Abstract
Aims. To investigate how circulating microvesicle phenotypes correlate with insulin sensitivity, body composition, plasma lipids, and hepatic fat accumulation. We hypothesized that changes elicited by testosterone replacement therapy are reflected in levels of microvesicles. Methods. Thirty-nine type 2 diabetic males with lowered testosterone levels were assigned to either testosterone replacement therapy or placebo and evaluated at baseline and after 24 weeks. Microvesicles were analysed by flow cytometry and defined as lactadherin-binding particles within the 0.1–1.0 μm gate. Microvesicles of platelet, monocyte, and endothelial cell origin were identified by cell-specific markers and their expression of CD36 was investigated. Results. Triglycerides correlated positively with all investigated microvesicle phenotypes in this study (p < 0.05), and indicators of hepatic fat accumulation, alanine aminotransferase, and gamma glutamyltransferase correlated with platelet and endothelial microvesicles and CD36-expressing microvesicles from platelets and monocytes (p < 0.05). BMI, waist circumference, and fat percentage correlated with CD36-expressing monocyte microvesicles (p < 0.05), while insulin sensitivity did not correlate with any microvesicle phenotypes. Microvesicle levels were unaffected by testosterone therapy. Conclusions. Metabolic syndrome components and hepatic fat accumulation correlated with microvesicle phenotypes, supporting the involvement of especially CD36 on monocytes in metabolic syndrome pathogenesis. Although testosterone therapy improved body composition measures, microvesicle phenotype levels were unaffected. This trial was registered at ClinicalTrials.gov (NCT01560546).
1. Introduction
The metabolic syndrome (MetSy) is constituted by a range of metabolic abnormalities including abdominal adiposity, insulin resistance, hypertension, and dyslipidaemia [1], all of which are associated with an increased risk for type 2 diabetes mellitus (T2D) [2] and cardiovascular disease (CVD) [3]. Given its increasing prevalence and the morbidity and mortality associated with MetSy, there is an urgent need to understand the underlying mechanisms of MetSy.
It is commonly agreed upon that insulin resistance is the central feature in describing the pathophysiology of MetSy [1]. The development of insulin resistance seems highly attributable to an overabundance of circulating fatty acids and ectopic lipid storage [4] leading to inflammation and oxidative stress [5]. Collectively, these alterations contribute to the development of the structural anomalies and cardiovascular complications associated with MetSy, including endothelial dysfunction, atherosclerosis, and thrombosis. Furthermore, low testosterone and sex hormone-binding globulin in men has been linked with the MetSy and T2D [6]. Testosterone replacement therapy (TRT) has previously been demonstrated to improve lean body mass, total fat mass, total cholesterol, and LDL cholesterol in men with T2D [7]. However, a more recent trial could not demonstrate any improvements in the plasma lipid profile nor glycaemic control in T2D patients receiving TRT [8]. Thus, the cardiometabolic benefits with special emphasis on ectopic lipid deposition of TRT remain uncertain.
Changes in levels of certain subgroups of circulating microvesicles (MVs) have previously been ascribed to MetSy [9] and several of its components including insulin resistance, hypertension, hyperlipidaemia, oxidative stress, and waist circumference [10]. MVs are a heterogeneous group of membrane-encapsulated particles that contain cellular components and are released by cells in latent, activated, and apoptotic states [11]. Therefore, a rapidly growing number of studies have recently started to recognize the role of MVs and their potential as biomarkers in a number of diseases. In addition, CD36 is a scavenger receptor and a fatty acid transporter present in most cell phenotypes including platelets [12], endothelial cells [13], and monocytes [14]. Increased levels of circulating CD36 have previously been associated with unhealthy fat distribution [15], atherosclerosis [16], insulin resistance [17], and fatty liver [18], all attributable to ectopic deposition of fat. Thus, measuring the expression of CD36 on MVs could yield important information regarding improvements in the abovementioned components.
The aim of this study was to address the hypotheses that levels of specific phenotypes of MVs correlate with components of MetSy and markers for hepatic fat accumulation and that improvements elicited by TRT affect MV phenotypes in aging, testosterone deficient males with T2D. Testosterone deficient adult males diagnosed with T2D were recruited and randomly assigned to TRT or placebo groups. Circulating MVs of platelet, endothelial, and monocyte origin were quantified in peripheral blood, and their expression of surface CD36 was determined as a surrogate marker for ectopic fat accumulation. Potential relationships were investigated between levels of MV subpopulations and body composition, hepatic fat accumulation, plasma lipids, and insulin resistance prior to the treatment regime, and changes in the levels of MV phenotypes over the trial period were finally compared between TRT and placebo groups.
2. Methods
2.1. Ethical Aspects
The present study was registered at ClinicalTrials.gov (NCT01560546) and approved by the local ethics committee and the Danish Health and Medicines Authority. All patients gave written informed consent prior to inclusion.
2.2. Subjects
This study was conducted on thirty-nine Caucasian men between 50 and 70 years of age, diagnosed with type 2 diabetes within the past ten years, treated with Metformin for ≥3 months, and with bioavailable testosterone levels <7.3 nmol/L. Exclusion criteria were glycated haemoglobin (HbA1C) ≥ 9.0%, BMI ≥ 40 kg/m2, severe hypertension, cardiovascular, lung, or kidney disease, primary or secondary hypogonadism, known malignant disease, haematocrit > 50%, PSA > 3 μg/L, abnormalities in routine blood tests, nocturia > 3 times/night, significant ECG abnormalities, severe active mental disease, current or previous drug abuse, or the desire to have children [8].
2.3. Study Design
This trial was double-blinded, randomized, and placebo-controlled by design and has been described previously [8]. Briefly, subjects were randomly assigned to either TRT or placebo groups and subjected to a 24-week treatment regime with either Testim® 1% testosterone gel or placebo, respectively. Testim 1% testosterone gel, a transdermal testosterone preparation, was supplied in tubes containing 50 mg testosterone each and applied to the shoulders and upper arms at the same time of day throughout the trial period. The initial dosage was 50 mg/day and was adjusted according to plasma testosterone concentration after three weeks to a maximum of 100 mg/day. Subjects were evaluated at baseline and after 24 weeks. During each visit, height, weight, waist and hip circumference, blood pressure, and biochemical characteristics were recorded, and venous blood samples were collected in the fasting state for flow cytometric analysis of MVs. In addition regional body composition was determined by dual energy X-ray absorptiometry (DXA). Insulin sensitivity was determined with the euglycaemic, hyperinsulinaemic clamp technique.
2.4. Fluorescent Labelling of MVs
Blood samples for flow cytometric analysis of MVs were collected into tubes containing sodium citrate anticoagulant at a 3.2% (0.105 M) final concentration and the first centrifugation cycle was initiated within one hour after collection. Samples were subjected to a two-step centrifugation protocol to obtain platelet-free plasma (PFP): an initial centrifugation step at 1800 ×g for 10 minutes at room temperature, followed by an additional centrifugation step of the supernatant at 3000 ×g for 15 minutes. PFP was stored at −80°C until analysis. 50 μL of freshly thawed plasma was transferred to TruCount® tubes (BD Bioscience, NJ, USA) in order to allow quantitation of measured events. Samples were incubated for 30 minutes at 4°C in the dark with 5 μL fluorescein isothiocyanate- (FITC-) conjugated bovine lactadherin (83 μg/mL, Hematologic Technologies Inc., VT, USA), 3 μL allophycocyanin- (APC-) conjugated anti-human CD41 (6 μg/mL IgG1, κ (clone HIP8, BioLegend, San Diego, CA, USA)), 10 μL phycoerythrin- (PE-) conjugated anti-human CD14 (60 μg/mL IgG2a (clone TÜK4, DAKO, Denmark)), 20 μL PE-conjugated anti-human CD62E (100 μg/mL IgG1, κ (clone 68-5H11, BD Pharmingen, New Jersey, USA)), and either 4 μL phycoerythrin- (PE-) conjugated anti-human CD36 (25 μg/mL IgG2a, κ (clone 5–271, BioLegend, San Diego, CA, USA)) or 20 μL APC-conjugated anti-human CD36 (6,25 μg/mL IgM, κ (clone CB38, BD Pharmingen, New Jersey, USA)), used when appropriate. Isotype controls matching each antibody were used as negative controls. After incubation, samples were diluted in 200 μL Dulbecco's phosphate buffered saline 0.0095 M PO4 (PBS) buffer (Lonza, Basel, Switzerland) that had been filtered through a sterile 0.2 μm Q-Max syringe filter (Frisenette, Knebel, Denmark).
2.5. Flow Cytometric Measurement of MVs
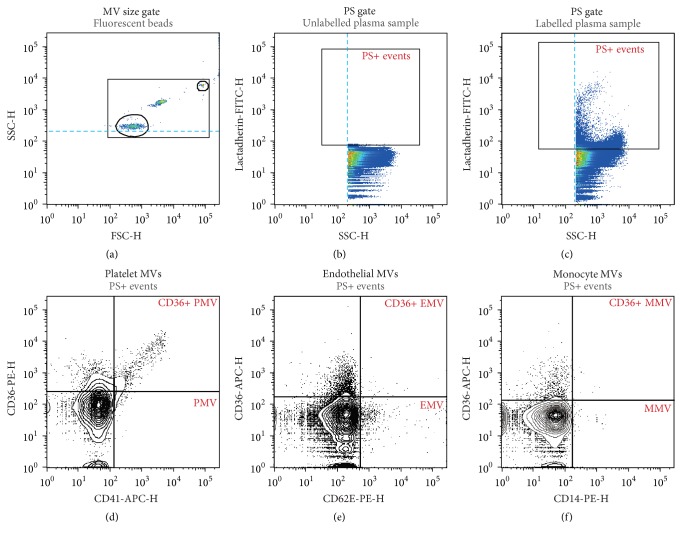
Flow cytometric analysis of plasma MV content was performed according to a recently described protocol [19] on a BD FACSAria™ III High Speed Cell Sorter (BD Biosciences, San Jose, CA, USA) at a maximal rate of 2 × 104 events per second until 106 events were collected in total. Data processing was performed as demonstrated in Figure 1. A size gate encompassing approximately 100 nm to 1000 nm was established in FlowJo™ software (v. 10.0.8, Tree Star, Inc., Oregon, USA) using the “Autogate” function around 200 nm and 900 nm fluorescent, size calibrated beads on log-scaled forward scatter height (FSC-H), and side scatter height (SSC-H). In addition, a discriminator was set at 200 on SSC-H to avoid exclusion of the smallest events. MVs were defined as phosphatidylserine positive (PS+) events based on binding of lactadherin-FITC within the established size gate. MVs of platelet origin were defined as CD41 positive events; monocyte MVs (MMVs) were defined as CD14 positive events; endothelial MVs (EMVs) were defined as CD62E positive events, and the expression of CD36 was investigated on PMVs, MMVs, and EMVs.
Figure 1.
Gating strategy for the flow cytometric microvesicle assay. (a) A 0.1–1.0 μm size gate was defined using silica beads. (b) Unlabelled plasma samples and isotype controls were used to establish gates in the fluorescence channels. (c) MVs were defined as phosphatidylserine expressing (PS+) particles based on their ability to bind lactadherin within the 0.1–1.0 μm gate. (d–f) CD41-APC, CD62E-PE, and CD14-PE antibodies were used to identify MVs of platelet (d), endothelial (e), and monocyte (f) origin, respectively, and their expression of CD36 was investigated using either CD36-PE or CD36-APC antibodies, as appropriate.
2.6. Data Analysis
All data analyses were conducted in R 3.2.2 (R Core Team, Vienna, Austria). The assumption of normality was tested with Shapiro-Wilk's W test for each parameter and visually confirmed. Observations were compared within the respective groups with paired Student's t-test or paired Wilcoxon signed ranks test where appropriate. Bivariable correlations between MV subpopulations and anthropometric, biochemical, and clamp parameters were studied using Spearman's ranked correlation coefficients (rS) due to nonnormal distribution of MV parameters. All p values reported are two-sided, and statistical significance was defined as p < 0.05.
3. Results
3.1. Characterization of Study Subjects
Demographic characteristics of the subjects were previously described by Magnussen et al. [8] and are summarized in Table 1. Of the thirty-nine subjects enrolled in this study, twenty were assigned to TRT and nineteen to placebo. In the initial assessment, no significant differences existed between TRT and placebo.
Table 1.
Baseline characteristics of study population.
| Placebo (n = 19) | Testosterone (n = 20) | p | |
|---|---|---|---|
| Age | 59,5 (6,3) | 61,4 (6) | 0,3367 |
| Smoking status (current/previous/never)† | 4/9/6 | 3/12/4 | |
| BMI (kg m−2) | 30,8 (3,8) | 30,6 (4) | 0,8879 |
| Waist circumference (cm) | 105,8 (10,8) | 106,9 (10) | 0,7577 |
| Total fat mass (kg) | 27,9 (6,5) | 26,9 (22,6; 34,8) | 0,7837 |
| Lean body mass (kg) | 61,7 (7,5) | 61,9 (8,9) | 0,9531 |
| Systolic blood pressure (mmHg) | 138,2 (12,8) | 137,7 (16,7) | 0,9151 |
| Diastolic blood pressure (mmHg) | 81,7 (8,2) | 80,5 (11,1) | 0,6948 |
| HbA1c (% Hb) | 6,50 (6,15; 6,80) | 6,52 (0,53) | 0,8108 |
| Total cholesterol (mmol L−1) | 3,8 (1,1) | 4,0 (0,7) | 0,5511 |
| LDL (mmol L−1) | 2,2 (0,8) | 2,3 (0,7) | 0,6904 |
| HDL (mmol L−1) | 0,9 (0,9; 1) | 1,0 (0,2) | 0,2057 |
| TG (mmol L−1) | 1,3 (1,16; 1,58) | 1,1 (0,85; 2,05) | 0,323 |
| ALT (IU L−1) | 31,0 (24,5; 43) | 35,7 (17,5) | 0,844 |
| GGT (IU L−1) | 39,0 (30; 51,5) | 31,0 (23,75; 39,25) | 0,1123 |
| Fasting glucose (mmol L−1) | 6,92 (1,01) | 7,14 (1,27) | 0,5602 |
| Disposal rate: base | 90,0 (12,8) | 88,6 (85,39; 109,02) | 0,3506 |
| Disposal rate: clamp | 179,4 (42,1) | 155,9 (136,34; 198,84) | 0,5877 |
| ΔRd‡ | 89,4 (40,6) | 89,8 (14,9) | 0,9655 |
| HGP: base | 83,2 (13,2) | 89,8 (14,9) | 0,1516 |
| HGP: clamp | 32,2 (24,57; 38,64) | 28,9 (22,02; 40,88) | 0,7284 |
| C-peptide: base | 1127,8 (318,7) | 933,5 (859,25; 1306) | 0,422 |
| Insulin: fasting | 103,8 (55,9) | 89,5 (47,8) | 0,4028 |
| Insulin clearance | 0,4 (0,1) | 0,3 (0,28; 0,33) | 0,1912 |
Data are depicted as mean (SD) or median (Q25%; Q75%). †Smoking status for 1 person in the testosterone group is unknown. ‡Difference in disposal rate at baseline and during clamp.
3.2. Body Composition and Markers for Hepatic Fat Accumulation
Correlations between components of MetSy and MV phenotypes are listed in Table 2. CD36+ MMVs were the only MV subpopulation found to be significantly correlated with BMI (rS = 0.35, p < 0.05), waist circumference (rS = 0.34, p < 0.05), and fat % (rS = 0.33, p < 0.05). No correlations between lean body mass and any MV phenotypes could be reported, however. Positive correlations were identified between MVs and ALT and between GGT and PMVs (rS = 0.43, p < 0.01), CD36+ PMVs (rS = 0.38, p < 0.05), and EMVs (rS = 0.33, p < 0.05). Furthermore, CD36+ MMVs correlated positively with both ALT (rS = 0.34, p < 0.05) and GGT (rS = 0.42, p < 0.01).
Table 2.
Correlations between MV subpopulations and parameters associated with components of MetSy and T2D at baseline.
| BMI | Waist | Total fat mass | Lean body mass | Fat % | HbA1c | Total cholesterol | LDL | HDL | TG | ALT | GGT | ΔRd† | HGP: base | HGP: clamp | C-peptide: base | Insulin: fasting | Insulin clearance | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactadherin+ | 0,18 | 0,08 | 0,09 | 0,20 | 0,00 | 0,30 | 0,15 | 0,06 | −0,16 | 0,43∗∗ | 0,34∗ | 0,27 | −0,21 | 0,12 | 0,25 | 0,30 | 0,36∗ | −0,04 |
| CD41+ | 0,06 | 0,09 | 0,08 | 0,21 | 0,00 | 0,14 | 0,48∗∗ | 0,32 | −0,18 | 0,58∗∗∗ | 0,17 | 0,43∗∗ | −0,07 | 0,16 | 0,27 | 0,14 | 0,24 | −0,19 |
| CD41+/CD36+ | −0,01 | 0,05 | 0,04 | 0,18 | −0,03 | 0,03 | 0,49∗∗ | 0,37∗ | −0,11 | 0,51∗∗ | 0,13 | 0,38∗ | 0,00 | 0,16 | 0,25 | 0,08 | 0,18 | −0,16 |
| CD62E+ | 0,12 | 0,04 | 0,08 | 0,31 | -0,06 | 0,08 | 0,30 | 0,14 | −0,06 | 0,38∗ | 0,16 | 0,33∗ | −0,18 | 0,13 | 0,14 | 0,25 | 0,31 | −0,03 |
| CD62E+/CD36+ | 0,03 | −0,04 | −0,08 | 0,27 | −0,22 | 0,18 | 0,30 | 0,14 | −0,01 | 0,41∗ | 0,01 | 0,19 | 0,05 | 0,19 | 0,13 | 0,10 | 0,17 | −0,04 |
| CD14+ | 0,18 | 0,10 | 0,18 | 0,18 | 0,14 | 0,10 | 0,20 | 0,09 | −0,23 | 0,50∗∗ | 0,19 | 0,26 | −0,19 | 0,14 | 0,21 | 0,30 | 0,41∗ | −0,19 |
| CD14+/CD36+ | 0,35∗ | 0,34∗ | 0,33∗ | 0,22 | 0,28 | −0,13 | 0,22 | 0,09 | −0,21 | 0,45∗∗ | 0,34∗ | 0,42∗∗ | −0,26 | 0,03 | 0,17 | 0,43∗∗ | 0,54∗∗∗ | −0,11 |
Spearman's ranked correlation coefficients (rS). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p< 0.001. †Difference in disposal rate at baseline and during clamp.
3.3. Plasma Lipid Profile
Positive correlations were identified between total cholesterol and PMVs (rS = 0.48, p < 0.01) and CD36+ PMVs (rS = 0.49, p < 0.01). Moreover, CD36+ PMVs were positively correlated with LDL cholesterol (rS = 0.37, p < 0.05). Triglycerides correlated positively with MVs (rS = 0.43, p < 0.01), PMVs (rS = 0.58, p < 0.001), CD36+ PMVs (rS = 0.51, p < 0.01), EMVs (rS = 0.38, p < 0.05), CD36+ EMVs (rS = 0.41, p < 0.05), MMVs (rS = 0.50, p < 0.01), and CD36+ MMVs (rS = 0.45, p < 0.01). No significant correlations were identified between HDL and MVs, however.
3.4. Insulin Sensitivity
Fasting insulin levels correlated positively with MVs (rS = 0.36, p < 0.05) and MMVs (rS = 0.41, p < 0.05), and both fasting levels of insulin (rS = 0.54, p < 0.001) and C-peptide (rS = 0.43, p < 0.01) correlated positively with CD36+ MMVs. No other significant correlations could be identified between MV phenotypes and parameters associated with insulin sensitivity.
3.5. TRT
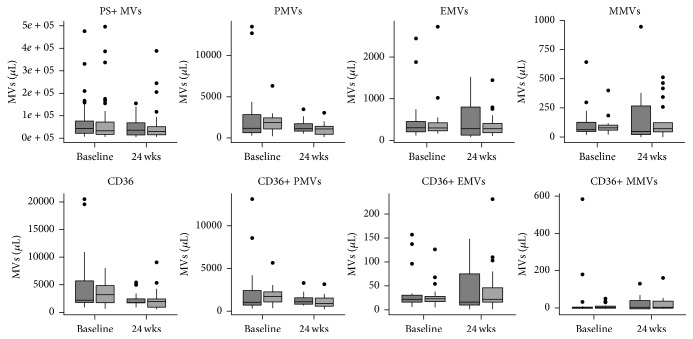
The effect of TRT on the patient population of this study has been described elsewhere [8]. In brief, TRT significantly increased lean body mass (p < 0.001) and decreased total fat mass (p = 0.0125), fat % (p < 0.001), and HDL (p = 0.006). No other parameters were affected by TRT. Furthermore, TRT did not affect any of the investigated MV phenotypes (Figure 2).
Figure 2.
Plasma levels of different MV phenotypes in placebo (n = 19, dark grey) and TRT (n = 20, light grey) groups at baseline and after 24 weeks of treatment. Outliers are represented as filled circles.
4. Discussion
The results of this study are twofold. First, specific phenotypes of MVs correlated with waist circumference, plasma triglycerides, total cholesterol, and LDL to a certain extent, and putative markers for hepatic fat accumulation, including ALT, and GGT. CD36+ MMVs were of particular significance, and the results of this study are in support of current evidence on the role of monocytes in the pathophysiology of MetSy and T2D. Second, although TRT resulted in improved lean body mass, total fat mass, and fat %, levels of circulating MVs remained unaltered.
By analysing relationships between levels of circulating MVs and components of MetSy and T2D, CD36+ MMVs were revealed to correlate with several measures of body composition. In obesity, adipose tissue monocytes undergo a phenotypic switch and are polarized toward a proinflammatory state [20]. Additionally, distinct differences in monocyte-derived macrophages and inflammatory cytokine profiles have been identified between different adipose tissue depots [5]. Furthermore, expression of CD36 on monocytes is increased in obesity [21], and a large body of evidence suggests that polarization of monocytes is mediated by CD36 [14]. Thus, it is possible that the increased expression of CD36 on the surface of monocytes and their polarization result in increased MV release and that the expression of CD36 on MMVs is equally increased.
Interestingly, PMVs and EMVs were uncorrelated with measures of body composition in the present study. Conversely, several studies have previously identified relationships between PMV and EMV phenotypes and different measures of body composition including BMI, waist circumference, and total fat mass [22]. These associations have previously been attributed to oxidative stress present in obesity [10], as BMI, waist circumference, and total fat mass correlate well with markers for oxidative stress [23]. However, these measures do not take into account the distribution of fat in the different adipose tissue compartments. Furthermore, the different adipose tissue compartments display distinct inflammatory characteristics [5]. Therefore, it would arguably be more accurate to analyse relationships between levels of MVs and the volumes of the different adipose tissue compartments.
In the present study, GGT was found to correlate positively with PMVs, CD36+ PMVs, EMVs, and CD36+ MMVs, and, additionally, ALT correlated positively with CD36+ MMVs. In the presence of hepatic lipid accumulation, it has been reported that hepatic gluconeogenesis and fatty acid oxidation are increased [24], which in turn could be related to liver damage due to a buildup of reactive oxygen species [25]. This results in increased secretion of circulating triglycerides and liver enzymes [26]. GGT and ALT are liver transaminases that are released into circulation in conditions that comprise damage of hepatocytes. One such condition is nonalcoholic fatty liver disease (NAFLD), and ALT and GGT are often used to predict the risk of incident T2D and CVD in these patients [27]. Thus, this result further supports the involvement of monocytes and their expression of CD36 in the pathogenesis of MetSy, T2D, and their related complications.
PMVs and CD36+ PMVs were found to correlate positively with total cholesterol, while only CD36+ PMVs correlated positively with LDL cholesterol. Studies have previously demonstrated that elevated levels of total cholesterol and LDL cholesterol in familial hypercholesterolemia are associated with increased thrombotic activity and platelet function [28]. Recently, plasma levels of total cholesterol have been associated with an increase in mean platelet volume [29], which reflects increased activation, metabolic reactivity, and coagulability [30]. Furthermore, as a consequence of oxidative stress present in MetSy and T2D, LDL becomes oxidized (oxLDL) and accumulates in the circulatory system. oxLDL has previously been implicated in activating platelets through mechanisms involving CD36 [31]. CD36 is expressed abundantly on the surface of platelets, and activation of platelets has been demonstrated to increase surface expression of CD36 by exocytosis of intracellular α-granules [32]. Furthermore, oxLDL has previously been demonstrated to increase EMV release [33]; however the finding that EMVs in the present study are unrelated to total cholesterol and LDL cholesterol suggests that this association could be influenced by confounding factors such as diabetes.
Another interesting observation was that all of the investigated MV phenotypes in the present study correlated with circulating triglycerides. One of the main sources of triglycerides in the circulatory system is lipoprotein particles with high concentrations of triglycerides such as very low density lipoproteins (VLDL) [34]. VLDL has been demonstrated to induce platelet hyperreactivity in an in vitro setting [35]. In addition, previous studies have demonstrated that increased levels of EMVs are highly associated with circulating triglycerides [22]. High levels of triglycerides have previously been implicated in impaired endothelial function [36] through mechanisms most likely associated with remnant lipoprotein particles [37] and the production of reactive oxygen species [38]. Taken together, it can be argued that dyslipidaemia affects platelets and endothelial cells and that this is reflected in levels of circulating MVs.
We identified positive associations between MMVs and CD36+ MMVs on the one hand and fasting insulin levels on the other. Additionally, CD36+ MMVs also correlated positively with basal levels of C-peptide. The development of insulin resistance has been highly ascribed to an overabundance of fatty acids as well as ectopic lipid storage [4]. Furthermore, the phenotypic shift observed in monocytes in obesity is thought to contribute to the development of insulin resistance and T2D (reviewed in [39]). This result therefore further supports the involvement of monocytes in the pathogenesis of MetSy and T2D. However, caution should be applied when interpreting results concerning insulin resistance in the participants of this study. Apart from MMVs, no other MV phenotypes were associated with measures of insulin resistance. A possible explanation for this is that the all participants were all prescribed treatment with Metformin, an inhibitor of hepatic glucose output [40]. Through its action in limiting hyperglycaemia, Metformin can therefore be considered a possible confounder that influences the levels of MVs.
In spite of TRT eliciting mild but significant improvements in lean body mass, total fat mass, and fat % in the present study, no significant changes could be observed in any of the studied MV subpopulations. It is important to note that, while TRT elicited mild changes in the abovementioned parameters, most parameters that were found to correlate with the investigated MV phenotypes were unaffected. Thus, it is unlikely that the MV phenotypes included in the present study are affected by TRT.
A number of limitations with this study should be considered. Although the number of participants were determined beforehand to be more than 15 in each group to result in a minimum relevant effect size of 1,3 kg in lean body mass [41] with a 5% probability for type 1 error and 10% probability of a type 2 error, the authors acknowledge that the number of participants in this study is a limitation. Therefore, while some of our findings were statistically significant, several of the null findings might be a consequence of the sample size. Another possible limitation of the present study is that correlations between baseline parameters cannot provide information about causality due to the cross-sectional nature of the analysis and should therefore purely be regarded as hypothesis generating. Finally, due to the highly homogenous nature of the study participants and the lack of a healthy control group, it is uncertain how the results of this study would translate to the overall population, and therefore generalization of these findings should be done with caution.
In conclusion, we demonstrated that the MV phenotypes investigated in this study correlated with several of the components of MetSy and T2D, where CD36+ MMVs were of particular importance. Our findings are therefore in support of the suggested role of monocytes as protagonists in the pathogenesis of the metabolic syndrome and T2D. It can be inferred that the activation of and shift in monocyte polarity from an anti-inflammatory to a proinflammatory phenotype upon increased ectopic fat deposition are mediated by CD36, which in turn leads to increased expression of CD36 on the plasma membrane [5, 14, 20, 21]. This activation accompanied by cell stress caused by the production of reactive oxygen species results in increased budding of the plasma membrane and release of MVs (reviewed in [11]). Taken together, increased numbers of CD36+ MMVs can therefore be proposed as possible blood-based biomarkers for ectopic fat accumulation in obesity, MetSy, and T2D. We additionally demonstrated that TRT did not result in altered levels of the investigated MV phenotypes, even though improvements were seen in lean body mass and total fat mass. It could, however, be argued that the changes in body composition elicited by TRT are mild, and it remains to be demonstrated whether TRT improves ectopic fat deposition and thereby cardiovascular health in patients with T2D. However, it cannot be excluded that the limitations of this study played a role in the latter result. Therefore, the authors of this study suggest that further studies are warranted in which the limitations of the present study are addressed to assess the direct effects of TRT on cardiovascular health in men with T2D.
Acknowledgments
The authors would like to thank Charlotte Christie Petersen for excellent technical assistance. In addition, the authors would like to extend their appreciation to the patients who participated in this study. This study was financially supported by the Novo Nordic Foundation (Grant no. 7713), the Danish Diabetes Academy, Odense University Hospital, the Institute of Clinical Research at the University of Southern Denmark, the Region of Southern Denmark, Consultant Council Scholarship Committee of Odense University Hospital, and the Christenson-Ceson Family Foundation.
Disclosure
Marianne Skovsager Andersen and Aase Handberg share senior authorship.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Eckel R. H., Grundy S. M., Zimmet P. Z. The metabolic syndrome. The Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Ford E. S., Li C., Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008;31(9):1898–1904. doi: 10.2337/dc08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gami A. S., Witt B. J., Howard D. E., et al. Metabolic syndrome and risk of incident cardiovascular events and death. Journal of the American College of Cardiology. 2007;49(4):403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Unger R. H. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144(12):5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 5.Kranendonk M. E. G., van Herwaarden J. A., Stupkova T., et al. Inflammatory characteristics of distinct abdominal adipose tissue depots relate differently to metabolic risk factors for cardiovascular disease: distinct fat depots and vascular risk factors. Atherosclerosis. 2015;239(2):419–427. doi: 10.1016/j.atherosclerosis.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 6.Brand J. S., Rovers M. M., Yeap B. B., et al. Testosterone, sex hormone-binding globulin and the metabolic syndrome in men: an individual participant data meta-analysis of observational studies. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0100409.e100409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gianatti E. J., Dupuis P., Hoermann R., et al. Effect of testosterone treatment on glucose metabolism in men with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37(8):2098–2107. doi: 10.2337/dc13-2845. [DOI] [PubMed] [Google Scholar]
- 8.Magnussen L. V., Glintborg D., Hermann P., Hougaard D. M., Højlund K., Andersen M. Effect of testosterone on insulin sensitivity, oxidative metabolism and body composition in aging men with type 2 diabetes on metformin monotherapy. Diabetes, Obesity and Metabolism. 2016;18(10):980–989. doi: 10.1111/dom.12701. [DOI] [PubMed] [Google Scholar]
- 9.Agouni A., Lagrue-Lak-Hal A. H., Ducluzeau P. H., et al. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. American Journal of Pathology. 2008;173(4):1210–1219. doi: 10.2353/ajpath.2008.080228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helal O., Defoort C., Robert S., et al. Increased levels of microparticles originating from endothelial cells, platelets and erythrocytes in subjects with metabolic syndrome: relationship with oxidative stress. Nutrition, Metabolism and Cardiovascular Diseases. 2011;21(9):665–671. doi: 10.1016/j.numecd.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Théry C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nature Reviews Immunology. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 12.Zimman A., Podrez E. A. Regulation of platelet function by class B scavenger receptors in hyperlipidemia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(12):2350–2356. doi: 10.1161/atvbaha.110.207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adachi H., Tsujimoto M. Endothelial scavenger receptors. Progress in Lipid Research. 2006;45(5):379–404. doi: 10.1016/j.plipres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy D. J., Kashyap S. R. Pathogenic role of scavenger receptor CD36 in the metabolic syndrome and diabetes. Metabolic Syndrome and Related Disorders. 2011;9(4):239–245. doi: 10.1089/met.2011.0003. [DOI] [PubMed] [Google Scholar]
- 15.Knøsgaard L., Thomsen S. B., Støckel M., Vestergaard H., Handberg A. Circulating sCD36 is associated with unhealthy fat distribution and elevated circulating triglycerides in morbidly obese individuals. Nutrition & Diabetes. 2014;4, article e114 doi: 10.1038/nutd.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen M. H., Irvine H., Vedel S., Raungaard B., Beck-Nielsen H., Handberg A. Elevated atherosclerosis-related gene expression, monocyte activation and microparticle-release are related to increased lipoprotein-associated oxidative stress in familial hypercholesterolemia. PLoS ONE. 2015;10(4) doi: 10.1371/journal.pone.0121516.e0121516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handberg A., Levin K., Højlund K., Beck-Nielsen H. Identification of the oxidized low-density lipoprotein scavenger receptor CD36 in plasma: a novel marker of insulin resistance. Circulation. 2006;114(11):1169–1176. doi: 10.1161/circulationaha.106.626135. [DOI] [PubMed] [Google Scholar]
- 18.Handberg A., Højlund K., Gastaldelli A., et al. Plasma sCD36 is associated with markers of atherosclerosis, insulin resistance and fatty liver in a nondiabetic healthy population. Journal of Internal Medicine. 2012;271(3):294–304. doi: 10.1111/j.1365-2796.2011.02442.x. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen M. H., Beck-Nielsen H., Andersen M. N., Handberg A. A flow cytometric method for characterization of circulating cell-derived microparticles in plasma. Journal of Extracellular Vesicles. 2014;3:1–12. doi: 10.3402/jev.v3.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lumeng C. N., Bodzin J. L., Saltiel A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. Journal of Clinical Investigation. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashyap S. R., Ioachimescu A. G., Gornik H. L., et al. Lipid-induced insulin resistance is associated with increased monocyte expression of scavenger receptor CD36 and internalization of oxidized LDL. Obesity. 2009;17(12):2142–2148. doi: 10.1038/oby.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amabile N., Cheng S., Renard J. M., et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. European Heart Journal. 2014;35(42):2972–2979. doi: 10.1093/eurheartj/ehu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furukawa S., Fujita T., Shimabukuro M., et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. Journal of Clinical Investigation. 2004;114(12):1752–1761. doi: 10.1172/JCI200421625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sunny N. E., Parks E. J., Browning J. D., Burgess S. C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metabolism. 2011;14(6):804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satapati S., Sunny N. E., Kucejova B., et al. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. Journal of Lipid Research. 2012;53(6):1080–1092. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yatsuya H., Nihashi T., Li Y., et al. Independent association of liver fat accumulation with insulin resistance. Obesity Research & Clinical Practice. 2014;8(4):e350–e355. doi: 10.1016/j.orcp.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Ghouri N., Preiss D., Sattar N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: a narrative review and clinical perspective of prospective data. Hepatology. 2010;52(3):1156–1161. doi: 10.1002/hep.23789. [DOI] [PubMed] [Google Scholar]
- 28.Mazeaud M. M., Driss F., Le Quan Sang K.-H., et al. Biochemical and functional alterations associated with hypercholesterolemia in platelets from hypertensive patients. Atherosclerosis. 1992;94(2-3):201–211. doi: 10.1016/0021-9150(92)90245-C. [DOI] [PubMed] [Google Scholar]
- 29.Icli A., Aksoy F., Nar G., et al. Increased mean platelet volume in familial hypercholesterolemia. Angiology. 2016;67(2):146–150. doi: 10.1177/0003319715579781. [DOI] [PubMed] [Google Scholar]
- 30.Boos C. J., Lip G. Y. Assessment of mean platelet volume in coronary artery disease—what does it mean? Thrombosis Research. 2007;120(1):11–13. doi: 10.1016/j.thromres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Wang H., Wang Z.-H., Kong J., et al. Oxidized low-density lipoprotein-dependent platelet- derived microvesicles trigger procoagulant effects and amplify oxidative stress. Molecular Medicine. 2012;18(2):159–166. doi: 10.2119/molmed.2011.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh A., Murugesan G., Chen K., et al. Platelet CD36 surface expression levels affect functional responses to oxidized LDL and are associated with inheritance of specific genetic polymorphisms. Blood. 2011;117(23):6355–6366. doi: 10.1182/blood-2011-02-338582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nomura S., Shouzu A., Omoto S., Nishikawa M., Iwasaka T., Fukuhara S. Activated platelet and oxidized LDL induce endothelial membrane vesiculation: clinical significance of endothelial cell-derived microparticles in patients with type 2 diabetes. Clinical and Applied Thrombosis/Hemostasis. 2004;10(3):205–215. doi: 10.1177/107602960401000302. [DOI] [PubMed] [Google Scholar]
- 34.Klop B., Elte J. W. F., Cabezas M. C. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Englyst N. A., Taube J. M., Aitman T. J., Baglin T. P., Byrne C. D. A novel role for CD36 in VLDL-enhanced platelet activation. Diabetes. 2003;52(5):1248–1255. doi: 10.2337/diabetes.52.5.1248. [DOI] [PubMed] [Google Scholar]
- 36.Lundman P., Eriksson M. J., Silveira A., et al. Relation of hypertriglyceridemia to plasma concentrations of biochemical markers of inflammation and endothelial activation (C-reactive protein, interleukin-6, soluble adhesion molecules, von Willebrand factor, and endothelin-1) American Journal of Cardiology. 2003;91(9):1128–1131. doi: 10.1016/S0002-9149(03)00165-6. [DOI] [PubMed] [Google Scholar]
- 37.Miller M., Stone N. J., Ballantyne C., et al. Triglycerides and cardiovascular disease: a scientific statement from the american heart association. Circulation. 2011;123(20):2292–2333. doi: 10.1161/cir.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 38.Stokes K. Y., Cooper D., Tailor A., Granger D. N. Hypercholesterolemia promotes inflammation and microvascular dysfunction: role of nitric oxide and superoxide. Free Radical Biology and Medicine. 2002;33(8):1026–1036. doi: 10.1016/s0891-5849(02)01015-8. [DOI] [PubMed] [Google Scholar]
- 39.Castoldi A., Naffah de Souza C., Câmara N. O. S., Moraes-Vieira P. M. The macrophage switch in obesity development. Frontiers in Immunology. 2016;6, article 637 doi: 10.3389/fimmu.2015.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song R. Mechanism of metformin: a tale of two sites. Diabetes Care. 2016;39(2):187–189. doi: 10.2337/dci15-0013. [DOI] [PubMed] [Google Scholar]
- 41.Isidori A. M., Giannetta E., Greco E. A., et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clinical Endocrinology. 2005;63(3):280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]