi electrons (π electrons) are removed from the upper level of the valence zone to lower energy levels, causing the molecule to behave like a semiconductor.120 Although PPY has many advantageous qualities that favor transmission of electrical charge, there are some properties of the polymer that present major disadvantages. A limiting factor in PPY’s conductivity is the disorder of its backbone,68 which can lead to slower deterioration of conductivity.68 Furthermore, PPY has very low solubility in most solvents and is brittle in its mechanical properties.19 One of the biggest disadvantages of PPY is its non-biodegradability.19 Various modification approaches have been attempted to improve on the aforementioned shortcomings of PPY including blending PPY with other synthetic polyesters such as PLGA, PLA, and PCL.19 Lack of available functional groups on the polymer backbone makes it difficult to alter the polymer properties through chemical modifications suitable for tissue engineering applications.113
Because the neuroregenerative benefits of PPY depend on its ability to conduct electricity, it is worthwhile to examine the electrical parameters that optimize its neuroregenerative capabilities. The PPY derivatives containing butane sulfonic acid, camphorsulfonic acid, and para-toluene sulfonic acid were found to be more electroactive than PPY.114 Increased PPY electrical conductivity originates from the presence of ionic groups in the form of acid in a biological environment through the flow of counterions. For PPY, in vitro models using rat PC12 cells have shown that the ES patterns that have been used to produce the best neurite outgrowth are 10 µA for two days114 or a voltage of 100 mV/cm115 for 2 h. Many of the benefits of PPY have been documented in both in vitro and in vivo studies.13–19, 68, 113–115 In vitro studies have shown that PPY is beneficial to neurite outgrowth for various cell types. Most commonly, PC12 cells (a neural crest-derived cell line that comes from rats) have been shown to benefit from ES through scaffolds that incorporate PPY to promote conductivity.13–18 Other studies have used dorsal root ganglia cells in order to show neurite outgrowth through ES of PPY.19–21 Polypyrrole scaffolds were able to support Schwann cell adhesion, survival, migration, and proliferation, and neurites outgrowth.121 Stewart et al. stimulated human neural stem cells on a PPY scaffold that was blended with the extracellular matrix protein laminin to show the synergistic effect of ES and tactile cues.104 Electrically stimulated human neural stem cells showed an increase in total neurite length per cell, mean neurite length, and maximum neurite length when compared to non-stimulated cells. Cells were stimulated for 8 h/day for three days (after an initial 24 h without stimulation) to allow for cellular adherence to scaffolds. Although the scaffold system was able to support the neurite outgrowth, it was found that there was heterogeneous electrical conductivity along the length of the scaffold that translated into uneven cell distribution and cells clustering at specific scaffold locations.104 For predictable tissue regeneration, it is highly desirable to design scaffolds that can provide uniform electrical conductance to provide uninterrupted ES to the regenerating nerve and promote uniform cell growth. As a better understanding of the effect of PPY on nerve regeneration continues to be revealed, different groups have given their interpretation of how the optimal electrically conductive NGC should be set up. Forcitini et al. showed that ES through PPY membranes led to increased protein adsorption, which was associated with increased NGF secretion from Schwann cells and increased speed of migration of Schwann cells.122 Presence of stimulatory proteins such as NGF on the surface of the scaffold and flow of electrical signal toward the distal end of the conduit during ES might provide optimal conditions for nerve regeneration.122 Such a gradient makes use of growth factors and ES allowing for directed Schwann cell migration and resulting in an increased rate of neural regeneration and functional recovery.122
Few studies report the use of PPY based scaffolds in an in vivo setting in the form of a single conduit15, 16 or multichannel conduit.123 Xu et al. reported the use of PPY containing poly (D, L-lactide) (PDLLA) NGC for the repair and regeneration of a 10 mm nerve gap in adult Sprague- Dawley rats.15 The blend scaffold containing 5% (wt) of PPY was able to induce longer neurites growth while maintaining the mechanical flexibility of PDLLA.15 The performance of the PPY blend scaffold was tested against PDLLA and autograft controls. There was no significant difference in the sciatic functional index (a commonly used behavioral test) between the PPY/PDLLA group and the autograft. Furthermore, the nerve conduction velocity of the PPY/PDLLA blend conduit was comparable to a healthy nerve.15 Histological images presented in Figs. 6 and 7 show many similarities between the regenerating nerves treated with PPY/PDLLA conduit and the autograft.15 Stewart et al. examined a stand-alone PPY nerve conduit and showed an increased quantity of myelinated fibers within and distal to the nerve graft.104 In the study, stand-alone PPY tubes were formed by electroplating a copper wire into aqueous 0.2 M PPY/0.2M sodium dodecyl benzene sulfonate with 10 mA applied for 60 min at 24°C. The PPY conduit was then removed from the wire by applying –10 V to the wire and platinum mesh and subsequently cut into 15 mm segments. In vivo results of implantation show that the growth conduits were biocompatible, as there was no inflammatory response or tissue damage. Furthermore, nerve growth and increased myelination was observed eight weeks after surgery, showing that the growth conduit was capable of supporting nerve regeneration/re-myelination. Fabrication of multichannel nerve conduit would allow for more complex/extensive nerve growth.
FIG. 6.
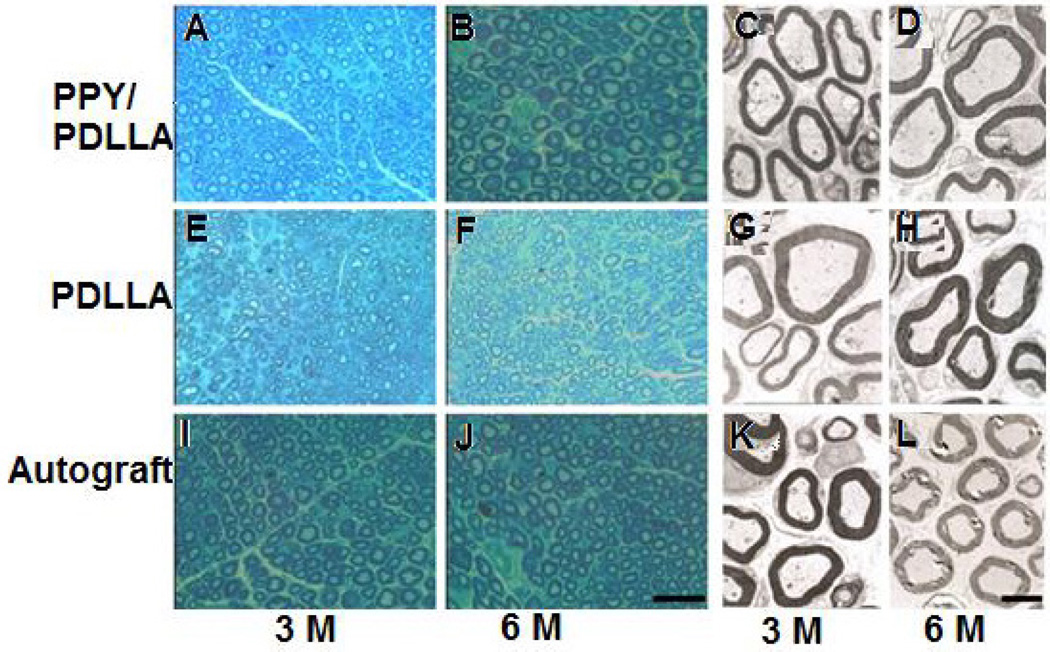
Representative histological images (A, B, E, F, I, and J) and transmission electron microscopy (TEM) micrographs (C, D, G, H, K, and L) obtained on the samples repaired with different scaffolds at two times of three and six months. Cross sections of the regenerated nerve stained with methylene blue clearly identify regenerated nerve fibers; the scale bar is 50 µm. TEM micrographs also revealed the similar findings; scale bar is 2 µm. Test scaffold PPY/ PDLLA and autograft (control) showed similarity in terms of regenerated myelinated fibers and structure. (Reprinted with permission from Elsevier.)15
FIG. 7.
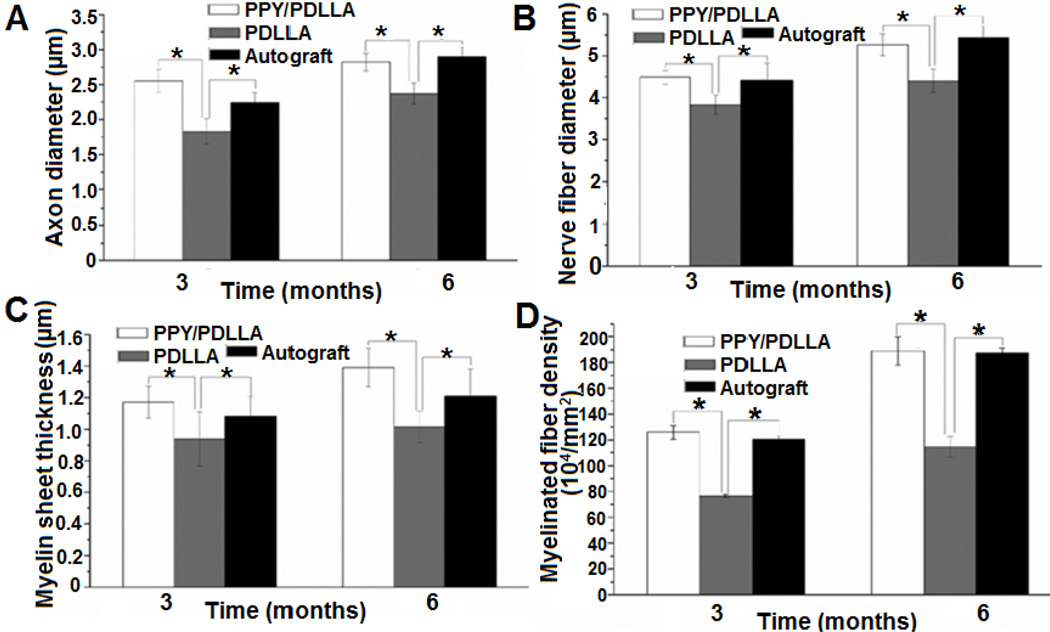
Quantification of the histological assessment of the regenerated nerve fibers three and six months after implantation. (A) Average axon diameter of regenerated myelinated nerve fibers. (B) Average diameter of the regenerated nerve fiber. (C) Average thickness of regenerated myelinated sheath. (D) Average density of regenerated myelinated nerve fibers (n = 6, *p < 0.05). In all of the assessments, no significant differences were observed between the PPY/ PDLLA and autograft. There tended to be a higher density of myelinated fibers and thicker myelin in fibers from the PPY/PDLLA group over the autograft at both three and six months. Nerves and axons in the autograft group, however, tended to have a greater than the PPY/PDLLA group at six months. (Reprinted with permission from Elsevier15.)
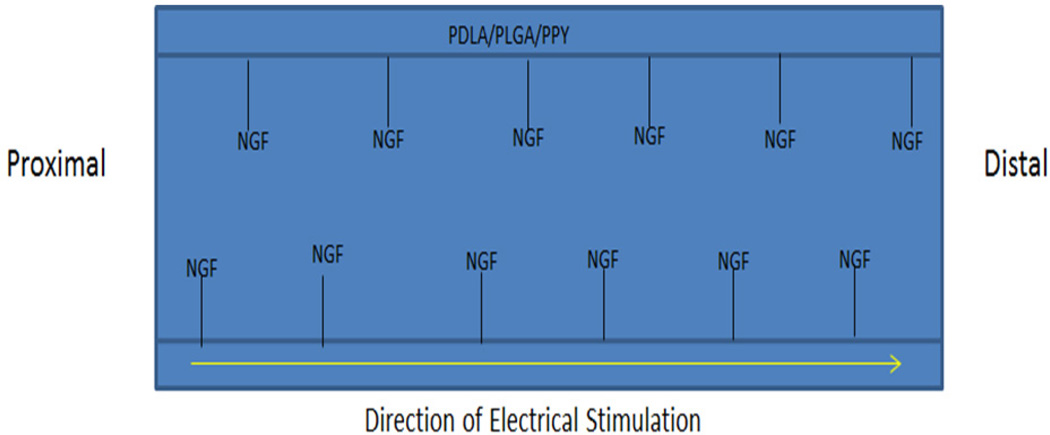
Other structural techniques have been shown to improve PPY’s effectiveness in stimulating nerve growth. One study investigated an NGC with PPY overlaid with unidirectional poly(D-lactide) (PDLA) fibers and was doped with para-toluene sulfonate.20 In brief, poly(d-lactide-co-glycolide) (75:25) was wet-spun into 30 µm sized fibers that were aligned onto gold-coated mylar sheets and wrapped around a collector spool. Next, the sheets were laid flat in order to facilitate galvanostatic deposition of PPY/ para-toluene sulfonate (pTS). The functionalized PPY matrix with pTS was then removed from the gold-mylar plate and formed into an NGC. Dorsal root ganglion neurons were then seeded onto scaffolds in four different groups: (i) PDLA/PPY/pTS with ES, (ii) PDLA/PPY/pTS without ES, (iii) PPY/ pTS with ES, and (iv) PPY/pTS without ES. The axonal growth followed the path of the PDLA fibers on both the stimulated and unstimulated scaffolds, whereas there was radial growth of axons on the plain PPY/pTS surfaces. The rate of axon growth was increased in the ES group and the maximal axon length in the PDLA/PPY/pTS with ES group was 60% greater than the axons in the PDLA/ PPY/pTS without ES, which had the next longest axons. Furthermore, there was a 27% increase in the distance traveled by Schwann cells in the PDLA/ PPY/pTS with ES group over the PDLA/PPY/pTS without ES group. Thus, optimizing all aspects of the NGC can have a profound effect on the nerve that is grown through the guidance scaffold. Nerve growth conduits can provide the controlled and conducive microenvironment to promote the rate of axonal growth greatly and improve its regenerative capacity. When the process of regeneration happens too slowly, the distal stump will die and regenerative efforts will be impossible and incomplete.
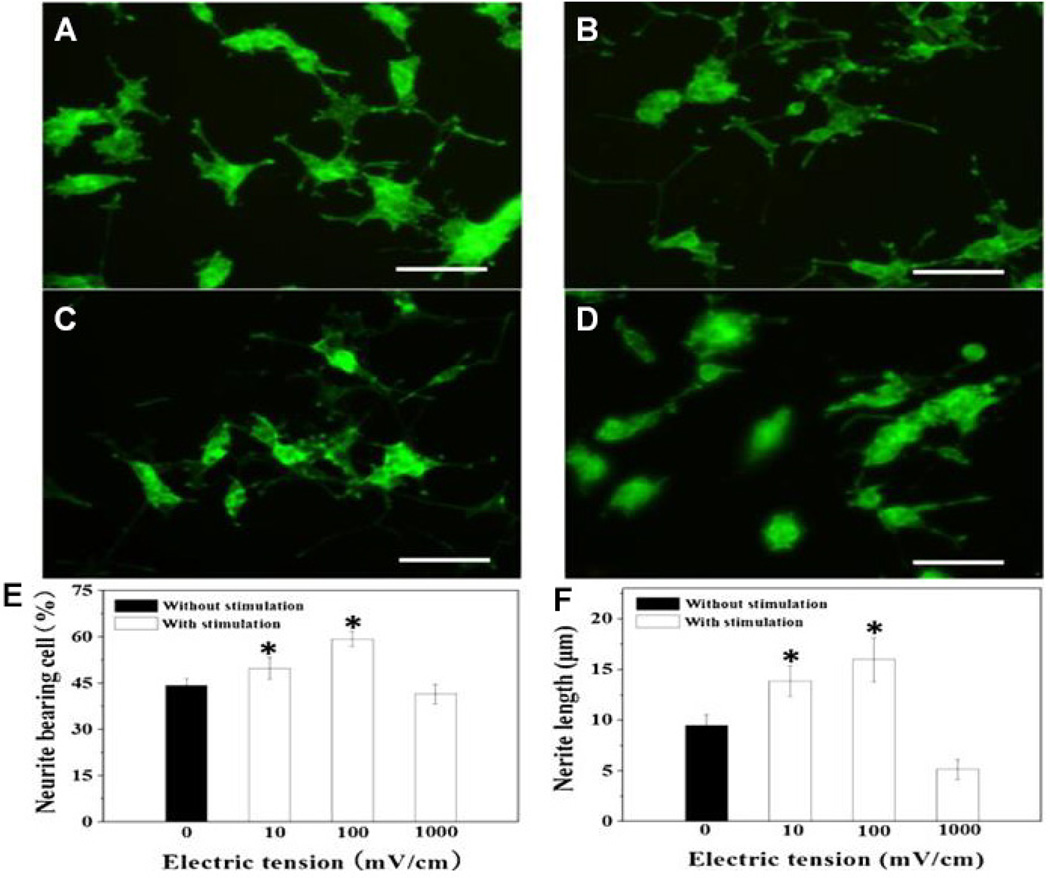
Polypyrrole containing porous cellulose gels under the ES resulted in remarkable neuronal phenotype expression by cultured PC12 cells.124 The cellulose base scaffold material, due to its fibrous and hydrophilic nature, offers greater biocompatibility and is thought to prevent excessive growth of connective tissue over lesions, which often poses a problem in peripheral nerve injury repair.124 Zeng et al. effectively applied ES to PPY scaffolds with rough scaffold surface containing NGF that led to accelerated axon elongation.18 The study also demonstrated the importance of selecting an appropriate level of ES. Scaffolds seeded with PC12 cells were electrically stimulated at different voltages (0, 10, 100, or 1000 mV/cm) for 2 h by a constant voltage source. Results showed that without ES, only 44% of PC12 cells grew neurites and the average neurite outgrowth was 9.5µm. Conversely, PC12 cells that were electrically stimulated at 100 mV/cm had 59% of their cells bearing neurites and the average neurite outgrowth was 16 µm. A voltage of 100 mV/cm was found to be the optimal level of ES, while other values, such as 10 mV/cm and 1000 mV/cm, did not provide ideal results; in the case of 1000 mV/cm, ES actually performed worse than the control group (see Fig. 8). These studies a suggestive of an optimal ES parameter that is specific for a scaffold system to drive neuronal phenotype development by specific cell type. Thus, scaffold optimization efforts should focus on altering the scaffold material chemistry, morphology, porosity, mechanical strength, and degradation features to enable ES for obtaining the best conditions for nerve regeneration. A summary of several such efforts are provided in Table 2 (in vitro studies), and Table 3 (in vivo studies). A desirable NGC prototype based on these studies is presented in Fig. 9.
FIG. 8.
Representative confocal images taken on fluorescent dye labeled PC12 cells cultured on NGF-conjugated PPY-PLLA fibers with and without ES: (A) control group with no ES; (B) with ES at 10 mV/cm; (C) with ES at 100 mV/cm. Applied ES changed cell morphology resulting in longer axons (B, C). The effect of neurites extension was inhibited at 1000 mV/cm ES as evidenced though shorter neurites protruding from its cell body (D). These images were analyzed and semiquantitative results are presented as (E) % of neurite bearing cells and average neurite length (F) following ES. These results suggest that PC12 cells stimulated at 100 mV/cm resulted highest number of neurite bearing cells and the largest average neurite length. (Reprinted with permission from Elsevier.)18
TABLE 2.
Examples of Polypyrrole Containing Nerve Growth Conduits In Vitro Characterization
| Polymer blend | Cell types used |
Electrical stimulation parameters | Neurite outgrowth length | Reference |
|---|---|---|---|---|
| PLGA immobilized with NGF |
PC12 | After 24 h in culture, a constant electrical potential of 10 mV/cm was applied across two electrodes for 2 h in the incubator using a potentiostat |
|
14 |
| PDLLA | PC12 | Cells were allowed to adhere to the cell plate for 24 h and a 100 mV potential was then applied across the wires for 2 h and the cells were cultured for an additional 24 h. |
|
15 |
| PCL | PC12 | Cells were cultured for 24 h before being electrically stimulated with 100 µA for 2 h. |
Neurites on average were 30% longer on PPy-PCL films than on unstimulated PPy-PCL films. |
16 |
| PSS | PC12 | Cells were stimulated at 10 µA either immediately (in the immediate stimulation group) or after 2 h (in the delayed stimulation group). |
The median neurite length for cells grown on PPY adsorbed with 0.25 mg/ml purified fibronectin when stimulated is 50% greater than that for PC12 cells grown on unstimulated PPY. |
17 |
| PLLA | PC12 | Cells were allowed to attach on NGF-conjugated PPY-PLLA fibers for 23 h and stimulated scaffolds for 10, 100, and 1000 mV/cm, depending on the treatment group, for 2 h by a constant voltage resource. |
Cells cultured on scaffolds with 100 mV/cm stimulation had best results with neurite outgrowth of 15.97 ± 2.14 µm and percent of neurite- bearing cells being 59.34 ± 2.46%. |
18 |
| PCL with PLGA coating |
DRG | Cells were allowed to adhere to the plates for 24 h and then received a 100 mV/cm AC electric field for 2 h/day. |
Increased axon length in the electrically stimulated group by 13% in neurons that were stimulated with an AC current and 21% in neurons that were stimulated with a DC current after three days of stimulation. Axon length of ES [DC] 996 ± 21 µm; axon length of ES [AC] 927 ± 15 µm; axon length of non-ES 821 ± 17 µm. |
115 |
| Laminin | Human neural stem cells |
Cells were allowed to adhere to the plate for 24 h before electrical stimulation, which was ± 0.25 mA/ cm2 using a biphasic waveform of 100 µs pulses with 20 µs interphase open circuit potential and a 3.78 ms short circuit (250Hz) for 8 h periods for three days. |
Electrically stimulated human neural stem cells comprised nodes or cluster or neurons joined by neurite networks. Increased total neurite length per cell, mean neurite length, and maximum neurite length compared to nonstimulated cells. |
104 |
TABLE 3.
Examples of Polypyrrole Containing Nerve Growth Conduits In Vivo Characterization
| Polymer blend |
Conduit type |
Transection length |
Animal study findings | Reference |
|---|---|---|---|---|
| PDLLA | Single conduit | 10 mm |
|
15 |
| PCL | Single conduit | 10 mm |
|
16 |
| Neat Polypyrrole | Multiconduit | 10 mm |
|
123 |
FIG. 9.
A prototype for an ideal nerve conduit fabricated from biodegradable polymers such as PDLA or PLGA incorporated with PPY to provide suitable mechanical strength, degradation rate, and ability to conduct current. The chemically tethered NGF on the polymer backbone provides a growth factor gradient to promote regeneration. Such a bioactive scaffold system in combination with ES may produce results that are comparable to autografts and may be ideal for regenerating long nerve gaps that are often difficult to heal.
B. Polyaniline
A nondegradable electrically conducting polymer polyaniline has been used as gas sensors,125–127 high-performance super capacitors,128, 129 and batteries,130 and in cotton fabrics as a way to provide protection from UV radiation.131 PANI has been also been explored as a scaffold material for tissue regeneration and drug delivery applications.59–63 Chemical stability and ability to withstand high temperature makes PANI an attractive material platform for a variety of applications.64, 132 Additionally, PANI possesses a wide range of electrical conductivity at a very low operational voltage.132 Like PPY, scaffolds derived from PANI have been also explored as a scaffold material in the possible application of bone,59, 60 cardiac,61–63 and skeletal muscle tissue engineering.133 Thermoset PANI is insoluble in any organic solvents, thus making it impossible to fabricate structures by thermal and solution casting methods once it is polymerized. Often pulverized nano- or micron-sized PANI particles are suspended in the other polymer solutions and fabricated into electrospun fiber matrices,64–66 hydrogels,134 or a combination of the two. 132
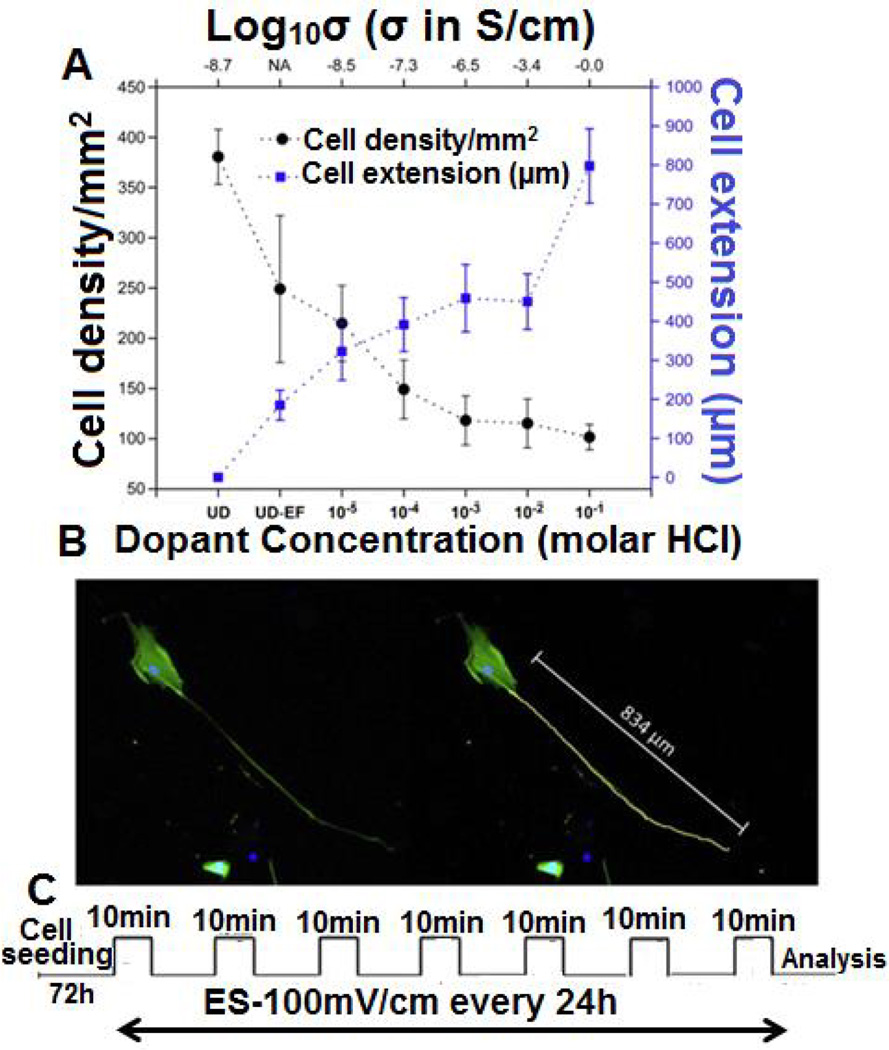
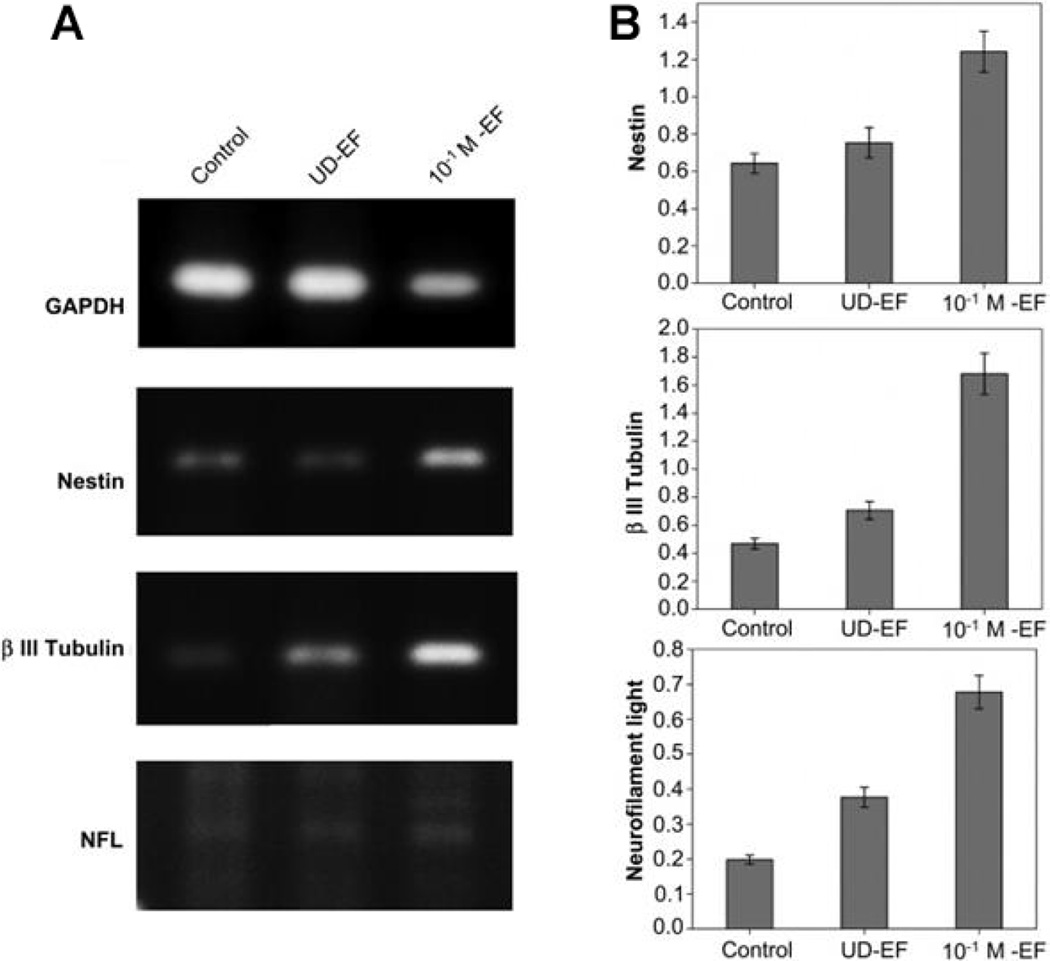
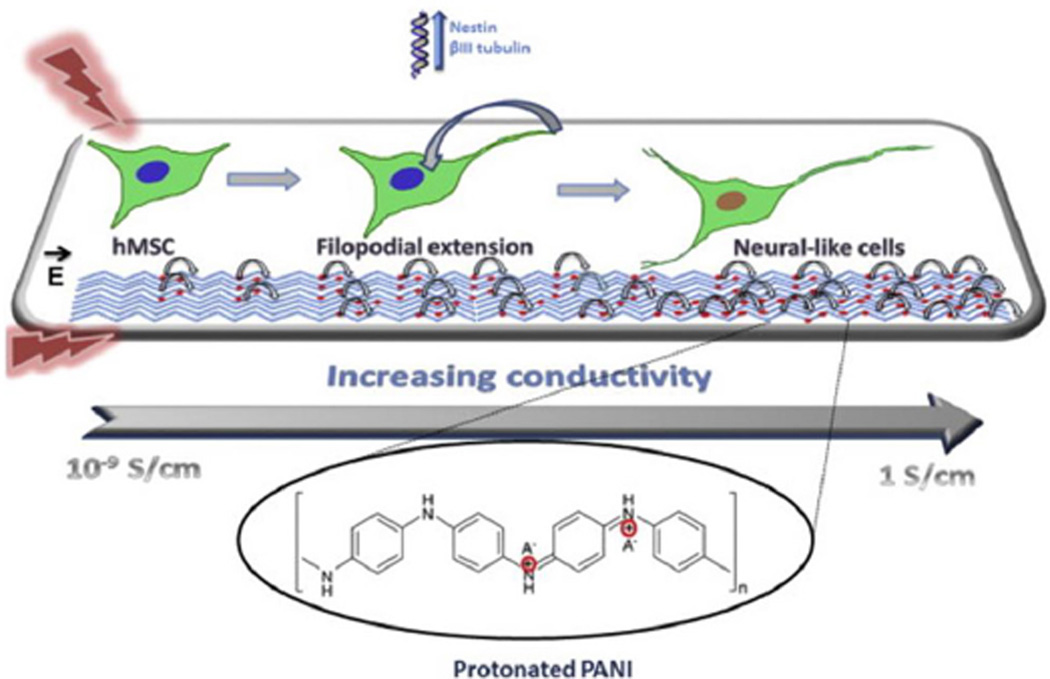
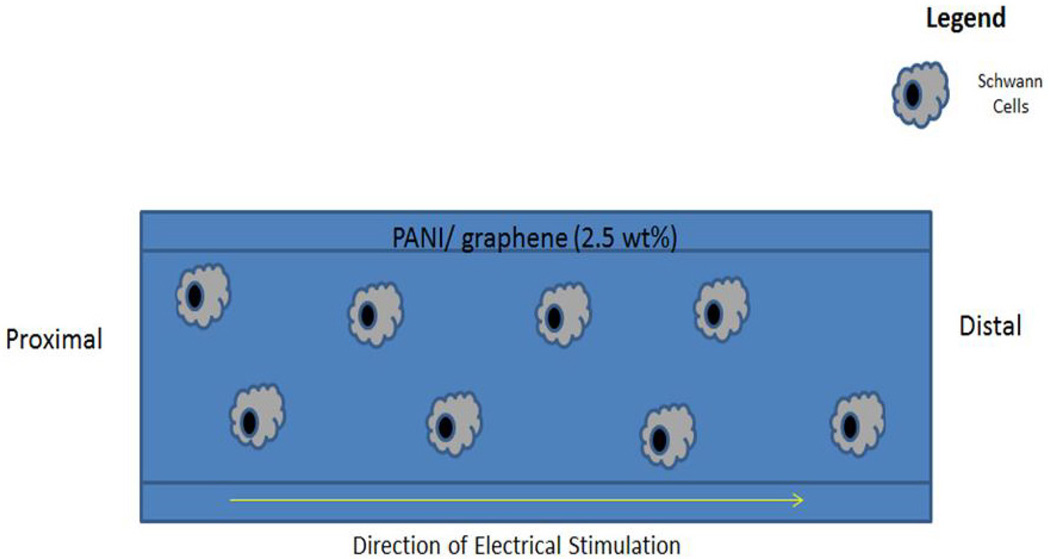
The difficulty in terms of processing and the nondegradable nature of PANI makes it less attractive for tissue engineering applications. Very few studies explored PANI as a candidate material for scaffolding applications in neural tissue regeneration.64, 90, 132 The general findings from these studies suggest increased neurite outgrowth on the PANI-containing scaffolds following ES.64, 90 In a study, PANI based scaffolds were shown to support neurite extension by applying 100 mV/cm at regular interval of 24 h in culture.90 A composite scaffold system comprised of chitosan/gelatin/PANI/graphene was able to support Schwann cells proliferation and differentiation.132 The least amount of PANI/ grapheme (2.5% wt) composition in the scaffold resulted in the highest cell attachment with a well-spread cell morphology.132 These findings provide evidence that PANI can support Schwann cells, which are very important to peripheral nerve regeneration. PANI films doped with hydrochloric acid (HCl) were used to evaluate their ability to support hMSCs neural phenotype development following ES.90 In brief, films seeded with hMSCs were stimulated with a direct current (DC) electric field of 10, 100, 500, or 1000 mV/cm for 10 min/day over a seven day period. Cells cultured only on electrically conducting substrates following ES resulted in axonlike, filopodial extensions, as illustrated in Fig. 10. It suggests that 0.1 M HCl doping with PANI and 100 mV/cm stimulation resulted in notable morphological changes in cells. The neurogenic hMSCs differentiation following ES was confirmed by the expression of nestin and βIII tubulin by immunostaining. The expression of these two neurogenic markers was higher in the elongated filopodia regions. The expression of neurogenic genes such as nestin, βIII tubulin, and neurofilament-light chain were also upregulated on the PANI substrates following stimulation as compared to controls (Fig. 11). The effect of applied voltage on neuronal phenotype development was studied by applying voltage in the range of 10–1000 mV/cm in an effort to find optimal ES parameters. An applied voltage of 100 mV/cm produced the most desirable neurogenic induction while 1000 mV/cm did not support cell differentiation. Thus, it is highly desirable to optimize the ES parameters for each scaffold system, as there is no well-defined voltage range that can induce neuronal hMSCs differentiation. Thus, it is also critically important that the scaffold carry homogeneous optimized ES to achieve reproducible cellular events. The use of a clinically relevant cell population such as hMSCs in combination with scaffolds and ES may enhance peripheral nerve regeneration.135, 136 The possible PANI scaffold prototype and associated cellular events are summarized in Figs. 12 and 13. While these in vitro experiments show that PANI may be useful in neural tissue engineering, in vivo application is limited due to several shortcomings as a biomaterial. Particularly, PANI is very brittle, rigid, and difficult to form into nerve guidance scaffolds, and nondegradabe.65 Thus, in order to effectively use PANI in nerve guidance conduits, it will be important to overcome these limitations. It has, however, proved to be a good polymer system to use in vitro to evaluate the mechanism by which ES promotes differentiation of stem cells into neurons. Table 4 summarizes the in vitro characterization of PANI as scaffold material in the direction of neural tissue regeneration.
FIG. 10.
(A) Effect of ES on cell density and cell elongation as a function of substrate electrical conductivity. hMSCs were cultured on polymeric matrices with different electrical conductivity for seven days and ES was applied at 100 mV/cm for 10 min/day. All data are shown as mean ± SD (n = 3–5) and are representative of at least three different samples. (B) Procedure to measure the long cytoskeletal protrusions. The total lengths (marked in yellow) of the protrusions were measured from the tip of the cell body to the end of the extension. All the length measurements were performed using the free-hand line tool in ImageJ software. (C) Sequence of electric field stimulation cycle applied to the hMSCs in culture. (Reprinted with permission from Elsevier.)90
FIG. 11.
Relative neural gene expression by hMSCs cultured on polymeric substrates of varied electrical conductivity (A, B) where glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used a house keeping gene. ES was applied at a rate of 100 mV/cm for 10 min/ day and harvested cellular constructs on day seven were analyzed for relative gene expression (B). Neural markers (Nestin and βIII Tubulin) were upregulated in highly conducting substrate (HCl doped PANI). The expression of Nestin, βIII Tubulin and neurofilament light were increased by 1.8-, 2.7-, and 1.7-fold, respectively. The values are represented as means ± SD. (Reprinted with permission from Elsevier.)90
FIG. 12.
Schematics representing the effect of substrate electrical conductivity on hMSCs following ES. Substrate electrical conductivity alters the cytoskeletal arrangement of hMSCs that leads to neural-like cell formation. With an increase in substrate electrical conductivity, charge transfer becomes more efficient, and that translates into elongation of filopodia. It is important to create an electrical conductivity gradient to drive the cell differentiation and provide a high degree of electronic conduction. (Reprinted with permission from Elsevier90.)
FIG. 13.
A prototype for an ideal nerve conduit fabricated from biodegradable polymers incorporated with PANI and graphene to provide suitable mechanical strength, degradation rate, and ability to conduct current. Schwann cells cultured on such substrates would be able to secrete growth factors and result in neurite extensions following ES. Scaffold system may also support the growth of any other neuronal progenitors and neuronal phenotype development.
TABLE 4.
Examples of Polyaniline Containing Nerve Growth Conduits In Vitro Characterization
| Polymer blend | Cell types used |
Neurite outgrowth length | Electrical stimulation parameters |
Reference |
|---|---|---|---|---|
| Poly (L-lactic aci-co-ε-caprolactone)/slik fibroin incorporated with NGF | PC12 |
|
Cells were seeded and allowed to adhere for 24 h and a constant voltage of 100 mV/cm was applied for 1 h/day | 65 |
| 33 PANI alone | hMSCs |
|
Cells were incubated for 72 h under field-free conditions and then the electric field of 10 mV/cm- 2V/cm, depending on the group, was applied for 10 min every 24 h. | 90 |
| PLLA | Rat nerve stem cells (C17.2) | Average neurite length of cells cultured on PLLA/PANI scaffolds after electrical stimulation was found to be 24 ± 4 μm compared to 15 ± 3 μm without electrical stimulation. | Cells were seeded and allowed to adhere for 24 h; they were then exposed to a steady electrical potential of 1.5 V for a period of 60 min | 64 |
| PCL | Nerve stem cells | The average neurite length for NSCs grown on PANI/PG with application of electrical stimulation for 1 h and without electrical stimulation was found to be 30 ± 1.1 and 22 ± 0.97 μm, respectively, whereas there was no difference in neurite outgrowth length between electrically stimulated and non-electrically stimulated groups for 15 and 30 min electrical stimulation studies. | Cells were seeded and incubated for 24 h and then exposed to steady potential of 1.5 V for 15, 30, and 60 min through a DC current source. | 66 |
| Polypropylene | PC12 | Functional PANI appeared to release an agent toxic to neurons with detrimental ramifications to survival. | No electrical stimulation | 171 |
C. Poly(3,4-Ethylenedioxythiophene)
Poly(3,4-ethylenedioxythiophene) (PEDOT) is a nondegradable electrically conducting polymer that has been extensively used for a variety of applications including drug delivery,137 solar cells,138–141 batteries,142 and proton exchange membrane fuel cells.143 PEDOT has also been explored as a scaffold material for nerve, bone,144 and liver tissue engineering.145 The bulk of the neural tissue engineering research surrounding PEDOT has been associated with its use in electrodes for nerve growth. Unlike PANI, PEDOT is soluble in a wide range of organic solvents and allows chemical modification, and makes it attractive for scaffolding applications.146, 147 The nondegradable nature of the material makes it less desirable for designing scaffolds for transient tissue regeneration applications. Thus, PEDOT is used as a model polymer system like PANI to study the effect of ES on cell differentiation. Collagen scaffolds containing PEDOT and PANI nanofibers were able to support PC12 cell growth and differentiation into nervelike cells in the absence of ES.57 The incorporation of PANI or PEDOT was to render scaffolds electrically conductive. A PANI-incorporated collagen matrix resulted in higher PC12 cell growth while a PEDOT-infused matrix resulted in greater neurogenic differentiation.57 For instance, PC12 cells expressed significantly higher levels of MAP2 and β-tubulin III neurogenic marker expression on PEDOT incorporated gels and resulted in higher dendrite and axon outgrowth as compared to PANI gels without ES. Differences in cell growth and neurogenic differentiation may be due to the changes in gel properties as a result of blending with these fibers and potential cell-cell communication effects. Abidian et al. fabricated an agarose hydrogel that had a PEDOT coating that was able to support growth of a peroneal nerve that showed similar morphology to that of the autograft group, albeit smaller and with less myelin.146 Conductive polymer PEDOT was electrodeposited inside the lumen of the agarose tube to create either fully coated PEDOT agarose conduits or partially coated PEDOT agarose conduits. These PEDOT coated hydrogel based conduits were then placed in a 10 mm nerve gap in a rat peroneal nerve and samples were harvested at 12 weeks. The measurements that were taken included extensor digitorum longus muscle contractile force measurements, muscle innervation by the peroneal nerve, and nerve histomorphometry. Overall, the results showed that the autograft outperformed all of the groups in every outcome measured. Interestingly, it also showed that both of the PEDOT groups outperformed the plain agarose group, with the partial PEDOT group performing the best. The better performance of partially coated PEDOT was attributed to an increased capacity for diffusion of nutrients and biomolecules through the partially coated lumen of the conduits. The nondegradable nature of PEDOT and absence of pores in the scaffold with complete coating may hinder the transport features and restrict nutrient exchange and metabolic waste removal. Cross-linked PEDOT:polystyrene sulfonate (PEDOT:PSS) blend matrices were created for a possible neural tissue engineering.58 The ionic component PSS in the blend contributes to the electrical conductance and erodes in the aqueous component. Cross-linking allows the controlled erosion of PSS from the matrix and the scaffold erosion properties depend on the amount of PSS in the matrix. Cell viability and proliferation studies with fibroblasts show that PEDOT:PSS scaffolds exhibited both good cell viability and cell proliferation over a four-day period. In the study, human neural progenitor cells were electrically stimulated over 12 days to show the effect of ES on these cells. The neural progenitor cells were exposed to pulsed stimulation with a frequency of 100 Hz for 24 h for the first four days and then over 12 h over the following eight days. Greater neurite outgrowth was observed on electrically stimulated PEDOT:PSS matrices than the control with ES. In comparison, PEDOT has high electrical conductance and is more electrochemically stable than PPY and PANI. Chemical modifications and creative blending approaches may further improve material properties and provide better erosion properties to the matrix.68
D. Effect of Electrical Stimulation on Schwann Cells
The connection between ES and Schwann cell function has been well documented. Not only has ES been shown to affect the growth and migration of neurons,148 but it has also been shown to affect the growth and migration of neural support cells such as Schwann cells, which are able to support axon regeneration through release of neurotrophic factors.76, 77, 94 When studying ES, it is important to understand the role of Schwann cells. When a nerve is transected, there are three stages of recovery that must occur for full recovery: (i) Wallerian degeneration, which is used to create a microenvironment conducive to axonal regrowth and reinnervation, (ii) axonal regeneration, and (iii) end-organ reinnervation.149 Schwann cells are vital to the process of axon regeneration as they serve as primary mediators for beginning the process of Wallerian degeneration.149 In the promotion of axonal regeneration, Schwann cells arrive rapidly at the site of nerve injury to assist in the process of clearing debris through phagocytosis and the recruitment of macrophages.149 They then proliferate on the endoneurial tubes of the extracellular matrix and form what are called bands of Bungner, which provide a path for the axon to regrow.149, 150 A summary of the important and crucial role Schwann cells play in nerve regeneration process is presented in Fig. 14.
FIG. 14.
Schematic illustration on the role of Schwann cells in nerve regeneration: (A) severed peripheral nerve, (B) Schwann cells recruited in the defect proliferate and release cytokines, which recruit macrophages to help clear cellular debris; (C) if Schwann cells are able to bridge the gap, they form Bands of Bungner, which help facilitate regeneration over the transection gap. Schwann cells are important to facilitation of peripheral nerve regeneration. Thus, understanding their interactions with the regenerating nerve is important in creating nerve conduits that will best facilitate peripheral nerve regeneration.
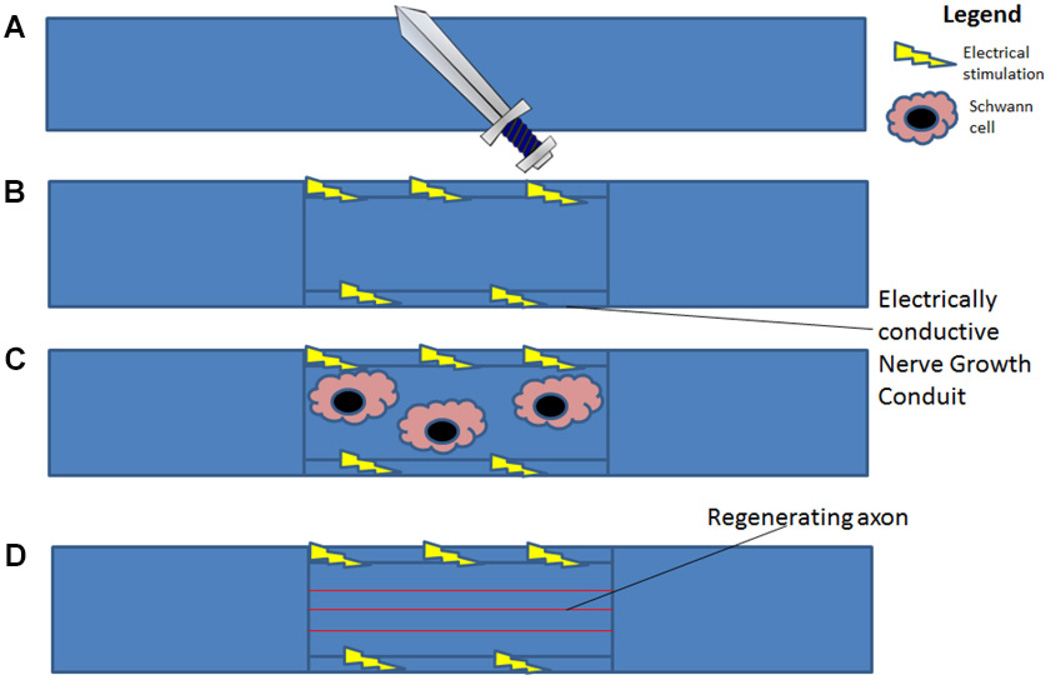
Studies have shown that ES can lead to increased secretion of neurotrophic factors such as BDNF and NGF from Schwann cells after ES.75–77 In addition, neural progenitors resulted in neurite outgrowth following ES.151 The use of Schwann cells without ES in the regeneration of long nerve defects has been reported to be positive152 in some cases and non-beneficial in others.153 However, the use of ES in conjunction with Schwann cells may produce positive effects in terms of nerve regeneration. For instance, electrically conductive scaffolds cocultured with Schwann cells and other clinically relevant stem cell populations such as hMSCs may result in an enhanced rate of nerve regeneration following ES. The growth factors secreted by Schwann cells following ES may further enhance the differentiation of hMSCs and other tissue-specific progenitors into neurons and accelerate the rate of nerve regeneration. Isolation and expansion of Schwann cells may pose limitations in terms of its clinical applicability as large number of cells are needed for implantation.154 In neural tissue engineering, efforts should also focus on the choice of cells, stimulation, and scaffold parameters to promote regeneration. Application of ES in conjunction with Schwann cells should consider parameters that favor their proliferation, neurite outgrowth, and secretion of neurotrophic factors. Figure 15 summarizes application of ES to NGCs seeded with Schwann cells in the promotion of peripheral nerve regeneration. In a tissue engineering approach, scaffolds combined with Schwann cells and hMSCs under ES secrete growth factors and may heal over greater transection lengths. Wallerian degeneration will be enhanced and proximal nerve stumps will be able to grow toward the distal nerve stump more quickly. Future studies should focus and identify ES parameters for different cell types to promote their regenerative performance. More studies are essential to understand the effects of ES on cytoskeletal arrangement and to adopt ES to guide Schwann cells to the distal stump.
FIG. 15.
Schematic illustration on the role of ES and Schwann cells in nerve regeneration: (A) transected nerve; (B) electrically conductive nerve conduit; (C) migration of Schwann cell at the defect site following ES; (D) Schwann cells secrete cytokines and aid in the nerve regeneration. Optimization of ES parameters may promote Schwann cells involvement in regeneration and have the potential to outperform the gold standard autograft.
V. FUTURE DIRECTIONS
Electrically conductive polymers are very promising structures for the advancement of neural tissue engineering. ES is a useful tool for manipulating Schwann cells and neural progenitors that play a major role in peripheral nerve regeneration. From these studies, it is apparent that electrically conducting scaffolds under ES can support the differentiation of stem cells into neuronlike cells but the mode of differentiation is not understood. Future studies should examine the mechanistic approaches of cell differentiation under the influence of ES and evaluate the role of differentiated cells during the regenerative process and their integration with the host tissue. Potential questions that should be addressed include: Will these cells be used as a primary source of regenerative tissue? Will they instead be used as sources of growth factor release to enhance native tissue regeneration? How will they be incorporated into the scaffold? What are the optimal ES settings, taking into account both the optimal settings for the native Schwann cells and the implanted MSCs? These are some of the questions that need to be studied in order to make the best use of hMSCs in future regenerative efforts. The development of a biodegradable scaffold system that provides the right combination of mechanical strength, pore and degradation properties, and predictable electrical conductivity may further drive research in neural tissue engineering. Electroactive polymers such as PPY, PANI, and PEDOT have several limitations for biomedical applications. Current efforts are focused on improving material properties, processing conditions, and electrical conductance by combining them with other materials. Presenting these materials in the oxidized form and maintaining their state is critical in obtaining a predictable electrical conductance in the physiological setting. These polymers often undergo reduction in physiologic conditions and their electrical conductivity significantly decreases. In an effort to improve their electrical conductivity, efforts are being made to include anionic dopants such as dodecylbezenesulfonate, PSS, tosylate, and perchlorate, to name a few. The presence of these dopants in conductive polymers changes their structure and also affects the orientation and arrangement of polymer chains, translating into a change in material properties in terms of crystallinity, mechanical strength, and electrical conductivity.155, 156 Scaffold fabrication and characterization should consider changes in scaffold properties in terms of morphology, roughness, phase separation, and erosion, which have been shown to affect cellular events.155, 156 Optimization also presents a challenge with dopants because the mechanical and electrical properties of the dopant must be balanced with the biological performance, which factors in the surface morphology and material chemistry.155 For example, use of a small amount of dopant may give rise to increased electrical conductivity and decreased matrix rigidity, and affect cell viability, which may not be desirable.155 These ionic dopants are water soluble and leach out of the scaffold following implantation in the aqueous conditions. With depleting dopant concentration, electrical conductivity of the scaffold reduces and the released dopants can be toxic. Thus, scaffold optimization and characterization efforts should consider these factors when designing an application. 155
A possible alternative is to use ionic polymers that present ionizable groups on the polymer backbone and conduct electricity in the biological environment though movement of ions.67 Recently, we have reported the application of the above concept wherein we created a series of sulfonated polymeric membranes and evaluated their ability to conduct electricity, and characterized them for initial cell compatibility.67 The electrically conductive property of the polymeric substrates is a result of the presence of a number of ionic groups on the polymer backbone. The electrical conductivity of polymeric sulfonated membranes in 20 mM physiological buffer solution was found to be 53.55, 35.39, and 29.51 mS/cm for three different membrane compositions studied.67 These values are far superior to many polymeric PANI, PPY, and PEDOT based systems. The ionic conductivity measured was directly proportional to the sulfonic acid content on the polymer backbone. An increasing amount of sulfonic acid functionality on the polymer backbone resulted in higher ion exchange capacity and higher water content. Thus, the sulfonated membranes showed improved matrix hydrophilicity and higher electrical conductivity ideally suited for nerve regeneration. These membranes supported adhesion and proliferation of human skin fibroblasts over 14 days and the proliferation rate was comparable to tissue culture polystyrene. The concept and the polymer modification methodology can be readily extended to other widely used biodegradable polymers.67 Ionically conducting polymers and structures may find application in the delivery of electrical stimulus to modulate tissue repair and regeneration after injury, especially in electroactive tissues such as nerves, skeletal muscle, cardiac muscle, and spine. More studies are needed to identify the benefits and drawbacks of the concept, and the clinical conditions that will most benefit. Our research group at the University of Connecticut is developing conductive natural and synthetic polymers, and the initial work is focused on the synthesis and characterization of ionically conductive biomedical polymers for ES mediated tissue regeneration. Thus, a possible future direction of electrically conducting polymers for NGCs is optimization of ionic polymers. Nerve grafts fabricated using ionically conducting polymers may provide highly desired scaffold properties in terms of superior mechanical and degradation features with improved biocompatibility and predictable electric current flow properties. Combined with electrical stimulation, these scaffolds may serve as ideal grafts for treating more challenging longer nerve defects beyond 30 mm.
VI. CONCLUSION
Nerve growth conduits are a promising alternative to the gold standard autograft for peripheral nerve regeneration. Incorporation of ES is an encouraging addition to NGC technology. Optimization of electrically conductive polymers in conjunction with cellular applications of tissue engineering has the ability to support nerve regeneration over long gaps. In order to best take advantage of ES parameters, new platforms such as ionically conducting polymers must be explored to provide ES for nerve and muscle regeneration. Electrically conductive NGCs may harness the full effect of ES and lead to better regenerative effects. Electrically conductive polymers in NGCs have proven to be important to the field of peripheral nerve regeneration and continued optimization of their properties should add valuable knowledge to the nerve tissue regenerative fields.
Acknowledgments
The authors acknowledge funding from the National Institute of Health (Grant No. 5R03NS058595), Department of Defense (Grant No. OR120140), and the National Science Foundation Awards No. IIP-1311907, No. IIP-1355327, and No. EFRI-1332329. The authors also acknowledge support from The Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences.
Glossary
- BDNF
brain-derived neurotrophic factor
- ES
electrical stimulation
- hMSC
human mesenchymal stem cell
- NGC
nerve growth conduit
- NGF
nerve growth factor
- NT-3
neurotrophin-3
- PANI
polyaniline
- PCL
polycaprolactone
- PDLA
poly-D-lactide
- PDLLA
poly-DL-lactide
- PEDOT
poly(3,4-ethylenedioxythiophene)
- PSS
polystyrene sulfonate
- PGA
polyglycolide
- PLA
polylactic acid
- PLGA
poly(lactic-co-glycolic acid)
- PLLA
poly-L-lactic acid
- PPY
polypyrrole
- pTS
para-toulene sulfonate
- vEGF
vascular endothelial growth factor
REFERENCES
- 1.Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87(5):381–385. doi: 10.1097/PHM.0b013e31815e6370. [DOI] [PubMed] [Google Scholar]
- 2.Grinsell D, Keating C. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:1–13. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arslantunali D, Dursun T, Yucel D, Hasirci N, Hasirci V. Peripheral nerve conduits: technology update. Med Devices (Auckland, NZ) 2014;7:405–424. doi: 10.2147/MDER.S59124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krebs C, Weinberg J, Akeeson . Lippincott’s Illustrated Reviews: Neuroscience. Baltimore (MD): Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 5.Pfister LA, Papaloïzos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007;12(2):65–82. doi: 10.1111/j.1529-8027.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- 6.Konofaos P, Ver Halen JP. Nerve repair by means of tubulization: past, present, future. J Reconstr Microsurg. 2013;29(3):149–164. doi: 10.1055/s-0032-1333316. [DOI] [PubMed] [Google Scholar]
- 7.Williams LR, Longo FM, Powell HC, Lundborg G, Varon S. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay. J Compar Neurol. 1983;218(4):460–470. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]
- 8.Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26(2):151–160. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 9.Chong JK. Nerve regeneration through preformed pseudosynovial tubes. Plast Reconstr Surg. 1980;66(6):866. [Google Scholar]
- 10.Seckel BR, Chiu TH, Sidman RL. Nerve regeneration through synthetic biodegradable nerve guides: regulation by the target organ. Plast Reconstr Surg. 1984;74(2):173–181. doi: 10.1097/00006534-198408000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Johnson EO, Soucacos PN. Nerve repair: experimental and clinical evaluation of biodegradable artificial nerve guides. Injury. 2008;39(3):30–36. doi: 10.1016/j.injury.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Rich KM, Alexander TD, Pryor JC, Hollowell JP. Nerve growth factor enhances regeneration through silicone chambers. Exp Neurol. 1989;105(2):162–170. doi: 10.1016/0014-4886(89)90115-5. [DOI] [PubMed] [Google Scholar]
- 13.Runge MB, Dadsetan M, Baltrusaitis J, Ruesink T, Lu L, Windebank AJ, Yaszemski MJ. Development of electrically conductive oligo (polyethylene glycol) fumarate-polypyrrole hydrogels for nerve regeneration. Biomacromolecules. 2010;11(11):2845–2853. doi: 10.1021/bm100526a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JY, Bashur CA, Milroy CA, Forciniti L, Goldstein AS, Schmidt CE. Nerve growth factor-immobilized electrically conducting fibrous scaffolds for potential use in neural engineering applications. IEEE Trans Nanobiosci. 2012;11(1):15–21. doi: 10.1109/TNB.2011.2159621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu H, Holzwarth JM, Yan Y, Xu P, Zheng H, Yin Y, Li S, Ma PX. Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials. 2014;35(1):225–235. doi: 10.1016/j.biomaterials.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durgam H, Sapp S, Deister C, Khaing Z, Chang E, Luebben S, Schmidt CE. Novel degradable co-polymers of polypyrrole support cell proliferation and enhance neurite out-growth with electrical stimulation. J Biomater Sci Polym Ed. 2010;21(10):1265–1282. doi: 10.1163/092050609X12481751806330. [DOI] [PubMed] [Google Scholar]
- 17.Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials. 2001;22(10):1055–1064. doi: 10.1016/s0142-9612(00)00344-6. [DOI] [PubMed] [Google Scholar]
- 18.Zeng J, Huang Z, Yin G, Qin J, Chen X, Gu J. Fabrication of conductive NGF-conjugated polypyrrole-poly(l-lactic acid) fibers and their effect on neurite outgrowth. Colloids Surf B. 2013;110:450–457. doi: 10.1016/j.colsurfb.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Runge MB, Dadsetan M, Baltrusaitis J, Knight AM, Ruesink T, Lazcano EA, Lu L, Windebank AJ, Yaszemski MJ. The development of electrically conductive polycaprolactone fumarate-polypyrrole composite materials for nerve regeneration. Biomaterials. 2010;31(23):5916–5926. doi: 10.1016/j.biomaterials.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quigley AF, Razal JM, Thompson BC, Moulton SE, Kita M, Kennedy EL, Clark GM, Wallace GG, Kapsa RM. A Conducting-polymer platform with biodegradable fibers for stimulation and guidance of axonal growth. Adv Mater. 2009;21(43):4393–4397. doi: 10.1002/adma.200901165. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y-S. Effects of electrical stimulation on peripheral nerve regeneration. BioMedicine. 2011;1(1):33–36. [Google Scholar]
- 22.Williams D. Benefit and risk in tissue engineering. Mater Today. 2004;7(5):24–29. [Google Scholar]
- 23.Shelke NB, James R, Laurencin CT, Kumbar SG. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym Adv Technol. 2014;25(5):448–460. [Google Scholar]
- 24.Tamaki T. Bridging long gap peripheral nerve injury using skeletal muscle-derived multipotent stem cells. Neural Regener Res. 2014;9(14):1333–1336. doi: 10.4103/1673-5374.137582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang P, Lu X, Chen J, Chen Z. Schwann cells originating from skin-derived precursors promote peripheral nerve regeneration in rats. Neural Regener Res. 2014;9(18):1696–1702. doi: 10.4103/1673-5374.141805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma S, Peng C, Wu S, Wu D, Gao C. Sciatic nerve regeneration using a nerve growth factor-containing fibrin glue membrane. Neural Regener Res. 2013;8(36):3416–3422. doi: 10.3969/j.issn.1673-5374.2013.36.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapur TA, Shoichet MS. Chemically-bound nerve growth factor for neural tissue engineering applications. J Biomater Sci Polym Ed. 2003;14(4):383–394. doi: 10.1163/156856203321478883. [DOI] [PubMed] [Google Scholar]
- 28.Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: Bioactive electrically conducting polymer for enhanced neurite extension. J Biomed Mater Res A. 2007;81(1):135–149. doi: 10.1002/jbm.a.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y-T, Haftel VK, Kumar S, Bellamkonda RV. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29(21):3117–3127. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calvey C, Zhou W, Stakleff KS, Sendelbach-Sloan P, Harkins AB, Lanzinger W, Willits RK. Short-term electrical stimulation to promote nerve repair and functional recovery in a rat model. J Hand Surg Am. 2014;40(2):314–322. doi: 10.1016/j.jhsa.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Baharvand H, Kiani S, Al-Deyab SS, Ramakrishna S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J Tissue Engin Regen Med. 2011;5(4):e17–e35. doi: 10.1002/term.383. [DOI] [PubMed] [Google Scholar]
- 32.Prasad Shastri V, Lendlein A. Engineering materials for regenerative medicine. MRS Bull. 2010;35(08):571–577. [Google Scholar]
- 33.Shastri VR, Schmidt CE, Kim T-H, Vacanti JP, Langer R. Polypyrrole—a potential candidate for stimulated nerve regeneration. MRS Online Proceedings Library. 1995;414 [Google Scholar]
- 34.Lin MY, Manzano G, Gupta R. Nerve allografts and conduits in peripheral nerve repair. Hand Clin. 2013;29(3):331–348. doi: 10.1016/j.hcl.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Shelke NB, Anderson M, Idrees SM, Nip J, Nip, Donde S, Gronowicz G, Kumbar SG. Polyester nano- and micro-technologies for tissue engineering. In: Ravikumar MNV, editor. Handbook of Polyester Drug Delivery Systems. Singapore: Pan Stanford Publishing; 2015. [Google Scholar]
- 36.James R, Toti US, Laurencin CT, Kumbar SG. Biomedical nanotechnology. New York (NY): Springer; 2011. Electrospun nanofibrous scaffolds for engineering soft connective tissues; pp. 243–258. [DOI] [PubMed] [Google Scholar]
- 37.Lee J-W, Serna F, Schmidt CE. Carboxy-endcapped conductive polypyrrole: biomimetic conducting polymer for cell scaffolds and electrodes. Langmuir. 2006;22(24):9816–9819. doi: 10.1021/la062129d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haan N, Song B. Therapeutic application of electric fields in the injured nervous system. Adv Wound Care (New Rochelle) 2014;3(2):156–165. doi: 10.1089/wound.2013.0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmons S, Ashley Z, Sutherland H, Russold MF, Li F, Jarvis JC. Functional electrical stimulation of denervated muscles: basic issues. Artif Organs. 2005;29(3):199–202. doi: 10.1111/j.1525-1594.2005.29034.x. [DOI] [PubMed] [Google Scholar]
- 40.Akai M, Hayashi K. Effect of electrical stimulation on musculoskeletal systems: a meta-analysis of controlled clinical trials. Bioelectromagnetics. 2002;23(2):132–143. doi: 10.1002/bem.106. [DOI] [PubMed] [Google Scholar]
- 41.Aaron RK, Ciombor DM. Therapeutic effects of electromagnetic fields in the stimulation of connective tissue repair. J Cell Biochem. 1993;52(1):42–46. doi: 10.1002/jcb.240520107. [DOI] [PubMed] [Google Scholar]
- 42.Williams HB. The value of continuous electrical muscle stimulation using a completely implantable system in the preservation of muscle function following motor nerve injury and repair: an experimental study. Microsurgery. 1996;17(11):589–596. doi: 10.1002/(SICI)1098-2752(1996)17:11<589::AID-MICR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 43.Hampton S, King L. Healing an intractable wound using bio-electrical stimulation therapy. Br J Nurs. 2005;14(15):S30–S32. [PubMed] [Google Scholar]
- 44.Araújo R, Franciulli P, Assis R, Souza R, Mochizuki L. Effects of laser, ultrasound and electrical stimulation on the repair of achilles tendon injuries in rats: a comparative study. Braz J Morphol Sci. 2007;24(3):187–191. [Google Scholar]
- 45.Mercóla JM, Kirsch D. The basis for micro current electrical therapy in conventional medical practice. J Advancement Med. 1995;8(2):107–120. [Google Scholar]
- 46.Stanish W, Rubinovich M, Kozey J, MacGillvary G. The use of electricity in ligament and tendon repair. Physician Sports Med. 1985;13:108–116. doi: 10.1080/00913847.1985.11708860. [DOI] [PubMed] [Google Scholar]
- 47.Stanish WD, Lai A. New concepts of rehabilitation following anterior cruciate reconstruction. Clin Sports Med. 1993;12(1):25–58. [PubMed] [Google Scholar]
- 48.Hang J, Kong L, Gu JW, Adair TH. VEGF gene expression is upregulated in electrically stimulated rat skeletal muscle. Am J Physiol. 1995;269(5 Pt 2):H1827–H1831. doi: 10.1152/ajpheart.1995.269.5.H1827. [DOI] [PubMed] [Google Scholar]
- 49.Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol. 1996;81(1):355–361. doi: 10.1152/jappl.1996.81.1.355. [DOI] [PubMed] [Google Scholar]
- 50.Kanno S, Oda N, Abe M, Saito S, Hori K, Handa Y, Tabayashi K, Sato Y. Establishment of a simple and practical procedure applicable to therapeutic angiogenesis. Circulation. 1999;99(20):2682–2687. doi: 10.1161/01.cir.99.20.2682. [DOI] [PubMed] [Google Scholar]
- 51.Annex BH, Torgan CE, Lin P, Taylor DA, Thompson MA, Peters KG, Kraus WE. Induction and maintenance of increased VEGF protein by chronic motor nerve stimulation in skeletal muscle. Am J Physiol. 1998;274(3 Pt 2):H860–H867. doi: 10.1152/ajpheart.1998.274.3.H860. [DOI] [PubMed] [Google Scholar]
- 52.Dawson JM, Hudlicka O. Can changes in microcirculation explain capillary growth in skeletal muscle? Int J Exp Pathol. 1993;74(1):65–71. [PMC free article] [PubMed] [Google Scholar]
- 53.Gavin TP, Spector DA, Wagner H, Breen EC, Wagner PD. Nitric oxide synthase inhibition attenuates the skeletal muscle VEGF mRNA response to exercise. J Appl Physiol. 2000;88(4):1192–1198. doi: 10.1152/jappl.2000.88.4.1192. [DOI] [PubMed] [Google Scholar]
- 54.Hudlicka O, Brown MD, Silgram H. Inhibition of capillary growth in chronically stimulated rat muscles by N(G)-nitro-l-arginine, nitric oxide synthase inhibitor. Microvasc Res. 2000;59(1):45–51. doi: 10.1006/mvre.1999.2193. [DOI] [PubMed] [Google Scholar]
- 55.Machado AFP, Santana EF, Tacani PM, Liebano RE. The effects of transcutaneous electrical nerve stimulation on tissue repair: a literature review. Can J Plast Surg. 2012;20(4):237. [PMC free article] [PubMed] [Google Scholar]
- 56.Haddad JB, Obolensky AG, Shinnick P. The biologic effects and the therapeutic mechanism of action of electric and electromagnetic field stimulation on bone and cartilage: new findings and a review of earlier work. J Altern Complement Med. 2007;13(5):485–490. doi: 10.1089/acm.2007.5270. [DOI] [PubMed] [Google Scholar]
- 57.Sirivisoot S, Pareta R, Harrison BS. Protocol and cell responses in three-dimensional conductive collagen gel scaffolds with conductive polymer nanofibres for tissue regeneration. Interface Focus. 2014;4(1):20130050. doi: 10.1098/rsfs.2013.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pires F, Ferreira Q, Rodrigues CA, Morgado J, Ferreira FC. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochimica Biophysica Acta (BBA)-General Subjects. 2015;1850(6):1158–1168. doi: 10.1016/j.bbagen.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 59.Min Y, Liu Y, Poojari Y, Wu J-C, Hildreth Iii BE, Rosol TJ, Epstein AJ. Self-doped polyaniline-based interdigitated electrodes for electrical stimulation of osteoblast cell lines. Synthetic Metals. 2014;198(0):308–313. [Google Scholar]
- 60.Rajzer I, Rom M, Menaszek E, Pasierb P. Conductive PANI patterns on electrospun PCL/gelatin scaffolds modified with bioactive particles for bone tissue engineering. Mater Lett. 2015;138(0):60–63. [Google Scholar]
- 61.Qazi TH, Rai R, Dippold D, Roether JE, Schubert DW, Rosellini E, Barbani N, Boccaccini AR. Development and characterization of novel electrically conductive PANI-PGS composites for cardiac tissue engineering applications. Acta Biomater. 2014;10(6):2434–2445. doi: 10.1016/j.actbio.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 62.Moura RM, de Queiroz AAA. Dendronized polyaniline nanotubes for cardiac tissue engineering. Artif Org. 2011;35(5):471–477. doi: 10.1111/j.1525-1594.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- 63.Borriello A, Guarino V, Schiavo L, Alvarez-Perez M, Ambrosio L. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. J Mater Sci Mater Med. 2011;22(4):1053–1062. doi: 10.1007/s10856-011-4259-x. [DOI] [PubMed] [Google Scholar]
- 64.Prabhakaran MP, Ghasemi-Mobarakeh L, Jin G, Ramakrishna S. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells. J Biosci Bioeng. 2011;112(5):501–507. doi: 10.1016/j.jbiosc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J, Qiu K, Sun B, Fang J, Zhang K, Hany E-H, Al-Deyab SS, Mo X. The aligned core-sheath nanofibers with electrical conductivity for neural tissue engineering. J Mater Chem B. 2014;2:7945–7954. doi: 10.1039/c4tb01185f. [DOI] [PubMed] [Google Scholar]
- 66.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng Part A. 2009;15(11):3605–3619. doi: 10.1089/ten.TEA.2008.0689. [DOI] [PubMed] [Google Scholar]
- 67.James R, Nagarale RK, Sachan VK, Badalucco C, Bhattacharya PK, Kumbar SG. Synthesis and characterization of electrically conducting polymers for regenerative engineering applications: sulfonated ionic membranes. Polym Advan Technol. 2014;25(12):1439–1445. [Google Scholar]
- 68.Balint R, Cassidy NJ, Cartmell SH. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10(6):2341–2353. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 69.Shiga T. Neutron Spin Echo Spectroscopy Viscoelasticity Rheology. Vol. 134. Berlin: Springer; 1997. Deformation and viscoelastic behavior of polymer gels in electric fields; pp. 131–163. [Google Scholar]
- 70.Pei Q, Inganläs O. Conjugated polymers and the bending cantilever method: electrical muscles and smart devices. Adv Mater. 1992;4(4):277–278. [Google Scholar]
- 71.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 72.Zhang N, Yan H, Wen X. Tissue-engineering approaches for axonal guidance. Brain Res Brain Res Rev. 2005;49(1):48–64. doi: 10.1016/j.brainresrev.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 73.de Ruiter GC, Malessy MJ, Yaszemski MJ, Windebank AJ, Spinner RJ. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus. 2009;26(2):E5. doi: 10.3171/FOC.2009.26.2.E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moroder P, Runge MB, Wang H, Ruesink T, Lu L, Spinner RJ, Windebank AJ, Yaszemski MJ. Material properties and electrical stimulation regimens of polycaprolactone fumarate-polypyrrole scaffolds as potential conductive nerve conduits. Acta Biomater. 2011;7(3):944–953. doi: 10.1016/j.actbio.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang J, Ye Z, Hu X, Lu L, Luo Z. Electrical stimulation induces calcium-dependent release of NGF from cultured Schwann cells. Glia. 2010;58(5):622–631. doi: 10.1002/glia.20951. [DOI] [PubMed] [Google Scholar]
- 76.Luo B, Huang J, Lu L, Hu X, Luo Z, Li M. Electrically induced brain-derived neurotrophic factor release from schwann cells. J Neurosci Res. 2014;92(7):893–903. doi: 10.1002/jnr.23365. [DOI] [PubMed] [Google Scholar]
- 77.Huang J, Hu X, Lu L, Ye Z, Zhang Q, Luo Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J Biomed Mater Res Part A. 2010;93(1):164–174. doi: 10.1002/jbm.a.32511. [DOI] [PubMed] [Google Scholar]
- 78.Lee AC, Vivian MY, Lowe JB, Brenner MJ, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184(1):295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 79.Terris DJ, Toft KM, Moir M, Lum J, Wang M. Brain-derived neurotrophic factor-enriched collagen tubule as a substitute for autologous nerve grafts. Arch Otolaryngol Head Neck Surg. 2001;127(3):294–298. doi: 10.1001/archotol.127.3.294. [DOI] [PubMed] [Google Scholar]
- 80.Mohanna P-N, Terenghi G, Wiberg M. Composite PHB-GGF conduit for long nerve gap repair: a long-term evaluation. Scand J Plast Reconstr Surg Hand Surg. 2005;39(3):129–137. doi: 10.1080/02844310510006295. [DOI] [PubMed] [Google Scholar]
- 81.Xu X, Yu H, Gao S, Mao H-Q, Leong KW, Wang S. Polyphosphoester microspheres for sustained release of biologically active nerve growth factor. Biomaterials. 2002;23(17):3765–3772. doi: 10.1016/s0142-9612(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 82.Ziv-Polat O, Shahar A, Levy I, Skaat H, Neuman S, Fregnan F, Geuna S, Grothe C, Haastert-Talini K, Margel S. The role of neurotrophic factors conjugated to iron oxide nanoparticles in peripheral nerve regeneration: in vitro studies. Biomed Res Int. 2014;2014:267808. doi: 10.1155/2014/267808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang S, Uludağ H. Nanoparticulate systems for growth factor delivery. Pharm Res. 2009;26(7):1561–1580. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- 84.Schlosshauer B, Müller E, Schröder B, Planck H, Müller H-W. Rat Schwann cells in bioresorbable nerve guides to promote and accelerate axonal regeneration. Brain Res. 2003;963(1):321–326. doi: 10.1016/s0006-8993(02)03930-6. [DOI] [PubMed] [Google Scholar]
- 85.Armstrong SJ, Wiberg M, Terenghi G, Kingham PJ. ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng. 2007;13(12):2863–2870. doi: 10.1089/ten.2007.0055. [DOI] [PubMed] [Google Scholar]
- 86.Bell JH, Haycock JW. Next generation nerve guides: materials, fabrication, growth factors, and cell delivery. Tissue Eng Part B. 2012;18(2):116–128. doi: 10.1089/ten.TEB.2011.0498. [DOI] [PubMed] [Google Scholar]
- 87.Lopes FRP, de Moura Campos LC, Corrêa JD, Balduino A, Lora S, Langone F, Borojevic R, Martinez AMB. Bone marrow stromal cells and resorbable collagen guidance tubes enhance sciatic nerve regeneration in mice. Exp Neurol. 2006;198(2):457–468. doi: 10.1016/j.expneurol.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 88.di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011;181:278–291. doi: 10.1016/j.neuroscience.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 89.Santiago LY, Clavijo-Alvarez J, Brayfield C, Rubin JP, Marra KG. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant. 2009;18(2):145–158. doi: 10.3727/096368909788341289. [DOI] [PubMed] [Google Scholar]
- 90.Thrivikraman G, Madras G, Basu B. Intermittent electrical stimuli for guidance of human mesenchymal stem cell lineage commitment towards neural-like cells on electroconductive substrates. Biomaterials. 2014;35(24):6219–6235. doi: 10.1016/j.biomaterials.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 91.Sun S, Titushkin I, Cho M. Regulation of mesenchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry. 2006;69(2):133–141. doi: 10.1016/j.bioelechem.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 92.Yamada E, Hashimoto S, Tachibana K, Okada M, Yamasaki K, Kondo H, Imoto K, Mochizuki S, Fujisato T, Ohsuga M. Effect of electric stimulation on adhesion and proliferation of cultured muscle cells. Proceedings of 12th World Multi-Conference on Systemics Cybernetics and Informatics.2008. [Google Scholar]
- 93.Blank M. Protein and DNA reactions stimulated by electromagnetic fields. Electromagn Biol Med. 2008;27(1):3–23. doi: 10.1080/15368370701878820. [DOI] [PubMed] [Google Scholar]
- 94.Feng JF, Liu J, Zhang XZ, Zhang L, Jiang JY, Nolta J, Zhao M. Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells. 2012;30(2):349–355. doi: 10.1002/stem.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jahanshahi A, Schönfeld L-M, Lemmens E, Hendrix S, Temel Y. In vitro and in vivo neuronal electrotaxis: a potential mechanism for restoration? Mol Neurobiol. 2014;49(2):1005–1016. doi: 10.1007/s12035-013-8575-7. [DOI] [PubMed] [Google Scholar]
- 96.Lu M-C, Tsai C-C, Chen S-C, Tsai F-J, Yao C-H, Chen Y-S. Use of electrical stimulation at different current levels to promote recovery after peripheral nerve injury in rats. J Trauma Acute Care Surg. 2009;67(5):1066–1072. doi: 10.1097/TA.0b013e318182351a. [DOI] [PubMed] [Google Scholar]
- 97.Haastert-Talini K, Schmitte R, Korte N, Klode D, Ratzka A, Grothe C. Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps. J Neurotrauma. 2011;28(4):661–674. doi: 10.1089/neu.2010.1637. [DOI] [PubMed] [Google Scholar]
- 98.Yeh CC, Lin YC, Tsai FJ, Huang CY, Yao C-H, Chen Y-S. Timing of applying electrical stimulation is an important factor deciding the success rate and maturity of regenerating rat sciatic nerves. Neurorehabil Neural Repair. 2010;24(8):730–735. doi: 10.1177/1545968310376758. [DOI] [PubMed] [Google Scholar]
- 99.Huang J, Zhang Y, Lu L, Hu X, Luo Z. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats. Eur J Neurosci. 2013;38(12):3691–3701. doi: 10.1111/ejn.12370. [DOI] [PubMed] [Google Scholar]
- 100.Bakhshi A, Bhalla G. Electrically conducting polymers: Materials of the twentyfirst century. J Sci Indust Res. 2004;63:715–728. [Google Scholar]
- 101.Bredas JL, Street GB. Polarons, bipolarons, and solitons in conducting polymers. Acc Chem Res. 1985;18(10):309–315. [Google Scholar]
- 102.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci U S A. 1997;94(17):8948–8953. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Subramanian A, Krishnan UM, Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: biomaterial mediated neural regeneration. J Biomed Sci. 2009;16(1):108. doi: 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stewart E, Kobayashi NR, Higgins MJ, Quigley AF, Jamali S, Moulton SE, Kapsa RM, Wallace GG, Crook JM. Electrical stimulation using conductive polymer polypyrrole promotes differentiation of human neural stem cells: a biocompatible platform for translational neural tissue engineering. Tissue Eng Part C. 2014;21(4):385–393. doi: 10.1089/ten.TEC.2014.0338. [DOI] [PubMed] [Google Scholar]
- 105.Cao J, Hu G, Peng Z, Du K, Cao Y. Polypyrrole-coated LiCoO2 nanocomposite with enhanced electrochemical properties at high voltage for lithium-ion batteries. J Power Sources. 2015;281(0):49–55. [Google Scholar]
- 106.Fan X, Yang Z, He N. Hierarchical nanostructured polypyrrole/graphene composites as supercapacitor electrode. RSC Adv. 2015;5(20):15096–15102. [Google Scholar]
- 107.Chee W, Lim H, Harrison I, Chong K, Zainal Z, Ng C, Huang N. Performance of flexible and binderless polypyrrole/graphene oxide/zinc oxide supercapacitor electrode in a symmetrical two-electrode configuration. Electrochim Acta. 2015;157(0):88–94. [Google Scholar]
- 108.Zhang Y, Zhen Z, Zhang Z, Lao J, Wei J, Wang K, Kang F, Zhu H. In situ synthesis of carbon nanotube/ graphene composite sponge and its application as compressible supercapacitor electrode. Electrochim Acta. 2015;157(0):134–141. [Google Scholar]
- 109.Xu J, Wang D, Yuan Y, Wei W, Gu S, Liu R, Wang X, Liu L, Xu W. Polypyrrole-coated cotton fabrics for flexible supercapacitor electrodes prepared using CuO nanoparticles as template. Cellulose. 2015;22(2):1355–1363. [Google Scholar]
- 110.Ayenimo JG, Adeloju SB. Inhibitive potentiometric detection of trace metals with ultrathin polypyrrole glucose oxidase biosensor. Talanta. 2015;137:62–70. doi: 10.1016/j.talanta.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 111.Seven B, Bourourou M, Elouarzaki K, Constant JF, Gondran C, Holzinger M, Cosnier S, Timur S. Impedimetric biosensor for cancer cell detection. Electrochem Commun. 2013;37:36–39. [Google Scholar]
- 112.Shamaeli E, Alizadeh N. Functionalized gold nanoparticle-polypyrrole nanobiocomposite with high effective surface area for electrochemical/pH dual stimuli-responsive smart release of insulin. Colloids Surf B. 2015;126:502–509. doi: 10.1016/j.colsurfb.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 113.Lee JY, Lee J-W, Schmidt CE. Neuroactive conducting scaffolds: nerve growth factor conjugation on active ester-functionalized polypyrrole. J R Soc Interface. 2008;6(38):801–810. doi: 10.1098/rsif.2008.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu Q, Xu S, Zhang K, Shan Y. Multi-porous electroactive poly(L-lactic acid)/polypyrrole composite micro/nano fibrous scaffolds promote neurite outgrowth in PC12 cells. Neural Regen Res. 2013;8(1):31–38. doi: 10.3969/j.issn.1673-5374.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nguyen HT, Sapp S, Wei C, Chow JK, Nguyen A, Coursen J, Luebben S, Chang E, Ross R, Schmidt CE. Electric field stimulation through a biodegradable polypyrrole-co-polycaprolactone substrate enhances neural cell growth. J Biomed Mater Res A. 2014;102(8):2554–2564. doi: 10.1002/jbm.a.34925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meng S, Rouabhia M, Zhang Z. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds. Bioelectromagnetics. 2013;34(3):189–199. doi: 10.1002/bem.21766. [DOI] [PubMed] [Google Scholar]
- 117.Pelto J, Björninen M, Pälli A, Talvitie E, Hyttinen J, Mannerström B, Suuronen Seppanen R, Kellomäki M, Miettinen S, Haimi S. Novel polypyrrole-coated polylactide scaffolds enhance adipose stem cell proliferation and early osteogenic differentiation. Tissue Eng Part A. 2013;19(7–8):882–892. doi: 10.1089/ten.tea.2012.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chu X-H, Xu Q, Feng Z-Q, Xiao J-Q, Li Q, Sun X-T, Cao Y, Ding Y-T. In vitro biocompatibility of polypyrrole/PLGA conductive nanofiber scaffold with cultured rat hepatocytes. Mater Res Exp. 2014;1(3):035402. [Google Scholar]
- 119.Ruhparwar A, Piontek P, Ungerer M, Ghodsizad A, Partovi S, Foroughi J, Szabo G, Farag M, Karck M, Spinks GM. Electrically contractile polymers augment right ventricular output in the heart. Artif Organs. 2014;38(12):1034–1039. doi: 10.1111/aor.12300. [DOI] [PubMed] [Google Scholar]
- 120.Efimov ON. Polypyrrole: a conducting polymer; its synthesis, properties and applications. Russian Chemical Reviews. 1997;66(5):443–457. [Google Scholar]
- 121.Wang X, Gu X, Yuan C, Chen S, Zhang P, Zhang T, Yao J, Chen F, Chen G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J Biomed Mater Res A. 2004;68(3):411–422. doi: 10.1002/jbm.a.20065. [DOI] [PubMed] [Google Scholar]
- 122.Forciniti L, Ybarra J, 3rd, Zaman MH, Schmidt CE. Schwann cell response on polypyrrole substrates upon electrical stimulation. Acta Biomater. 2014;10(6):2423–2433. doi: 10.1016/j.actbio.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 123.George PM, Saigal R, Lawlor MW, Moore MJ, LaVan DA, Marini RP, Selig M, Makhni M, Burdick JA, Langer R, Kohane DS. Three-dimensional conductive constructs for nerve regeneration. J Biomed Mater Res A. 2009;91(2):519–527. doi: 10.1002/jbm.a.32226. [DOI] [PubMed] [Google Scholar]
- 124.Shi Z, Gao H, Feng J, Ding B, Cao X, Kuga S, Wang Y, Zhang L, Cai J. In situ synthesis of robust conductive cellulose/polypyrrole composite aerogels and their potential application in nerve regeneration. Angew Chem. 2014;126(21):5484–5488. doi: 10.1002/anie.201402751. [DOI] [PubMed] [Google Scholar]
- 125.Yunusa Z, Hamidon MN, Ismail A, Isa MM, Yaacob MH, Rahmanian S, Ibrahim SA, Shabaneh AA. Development of a hydrogen gas sensor using a double saw resonator system at room temperature. Sensors. 2015;15(3):4749–4765. doi: 10.3390/s150304749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bai S, Sun C, Wan P, Wang C, Luo R, Li Y, Liu J, Sun X. Transparent conducting films of hierarchically nanostructured polyaniline networks on flexible substrates for high-performance gas sensors. Small. 2015;11(3):306–310. doi: 10.1002/smll.201401865. [DOI] [PubMed] [Google Scholar]
- 127.Chen D, Lei S, Chen Y. A single polyaniline nanofiber field effect transistor and its gas sensing mechanisms. Sensors. 2011;11(7):6509–6516. doi: 10.3390/s110706509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lim JW, Haq AU, Kim SO. Direct growth of polyaniline chains from nitrogen site of N-doped carbon nanotubes for high performance supercapacitor. Adv Sci Technol. 2014;93:164–167. [Google Scholar]
- 129.Huang H, Gan M, Ma L, Yu L, Hu H, Yang F, Li Y, Ge C. Fabrication of polyaniline/graphene/titania nanotube arrays nanocomposite and their application in supercapacitors. J Alloys Compd. 2015;630(0):214–221. [Google Scholar]
- 130.Lee S-N, Baek S, Amaresh S, Aravindan V, Chung KY, Cho BW, Yoon W-S, Lee Y-S. Cu-Li2MnSiO4-polyaniline composite hybrids as high performance cathode for lithium batteries. J Alloy Compd. 2015;630(0):292–298. [Google Scholar]
- 131.Tang X, Tian M, Qu L, Zhu S, Guo X, Han G, Sun K, Hu X, Wang Y, Xu X. Functionalization of cotton fabric with graphene oxide nanosheet and polyaniline for conductive and UV blocking properties. Synt Met. 2015;202(0):82–88. [Google Scholar]
- 132.Baniasadi H, Ramazani SA, Mashayekhan S. Fabrication and Characterization of Conductive Chitosan/Gelatin-based Scaffolds for Nerve Tissue Engineering. Int J Biol Macromol. 2015;74(0):360–366. doi: 10.1016/j.ijbiomac.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 133.Chen M-C, Sun Y-C, Chen Y-H. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013;9(3):5562–5572. doi: 10.1016/j.actbio.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 134.Guarino V, Alvarez-Perez MA, Borriello A, Napolitano T, Ambrosio L. Conductive PANi/PEGDA macroporous hydrogels for nerve regeneration. Adv Healthc Mater. 2013;2(1):218–227. doi: 10.1002/adhm.201200152. [DOI] [PubMed] [Google Scholar]
- 135.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36(4):568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 136.Abdallah B, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Therapy. 2008;15(2):109–116. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- 137.Boehler C, Asplund M. A detailed insight into drug delivery from PEDOT based on analytical methods: Effects and side effects. J Biomed Mater Res A. 2015;103(3):1200–1207. doi: 10.1002/jbm.a.35252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ganeshan D, Chen S-C, Yin Z, Zheng Q. An anode buffer layer with size-controlled Ag nanoparticles for polymer solar cells with improved efficiencies. RSC Adv. 2015;5(21):16153–16161. [Google Scholar]
- 139.Chen P-Y, Li C-T, Lee C-P, Vittal R, Ho K-C. PEDOT-decorated nitrogen-doped graphene as the transparent composite film for the counter electrode of a dye-sensitized solar cell. Nano Energy. 2015;12(0):374–385. [Google Scholar]
- 140.Xiao S, Chen L, Tan L, Huang L, Wu F, Chen Y. Sulfonate Poly(aryl ether sulfone)-Modified PEDOT:PSS as hole transport layer and transparent electrode for high performance polymer solar cells. J Phys Chem C. 2015;119(4):1943–1952. [Google Scholar]
- 141.Wang H, Kumar V. Transparent and conductive polysiloxanes/PEDOT: PSS nanocomposite thin films with a “water-impermeable” property to significantly enhance stability of organic-inorganic hybrid solar cells. RSC Adv. 2015;5(13):9650–9657. [Google Scholar]
- 142.Shah MSAS, Muhammad S, Park JH, Yoon W-S, Yoo PJ. Incorporation of PEDOT: PSS into SnO2/reduced graphene oxide nanocomposite anodes for lithium-ion battery to achieve ultra-high capacity and cyclic stability. RSC Adv. 2015;5:13964–13971. [Google Scholar]
- 143.Kim H, Lee Y-J, Park G-G, Park S-H, Choi Y-Y, Yoo Y. Fabrication of carbon paper containing PEDOT: PSS for use as a gas diffusion layer in proton exchange membrane fuel cells. Carbon. 2015;85:422–428. [Google Scholar]
- 144.Shahini A, Yazdimamaghani M, Walker KJ, Eastman MA, Hatami-Marbini H, Smith BJ, Ricci JL, Madihally SV, Vashaee D, Tayebi L. 3D conductive nanocomposite scaffold for bone tissue engineering. Int J Nanomed. 2014;9:167–181. doi: 10.2147/IJN.S54668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tahmasbi Rad A, Ali N, Kotturi HSR, Yazdimamaghani M, Smay J, Vashaee D, Tayebi L. Conducting scaffolds for liver tissue engineering. J Biomed Mater Res A. 2014;102(11):4169–4181. doi: 10.1002/jbm.a.35080. [DOI] [PubMed] [Google Scholar]
- 146.Abidian MR, Daneshvar ED, Egeland BM, Kipke DR, Cederna PS, Urbanchek MG. Hybrid conducting polymer-hydrogel conduits for axonal growth and neural tissue engineering. Adv Healthc Mater. 2012;1(6):762–767. doi: 10.1002/adhm.201200182. [DOI] [PubMed] [Google Scholar]
- 147.Kung TA, Langhals NB, Martin DC, Johnson PJ, Cederna PS, Urbanchek MG. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast Reconstr Surg. 2014;133(6):1380–1394. doi: 10.1097/PRS.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 148.Wrobel MR, Sundararaghavan HG. Directed migration in neural tissue engineering. Tissue Eng Part B Rev. 2013;20(2):93–105. doi: 10.1089/ten.TEB.2013.0233. [DOI] [PubMed] [Google Scholar]
- 149.Menorca RM, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29(3):317–330. doi: 10.1016/j.hcl.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ribeiro-Resende VT, Koenig B, Nichterwitz S, Oberhoffner S, Schlosshauer B. Strategies for inducing the formation of bands of Büngner in peripheral nerve regeneration. Biomater. 2009;30(29):5251–5259. doi: 10.1016/j.biomaterials.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 151.Koppes AN, Nordberg AL, Paolillo GM, Goodsell NM, Darwish HA, Zhang L, Thompson DM. Electrical stimulation of Schwann cells promotes sustained increases in neurite outgrowth. Tissue Eng Part A. 2013;20(3-4):494–506. doi: 10.1089/ten.tea.2013.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jiang X, Lim SH, Mao H-Q, Chew SY. Current applications and future perspectives of artificial nerve conduits. Exp Neurol. 2010;223(1):86–101. doi: 10.1016/j.expneurol.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 153.Dai L-G, Huang G-S, Hsu S-h. Sciatic nerve regeneration by cocultured Schwann cells and stem cells on microporous nerve conduits. Cell Transplant. 2013;22(11):2029–2039. doi: 10.3727/096368912X658953. [DOI] [PubMed] [Google Scholar]
- 154.Rodrigues MCO, Rodrigues AA, Glover LE, Voltarelli J, Borlongan CV. Peripheral nerve repair with cultured schwann cells: getting closer to the clinics. Scientific World J. 2012;2012:1–10. doi: 10.1100/2012/413091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Baek S, Green RA, Poole-Warren LA. Effects of dopants on the biomechanical properties of conducting polymer films on platinum electrodes. J Biomed Mater Res A. 2014;102(8):2743–2754. doi: 10.1002/jbm.a.34945. [DOI] [PubMed] [Google Scholar]
- 156.Fonner JM, Forciniti L, Nguyen H, Byrne JD, Kou Y-F, Syeda-Nawaz J, Schmidt CE. Biocompatibility implications of polypyrrole synthesis techniques. Biomed Mater. 2008;3(3):034124. doi: 10.1088/1748-6041/3/3/034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8(110):1–13. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hudson TW, Evans GR, Schmidt CE. Engineering strategies for peripheral nerve repair. Clin Plast Surg. 1999;26(4):617–628. ix. [PubMed] [Google Scholar]
- 159.Oh SH, Kim JH, Song KS, Jeon BH, Yoon JH, Seo TB, Namgung U, Lee IW, Lee JH. Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials. 2008;29(11):1601–1609. doi: 10.1016/j.biomaterials.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 160.Bini T, Gao S, Xu X, Wang S, Ramakrishna S, Leong K. Peripheral nerve regeneration by microbraided poly (L-lactide-co-glycolide) biodegradable polymer fibers. J Biomed Mater Res A. 2004;68(2):286–295. doi: 10.1002/jbm.a.20050. [DOI] [PubMed] [Google Scholar]
- 161.Evans G, Brandt K, Widmer M, Lu L, Meszlenyi R, Gupta P, Mikos A, Hodges J, Williams J, Gürlek A. In vivo evaluation of poly (L-lactic acid) porous conduits for peripheral nerve regeneration. Biomaterials. 1999;20(12):1109–1115. doi: 10.1016/s0142-9612(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 162.Chang CJ. Effects of nerve growth factor from genipin-crosslinked gelatin in polycaprolactone conduit on peripheral nerve regeneration—in vitro and in vivo. J Biomed Mater Res A. 2009;91(2):586–596. doi: 10.1002/jbm.a.32252. [DOI] [PubMed] [Google Scholar]
- 163.Yan Q, Yin Y, Li B. Use new PLGL-RGD-NGF nerve conduits for promoting peripheral nerve regeneration. Biomed Eng Online. 2012;11(36):1–16. doi: 10.1186/1475-925X-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chang C-J. The effect of pulse-released nerve growth factor from genipin-crosslinked gelatin in Schwann cell–seeded polycaprolactone conduits on large-gap peripheral nerve regeneration. Tissue Eng Part A. 2008;15(3):547–557. doi: 10.1089/ten.tea.2007.0342. [DOI] [PubMed] [Google Scholar]
- 165.Fu KY, Dai LG, Chiu IM, Chen JR, Hsu SH. Sciatic nerve regeneration by microporous nerve conduits seeded with glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor gene transfected neural stem cells. Artif Organs. 2011;35(4):363–372. doi: 10.1111/j.1525-1594.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- 166.Frattini F, Lopes FR, Almeida FM, Rodrigues RF, Boldrini LC, Tomaz MA, Baptista AF, Melo PA, Martinez AM. Mesenchymal stem cells in a polycaprolactone conduit promote sciatic nerve regeneration and sensory neuron survival after nerve injury. Tissue Eng Part A. 2012;18(19–20):2030–2039. doi: 10.1089/ten.TEA.2011.0496. [DOI] [PubMed] [Google Scholar]
- 167.Daly WT, Yao L, Abu-rub MT, O’Connell C, Zeugolis DI, Windebank AJ, Pandit AS. The effect of intraluminal contact mediated guidance signals on axonal mismatch during peripheral nerve repair. Biomaterials. 2012;33(28):6660–6671. doi: 10.1016/j.biomaterials.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 168.Koh H, Yong T, Teo W, Chan C, Puhaindran M, Tan T, Lim A, Lim B, Ramakrishna S. In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration. J Neural Eng. 2010;7(4):046003. doi: 10.1088/1741-2560/7/4/046003. [DOI] [PubMed] [Google Scholar]
- 169.Elzinga K, Tyreman N, Ladak A, Savaryn B, Olson J, Gordon T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp Neurol. 2015;269:142–153. doi: 10.1016/j.expneurol.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 170.Huang J, Lu L, Zhang J, Hu X, Zhang Y, Liang W, Wu S, Luo Z. Electrical stimulation to conductive scaffold promotes axonal regeneration and remyelination in a rat model of large nerve defect. PLoS One. 2012;7(6):e39526. doi: 10.1371/journal.pone.0039526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cullen DK, Patel AR, Doorish JF, Smith DH, Pfister BJ. Developing a tissue-engineered neural-electrical relay using encapsulated neuronal constructs on conducting polymer fibers. J Neural Eng. 2008;5(4):374–384. doi: 10.1088/1741-2560/5/4/002. [DOI] [PubMed] [Google Scholar]