Abstract
Background. Malaria and urogenital schistosomiasis are coendemic in Mount Cameroon Area. This study investigated the prevalence of S. haematobium, P. falciparum, and coinfections and their effect on anaemia in pregnancy. Methods. Pregnant women reporting for antenatal care (ANC) clinic visit in Munyenge were enrolled. S. haematobium and P. falciparum infections were determined by urine filtration and microscopy, respectively. Haemoglobin (Hb) levels were measured using haemoglobinometer. Of 250 women, 46.8%, 39.2%, and 15.2% had S. haematobium, P. falciparum, and coinfections, respectively. Schistosomes infection was higher in younger women (≤25 years) and those who bathe in and had domestic contact with stream compared with older age (>25 years) women and those who had only domestic contact with stream. Lower infection rate was associated with less water contact (≤2 times/day) compared with more water contact (>2 times/day). Compared with no sulphadoxine-pyrimethamine (SP) usage, malaria parasitaemia was less among women who used SP. Stream usage increased risk of coinfection while less water contact and SP usage decreased its risk. All coinfected cases were anaemic and coinfection accounted for 93.8% of severe anaemia. Conclusion. Coinfection contributes to anaemia severity. Less water contact and SP usage will reduce coinfection in pregnancy in Munyenge.
1. Background
Malaria parasite and helminth infections are the most prevalent parasitic diseases in developing countries and their epidemiologic coexistence is frequently observed, particularly in Africa [1]. The overlap of malaria parasite and helminth infections is influenced by high frequencies of the parasites in the same population, similar geographical distribution of parasites, shared risk factors, common transmission methods [1], and genetic and immunological predisposition [2]. Findings from epidemiological studies suggest that interactions between malaria parasite and helminth infections can be antagonistic [3, 4] or synergistic [5, 6]. Some studies have proposed an immunologic hypothesis based upon the type T cell response (Th1 or Th2) induced by each parasite [2, 7]. Synergistic T cell responses could decrease the pathological impact of the infections, whereas antagonistic T cell responses could exacerbate disease. Coinfection may have considerable health consequences leading to more severe clinical symptoms and pathology than infection with single parasite species [8, 9]. For example, coinfections of Plasmodium falciparum with hookworms and schistosomes tend to exacerbate hepatosplenic, anaemia, and malnutrition morbidities [8].
Plasmodium falciparum inflicts the greatest burden and about 90% of the populations infected with malaria live in sub-Saharan Africa. Pregnant women are particularly vulnerable to P. falciparum especially in first pregnancy [10] and protective interventions against malaria include intermittent preventive treatment with sulphadoxine-pyrimethamine (IPTp-SP) and use of insecticide treated bed nets (ITNs) [11]. Besides malaria, schistosomiasis is the second important parasitic disease in terms of socioeconomic and public health importance [12]. More than 90% of the roughly 200 million cases of schistosomiasis occur in Africa [13] of which approximately two-thirds are caused by Schistosoma haematobium [14], the etiologic agent of urogenital schistosomiasis. Schistosomiasis is endemic in rural areas where there is a lack of safe water supply, poverty, ignorance, and poor hygienic practices [15]. Forty million women of child bearing age are infected with younger and pregnant women being at greater risk of infection [16, 17]. Domestic activities such as washing clothes and fetching water in infected water expose women and children to infection [18]. In Cameroon, schistosomiasis is endemic in the northern regions [19], Centre, East, West [20], Littoral, North West, South, and Southwest Regions [21]. Urogenital schistosomiasis is endemic in Southwestern Cameroon where Barombi Kotto [22] and Munyenge [23] are identified transmission foci. While most of these studies have focused on school-age children and the community, epidemiological data on the prevalence of urogenital schistosomiasis and its burden among pregnant women living in these endemic foci is lacking.
Studies have demonstrated that schistosomiasis infection in pregnant women results in severe anaemia [16], low birth weight, and maternal mortality [24–26]. However, the aetiology of anaemia is multifactorial involving complex interaction between nutrition status, infectious disease (malaria, human immunodeficiency virus (HIV), and helminths), and other factors (sociodemographic and economic) [27]. These conditions are integrally linked and subsequently lead to adverse pregnancy outcomes [27]. S. haematobium have been linked to placental inflammation leading to poor birth outcomes as a result of placental malfunction. Data suggest that infected women have a higher rate of spontaneous abortions and a higher risk for ectopic pregnancies [28, 29]. Conversely, studies have demonstrated the biologic plausibility that female genital schistosomiasis may make women more susceptible to HIV [30]. Praziquantel (PZQ) is recommended in pregnancy [31].
Pregnant women living in Munyenge, Mount Cameroon Area, may be exposed to coinfection with S. haematobium and P. falciparum and thus may experience anaemia severity. The specific aims of this study were to (i) determine the prevalence and intensity of S. haematobium, P. falciparum, and coinfections among pregnant women reporting for ANC clinic visit at the Munyenge Health Centre, (ii) determine the factors associated with prevalence and intensity of these infections, and (iii) assess the relative effect of S. haematobium, P. falciparum, and their coinfection on anaemia prevalence and severity in pregnancy.
2. Methods
2.1. Study Design
Malaria and urogenital schistosomiasis are coendemic in some areas of Mount Cameroon Area, Southwestern Cameroon. Munyenge is a rural community situated at the foot of mount Cameroon at an altitude of 261 above sea level. This village is endemic foci for the transmission of S. haematobium [23] and recent reports of a community-based study show an overall prevalence of 40% [32]. In the Mount Cameroon Area, malaria parasite transmission is perennial [33] and P. falciparum accounts for 60% of malaria parasite infection among pregnant women [34]. A cross-sectional study aimed to determine the prevalence of S. haematobium, P. falciparum, and coinfections and evaluate their relative effect on anaemia prevalence and severity among pregnant women is justified.
A sample size of 263 pregnant was determined to be adequate to detect a 5% change in prevalence. Sample size calculation was based on the estimate of the prevalence of S. haematobium infection in Munyenge according to a baseline epidemiological survey carried out in 2012 by Ntonifor et al. [23]. The sample size was determined using the formula n = z2pq/d2 [35], where n is the sample size required, z = 1.96 is confidence level test statistic at the desired level of significance, p = 78% is the proportion of urogenital schistosomiasis prevalence, q = 1 − p is the proportion of urogenital schistosomiasis negative, and d is the acceptable error willing to be committed. However, due to logistics, we had a sample size of 250 pregnant women which is well within 90–95% of the expected sample size calculated.
The study was carried out in the Munyenge Health Area which is about 27 km from Muyuka town. It is bounded to the West by Likoko native, to the East by Masone, to the North by Mount Cameroon, and to the North East by Mbonge subdivision (Figure 1). Munyenge has a heterogenous population of 15,000 inhabitants consisting of individuals from several cultural backgrounds including natives from Oroko, Wimbum, kom, Mettas, Ibus, Nhies, Ndop, and Isimbis. This area is found in the rain forest of the Southwest Region with rich volcanic soil encouraging farming activities. The main occupation of the people is farming, with cocoa and plantains being their main cash crops [32]. Munyenge Health Area has four streams with outlet springs (providing natural water sources) situated in the middle of the village. These springs are habitats for the Bulinus spp. intermediate host and thus constitute the main transmission foci of S. haematobium in the community. Bulinus snails are found on vegetation and rocks surrounding the water point and on vegetation within the streams. These springs include the “coast timber” and “KCB” (men and women). The coast timber is a small catchment for spring water located within the community where pupils play often while the KCB “man” and “woman” as the name suggests are bathing springs for males and females, respectively, which are found further from the community. Munyenge is characterised by the absence of pipe borne water and the use of fresh water sources for household activities is common. All members of the community use these springs for drinking water, bathing, washing of clothes, and household utensils [32]. Munyenge has a temperature range of 24°C–27°C which favours high release of cercariae into the waters. These conditions make it certain that the people will continue to be infected and reinfected.
Figure 1.
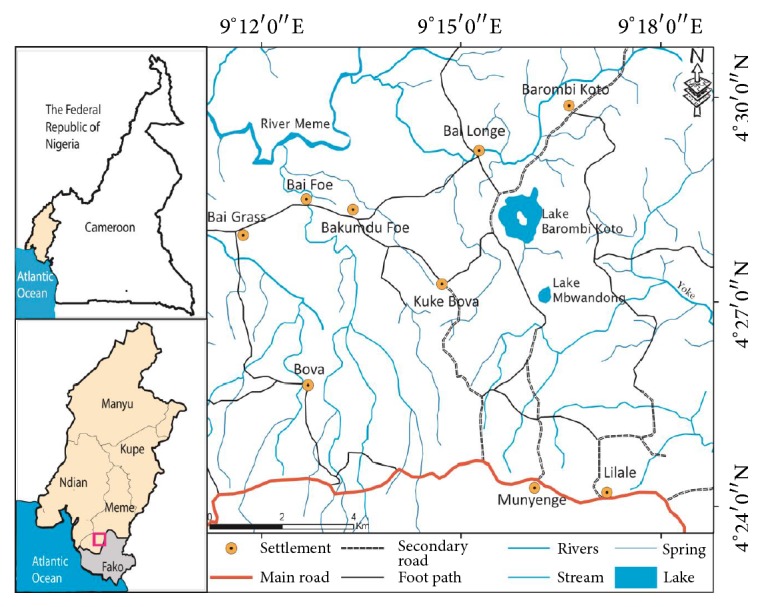
Map showing the location of Munyenge in Mount Cameroon Area.
3. Data Collection
Pregnant women reporting for ANC clinic visit at the Munyenge Health Centre between June and September 2014, who had lived in Munyenge for at least two months and gave their consent, were enrolled consecutively. Pregnant women were interviewed by a field researcher using a questionnaire which recorded: demographic information (age, residence), gynaecologic/obstetric history (gravidity status, gestational age, and pregnancy complications), socioeconomic indicators (educational level, occupation), and questions related to malaria (IPTp-SP uptake, ITN usage) and schistosomiasis (main household water source, frequency of contact with open water source, and domestic activities carried out in the stream). In addition to the questionnaire interview, women were asked to collect urine in supplied 20 mL screw top plastic containers between 10:00 am and 2:00 pm. The samples were stored in a cool box during transportation to the University of Buea, Medical Research Laboratory, and processed within 24 hours of collection.
3.1. Laboratory Analyses
3.1.1. Anaemia Status Determination
Haemoglobin levels were determined from finger-prick blood samples using a portable battery-operated photometer (HemoCue®) (HemoCue 201+ system, HemoCue, Angelholm, Sweden) (URIT-12). Hb concentration was expressed in g/dL. Anaemia was defined as an Hb value < 11.0 g/dL [36]. Anaemia severity was defined as follows: mild anaemia (Hb: 10–10.9 g/dL), moderate anaemia (Hb: 7–9.9 g/dL), and severe anaemia (Hb < 7.0 g/dL) [36].
3.1.2. Parasitological Examination
Malaria parasites were identified on thick and thin blood smears stained with 5% Giemsa. The smears were observed for 30 minutes under the ×100 (oil immersion) objective of a UNICO® light microscope [37]. Malaria parasite density was estimated by counting parasites against 200 leucocytes in thick smears, assuming a white cell count of 8000 leucocytes per μL of blood [38]. Malaria parasite density was classified into <500, 501–5,000, and >5,000 for low, moderate, and high parasitaemia, respectively [38]. S. haematobium eggs were identified in urine samples using the filtration technique [39]. In brief, 10 mls of urine was filtered using membrane filters (Sterlitech Polycarbonate (PCTE) membrane filters, USA) and the egg count was recorded per 10 mls of urine. The infection intensity was classified as light (<50 eggs/10 mL of urine) or heavy (≥50 eggs/10 mL of urine) as defined by the World Health Organization (WHO) [40]. Microhaematuria was used as proxy-diagnosis of urogenital schistosomiasis, an accepted marker in the rapid diagnosis of S. haematobium infection in urine [41]. Samples were tested for microhaematuria using urine reagent strips (Uripath, Plasmatec Laboratory, UK) (Combi-11) as per manufacturer instructions. Results were expressed as negative or in levels of positivity (+, ++, or +++) and not including traces. A pregnant woman was infected with S. haematobium when she was diagnosed positive by microscopic examination and/or urine reagent strip.
3.2. Statistical Analysis
The data was analyzed using SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA). Proportions of S. haematobium, P. falciparum, and coinfection and anaemia status were compared between different groups (age groups, gravidity status, educational level, occupational status, stream usage and activities, and IPTp-SP uptake) using Pearson Chi-square test. Crude odds ratios were estimated and factors associated with infections and anaemia to be included in the multivariate logistic regression model were identified. Variables that had a P value < 0.20 in bivariate analysis were included in the multivariate logistic regression model. Using the enter method, variables that showed independent association with infections and anaemia status at a significance level of P < 0.05 were retained in the model. Mean Hb levels were compared between groups using Analysis of Variance test (ANOVA) and Student's t-test. Independent factors associated with Hb levels were obtained using multilinear regression analysis. P values < 0.05 were considered significant.
3.3. Ethical Considerations
The study protocol and design including the consent procedures were approved by the Institutional Ethics Review Board of the Faculty of Health Science, University of Buea, and the Buea Regional Delegation of Public Health. Prior to conducting the study, the aim of the study and procedures to be used to collect data were explained to the pregnant women at the ANC clinic. Written (from those who can read and write) or verbal (from those who cannot read and write) informed consent from all study participants was obtained. Each pregnant woman who agreed to participate in the study was enrolled and given urine sample container to collect urine. Participation was voluntary and study participants were assured of confidentiality and anonymity of data.
4. Results
4.1. Characteristics of the Study Population
In this cross-sectional study, a total of 250 pregnant women reporting for antenatal care was enrolled. The mean age of the study participants was 25 ± 5.21 years (range: 14–40 years). The majority (75.2%) of the women were married. All the study participants had at least a primary education and about 50% had obtained some form of secondary education. Although a higher proportion of the women had been enrolled for first ANC in the second trimester, more than 40% had late clinic registration. About two-thirds of women reported taking at least one dose of IPTp-SP and 46% reported having slept under a bed net the previous night. The stream was the main source of water (99% stream usage) for domestic use and bathing. The characteristics of the study sample are shown in Table 1.
Table 1.
Characteristics of the study participants.
| Characteristics | Number examined (N) | (%) |
|---|---|---|
| Age group (years) | ||
| ≤20 | 56 | 22.4 |
| 21–25 | 79 | 31.6 |
| 26–30 | 71 | 28.4 |
| >30 | 44 | 17.6 |
|
| ||
| Marital status | ||
| Single | 62 | 24.8 |
| Married | 188 | 75.2 |
|
| ||
| Gravidity | ||
| Primigravidae | 69 | 27.6 |
| Secundigravidae | 71 | 28.4 |
| Multigravidae | 110 | 44.0 |
|
| ||
| Trimester of first ANC | ||
| First | 10 | 4.0 |
| Second | 125 | 50.0 |
| Third | 115 | 46.0 |
|
| ||
| Educational level | ||
| Primary | 123 | 49.2 |
| Secondary | 127 | 50.8 |
|
| ||
| Occupation | ||
| House wife | 52 | 20.8 |
| Business | 93 | 35.2 |
| Farmer | 72 | 28.8 |
| Student | 33 | 13.2 |
|
| ||
| Stream usage | ||
| Yes | 248 | 99.2 |
| No | 2 | 0.8 |
|
| ||
| Activities in the stream | ||
| Domestic contact and bathing | 126 | 50.4 |
| Domestic contact only | 124 | 49.6 |
|
| ||
| Frequency to streams/day |
||
| 1 to 2 times | 122 | 48.8 |
| 3 to 4 times | 57 | 22.8 |
| 5+ times | 71 | 28.4 |
|
| ||
| IPTp-SP uptake | ||
| Yes | 169 | 67.6 |
| No | 81 | 32.4 |
|
| ||
| ITN use | ||
| Yes | 115 | 46.0 |
| No | 135 | 54.0 |
4.2. Prevalence and Intensity of Infection
4.2.1. S. haematobium Infection
Of the 250 volunteer pregnant women enrolled, 117 (46.8%; 95% CI: 41–53) were positive for S. haematobium infection among whom 53 (45.3%) had heavy (≥50 eggs/10 mL of urine) infection while 54.7% (64) had light (<50 eggs/10 mL of urine) infection. The prevalence of microhaematuria was 9.6% (24/250). Using microscopic urine examination as gold standard, the specificity and sensitivity of microhaematuria in the diagnosis of S. haematobium infection were 100% (95% CI: 97.2–100) and 20.5% (95% CI: 14.2–28.7), respectively. Microhaematuria was strongly related to egg density categories where microhaematuria was common (χ2 = 8.23; P = 0.004) among women with heavy egg load (72.7%) than in those with light infection (27.3%).
4.2.2. P. falciparum Infection
The overall prevalence of P. falciparum parasitaemia among the study participants was 39.2% (98) (95% CI: 33.4–45.4). Of the 98 pregnant women infected with P. falciparum, the proportions of low (<500 parasites/μL of blood), moderate (501–5000), and high (>5000) parasitaemia were 43.9% (43), 52.0% (61), and 4.1 (4), respectively. About 15% (38) (95% CI: 11.3–20.2) of the pregnant women carried concurrent infections with S. haematobium and P. falciparum. Seventy-nine (31.6%) and sixty (24%) women had single infection with S. haematobium and P. falciparum, respectively.
4.2.3. Factors Associated with Prevalence and Intensity of S. haematobium Infection
S. haematobium infection was associated with age, gravidity status, water contact frequency, type of activity carried out in the stream, and P. falciparum parasitaemia in bivariate analysis (Table 2). The prevalence of infection did not differ significantly with marital status, educational level, and occupational status. In multivariate analysis (controlling for age and gravidity status as confounders), younger age groups, ≤20 (aOR = 15.2 95% CI: 1.7–138.3) and 21–25 years (aOR = 7.3; 95% CI: 1.2–44.3), and bathing and domestic contact with stream (aOR = 33.5; 95% CI 9.7–115.9) were risk factors associated with S. haematobium infection. On the other hand, less water contact frequency (1 to 2 times per day) (aOR = 2.8E − 10; 95% CI: 9.4E − 11–8.5E − 10) was associated with decreased risk of infection. Surprisingly, primigravidity (OR = 0.2; 95% CI: 0.03–0.9) and secundigravidity (OR = 0.1; 95% CI 0.02–0.4) were less likely at risk. Intensity of infection was associated with malaria parasitaemia where light egg density infection was less common (aOR = 0.4; 95% CI: 0.2–0.7; P = 0.004) in malaria positive women (21.9%; 14/64) than in malaria negative women (78.1%; 50/64).
Table 2.
Risk factors associated with S. haematobium infection among pregnant women in Munyenge.
| Factors | Category | S. haematobium positive% (n) | Unadjusted OR (95% CI) | #Adjusted OR (95% CI) | P value |
|---|---|---|---|---|---|
| Age (years) | ≤20 | 55.4 (31) | 3.0 (1.3–6.8) | 15.2 (1.7–138.3) | 0.016 |
| 21–25 | 53.2 (42) | 2.7 (1.2–5.9) | 7.3 (1.2–44.3) | 0.031 | |
| 26–30 | 43.7 (31) | 1.9 (0.8–4.1) | 1.1 (0.2–6.1) | 0.885 | |
| >30 | 29.5 (13) | REF | REF | ||
| χ 2; P value | 8.48; 0.037 | ||||
|
| |||||
| Gravidity | Primigravidity | 63.8 (44) | 2.7 (1.6–5.5) | 0.2 (0.03–0.9) | 0.034 |
| Secundigravidity | 45.1 (32) | 1.4 (0.6–2.5) | 0.1 (0.02–0.4) | 0.001 | |
| Multigravidity | 37.3 (41) | REF | REF | ||
| χ 2; P value | 12.08; 0.002 | ||||
|
| |||||
| Marital status | Single | 48.4 (30) | 1.1 (0.6–1.9) | NA | |
| Married | 46.3 (87) | REF | |||
| χ 2; P value | 0.83; 0.773 | ||||
|
| |||||
| Educational level | Primary |
49.6 (61) |
1.3 (0.8–2.1) |
NA | NA |
| Secondary | 44.1 (56) |
REF | |||
| χ 2; P value | 0.74; 0.384 | ||||
|
| |||||
| Occupation | Housewife | 42.3 (22) | 1.3 (0.6–2.7) | 2.5 (0.4–15.7) | 0.333 |
| Business | 52.7 (49) | 1.8 (1.1–3.7) | 4.3 (0.9–21.9) | 0.079 | |
| Student | 60.6 (20) | 2.7 (1.2–6.4) | 1.6 (0.3–9.0) | 0.587 | |
| Farmer | 36.1 (26) | REF | REF | ||
| χ 2; P value | 7.55; 0.056 | ||||
|
| |||||
| Activities in the stream | Domestic contact and bathing |
84.1 (106) | 49.2 (22.4−107.7) | 33.5 (9.7–115.9) | <0.001 |
| Domestic contact only |
8.9 (11) | REF | REF | ||
| χ 2; P value | 142.16; <0.001 | ||||
|
| |||||
| Frequency to the stream/day | 1 to 2 times | 13.1 (16) | 0.14 (0.07–0.3) | 2.8E − 10 (9.4E − 11–8.5E − 10) | <0.001 |
| 3 to 4 times | 52.6 (30) | REF | 6.7E − 10 (6.7E − 10–6.7E − 10) | ||
| 5+ times | 100 (71) | — | REF | ||
| χ 2; P value | 137.09; <0.001 | ||||
|
| |||||
| Malaria parasitaemia |
Positive | 38.8 (38) | 0.6 (0.4–1.0) | 0.4 (0.14–1.2) | 0.098 |
| Negative | 52 (79) | REF | REF | ||
| χ 2; P value | 4.17; 0.041 | ||||
χ 2: Pearson Chi-square test; OR: odd ratio.
#OR adjusted using multivariate regression analysis.
4.2.4. Factors Associated with Prevalence of P. falciparum Infection
The prevalence of P. falciparum infection was associated (χ2 = 17.82; P < 0.01) with IPTp-SP uptake where malaria parasite infection was greater in women who had not taken IPTp-SP (58.0%) than in those who had at least one SP dose (30.2%). The occurrence of P. falciparum infection did not differ significantly with maternal age, gravidity status, or ITN usage.
4.2.5. Factors Associated with Prevalence of Coinfection with S. haematobium and P. falciparum
Coinfection was associated with the type of activity carried out in the stream and water contact frequency as well as IPTp-SP uptake. Bathing and domestic contact with stream (aOR = 13.3; 95% CI 2.2–79.5) increased risk of coinfection among pregnant women; meanwhile, less water contact frequency (1 to 2 times per day (aOR = 0.1; 95% CI: 0.01–0.4) and 3 to 4 times per day (aOR = 0.3; 95% CI: 0.1–0.9)) decreased risk of coinfection. Women who had at least one SP dose were less likely (aOR = 0.06; 95% CI: 0.02–0.2) to be coinfected (Table 3).
Table 3.
Risk factors associated with coinfection with S. haematobium and P. falciparum among pregnant women in Munyenge.
| Factors | Category | Presence of coinfection% (n) | Unadjusted OR (95% CI) |
#Adjusted OR (95% CI) |
P value |
|---|---|---|---|---|---|
| Age (years) | ≤20 | 25 (14) | 4.6 (1.2–17) | 2.8 (0.3–22.7) | 0.338 |
| 21–25 | 17.7 (14) | 2.9 (0.8–10.9) | 1.5 (0.2–9.7) | 0.662 | |
| 26–30 | 9.9 (7) | 1.5 (0.4–6.1) | 0.3 (0.04–2.2) | 0.247 | |
| >30 | 6.8 (3) | REF | REF | ||
| χ 2; P value | 8.53; <0.001 | ||||
|
| |||||
| Gravidity | Primigravidity | 23.6 (16) | 3.4 (1.4–8.2) | 0.4 (0.1–1.7) | 0.198 |
| Secundigravidity | 18.3 (13) | 2.5 (1.0–6.2) | 0.9 (0.2–3.5) | 0.893 | |
| Multigravidity | 8.2 (9) | REF | REF | ||
| χ 2; P value | 8.15; 0.017 | ||||
|
| |||||
| Marital status | Single | 17.7 (11) | 1.3 (0.6–2.8) | NA | |
| Married | 14.4 (27) | REF | |||
| χ 2; P value | 0.41; 0.52 | ||||
|
| |||||
| Educational level | Primary Secondary χ2; P value |
13.8 (17) 6.5 (31) 0.36; 0.55 |
1.4 (0.7–2.9) REF |
NA | |
|
| |||||
| Occupation | Housewife | 13.5 (7) | 1.2 (0.4–3.7) | NA | |
| Business | 17.2 (16) | 2.1 (0.8–5.3) | |||
| Student | 21.2 (7) | 3.0 (1.0–9.2) | |||
| Farmer | 11.1 (8) | REF | |||
| χ 2; P value | 2.27; 0.518 | ||||
|
| |||||
| Activities in the stream | Domestic contact and bathing |
26.6 (36) | 24.4 (5.7–104) | 13.3 (2.2–79.5) | 0.005 |
| Domestic contact only | 1.6 (2) | REF | REF | ||
| χ 2; P value | 35.24; <0.001 | ||||
|
| |||||
| Frequency to the stream/day | 1 to 2 times | 2.5 (3) | 0.04 (0.01–0.15) | 0.1 (0.01–0.4) | 0.002 0.027 |
| 3 to 4 times | 15.8 (9) | 0.3 (0.1–0.8) | 0.3 (0.1–0.9) | ||
| 5+ times | 36.6 (26) | REF | REF | ||
| χ 2; P value | 40.65; <0.001 | ||||
|
| |||||
| IPTp-SP uptake |
Yes | 9.5 (16) | 0.3 (0.1–0.6) | 0.06 (0.02–0.2) | <0.001 |
| No | 27.2 (22) | REF | REF | ||
| χ 2; P value | 13.29; <0.001 | ||||
χ 2: Pearson Chi-square test; OR: odd ratio.
#Adjusted OR using multivariate regression analysis.
4.3. Haemoglobin Levels and Anaemia
The mean (±SD) haemoglobin level of the pregnant women enrolled in the study was 9.0 ± 1.6 g/dL (range: 6.1–13.7 g/dL). Coinfection significantly reduced Hb levels of pregnant women in the study area where levels in coinfected individuals were significantly lower (P < 0.001) when compared with levels seen with single infections (S. haematobium and P. falciparum) and no infection (Table 4). In addition, Hb levels were significantly lower among women coinfected with P. falciparum and heavy S. haematobium infections than in individuals coinfected with P. falciparum and light S. haematobium infection and those with no infection (Table 5). Although age, marital status, educational level, occupational status, infection status, and IPTp-SP uptake were identified as factors associated with Hb levels, IPTp-SP was seen as the only independent predictor of Hb levels taking into consideration all possible confounding variables (Table 4).
Table 4.
Factors associated with mean (±SD) haemoglobin levels among pregnant women in Munyenge Health Area.
| Factors | Category | Mean (±SD) Hb levels | Test-value | Unadjusted P value |
t-test |
&Adjusted P value |
|---|---|---|---|---|---|---|
| Age (years) | ≤20 | 8.6 ± 1.7 | ∗ F = 3.35 | 0.02 | 2.02 | 0.045 |
| 21–25 | 9.1 ± 1.7 | |||||
| 26–30 | 9.0 ± 1.4 | |||||
| >30 | 9.5 ± 1.5 | |||||
|
| ||||||
| Gravidity | Primigravidity | 8.9 ± 1.9 | F = 0.75 | 0.474 | NA | NA |
| Secundigravidity | 8.9 ± 1.4 | |||||
| Multigravidity | 9.1 ±1.5 | |||||
|
| ||||||
| Marital status | Single | 8.5 ± 1.4 | $ t = −2.70 | 0.007 | 1.79 | 0.075 |
| Married | 9.2 ± 1.6 | |||||
|
| ||||||
| Educational level | Primary | 9.3 ± 1.7 | t = 2.42 | 0.016 | −1.62 | 0.106 |
| Secondary | 8.8 ± 1.5 | |||||
|
| ||||||
| Occupation | Housewife | 9.6 ± 1.8 | F = 10.16 | <0.001 | 0.19 | 0.852 |
| Business | 8.7 ± 1.3 | |||||
| Student | 8.0 ± 1.2 | |||||
| Farmer | 9.4 ± 1.7 | |||||
|
| ||||||
| Infection status | S. haematobium only | 9.5 ± 1.6 | F = 31.61 | <0.001 | −1.22 | 0.225 |
| P. falciparum only | 9.1 ± 1.2 | |||||
| Coinfection | 7.0 ± 1.0 | |||||
| No infection | 9.5 ± 1.4 | |||||
|
| ||||||
| IPTp-SP uptake | Yes | 9.2 ± 1.6 | t = 2.95 | 0.004 | −2.60 | 0.01 |
| No | 8.6 ± 1.5 | |||||
∗Analysis of variance test (ANOVA).
$Student's t-test.
&Adjusted P values using multilinear regression analysis.
NA: not applicable: variables with P > 0.2 in bivariate analysis were not included in regression analysis.
Table 5.
Association between S. haematobium intensity, P. falciparum infection, and mean (±SD) haemoglobin levels and anaemia severity.
| S. haematobium egg intensity | P. falciparum infection status | N | Mean (±SD) Hb levels |
Anaemia severity (% (n)) | |||
|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | &Significance level | ||||
| Light | Positive | 14 | 7.8 ± 1.2 | 0 (0) | 50 (7) | 50 (7) | χ 2 = 29.71; P < 0.001 |
| Negative | 50 | 9.4 ± 1.7 | 14 (7) | 72 (36) | 0 (0) | ||
|
| |||||||
| Heavy | Positive | 24 | 6.6 ± 0.3 | 0 (0) | 4.2 (1) | 95.8 (23) | χ 2 = 41.72; P < 0.001 |
| Negative | 29 | 9.3 ± 1.3 | 6.9 (2) | 72.4 (21) | 6.9 (2) | ||
|
| |||||||
| Negative | Positive | 60 | 8.3 ± 1.5 | 16.7 (10) | 76.7 (46) | 0 (0) | χ 2 = 4.2; P = 0.122 |
| Negative | 73 | 9.5 ± 1.5 | 19.2 (14) | 63.0 (46) | 0 (0) | ||
|
| |||||||
| ∗Significance level | F = 12.40; P < 0.001 | ||||||
∗Analysis of variance test (ANOVA).
&Pearson Chi-Square test.
Anaemia prevalence was 88.8% (222/250) with anaemia severity as follows: mild (13.2%; n = 33), moderate (62.8%; n = 157), and severe (12.8%; n = 32). All cases diagnosed with coinfection were anaemic (Table 6). Coinfection accounted for 93.8% (30/32) of all severe anaemia cases with majority 71.9% (23/32) of the severe anaemic cases coinfected with P. falciparum and heavy density S. haematobium infections (Table 5). Uptake of IPTp-SP (43.8%; 14/32) was associated (χ2 = 11.32; P = 0.01) with reduced percentage of severe anaemia compared with that observed among women with no SP (56.3%; 18/32). Risk factors found to be associated with increased odds of anaemia were P. falciparum infection (OR = 4.0, 95% CI: 1.0–14.5) and occupation (business) (OR = 20.1, 95% CI: 4.0–101) (Table 6).
Table 6.
Risk factors associated with anaemia among pregnant women in Munyenge Health Area.
| Factors | Category | Anaemia prevalence |
#Adjusted OR (95% CI) |
P value |
|---|---|---|---|---|
| Age (years) | < or = 20 | 92.9 (52) | NA | |
| 21–25 | 87.3 (69) | |||
| 26–30 | 90.1 (64) | |||
| >30 | 84.1 (37) | |||
| χ 2; P value | 2.21; .531 | |||
|
| ||||
| Gravidity | Primigravidity | 87 (60) | NA | |
| Secundigravidity | 94.4 (67) | |||
| Multigravidity | 86.4 (95) | |||
| χ 2; P value | 3.10; 0.212 | |||
|
| ||||
| Marital status | Single | 93.5 (58) | 0.8 (0.2–3.2) |
0.79 |
| Married |
87.2 (164) |
REF | ||
| χ 2; P value | 1.87; 0.17 | |||
|
| ||||
| Educational level | Primary | 80.5 (99) | 0.1 (0.04–0.5) | 0.001 |
| Secondary | 96.9 (123) | REF | ||
| χ 2; P value | 16.82; <0.001 | |||
|
| ||||
| Occupational status | Housewife | 80.8 (42) | 1.5 (0.5–4.0) | 0.458 |
| Business | 97.8 (91) | 20.1 (4.0–101) | 0.001 | |
| Student | 100 (33) | 6.3E8 (6.3E8–6.3E8) | — | |
| Farmer | 77.8 (56) | REF | ||
| χ 2; P value | 23.99; <0.001 | |||
|
| ||||
| P. falciparum infection status | Positive | 95.9 (94) | 4.0 (1.1–14.5) | 0.037 |
| Negative | 84.2 (128) | REF | ||
| χ 2; P value | 8.21; 0.004 | |||
|
| ||||
| IPTp-SP uptake | Yes | 86.4 (146) | 1.1 (0.3–3.8) | 0.866 |
| No | 93.8 (76) | REF | ||
| χ 2; P value | 3.05; 081 | |||
|
| ||||
| S. haematobium infection status | Positive | 90.6 (106) | NA | |
| Negative |
87.2 (116) |
|||
| χ 2; P value | 0.71; 0.40 | |||
|
| ||||
| Coinfection status | Presence |
100 (38) |
14.5E8 (0.0 - ) | 0.998 |
| Absence | 86.8 (184) | REF | ||
| χ 2; P-value | 5.65; 0.017 | |||
#Adjusted OR using multivariate regression analysis.
NA: not applicable: variables with P > 0.2 in bivariate analysis were not included in multivariate analysis.
5. Discussion
To our knowledge, this is the first study carried out on urogenital schistosomiasis among pregnant women in Cameroon. This study determined the prevalence of S. haematobium, P. falciparum, and coinfection, factors associated with these infections and assessed their relative effect on anaemia prevalence and severity among pregnant women in Munyenge. S. haematobium and P. falciparum infections are common among pregnant women living in Munyenge and their coinfection exacerbates anaemia.
The prevalence of S. haematobium infection among pregnant women in our study was 46.8%. The high prevalence reflects high exposure to infection among pregnant women living in Munyenge due to absolute dependence on natural water sources for domestic activities and bathing. Compared to the level of infection in the present study, lower prevalence of urogenital schistosomiasis among pregnant women has been reported in Nigeria by Eyo et al. [42] (23.8%) and Salawu and Odaibo [43] (20.8%). Differences in the method used for the detection of S. haematobium infection may partly explain the observed differences in rates. Although these studies attributed the lower prevalence levels of urinary schistosomiasis among pregnant women to a taboo restricting pregnant women from visiting natural water bodies [42], compared with urine filtration method used in our study, the lower sensitive centrifugation method use in the diagnosis of S. haematobium infection in the Nigerian studies may have underestimated true infection levels. Malaria is common among pregnant women in the study area with a prevalence of 39.2%. The only factor seen to be associated with malaria parasite infection in this study site was IPTp uptake. The effectiveness of IPTp-SP in the prevention of malaria in pregnancy is well established [34, 44].
For transmission of schistosomiasis to take place, the schistosomes parasite requires an avenue where it is in direct contact with the human host [9]. Pregnant women living in Munyenge get in contact with infection during activities such as laundry, plate washing, and water fetching for domestic use. In addition to domestic activities, bathing in streams poses a greater risk of infection among pregnant women in this area. Analyses from other studies have shown that regularly bathing in water sources contaminated with the developmental stages of the schistosomes parasite was associated with prevalence and intensity of schistosomiasis [45–47]. Moreover, increased risk of infection associated with the number and duration of water contact with infested waters per day has also been reported [47]. Women who reported surface-water contact at least 3 to 5 times per day were at greater risk of infection due to longer period of contact with contaminated water. Health education to instruct pregnant women to make less surface-water contact frequency and the implication of voiding their bladder in water bodies is paramount. These behavioural changes will significantly reduce the risk of S. haematobium infection among pregnant women and contamination of water sources in this setting. Ultimately, provision of portable water and improved sanitation system will play a major role in decreasing disease transmission and incidence.
Age, as observed in most schistosomiasis surveys, was a major determinant of schistosomes infection among pregnant women in our study area. The highest prevalence values of urogenital schistosomiasis were recorded in younger women (≤25 years). Individuals within ≤20 age group were found to be at a greater risk of S. haematobium infection with prevalence of 55.4%. This is in agreement with trends established in schistosomiasis surveys carried out in Cameroon [32, 48] and other parts of Africa [42, 43]. Alternatively, the decrease risk of infection observed in older age groups (>25 years) conformed to earlier reports [32, 42]. Studies have reported that age-acquired immunity to reinfection and changes in water contact patterns contribute to the declining trend in prevalence with increasing age [49]. Older women are less likely to be engaged in water contact behaviours compared to younger women. Age dependent immunity to S. haematobium has been shown to affect mean egg output of infected persons [49]. Socioeconomic status of the women was not an independent factor associated with S. haematobium prevalence in this high-risk community. Similarly, reports from other rural settings endemic for schistosomiasis failed to identify any socioeconomic variables that are strongly associated with schistosomiasis prevalence [50, 51]. The absence of association between socioeconomic variables and infection prevalence may be attributed to general poverty and uniformity in high exposure risk in the population [50].
The overall prevalence of coinfection with S. haematobium and P. falciparum infection was 15.2% suggesting coendemicity of both infections in the study area. Similarly, Yatich et al. [52] reported a helminth and malaria coinfection prevalence of 16.6% among pregnant women in Ghana. The impact of helminth infections on malaria parasitaemia and disease during coinfection is an established phenomenon although much is still unknown and contradictions persist [53, 54]. We observed that light S. haematobium infection was less common (aOR = 0.4) among pregnant women coinfected with P. falciparum suggesting a negative interaction between both parasites [4]. In accordance with findings of Getie et al. [55], schistosomiasis coinfection could affect Plasmodium parasitemia and vice versa, depending on the intensity of the ova in coinfected persons. Nonetheless, a further study is needed to explore the underlying mechanisms of interaction between malaria parasitaemia and S. haematobium.
Schistosomiasis causes long term morbidity such as anaemia. Our study showed that the magnitude of S. haematobium egg counts is significantly related to haemoglobin concentration confirming that urogenital schistosomiasis contributes to anaemia [24, 25]. In this study, anaemia was more pronounced in women with heavy infection intensity than in those with light infection. Coinfection of helminth infections and P. falciparum increases anaemia severity [8, 9]. Coinfection among pregnant women lowers Hb concentration compared with single infection. This is in agreement with findings of Okafor and Elenwo [56]. More so, coinfected women with heavy intensity S. haematobium infection had the lowest mean Hb levels (6.6 g/dL) and this subpopulation of women contributed to about 72% of all severe anaemic cases. The combined presence and interaction of S. haematobium and P. falciparum infections is partly responsible for the low haemoglobin concentration in women with concurrent infection. Malaria causes anaemia by destruction and removal of parasitized red blood cells and shortening of the life span of nonparasitized red cells as well as decreasing the rate of erythrocyte production in bone marrow [57]. The mechanism by which schistosomiasis causes anaemia is not fully understood but it is suggested that helminth infections could contribute to increase in the prevalence of inflammatory syndromes impairing erythropoiesis and interfering with mobilization of reticuloendothelial iron storages and shortening erythrocyte survival [58]. Similar to previous reports of a study in Uganda [59], malaria parasite infection was an independent factor associated with increase anaemia risk.
The risk of coinfection was associated with stream usage (bathing and domestic contact with stream) while less water contact and SP usage decreased risk of infection. This finding suggests that intervention strategies focusing on combating malaria and schistosomiasis, respectively, by increasing the uptake of IPTp-SP/doses and less water contact among pregnant women living in Munyenge represents the most appropriate prevention of coinfection with consequent increase in Hb levels.
This study had one limitation. We did not investigate the prevalence of HIV infection among the study participants. It has been shown that coinfections with helminths and malaria cause considerable morbidity in the host particularly in the presence of HIV infection [60].
To conclude, the study has indicated that S. haematobium and P. falciparum infections are common among pregnant women living in Munyenge and their coinfection is influenced by high frequencies of these parasites in the same population. The study also revealed that younger age and bathing and domestic contact with stream are independently associated with prevalence of S. haematobium infection while no IPTp-SP was associated with P. falciparum infection. Stream usage increased risk of coinfection while less water contact and SP usage decreased its risk. The fact that light S. haematobium infection was less common in P. falciparum infected women suggests that Plasmodium falciparum parasitaemia may be associated with intensity of urogenital schistosomiasis in coinfected individuals. Anaemia is a severe public health problem in pregnancy in Munyenge and coinfection with S. haematobium and P. falciparum exacerbates anaemia. Less water contact frequency and increase uptake of IPTp-SP/doses will significantly reduce risk of coinfection and consequently anaemia severity in pregnancy in this setting.
Acknowledgments
The authors are grateful to all the pregnant women who gave their consent to participate in the study. Special thanks are due to the chief medical officer, nurses, and laboratory technician of the Munyenge Health Centre for their cooperation and contribution. This study received financial support from the Ministry of Higher Education University Research Modernisation grant given to authors Judith K. Anchang-Kimbi and Eric Akum Achidi.
Abbreviations
- IPTp-SP:
Intermittent preventive treatment in pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP)
- ITN:
Insecticide treated bed nets
- HIV:
Human immunodeficiency virus
- ANC:
Antenatal care.
Competing Interests
The authors declare that they do not have any competing interests.
Authors' Contributions
Judith K. Anchang-Kimbi conceived and designed the study, analyzed the data, and wrote the manuscript. Dillys Mansoh Elad participated in the design of the study, performed the experiments, and made inputs in manuscript write-up. Gemain Taiwe Sotoing and Eric Akum Achidi supervised, reviewed, and provided inputs to the manuscript. All authors read and approved the final manuscript.
References
- 1.Petney T. N., Andrews R. H. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. International Journal for Parasitology. 1998;28(3):377–393. doi: 10.1016/s0020-7519(97)00189-6. [DOI] [PubMed] [Google Scholar]
- 2.Cox F. E. G. Concomitant infections, parasites and immune responses. Parasitology. 2001;122, supplement 1:S23–S38. doi: 10.1017/s003118200001698x. [DOI] [PubMed] [Google Scholar]
- 3.Nacher M., Singhasivanon P., Yimsamran S., et al. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. Journal of Parasitology. 2002;88(1):55–58. doi: 10.1645/0022-3395(2002)088[0055:IHIAAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Le Hesran J.-Y., Akiana J., Ndiaye E. H. M., Dia M., Senghor P., Konate L. Severe malaria attack is associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2004;98(7):397–399. doi: 10.1016/j.trstmh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Nacher M., Gay F., Singhasivanon P., et al. Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite Immunology. 2000;22(3):107–113. doi: 10.1046/j.1365-3024.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 6.Briand V., Watier L., Le Hesran J.-Y., Garcia A., Cot M. Coinfection with Plasmodium falciparum and Schistosoma haematobium: protective effect of schistosomiasis on malaria in Senegalese children? American Journal of Tropical Medicine and Hygiene. 2005;72(6):702–707. [PubMed] [Google Scholar]
- 7.Christensen N. Ø., Furu P., Kurtzhals J., Odaibo A. Heterologous synergistic interactions in concurrent experimental infection in the mouse with Schistosoma mansoni, Echinostoma revolution, Plasmodium yoelii, Babesia microti, and Trypanosoma brucei. Parasitology Research. 1988;74(6):544–551. doi: 10.1007/BF00531632. [DOI] [PubMed] [Google Scholar]
- 8.Raso G., Luginbühl A., Adjoua C. A., et al. Multiple parasite infections and their relationship to self-reported morbidity in a community of rural Côte d'Ivoire. International Journal of Epidemiology. 2004;33(5):1092–1102. doi: 10.1093/ije/dyh241. [DOI] [PubMed] [Google Scholar]
- 9.Brooker S., Akhwale W., Pullan R., et al. Epidemiology of Plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. American Journal of Tropical Medicine and Hygiene. 2007;77(6):88–98. [PMC free article] [PubMed] [Google Scholar]
- 10.Shulman C. E., Dorman E. K. Reducing childhood mortality in poor countries: importance and prevention of malaria in pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97(1):30–35. doi: 10.1016/s0035-9203(03)90012-5. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organisation. Malaria Prevention and Control during Pregnancy in the African Region. Brazzaville, Congo: WHO/AFRO; 2004. [Google Scholar]
- 12.Engels D., Chitsulo L., Montresor A., Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Tropica. 2002;82(2):139–146. doi: 10.1016/S0001-706X(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotez P. J., Kamath A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLOS Neglected Tropical Diseases. 2009;3(8, article e412) doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Der Werf M. J., De Vlas S. J., Brooker S., et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Tropica. 2003;86(2-3):125–139. doi: 10.1016/S0001-706X(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 15.Nour M. N. Schistosomias: health effect on women. Reviews in Obstetrics & Gynecology. 2010;3(1):28–32. [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman J. F., Mital P., Kanzaria H. K., Olds G. R., Kurtis J. D. Schistosomiasis and pregnancy. Trends in Parasitology. 2007;23(4):159–164. doi: 10.1016/j.pt.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 17. http://www.who.int/schistosomiasis/strategy/en/
- 18.Ntonifor H. N., Ajayi J. A. Water contact and Schistosoma haematobium infection. A case study of some communities in Toro Local Government council Area (TLGCA) of Bauchi State. Nigeria Journal of Natural and Applied Sciences. 2005;1(1):54–59. [Google Scholar]
- 19.Saotoing P., Vroumsia T., Njan A. M., Tchuenguem F. N., Messi J. Epidemiological survey of schistosomiasis due to Schistosoma haematobium in some primary schools in the town of Maroua, far north region Cameroon. International Journal of Tropical Medicine. 2011;6(2):19–24. doi: 10.3923/ijtmed.2011.19.24. [DOI] [Google Scholar]
- 20.Tchuem Tchuenté L. A., Ngassam R. I. K., Sumo L., et al. Mapping of schistosomiasis and soil- transmitted helminthiasis in the regions of centre, east and west cameroon. PLoS Neglected Tropical Diseases. 2012;6(3):p. e1553. doi: 10.1371/journal.pntd.0001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tchuem Tchuenté L. A., Dongmo N. C., Ngassam P., et al. Mapping of schistosomiasis and soil- transmitted helminthiasis in the regions of Littoral, North-West, South and South-West Cameroon and recommendations for treatment. BMC Infectious Disease. 2013;13, article 602 doi: 10.1186/1471-2334-13-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ndamukong K. J. N., Ayuk M. A., Dinga J. S., Akenji T. N., Ndiforchu V. A., Titanji V. P. K. Prevalence and intensity of urinary schistosomiasis in primary school children of the Kotto Barombi health area, Cameroon. East African Medical Journal. 2001;78(6):287–289. doi: 10.4314/eamj.v78i6.9018. [DOI] [PubMed] [Google Scholar]
- 23.Ntonifor H. N., Mbunkur G. N., Ndaleh N. W. Epidemiological survey of urinary schistosomiasis in some primary schools in a new focus behind Mount Cameroon (Munyenge), South West Region, Cameroon. East African Medical Journal. 2012;89(3):82–88. [PubMed] [Google Scholar]
- 24.Friedman J. F., Kanzaria H. K., McGarvey S. T. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends in Parasitology. 2005;21(8):386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Ajanga A., Lwambo N. J. S., Blair L., Nyandindi U., Fenwick A., Brooker S. Schistosoma mansoni in pregnancy and associations with anaemia in northwest Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100(1):59–63. doi: 10.1016/j.trstmh.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Helling-Giese G., Kjetland E. F., Gundersen S. G., et al. Schistosomiasis in women: manifestations in the upper reproductive tract. Acta Tropica. 1996;62(4):225–238. doi: 10.1016/s0001-706x(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 27.Steketee R. W., Nahlen B. L., Parise M. E., Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. American Journal of Tropical Medicine and Hygiene. 2001;64(1-2):28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 28.Laxman V. V., Adamson B., Mahmood T. Recurrent ectopic pregnancy due to Schistosoma hematobium. Journal of Obstetrics and Gynaecology. 2008;28(4):461–462. doi: 10.1080/01443610802164896. [DOI] [PubMed] [Google Scholar]
- 29.Bahrami S., Alatassi H., Slone S. P., O'Connor D. M. Tubal gestation and schistosomiasis: a case report. Journal of Reproductive Medicine for the Obstetrician and Gynecologist. 2006;51(7):595–598. [PubMed] [Google Scholar]
- 30.Mbabazi P. S., Andan O., Fitzgerald D. W., Chitsulo L., Engels D., Downs J. A. Examining the relationship between urogenital schistosomiasis and HIV infection. PLoS Neglected Tropical Diseases. 2011;5(12) doi: 10.1371/journal.pntd.0001396.e1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tweyongyere R., Mawa P. A., Emojong N. O., et al. Effect of praziquantel treatment of Schistosomamansoni during pregnancy on intensity of infection and antibody responses to schistosome antigens: results of a randomised, placebo-controlled trial. BMC Infectious Diseases. 2009;9, article 32 doi: 10.1186/1471-2334-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ntonifor H. N., Green A. E., Bopda M. O. S., et al. Epidemiology of urinary schistosomiasis and soil transmitted helminthiasis in a recently established focus behind Mount Cameroon. International Journal of Current Microbiology and Applied Sciences. 2015;4(3):1056–1066. [Google Scholar]
- 33.Wanji S., Kengne-Ouafo A. J., Joan Eyong E. E., et al. Genetic diversity of Plasmodium falciparum merozoite surface protein-1 block 2 in sites of contrasting altitudes and malaria endemicities in the Mount Cameroon Region. American Journal of Tropical Medicine and Hygiene. 2012;86(5):764–774. doi: 10.4269/ajtmh.2012.11-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anchang-Kimbi J. K., Achidi E. A., Nkegoum B., Sverremark-Ekström E., Troye-Blomberg M. Diagnostic comparison of malaria infection in peripheral blood, placental blood and placental biopsies in Cameroonian parturient women. Malaria Journal. 2009;8(1, article 126) doi: 10.1186/1475-2875-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryan F. J. The Design and Analysis of Research Studies. Cambridge, UK: University of Otago, Dunedin, New Zealand; Cambridge University Press; 1992. [Google Scholar]
- 36.World Health Organisation. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva, Switzerland: WHO; 2011. (Vitamin and mineral Nutrition Information System). [Google Scholar]
- 37.Cheesbrough M. District Laboratory Practice in Tropical Countries. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- 38.Moody A. Rapid diagnostic tests for malaria parasites. Clinical Microbiology Reviews. 2002;15(1):66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christensen N. O., Gotsche G., Frandsen F. Parasitological Techniques for Use in Routine Laboratory Maintainance of Schistosomes and Used in Studies on the Epidemiology of Human and Bovine Schistosomiasis. Danish Bilhaziasis Laboratory Manual; 1984. [Google Scholar]
- 40. World Health Organization—Tropical Disease Research, TDR strategic direction: Schistosomiasis, WHO-TDR, 2002.
- 41.World Health Organization. Guidelines for the Evaluation of Soil Transmitted Helminthiasis and Schistosomiasis at Community Level: A Guide for Managers of Control Programme. Geneva, Switzerland: WHO; 1993. [Google Scholar]
- 42.Eyo J. E., Onyishi G. C., Okafor F. C. Urinary schistosomiasis among pregnant women in some endemic tropical semi-urban communities of Anambra State, Nigeria. Tropical Biomedicine. 2012;29(4):575–579. [PubMed] [Google Scholar]
- 43.Salawu O. T., Odaibo A. B. Schistosomiasis among pregnant women in rural communities in Nigeria. International Journal of Gynecology and Obstetrics. 2013;122(1):1–4. doi: 10.1016/j.ijgo.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 44.Tan K. R., Katalenich B. L., Mace K. E., et al. Efficacy of sulphadoxine-pyrimethamine for intermittent preventive treatment of malaria in pregnancy, Mansa, Zambia. Malaria Journal. 2014;13(1, article 227) doi: 10.1186/1475-2875-13-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bethony J., Williams J. T., Kloos H., et al. Exposure to Schistosoma mansoni infection in a rural area in Brazil. II: household risk factors. Tropical Medicine and International Health. 2001;6(2):136–145. doi: 10.1046/j.1365-3156.2001.00685.x. [DOI] [PubMed] [Google Scholar]
- 46.Sousa-Figueiredo J. C., Gamboa D., Pedro J. M., et al. Epidemiology of malaria, schistosomiasis, geohelminths, anemia and malnutrition in the context of a demographic surveillance system in northern Angola. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0033189.e33189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anto F., Asoala V., Adjuik M., et al. Water contact activities and prevalence of schistosomiasis infection among school-age children in communities along an irrigation scheme in Rural Northern Ghana. Journal of Bacteriology & Parasitology. 2013;4, article 177 doi: 10.4172/2155-9597.1000177. [DOI] [Google Scholar]
- 48.Tchuem Tchuenté L.-A., Behnke J. M., Gilbert F. S., Southgate V. R., Vercruysse J. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Tropical Medicine and International Health. 2003;8(11):975–986. doi: 10.1046/j.1360-2276.2003.01120.x. [DOI] [PubMed] [Google Scholar]
- 49.Etard J.-F., Audibert M., Dabo A. Age-acquired resistance and predisposition to reinfection with Schistosoma haematobium after treatment with praziquantel in Mali. The American Journal of Tropical Medicine and Hygiene. 1995;52(6):549–558. doi: 10.4269/ajtmh.1995.52.549. [DOI] [PubMed] [Google Scholar]
- 50.Gazzinelli A., Velasquez-Melendez G., Crawford S. B., LoVerde P. T., Correa-Oliveira R., Kloos H. Socioeconomic determinants of schistosomiasis in a poor rural area in Brazil. Acta Tropica. 2006;99(2-3):260–271. doi: 10.1016/j.actatropica.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kapito-Tembo A. P., Mwapasa V., Meshnick S. R., et al. Prevalence distribution and risk factors for Schistosoma hematobium infection among school children in Blantyre, Malawi. PLoS Neglected Tropical Diseases. 2009;3(1, article e361) doi: 10.1371/journal.pntd.0000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yatich N. J., Yi J., Agbenyega T., et al. Malaria and intestinal helminth co-infection among pregnant women in Ghana: prevalence and risk factors. The American Journal of Tropical Medicine and Hygiene. 2009;80(6):896–901. [PubMed] [Google Scholar]
- 53.Nacher M. Interactions between worms and malaria: good worms or bad worms? Malaria Journal. 2011;10, article no. 259 doi: 10.1186/1475-2875-10-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adegnika A. A., Kremsner P. G. Epidemiology of malaria and helminth interaction: a review from 2001 to 2011. Current Opinion in HIV and AIDS. 2012;7(3):221–224. doi: 10.1097/coh.0b013e3283524d90. [DOI] [PubMed] [Google Scholar]
- 55.Getie S., Wondimeneh Y., Getnet G., et al. Prevalence and clinical correlates of Schistosoma mansoni co-infection among malaria infected patients, Northwest Ethiopia. BMC Research Notes. 2015;8(1, article no. 480) doi: 10.1186/s13104-015-1468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okafor E., Elenwo A. Haemoglobin status of children with mixed infection of malaria and urinary schistosomiasis in Odau Community, Rivers state, Nigeria. Journal of Agriculture and Social Research. 2008;7(1):56–62. doi: 10.4314/jasr.v7i1.2844. [DOI] [Google Scholar]
- 57.McDevitt M. A., Xie J., Gordeuk V., Bucala R. The anemia of malaria infection: role of inflammatory cytokines. Current Hematology Reports. 2004;3(2):97–106. [PubMed] [Google Scholar]
- 58.Shaw J. G., Friedman J. F. Iron deficiency anemia: focus on infectious diseases in lesser developed countries. Anemia. 2011;2011:10. doi: 10.1155/2011/260380.260380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green H. K., Sousa-Figueiredo J. C., Basáñez M.-G., et al. Anaemia in Ugandan preschool-aged children: the relative contribution of intestinal parasites and malaria. Parasitology. 2011;138(12):1534–1545. doi: 10.1017/s0031182011001016. [DOI] [PubMed] [Google Scholar]
- 60.Ivan E., Crowther N. J., Mutimura E., Osuwat L. O., Janssen S., Grobusch M. P. Helminthic infections rates and malaria in HIV-infected pregnant women on anti-retroviral therapy in Rwanda. PLoS Neglected Tropical Diseases. 2013;7(8) doi: 10.1371/journal.pntd.0002380.e2380 [DOI] [PMC free article] [PubMed] [Google Scholar]


