Abstract
Early exposure to stressful life events plays a significant role in adolescent depression. Clinical studies have identified a number of factors that increase the risk of depression, including sex of the subject, duration of the stressor, and genetic polymorphisms that elevate serotonin levels. In this study we used the maternal separation (MS) model to investigate to what extent these factors interacted during development to manifest in depressive-like behavior in male and female rats. The triadic model of learned helplessness parses depressive-like behavior into aspects of controllable, uncontrollable, and motivational behaviors. This model was used to investigate how the timing of MS between the ages of postnatal day (P) 2–9 and P9–16 interacted with either simultaneous vehicle (saline; 1 ml/kg; i.p.) or fluoxetine (10 mg/kg) exposure, which was used to enhance serotonin levels; these experiments also compared the effect of a vehicle injection during these developmental periods to a no injection control. Vehicle injections alone increased helplessness in the controllable condition in male rats when injected between P9–16 only, and did not interact further with MS. MS at both ages decreased controllability in male adolescents; females demonstrated an increase in controllability after MS. Elevated serotonin at P2–9 increased escape latencies in male and female control and MS subjects. Fluoxetine exposure at P9–16 increased helplessness in controls. Fluoxetine decreased helplessness in MS males independent of age, but increases helplessness in MS females. This study highlights the importance of age of MS (MS between P2–9 increases helplessness in males more than females), the duration of the stressor (previous results show females are effected by longer MS [P2–20], but not shorter [this study]), and that elevated serotonin increases escape latencies to a greater extent in females.
Keywords: abuse, adolescence, depression, fluoxetine, maternal separation, stress
INTRODUCTION
Early childhood traumatic events (physical and sexual abuse, neglect, loss of a caregiver, or natural disaster) are associated with the emergence of depression during adolescence (Putnam, 2003; Kendler et al., 2004; Andersen and Teicher, 2008; Teicher et al., 2009). Not every child who experiences an adverse environment will become depressed (Copeland et al., 2007), but those that do become depressed are more likely to experience more adverse outcomes if the duration of abuse was longer (Teicher et al., 2009). While clinical studies have highlighted a number of other risk factors for abuse-associated depression, including age of abuse, duration of abuse, gender, and elevated serotonin levels, preclinical research lags behind in comparable investigations. The current study was designed to determine how these factors modulate depressive-like behavior in a rat model of early life stress.
First, the timing of exposure to an adverse event/environment during a sensitive period of brain development has a selective impact on both behavior and the brain (Andersen and Teicher, 2008; Andersen et al., 2008). For example, abusive episodes that occur before 12 years of age are associated with depression, whereas episodes that occur after 12 years are more frequently associated with posttraumatic stress disorder (Schoedl et al., 2010). Neuroanatomical studies show that abuse prior to puberty has more selective effects on the hippocampus, whereas abuse after puberty appears more selective for the prefrontal cortex (Andersen and Teicher, 2008). While previous animal research parallels these neuroanatomical findings (Leussis and Andersen, 2008; Leussis et al., 2012), different windows of early stress exposure have only been examined in a single animal study (Lehmann et al., 1999). Animal models of early life stress include the maternal separation model (MS) of removing the pups from the dam (Plotsky and Meaney, 1993; Mourlon et al., 2010; Reus et al., 2011; Leussis et al., 2012), communal nesting (Macri et al., 2010), or reducing nest bedding (Raineki et al., 2012). Variations exist within the MS literature as well. Paradigms of MS include removal of the whole litter from the dam or individual isolation; separation for 24 h only at postnatal day 2 (P2), 3 h a day between P2–9, or 4 h a day between P2–20. Social isolation of animals after weaning is a model for later, adolescent stress. Because a myriad of MS paradigms use shorter separation periods than our extended P2–20 period, one of the goals of the study was to determine whether a shorter MS duration would influence depressive-like behavior, as well as address whether the age of onset (e.g., P2 versus P9) produces depressive-like behavior.
The second goal of these studies was to determine what role gender plays in the manifestation of depressive-like behaviors. While women are more susceptible to depressive disorders, males are more vulnerable to the sequelae of abuse. Neuroanatomical studies show that males have greater decreases in hippocampus and corpus callosum size than females (De Bellis and Keshavan, 2003; Teicher et al., 2004). Boys with a history of maltreatment demonstrated higher diurnal cortisol levels than girls with a history of maltreatment (Doom et al., 2013). Finally, a unique sexually dimorphic relationship exists between the type of early life experiences and outcome. In a prospective study of at-risk children and adolescents, Duggal and colleagues found that depressive symptoms in female teens are more associated with maternal depressive behavior, whereas males were more strongly affected by changes in supportive early care (Duggal et al., 2001). In the MS model, maternal behavior can become more disrupted by the separation and causes the dams to show more disorganized care-taking behavior (Macri et al., 2004), suggesting that the males may be more vulnerable in this model. Research shows that MS between P2–20 produces sex-dependent effects when depressive-like behaviors were parsed into different aspects of learned helplessness (LH) with the triadic model developed by Maier and colleagues (Maier and Watkins, 2005; Pryce et al., 2011). Long exposure MS (e.g., P2–20) significantly impaired the controllability aspects of depression in adolescent males, but had less of an effect in MS females during adolescence (Leussis et al., 2012). MS did not differentially affect escape latencies or failures in the condition where the loss of control over a stressor is paramount, but females were impaired in their first exposure to a stressor, which may indicate changes in motivation to escape (Pryce et al., 2011).
The third aspect to be investigated in the current study is what role, if any, elevated levels of serotonin play to enhance or attenuate the effects of MS – when they occur simultaneously. Clinical studies have shown that genetic predisposition may increase the vulnerability to depression in abuse cases. For example, changes in the serotonin transporter that render it less sensitive (e.g., the serotonin transporter linked promotor region; 5-HTTLRP) have been associated with increased adverse outcomes in abused populations (Caspi et al., 2002, 2003; Somaini et al., 2011). Increased serotonin levels caused by fluoxetine exposure during pregnancy or the nursing period have been shown to impact physiological mechanisms (Morrison et al., 2005), but little is known about the impact on depressive behavior later in life. Manipulations of serotonin levels early in life may or may not lead to elevated depressive-like behavior in mice (Ansorge et al., 2004) or rats (Karpova et al., 2009), respectively. In the current study, the interaction between simultaneous MS and elevated serotonin levels was investigated in rats.
The following studies were undertaken to further understand what factors of MS and the underlying neurobiology contribute to increased LH during adolescence. First, we investigated the effects of both duration and developmental timing of MS exposure. Preclinical work has shown that MS during early life (P4 for 24 h in rats) increases active avoidance in males, MS at P9 and 18 has minimal effect, and females are not affected regardless of the stage of MS exposure (Lehmann et al., 1999). While the active avoidance model might be one of the most common paradigms to test depressive-like behavior, this paradigm examines the loss of control over a stressor only. Depression can manifest at multiple levels, including a loss of resilience or anhedonia/attenuated motivation (Pryce et al., 2011). The triadic model incorporates these aspects, respectively, into the design by including an escapable group (where training on day 1 allows the animal to develop resilience) and a no shock group. The second advantage of the model is that training and testing in the triadic model take place in two different environments, unlike the active avoidance or the forced swim model. The studies presented here investigated the effects of MS between P2–9 (MS2) and MS between 9–16 (MS9) on LH using the triadic model. Second, we investigated how serotonin interacts with MS by exposing subjects to the selective serotonin reuptake inhibitor fluoxetine. Preclinical studies are equivocal, and mice (Ansorge et al., 2004), but not rats (Karpova et al., 2009), show differential effects of serotonin on depressive behaviors (reviewed by Wieck et al. (2013)). Here, we used fluoxetine to increase extracellular levels of serotonin to determine if affective behavior is uniquely modulated by exposure to stress and/or serotonin during specific developmental periods. For this second study, fluoxetine was administered during MS2 and MS9 exposure windows and subjects were tested for LH in adolescence.
EXPERIMENTAL PROCEDURES
Subjects
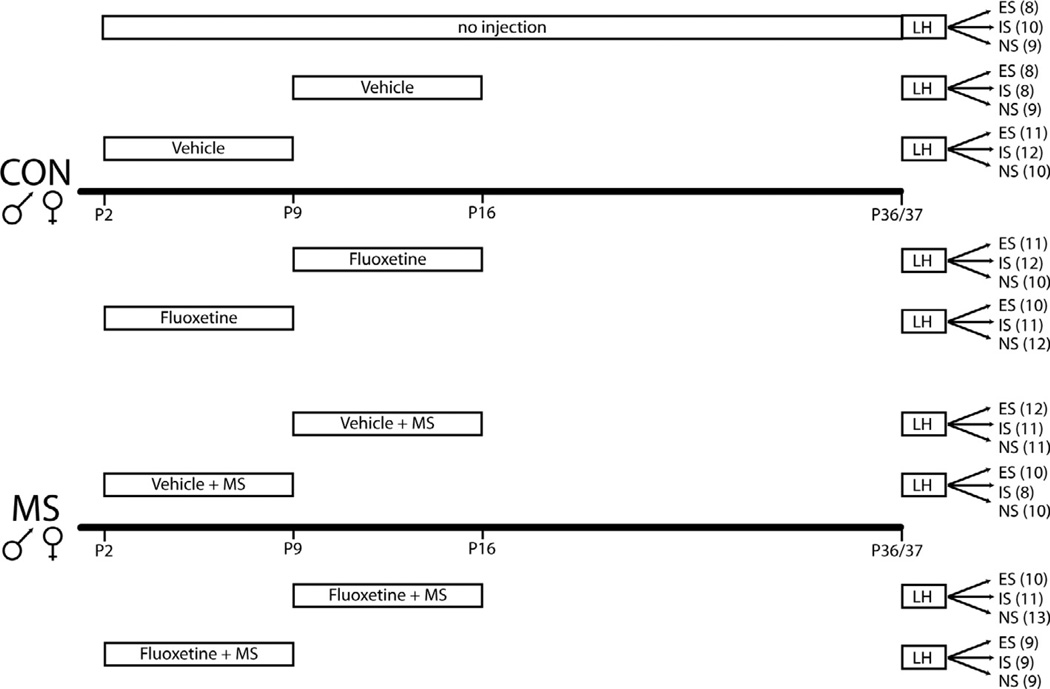
Pregnant multiparous Sprague–Dawley females (250–275 g) were obtained from the Charles River Laboratories (Wilmington, MA, USA) on day 13 of gestation. With the day of birth designated as postnatal day 0 (P0), litters were culled to 10 pups (five males and five females) on P2. Rats were housed with food and water available ad libitum in constant temperature and humidity conditions on a 12-h light/dark cycle (light period 0700–1900). Rats were weaned on P21–22, and group-housed in same-sex caging with 3–4 rats/cage until experimentation. Only one rat per litter was used per condition to avoid litter effects. Subjects were tested during early adolescence (P36–38) and the sample size was n = 4–7/condition. The experimental groups and sample sizes per condition are illustrated in Fig. 1 and their weight gain for the groups is shown in Fig. 2. All these experiments were conducted in accordance with the 1996 Guide for the Care and Use of Laboratory Animals (NIH) and were approved by the Institutional Animal Care and Use Committee at the McLean Hospital.
Fig. 1.
The experimental design. Animals were assigned to a No injection condition, a control condition (CON) or maternal separation (MS) condition. They were then treated with saline vehicle or fluoxetine (with the exception of the No injection condition) and tested for depressive behavior under escapable (ES), inescapable (IS) or a no shock (NS) condition. The number of subjects is shown in parentheses.
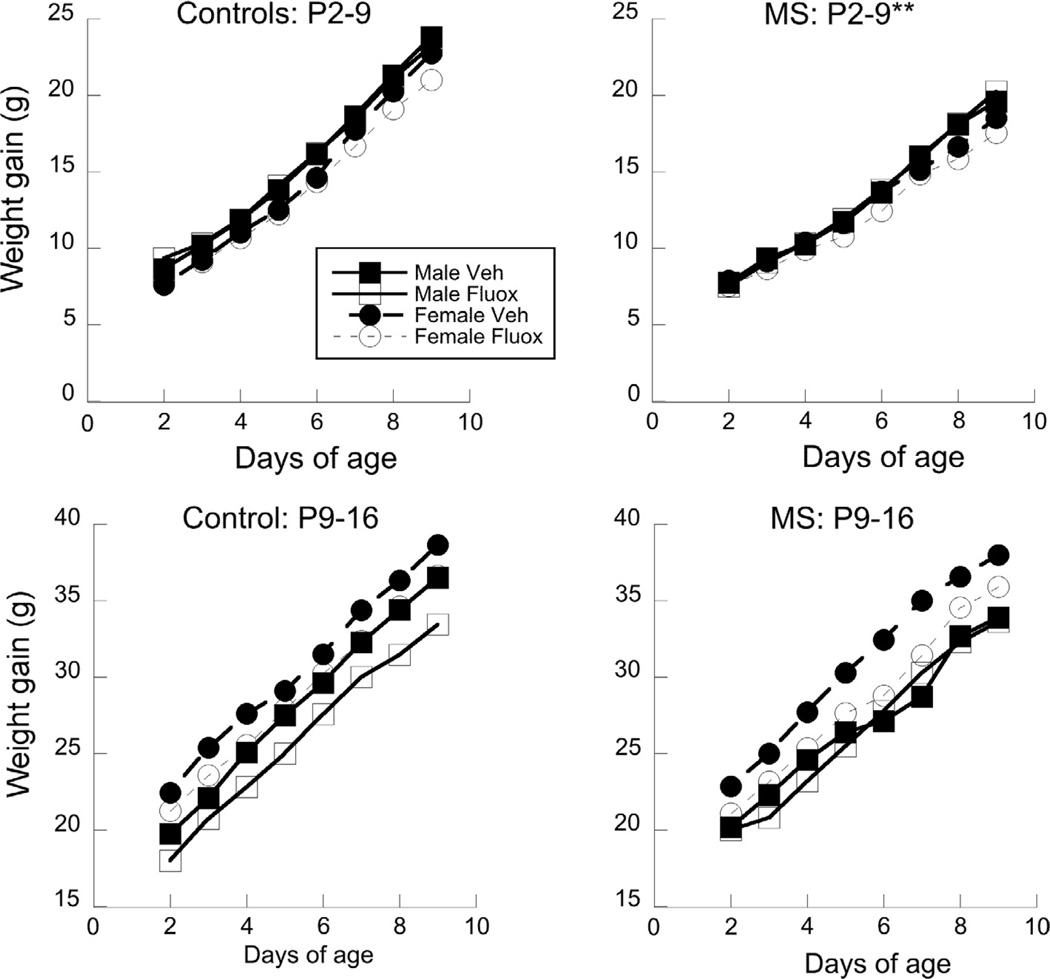
Fig. 2.
Weight gain across nine days of MS (or control conditions) is shown across Sex and Drug. **P < 0.05 indicating a significant difference between MS and Control. Means only are presented for clarity.
Effect of injection
Litters were randomly assigned to an animal facility reared control group (No injection Group), or one of the two injection groups. Pups received a saline injection (1 ml/kg, i.p., delivered with a Hamilton syringe) once daily between P2–9 (CON2) or between P9–16 (CON9). Pups were temporarily removed from the dam to receive the injection and placed immediately back into the home cage. The No injection group underwent routine cage changes.
Effect of age of MS
Additional litters were randomly assigned to an animal facility reared control group (CON Group) or one of two MS groups. Pups were individually isolated for 4 h per day in isolation cups placed in a 37 °C water bath between P2–9 (MS2) or between P9–16 (MS9). The dam was kept nearby in the colony room. This procedure is identical to procedures used previously by this laboratory (Andersen et al., 1999; Andersen and Teicher, 2004) and similar to others (Plotsky and Meaney, 1993). All subjects in this experiment, both CON and MS, underwent routine weekly changes in cage bedding and were weighed daily before receiving either a saline (1 ml/kg, i.p.) or fluoxetine (10 mg/kg, i.p.) injection. MS subjects received their injection prior to separation. The fluoxetine dose is based on studies in mice where 10 mg/kg increased depressive-like behavior in adulthood (Ansorge et al., 2004).
Learned helplessness (LH)
Rats were tested in the LH triadic paradigm between P36 and P38 to assess the role of controllability of stress on behavior (Maier and Watkins, 2005). Subjects were assigned to a triad consisting of (1) an inescapable shock (IS) subject; (2) an escapable shock (ES) subject; and (3) a no shock (NS) control subject according to our previous methods (Leussis and Andersen, 2008; Leussis et al., 2012). One hundred trials of a tail shock (1.0 mA for trials 1–30, 1.3 mA for trials 31–60, and 1.6 mA for trials 61–100) in a wheel-turn box condition (Drugan, 2001). Unsignaled shocks were delivered on a variable time 45-s schedule (range 30–60 s). While the ES subject could turn a wheel to terminate the shock, the IS subject was yoked to the ES subject and thus received the same amount of shock as the ES subject. The IS subject could not terminate the shock, which continued until the ES rat completed the wheel-turn response or a maximum of 30 s. The NS rat was restrained in a wheel-turn box but received no shock and was used to assess generalized deficits in learning or motivation on Day 2. On Day 2, each subject was placed into a shuttlebox (Med Associates Inc., St. Albans, VT, USA). Rats from all three conditions could terminate a 1 mA footshock by shuttling to the other side for trials 1–5, or by shuttling to the other side and back again for trials 6–30. This response was cued by a tone that preceded the shock by 2 s. The shock remained on for 30 s, or until terminated by the appropriate behavioral response. Consistent with previous studies (Drugan, 2001) data from the first five trials were not used in the analyses as subjects were learning the appropriate behavioral response. The number of escape failures and the mean latency to escape was reported for trials 6–30. Groups were counterbalanced on Day 1 to account for any learning differences in escape responding.
RESULTS
Changes in weight gain following LH and drug
Weight analyses were conducted separately between P2 and P9 groups. In the P2 groups, weight gain differentially changed across maturation age, which interacted with both MS and Drug (F(7, 69) = 32.96 and 9.12, P < 0.001). These data are shown in Fig. 2. In the P9 groups, an interaction between Age × MS was observed (F(7, 70) = 2.05, P < 0.05). However, the main effect relationships were stronger than the P2 group. Drug had a significant effect (F(1, 70) = 10.25, P < 0.01), where fluoxetine animals gained less weight than vehicle controls. Sex was also a significant variable (F(1, 70) = 4.72, P < 0.05) such that females showed more weight gain than the males.
The interaction of age and saline injections on LH
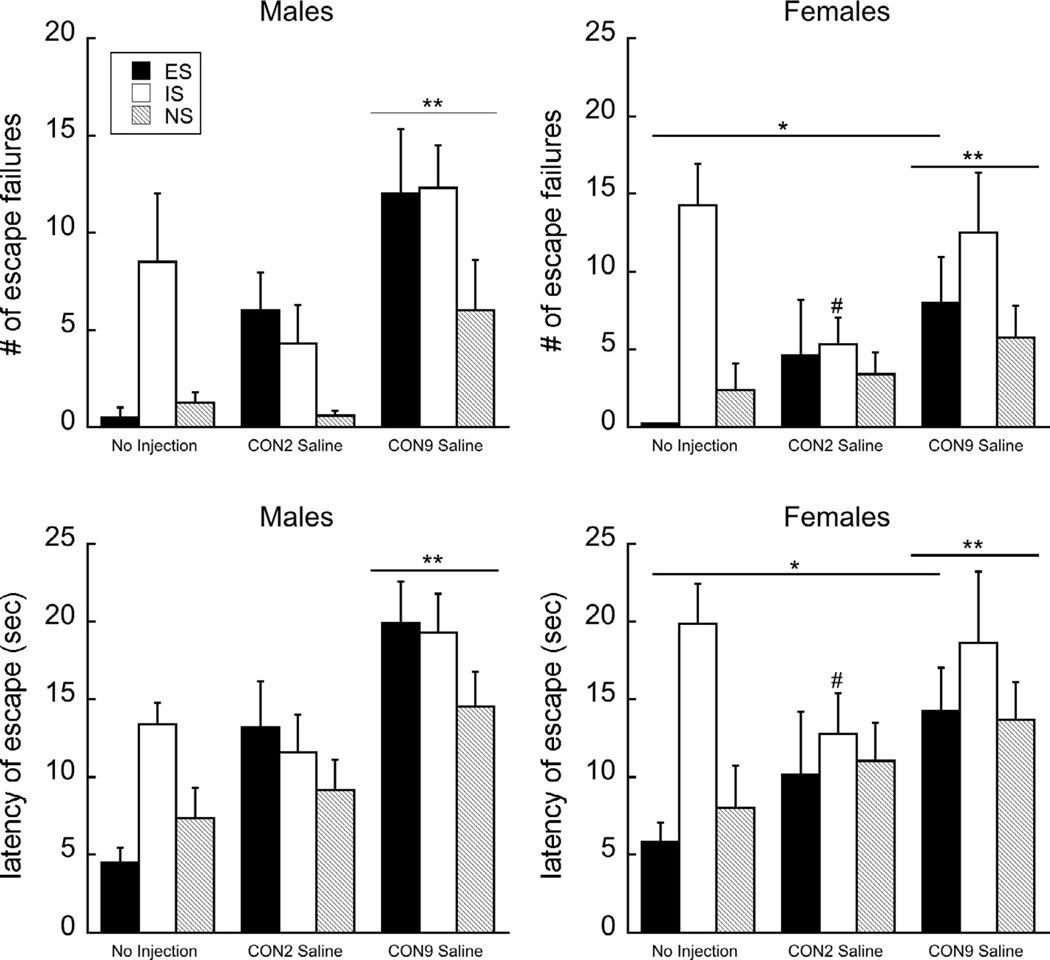
To initially determine whether saline injections effect depressive-like behavior, we used a three factor analysis of variance (ANOVA) to compare Injection (CON2, CON9, to No injection group) × Sex × Triad (ES, IS, NS). A two-way interaction of Injection × Triad was observed (F(4, 60) = 3.11 and 2.56, P < 0.05 for the number of escape failures and escape latency, respectively; Fig. 3). Bonferroni post hoc corrected comparisons revealed that CON2 saline injections did not significantly differ from No injections (P = 1.0), while CON9 significantly differed from both the CON2 and the No injection group for both failures and latencies (P < 0.005s). Within the triad, ES was significantly elevated by the saline injection at CON9 relative to the No injection group only (Bonferroni corrected P’s = 0.005 and 0.003 for failures and latency). The IS group at CON2 was significantly lower than either group (P < 0.05 for both measures), whereas no effect was observed in the NS group. Sex did not significantly interact at any level (P’s > 0.2–0.9).
Fig. 3.
The effect of injection on depressive-like behavior. In both males (left) and females (right), the timing of a saline injection at either P2–9 in Control subjects (CON2) or P9–16 (CON9) had differential effects on behavior. *P < 0.05; showing comparisons between the ES group specifically; #P < 0.05 indicates a difference between CON2 IS and the other IS groups; **P < 0.05 indicates that the CON9 group, independent of triad condition, differed from the No injection and the CON2 groups. Means ± SEM are presented.
The influence of MS
To determine the general influence of MS, we first analyzed the effect of MS in saline-injected animals only. The interaction MS (MS, non-MS) × Age × Sex × Triad was significant for escape failures (F(14,96) = 1.81, P < 0.05), while only the two-way interaction (MS × Age: F(1, 96) = 4.44, P < 0.04) was significant. As the main effect for Age was significant for both measures (F(1,96) = 10.74 and 6.54, P < 0.02), we examined the different age groups separately.
MS at P2
Escape failure and latency were both significantly effected by a MS × Sex interaction (F(1, 49) = 6.85 and 4.26, P < 0.05) with only females showing that MS significantly reduced escape failures relative to controls (F(1, 23) = 5.21, P < 0.03). The influence of MS plus saline injection at P2:
We compared the MS, saline-injected animals to non-injected, control subjects to reveal whether the decrease in escape failures or latencies in MS subjects is a general effect or just in comparison to saline-injected animals. A significant MS × Sex × Triad interaction for escape failures was found (F(4,42) = 3.03, P < 0.03), while the two-way interactions were evident for escape latencies (MS × Sex and MS × Triad: F(2, 42) = 8.29 and 13.23, P < 0.001). The female MS–IS group (and saline-injected) showed less escape failures and shorter latency compared to non-injected, non-MS controls (F(1, 6) = 21.26 and 14, P < 0.01). The male ES group was more impaired in terms of escape behavior and latency after MS relative to the non-injected subjects (F(1, 7) = 16.57 and 26.48, P < 0.007).
The influence of MS at P9
A main effect for MS was found with less escape failures as well as a lower escape latency in MS subjects relative to CON9 subjects (F(1, 47) = 10.15 and 10.82, P < 0.003). The influence of MS plus saline injection at P9: The maternally separated (MS9), saline-injected animals to non-injected, subjects were compared. A MS × Sex × Triad interaction was found for escape failures (F(4, 49) = 2.55, P = 0.05) and a MS × Triad interaction for latencies (F(2, 49) = 4.85, P < 0.02). Females in the IS group conduct less escape failures after MS (and saline injection) (F(1, 7) = 33.93 and 29.64, P < 0.002).
The interaction of MS, age, sex, and fluoxetine on LH
The initial analysis of this five-way interaction was not significant (P > 0.5). However, a four-way interaction of MS × Age × Sex × Triad was significant for escape failures (F(2,230) = 2.9, P = 0.05), but not for latency (P = 0.13; Figs. 4 and 5). The three-way interaction of MS × Age × Sex was significant for both measures (F(1,230) = 4.61 and 4.75, P < 0.05, failures and latency respectively; Bonferroni corrected). In an effort to further understand this complex relationship, the data were analyzed separately for males and females.
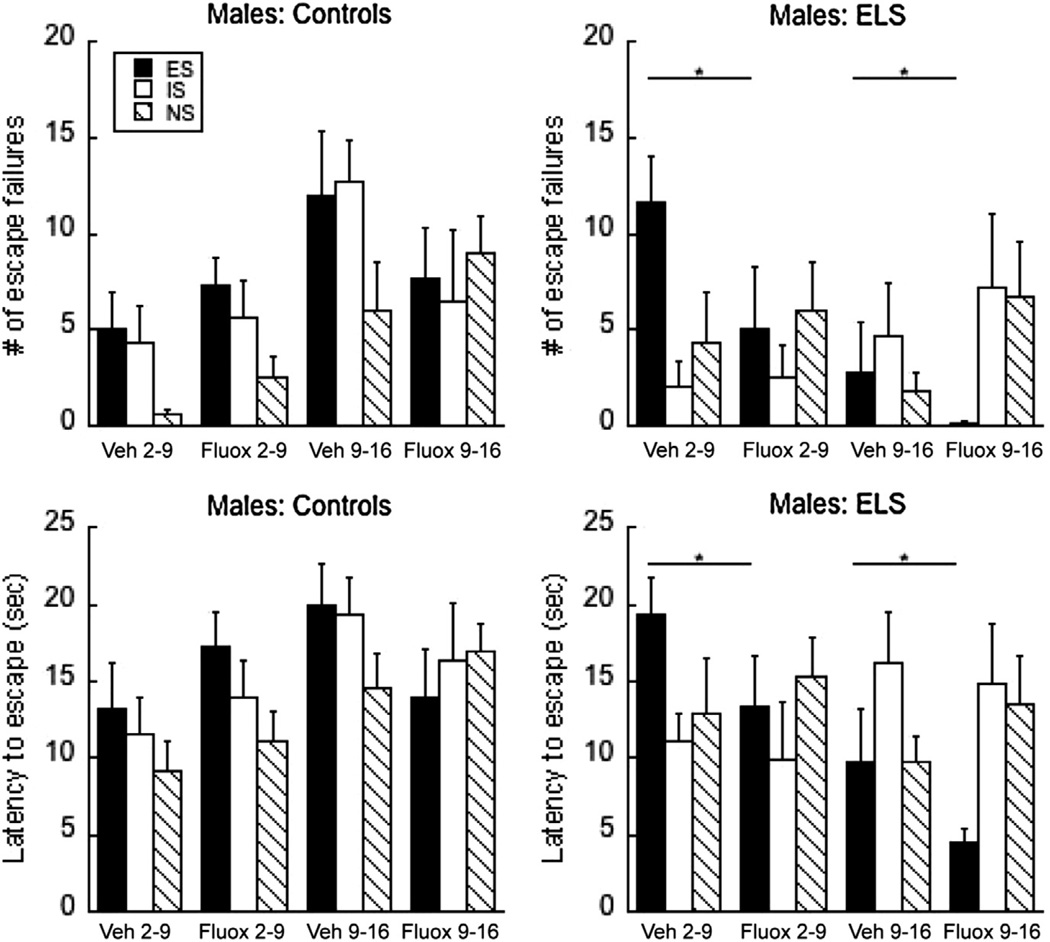
Fig. 4.
The effect of vehicle or fluoxetine on MS2 and MS9 on adolescent learned helplessness in males. Escape failures (top) or escape latencies (bottom) following simultaneous exposure to vehicle or 10 mg/kg fluoxetine in Controls or during the MS paradigm. *P < 0.05 within the ES condition alone. Means ± SEM are presented.
Fig. 5.
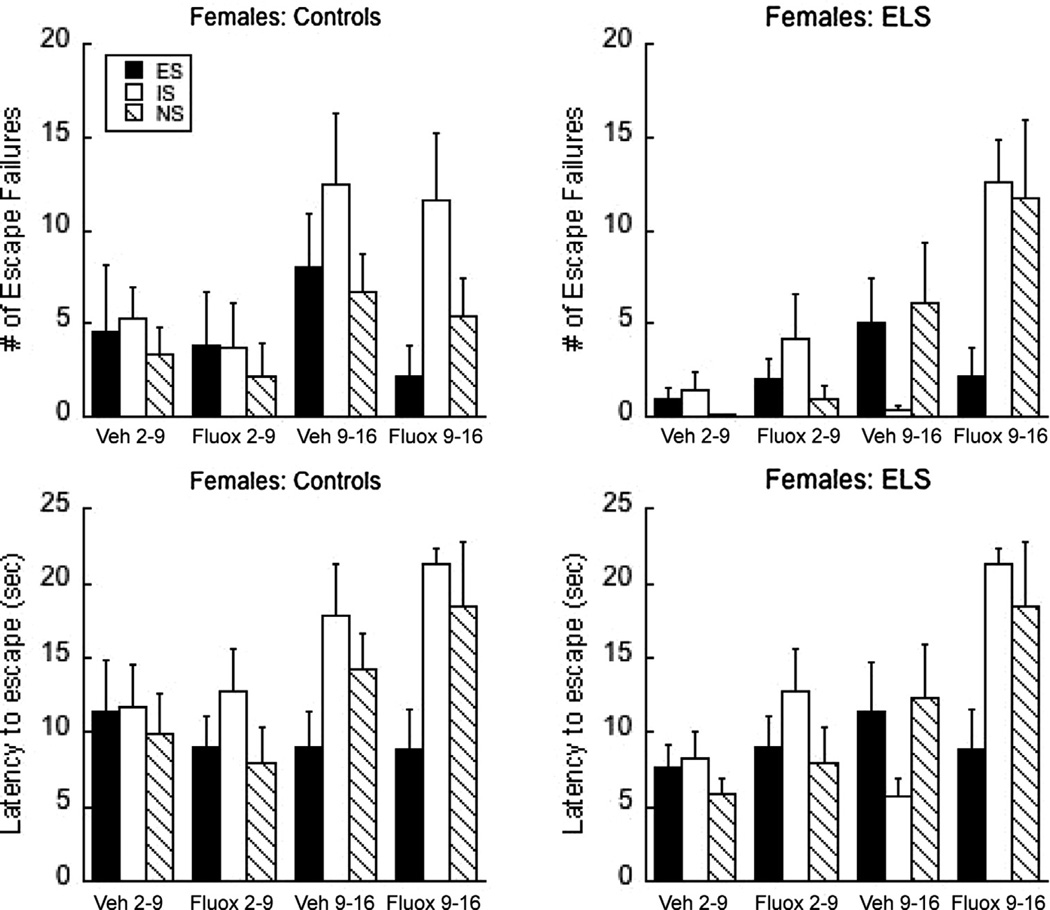
The effect of vehicle or fluoxetine on MS2 and MS9 on adolescent learned helplessness in females. Escape failures (top) or escape latencies (bottom) following simultaneous exposure to vehicle or 10 mg/kg fluoxetine in Controls or during the MS paradigm. Means ± SEM are presented.
Interaction of MS, age, and fluoxetine in males
The analysis of the males revealed a three-way interaction of MS × Age × Drug (F(2,104) = 3.01, P < 0.05 for escape failures; latency achieved a P = 0.07). Multiple two-way interactions were significant, most notably MS × Age (F(1,104) = 5.83; 7.57, P’s < 0.02), and Age × triad (F(2,104) = 3.74, 3.68, P < 0.03). Finally, Drug × triad was also significant (F(2,104) = 3.52, 3.78, P < 0.03). In order to determine which part of the triad is influenced by Drug, as shown in Fig. 4, each part of the triad was analyzed separately, and the P values were adjusted for post hoc analyses.
ES in males
As evident in the ES group, fluoxetine significantly improved both escape failures and latencies (F(1, 33) = 5.89 and 5.44, P = 0.02). In addition, an MS × Age interacted for both measures (P < 0.005). CON9 animals were more impaired in the saline condition compared with CON2 saline animals in the ES condition; fluoxetine effectively reduced LH in the MS group only, independent of Age (Drug: P < 0.05).
IS and NS in males
In the IS group fluoxetine had no significant effect (P > 0.5) but the age of manipulation significantly influenced escape failures and latencies with animals treated at P9 showing more helplessness than at P2 (P < 0.01). None of the manipulations exerted a significant effect on NS in the males.
Interaction of MS, Age, and fluoxetine in females
None of the interactions were significant, although a number of main effects were significant (Fig. 5). In the females, a main effect of Age was observed (F(1,125) = 20.28 and 13.05, P < 0.0001 for failures and latency respectively). A main effect of Triad was also significant (F(2,125) = 5.10 and 5.08, P < 0.01). The effects of MS marginally interacted with Drug for escape failures (F(1,125) = 3.46, P = 0.06). In females, exposure to fluoxetine improves escape behavior in CON animals but impairs escapes in MS animals.
DISCUSSION
Exposure to early adversity can alter behavior later in life. This study was undertaken to determine the factors that influence complex depressive-like behaviors using the triadic design of learned helplessness. In the set of experiments described, we found that saline injections once a day are sufficient to increase helplessness in the ES condition (Fig. 3). The effect was greatest when saline was administered between P9–16 (CON9) in comparison with No injection or administration between P2–9 (CON2). In contrast, helplessness was reduced in the CON2 IS condition compared to non-injected and CON9 animals.
We further investigated if additional stress induced by MS further influences behavior. Interestingly, MS had no adverse effects but seems to be beneficial when compared to animals that were exposed to injection stress alone. Finally, to better determine the sex-dependency of these effects, comparisons were made between the non-injected subjects to both CON and MS subjects. While stress increases depressive-like behavior in ES males, IS females show reduced depressive-like behavior. This reduction of depressive-like behavior in females can also be found in the IS group from MS9.
In contrast, exposure to MS with simultaneous saline or fluoxetine administration also yielded sex-dependent results. MS2 in males increased helplessness (in saline-injected subjects), and this effect was attenuated by fluoxetine; fluoxetine was ineffective in CON2 subjects. In females, age also exerted a significant overall effect, such that behavior overall was more impaired when the manipulations occurred at P9. In contrast to the males, fluoxetine exposure improves escape behavior in CON animals, but impairs it in MS animals.
The MS paradigm has been used to understand how exposure to early adversity can alter behavior later in life. Multiple variations of this paradigm have been used that range from 24 h, single-day separations to our more strenuous 4 h/day for 19 days (Brenhouse and Andersen, 2011a; Leussis et al., 2012). Even separation for 15 min or just moving the pups within the cage changes behavior later in adulthood (Jaworski et al., 2005). In the same way, P9–16 pups taken away from the dam for injections for about 5 min show depressive-like behavior. This time may have been sufficient to disorganize care-taking behavior that when compounded with the injection stress induced behavioral changes. Additional MS had no further adverse effects, and instead, decreased depressive-like behavior. A possible explanation could be that the short separation for the injection is more stressful for the dam and pups than a longer separation after the injection. Comparison of the maternally separated (MS) and CON animals to non-treated animals revealed that the protective effect is evident. This effect is sex dependent. ES males show increased depressive-like behavior after maternal separation between P2–9 (MS2). This increase can also be seen when comparing vehicle-injected MS and non-MS males, but as the vehicle injection itself impairs the animals, the difference is not significant. Females in the uncontrollable condition, however, show decreased depressive-like behavior after separation between P2–9 or P9–16.
Given the delayed development of the cortex (Brenhouse and Andersen, 2011b) that underlies ES behavior (Amat et al., 2005), we predicted that MS later in development would have greater impact than earlier MS. This increase in impact with increasing age of treatment could be seen after vehicle injection. Both sexes show impairments in the ES group, slightly after P2–9 treatment and significantly after P9–16 treatment. The additional MS had seemingly “protective” effects on MS9 males and females. Similar effects have previously been reported for longer maternal separation (Carrera et al., 2009) and shorter separation paradigms (Milde et al., 2004). Due to this protective effect, we only find an increase in helplessness in MS2 males; in contrast, a reduction in helplessness is evident in MS9 males and MS2 and MS9 females. Our longer separation paradigm between P2–20 produced depressive effects in both males and females (Leussis et al., 2012). It is possible that this MS protective effect may result from the shortened duration of the separation and is found in males only when the separation occurred later. The very short stressor, namely vehicle injection, however, was not able to induce protective effects but increased helplessness. It is also important to note that early life stress induces depressive-like behavior in the ES (males) and IS (females) conditions. Changes in the prefrontal cortex mediate both of these behaviors (Amat et al., 2005), and we have previously demonstrated MS effects in this region (Brenhouse and Andersen, 2011a; Brenhouse and Andersen, 2011b). Protective effects were observed in the IS group, where typically exposure to an uncontrollable stressor on day one induces helplessness. Early experience with a mild, but repeated, stressor might have helped females to cope with this stressor later in life.
Consistent with our earlier findings of depressive-like behavior following MS2–20 (Leussis et al., 2012), male rats in the ES condition were affected more adversely than females.
Females are more resistant to stressful events at the level of controllability as we (Leussis and Andersen, 2008; Leussis et al., 2012) and others (Dalla et al., 2008) have found. Female pups in general receive less maternal care than their male counterparts and thus may be less sensitive to MS. Within this species-relevant context, the likelihood that MS had a diminished impact may be more predictable than what occurs in human beings. Since male rat pups are more dependent on maternal care earlier in life, MS2 could have a greater effect than later stress exposure (e.g., MS9). Longer periods of MS (P2–20) significantly altered depressive behavior in both males and females to varying degrees (Leussis et al., 2012). While the longer duration of exposure resulted in elevated helplessness in the NS females in the earlier study, the shorter duration had a non-significant protective impact in females in this study. These results are consistent with an earlier study examining active avoidance, which is similar in scope to the IS condition (Lehmann et al., 1999). These data are also somewhat consistent with the clinical literature, where a single episode of abuse is associated with more favorable outcomes than repeated exposures (Copeland et al., 2007). Unfortunately, reality shows that females are more likely to experience higher rates of re-victimization explaining the high rates of depression in females (Koenen and Widom, 2009).
Examination of clinical, early sexual abuse data suggests that abusive episodes spanning across 1 year or less are associated with adolescent teen depression (Teicher et al., 2009) and sexual abuse accelerates the onset of depression into an earlier age than is typical (Gladstone et al., 2004). Our animal data suggest that females may require additional risk factors to be present or experience a greater amount of stress (e.g., MS may not be as significant in female rat pups as is to males) to increase depressive risk. This factor was not assessed in our earlier clinical studies. One such risk factor that has been identified in previous studies is elevated serotonin levels during development (e.g., 5-HT and Monoamineoxidase A polymorphisms) (Schulze et al., 2000; Caspi et al., 2003; Dannlowski et al., 2008; Stein et al., 2008). We used fluoxetine exposure to elevate serotonin during hypothesized sensitive periods. Fluoxetine impaired depressive-like behavior in the MS females, while improving escapes in the CON subjects. MS increases serotonin levels (Andersen et al., 1999), which may have blocked any potential serotonin enhancement by fluoxextine.
In males, however, a different picture emerges. Consistent with the results shown here, fluoxetine reduced helplessness in male rats (Karpova et al., 2009; Wieck et al., unpublished observation), but not in mice (Ansorge et al., 2004). Simultaneous fluoxetine with MS2 or MS9 facilitated escapes in the males in the controllability (ES) condition, while neither impairing nor facilitating helplessness in the IS and NS conditions. Early fluoxetine treatment may provide an effective early intervention for early life stress if we know which animal model best reflected the human condition.
CONCLUSION
Our results show that longer exposure to MS is associated with greater depressive-like behavior (comparing the results from this paper and Leussis et al., 2012). MS2 produces greater impairment in males than in MS9, females are less sensitive to MS than males, but are more sensitive to the adverse effects of fluoxetine exposure while females show greater protective effects. Fluoxetine exposure during MS was not only able to prevent the impairments but also the protective effects of the separation. These studies provide a foundation of behavioral sequelae that are relevant to the human condition, but more mechanistic work is truly needed to understand how similar exposures of MS and genes/drugs can selectively alter different aspects of depressive-like behaviors in a sex-dependent manner.
Acknowledgments
We would like to thank Wheaton College professor Rolf Nelson for sending excellent student interns to our laboratory (J.D.), the Leopoldina Fellowship Program LPDS-40, and funding from the Simches family for their continued Support.
Abbreviations
- ES
escapable shock
- IS
inescapable shock
- LH
learned helplessness
- MS
maternal separation
- NS
no shock
- P
postnatal day
REFERENCES
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Lyss PJ, Dumont NL, Teicher MH. Enduring neurochemical effects of early maternal separation on limbic structures. Ann N Y Acad Sci. 1999;877:756–759. doi: 10.1111/j.1749-6632.1999.tb09317.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011a;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol Psychiatry. 2011b;70:434–440. doi: 10.1016/j.biopsych.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera O, Cerrato M, Sanchez A, Gutierrez E. Long maternal separation has protective effects in rats exposed to activity-based anorexia. Dev Psychobiol. 2009;51:616–624. doi: 10.1002/dev.20396. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Keeler G, Angold A, Costello EJ. Traumatic events and posttraumatic stress in childhood. Arch Gen Psychiatry. 2007;64:577–584. doi: 10.1001/archpsyc.64.5.577. [DOI] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2008;33(7):1559–1569. doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Deckert J, Hohoff C, Kugel H, Arolt V, Heindel W, Kersting A, Baune BT, Suslow T. 5-HTTLPR biases amygdala activity in response to masked facial expressions in major depression. Neuropsychopharmacology. 2008;33(7):1559–1569. doi: 10.1038/sj.npp.1301411. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS. Sex differences in brain maturation in maltreatment-related pediatric posttraumatic stress disorder. Neurosci Biobehav Rev. 2003;27:103–117. doi: 10.1016/s0149-7634(03)00013-7. [DOI] [PubMed] [Google Scholar]
- Doom JR, Cicchetti D, Rogosch FA, Dackis MN. Child maltreatment and gender interactions as predictors of differential neuroendocrine profiles. Psychoneuroendocrinology. 2013;38(8):1442–1454. doi: 10.1016/j.psyneuen.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugan RC. Current protocols in neuroscience. 8.10B.1–8.10B.12. John Wiley & Sons, Inc; 2001. Rodent models of depression: learned helplessness using a triadic design in rats; pp. 8.10B.11–18.10B.12. [DOI] [PubMed] [Google Scholar]
- Duggal S, Carlson EA, Sroufe LA, Egeland B. Depressive symptomatology in childhood and adolescence. Dev Psychopathol. 2001;13:143–164. doi: 10.1017/s0954579401001109. [DOI] [PubMed] [Google Scholar]
- Gladstone GL, Parker GB, Mitchell PB, Malhi GS, Wilhelm K, Austin MP. Implications of childhood trauma for depressed women: an analysis of pathways from childhood sexual abuse to deliberate self-harm and revictimization. Am J Psychiatry. 2004;161:1417–1425. doi: 10.1176/appi.ajp.161.8.1417. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Francis DD, Brommer CL, Morgan ET, Kuhar MJ. Effects of early maternal separation on ethanol intake, GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology (Berl) 2005;181:8–15. doi: 10.1007/s00213-005-2232-4. [DOI] [PubMed] [Google Scholar]
- Karpova N, Lindholm J, Pruunslid P, Timmusk T, Castren E. Long-lasting behavioral and molecular alterations induced by early postnatal fluoxetine are restored by chronic fluoxetine treatment in mice. Eur Neuropsychopharmacol. 2009;19:97–108. doi: 10.1016/j.euroneuro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med. 2004;34:1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Widom CS. A prospective study of sex differences in the lifetime risk of posttraumatic stress disorder among abused and neglected children grown up. J Trauma Stress. 2009;22:566–574. doi: 10.1002/jts.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav. 1999;64:705–715. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Freund N, Brenhouse HC, Thompson BS, Andersen SL. Depressive-like behavior in adolescents after maternal separation: sex differences, controllability, and GABA. Dev Neurosci. 2012;34:210–217. doi: 10.1159/000339162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S, Laviola G, Leussis MP, Andersen SL. Abnormal behavioral and neurotrophic development in the younger sibling receiving less maternal care in a communal nursing paradigm in rats. Psychoneuroendocrinology. 2010;35(3):392–402. doi: 10.1016/j.psyneuen.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Macri S, Mason GJ, Wurbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPA and fear responses in rats. Eur J Neurosci. 2004;20:1017–1024. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Milde AM, Enger O, Murison R. The effects of postnatal maternal separation on stress responsivity and experimentally induced colitis in adult rats. Physiol Behav. 2004;81:71–84. doi: 10.1016/j.physbeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Riggs KW, Rurak DW. Fluoxetine during pregnancy: impact on fetal development. Reprod Fertil Dev. 2005;17:641–650. doi: 10.1071/rd05030. [DOI] [PubMed] [Google Scholar]
- Mourlon V, Baudin A, Blanc O, Lauber A, Giros B, Naudon L, Dauge V. Maternal deprivation induces depressive-like behaviours only in female rats. Behav Brain Res. 2010;213:278–287. doi: 10.1016/j.bbr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Azzinnari D, Spinelli S, Seifritz E, Tegethoff M, Meinlschmidt G. Helplessness: a systematic translational review of theory and evidence for its relevance to understanding and treating depression. Pharmacol Ther. 2011;132:242–267. doi: 10.1016/j.pharmthera.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Putnam FW. Ten-year research update review: child sexual abuse. J Am Acad Child Adolesc Psychiatry. 2003;42:269–278. doi: 10.1097/00004583-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Raineki C, Cortes MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32:7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus GZ, Stringari RB, Ribeiro KF, Cipriano AL, Panizzutti BS, Stertz L, Lersch C, Kapczinski F, Quevedo J. Maternal deprivation induces depressive-like behaviour and alters neurotrophin levels in the rat brain. Neurochem Res. 2011;36:460–466. doi: 10.1007/s11064-010-0364-3. [DOI] [PubMed] [Google Scholar]
- Schoedl AF, Costa MC, Mari JJ, Mello MF, Tyrka AR, Carpenter LL, Price LH. The clinical correlates of reported childhood sexual abuse: an association between age at trauma onset and severity of depression and PTSD in adults. J Child Sex Abus. 2010;19:156–170. doi: 10.1080/10538711003615038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze TG, Muller DJ, Krauss H, Scherk H, Ohlraun S, Syagailo YV, Windemuth C, Neidt H, Grassle M, Papassotiropoulos A, Heun R, Nothen MM, Maier W, Lesch KP, Rietschel M. Association between a functional polymorphism in the monoamine oxidase A gene promoter and major depressive disorder. Am J Med Genet. 2000;96:801–803. [PubMed] [Google Scholar]
- Somaini L, Donnini C, Manfredini M, Raggi MA, Saracino MA, Gerra ML, Amore M, Leonardi C, Serpelloni G, Gerra G. Adverse childhood experiences (ACEs), genetic polymorphisms and neurochemical correlates in experimentation with psychotropic drugs among adolescents. Neurosci Biobehav Rev. 2011;35:1771–1778. doi: 10.1016/j.neubiorev.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Stein MB, Schork NJ, Gelernter J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology. 2008;33(2):312–319. doi: 10.1038/sj.npp.1301422. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Polcari A, Andersen SL. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. J Clin Psychiatry. 2009;70:684–691. doi: 10.4088/jcp.08m04235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieck A, Brenhouse HC, Andersen SL. Developmental influences of 5-HT on depression: roles of BDNF, stress, and inflammatory processes. In: Hall FS, editor. Serotonin: biosynthesis, regulation and health implications. NOVA; 2013. pp. 147–168. [Google Scholar]