Abstract
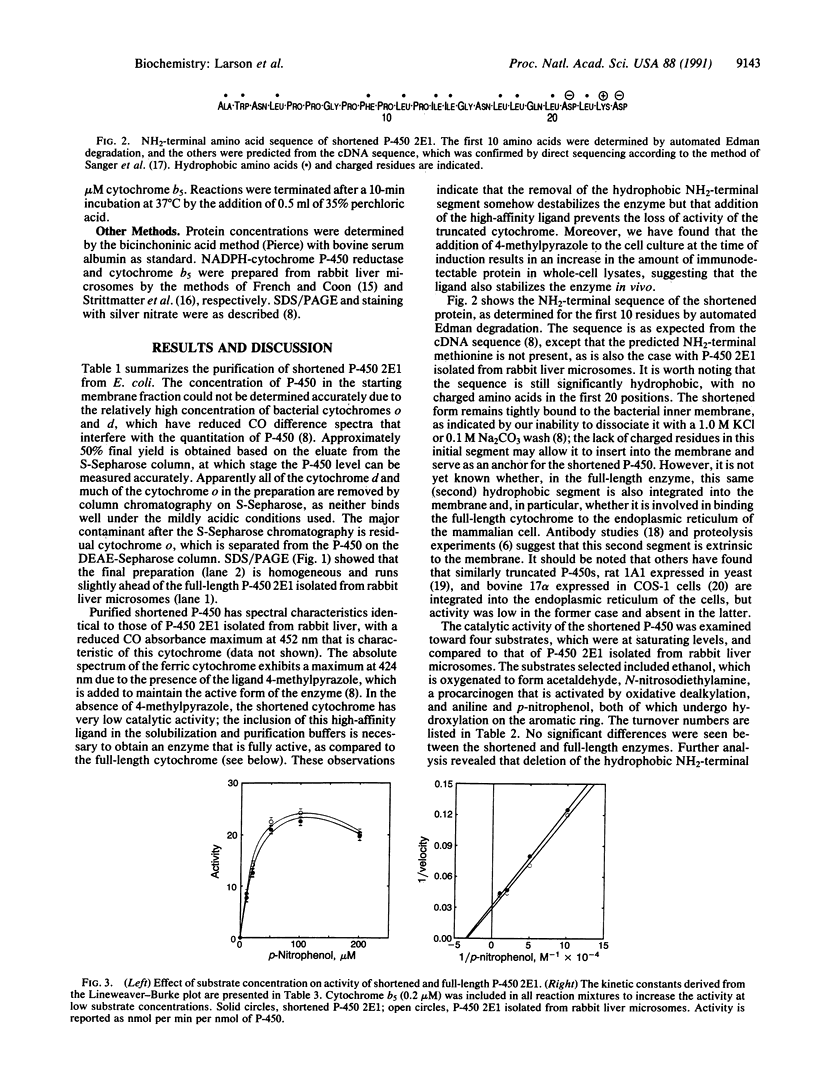
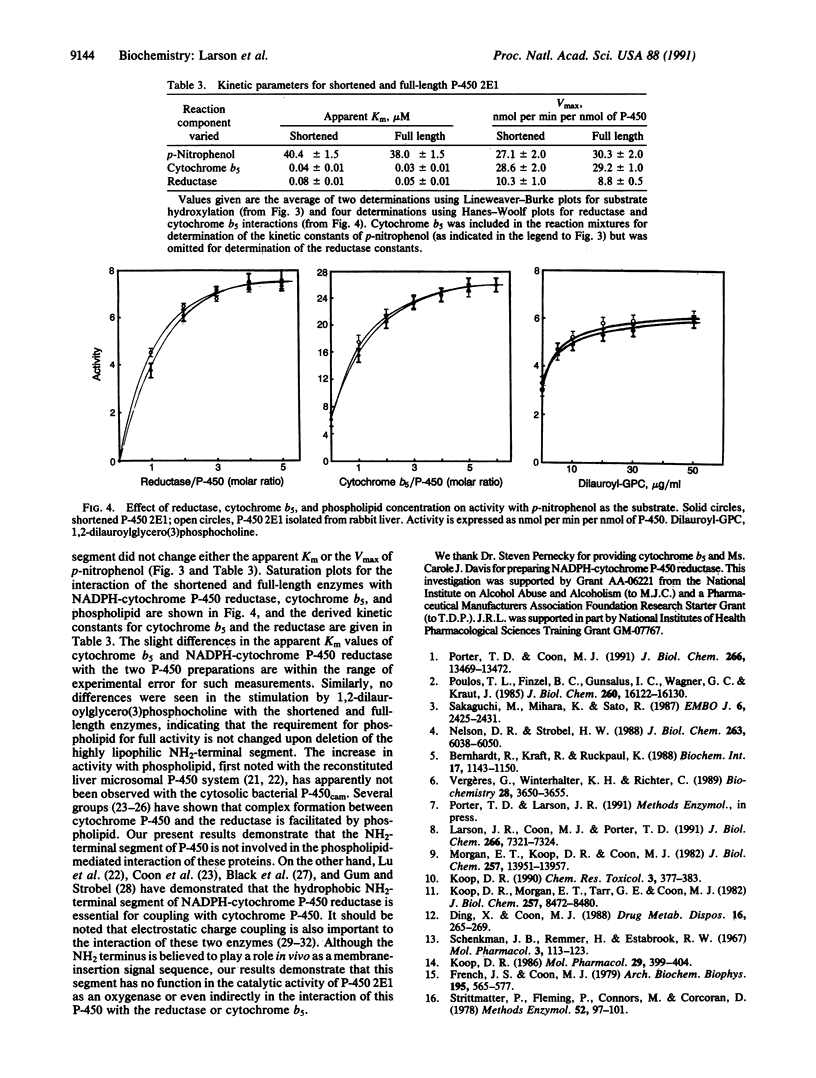
As reported previously, alcohol-inducible cytochrome P-450 2E1 lacking the hydrophobic NH2-terminal segment is located primarily in the inner cell membrane when expressed in Escherichia coli and is active with a typical substrate. To study the catalytic properties in detail, we have purified the truncated P-450 lacking residues 3-29 to electrophoretic homogeneity from the solubilized bacterial membrane fraction in the presence of 4-methylpyrazole as a stabilizing agent. The resulting heme protein with a specific content of 15.8 nmol of P-450 per mg of protein has a reduced CO difference spectrum identical to that of the full-length enzyme, with a Soret maximum at 452 nm. The rates of catalysis of four reactions in the reconstituted enzyme system, including the oxygenation of ethanol to give acetaldehyde, the oxidative dealkylation of N-nitrosodiethylamine to give ethylene and acetaldehyde, and the ring hydroxylation of aniline and p-nitrophenol, are the same with the shortened and full-length enzymes. The apparent Km of p-nitrophenol is also the same, as is that for NADPH-cytochrome P-450 reductase and for cytochrome b5, which stimulates p-nitrocatechol formation about 3-fold. Moreover, the requirement for phosphatidylcholine for full catalytic activity is unchanged despite the absence of the NH2-terminal segment. Although this highly hydrophobic segment is believed to play a role in the intact cell as a membrane-insertion signal sequence, we conclude that it has no function in the catalytic activity of the cytochrome as an oxygenase, including interactions with the other components of the enzyme system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhardt R., Kraft R., Otto A., Ruckpaul K. Electrostatic interactions between cytochrome P-450 LM2 and NADPH-cytochrome P-450 reductase. Biomed Biochim Acta. 1988;47(7):581–592. [PubMed] [Google Scholar]
- Bernhardt R., Kraft R., Ruckpaul K. A simple determination of the sideness of the NH2-terminus in the membrane bound cytochrome P-450 LM2. Biochem Int. 1988 Dec;17(6):1143–1150. [PubMed] [Google Scholar]
- Black S. D., French J. S., Williams C. H., Jr, Coon M. J. Role of a hydrophobic polypeptide in the N-terminal region of NADPH-cytochrome P-450 reductase in complex formation with P-450LM. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1528–1535. doi: 10.1016/0006-291x(79)91238-5. [DOI] [PubMed] [Google Scholar]
- Causey K. M., Eyer C. S., Backes W. L. Dual role of phospholipid in the reconstitution of cytochrome P-450 LM2-dependent activities. Mol Pharmacol. 1990 Jul;38(1):134–142. [PubMed] [Google Scholar]
- Clark B. J., Waterman M. R. The hydrophobic amino-terminal sequence of bovine 17 alpha-hydroxylase is required for the expression of a functional hemoprotein in COS 1 cells. J Biol Chem. 1991 Mar 25;266(9):5898–5904. [PubMed] [Google Scholar]
- De Lemos-Chiarandini C., Frey A. B., Sabatini D. D., Kreibich G. Determination of the membrane topology of the phenobarbital-inducible rat liver cytochrome P-450 isoenzyme PB-4 using site-specific antibodies. J Cell Biol. 1987 Feb;104(2):209–219. doi: 10.1083/jcb.104.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X. X., Coon M. J. Cytochrome P-450-dependent formation of ethylene from N-nitrosoethylamines. Drug Metab Dispos. 1988 Mar-Apr;16(2):265–269. [PubMed] [Google Scholar]
- French J. S., Coon M. J. Properties of NADPH-cytochrome P-450 reductase purified from rabbit liver microsomes. Arch Biochem Biophys. 1979 Jul;195(2):565–577. doi: 10.1016/0003-9861(79)90383-7. [DOI] [PubMed] [Google Scholar]
- French J. S., Guengerich F. P., Coon M. J. Interactions of cytochrome P-450, NADPH-cytochrome P-450 reductase, phospholipid, and substrate in the reconstituted liver microsomal enzyme system. J Biol Chem. 1980 May 10;255(9):4112–4119. [PubMed] [Google Scholar]
- Gum J. R., Strobel H. W. Isolation of the membrane-binding peptide of NADPH-cytochrome P-450 reductase. Characterization of the peptide and its role in the interaction of reductase with cytochrome P-450. J Biol Chem. 1981 Jul 25;256(14):7478–7486. [PubMed] [Google Scholar]
- Koop D. R. Hydroxylation of p-nitrophenol by rabbit ethanol-inducible cytochrome P-450 isozyme 3a. Mol Pharmacol. 1986 Apr;29(4):399–404. [PubMed] [Google Scholar]
- Koop D. R. Inhibition of ethanol-inducible cytochrome P450IIE1 by 3-amino-1,2,4-triazole. Chem Res Toxicol. 1990 Jul-Aug;3(4):377–383. doi: 10.1021/tx00016a017. [DOI] [PubMed] [Google Scholar]
- Koop D. R., Morgan E. T., Tarr G. E., Coon M. J. Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits. J Biol Chem. 1982 Jul 25;257(14):8472–8480. [PubMed] [Google Scholar]
- Larson J. R., Coon M. J., Porter T. D. Alcohol-inducible cytochrome P-450IIE1 lacking the hydrophobic NH2-terminal segment retains catalytic activity and is membrane-bound when expressed in Escherichia coli. J Biol Chem. 1991 Apr 25;266(12):7321–7324. [PubMed] [Google Scholar]
- Lu A. Y., Junk K. W., Coon M. J. Resolution of the cytochrome P-450-containing omega-hydroxylation system of liver microsomes into three components. J Biol Chem. 1969 Jul 10;244(13):3714–3721. [PubMed] [Google Scholar]
- Morgan E. T., Koop D. R., Coon M. J. Catalytic activity of cytochrome P-450 isozyme 3a isolated from liver microsomes of ethanol-treated rabbits. Oxidation of alcohols. J Biol Chem. 1982 Dec 10;257(23):13951–13957. [PubMed] [Google Scholar]
- Müller-Enoch D., Churchill P., Fleischer S., Guengerich F. P. Interaction of liver microsomal cytochrome P-450 and NADPH-cytochrome P-450 reductase in the presence and absence of lipid. J Biol Chem. 1984 Jul 10;259(13):8174–8182. [PubMed] [Google Scholar]
- Nadler S. G., Strobel H. W. Role of electrostatic interactions in the reaction of NADPH-cytochrome P-450 reductase with cytochromes P-450. Arch Biochem Biophys. 1988 Mar;261(2):418–429. doi: 10.1016/0003-9861(88)90358-x. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Strobel H. W. On the membrane topology of vertebrate cytochrome P-450 proteins. J Biol Chem. 1988 May 5;263(13):6038–6050. [PubMed] [Google Scholar]
- Porter T. D., Coon M. J. Cytochrome P-450. Multiplicity of isoforms, substrates, and catalytic and regulatory mechanisms. J Biol Chem. 1991 Jul 25;266(21):13469–13472. [PubMed] [Google Scholar]
- Poulos T. L., Finzel B. C., Gunsalus I. C., Wagner G. C., Kraut J. The 2.6-A crystal structure of Pseudomonas putida cytochrome P-450. J Biol Chem. 1985 Dec 25;260(30):16122–16130. [PubMed] [Google Scholar]
- Sakaguchi M., Mihara K., Sato R. A short amino-terminal segment of microsomal cytochrome P-450 functions both as an insertion signal and as a stop-transfer sequence. EMBO J. 1987 Aug;6(8):2425–2431. doi: 10.1002/j.1460-2075.1987.tb02521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Tateishi T., Hatano M., Fujii-Kuriyama Y. Probing the role of lysines and arginines in the catalytic function of cytochrome P450d by site-directed mutagenesis. Interaction with NADPH-cytochrome P450 reductase. J Biol Chem. 1991 Feb 25;266(6):3372–3375. [PubMed] [Google Scholar]
- Strittmatter P., Fleming P., Connors M., Corcoran D. Purification of cytochrome b5. Methods Enzymol. 1978;52:97–101. doi: 10.1016/s0076-6879(78)52010-7. [DOI] [PubMed] [Google Scholar]
- Tamburini P. P., Schenkman J. B. Differences in the mechanism of functional interaction between NADPH-cytochrome P-450 reductase and its redox partners. Mol Pharmacol. 1986 Aug;30(2):178–185. [PubMed] [Google Scholar]
- Vergères G., Winterhalter K. H., Richter C. Identification of the membrane anchor of microsomal rat liver cytochrome P-450. Biochemistry. 1989 May 2;28(9):3650–3655. doi: 10.1021/bi00435a005. [DOI] [PubMed] [Google Scholar]
- Yabusaki Y., Murakami H., Sakaki T., Shibata M., Ohkawa H. Genetically engineered modification of P450 monooxygenases: functional analysis of the amino-terminal hydrophobic region and hinge region of the P450/reductase fused enzyme. DNA. 1988 Dec;7(10):701–711. doi: 10.1089/dna.1988.7.701. [DOI] [PubMed] [Google Scholar]




