Abstract
Exposure to adversity during development is an identified risk factor for depression later in life. In humans, early adversity accelerates the onset of depressive symptoms, which manifest during adolescence. Animal studies have used maternal separation as a model of early adversity to produce adult depressive-like behaviors, but have yet to examine these behaviors during adolescence. Moreover, the nature of depressive-like behaviors has not been well characterized in this model. Here, we used the triadic model of learned helplessness to understand controllability, helplessness, and motivational factors following maternal separation in male and female adolescent rats. We found sex-dependent changes in the effects of separation, with males demonstrating loss of controllability in an escapable shock condition, whereas females demonstrated motivational impairment in a no-shock condition. The effect, however, did not endure as adult females were no longer helpless. Reductions in parvalbumin, a GABAergic marker, in the prefrontal cortex of separated subjects relative to age-matched controls were evident and paralleled depressive-like behavior. Understanding the risk factors for depression, the nature of depressive-like behaviors, and their unique sex dependency may ultimately provide insight into improved treatments.
Keywords: Abuse, Adolescent, Anhedonia, Depression, Helplessness, Sex differences, Stress
Introduction
Approximately 15–20% of teens will experience depression before they reach adulthood [1], and 8.3% of those individuals will suffer for at least 8–12 months. Of these individuals, 5% will meet criteria for major depressive disorder and another 3% will have dysthymia, a mild, long-lasting form of depression. With the onset of puberty, the incidence of depression rises and sex differences emerge. Females are two times more likely to experience depression than boys, although this sex difference does not appear to be due to gonadal hormones (reviewed in Andersen and Teicher [2]). Reductions in GABA activity have been reported in teen depression [3] and in suicide victims with depression when compared to suicide victims without depression [4]. The nature of these depressive episodes is marked by more anhedonia in teens relative to older individuals, as well as other qualitative differences [5]. These differences highlight the need to identify unique treatments for younger individuals. Since the cause often informs the cure [6], it is equally important to understand how early environmental experiences can lead to depressive illness in adolescence [2, 7].
Exposure to early adverse experiences significantly increases the likelihood of depression. Experiences such as abuse (both physical and sexual), unstable caregiving or neglect, exposure to illness, or a family history contribute substantially to the risk of adolescent depression. Importantly, exposure to adverse experiences accelerates depression onset to much earlier in life, i.e., adolescence, than the normal onset of depression, which is closer to 25 years of age [2, 8, 9].
Animal models of depression have used a variety of developmental environmental stressors to produce depressive-like features in adulthood. Such models include the maternal separation model (MS) of removing the pups from the dam [10], social isolation stress for relatively short periods of time [11], chronic social instability stress [12], early weaning [13], and resident-intruder paradigms [14]. However, the majority of MS models have focused on adult outcomes [15, 16]. Reports on the effects of MS on adult forced swim test behavior are inconsistent, and may be difficult to interpret since forced swim immobility provides limited insight into the complicated nature of depressive-like behaviors [17]. Given that exposure to early adversity is associated with an earlier onset of depressive-like symptoms, the current study had two main goals: (1) to investigate whether MS could increase depressive-like behaviors during adolescence, and (2) to investigate whether MS influences different aspects of depressive-like behavior. For this latter goal, we used the triadic model of learned helplessness (LH) [17, 18]. The triadic model allows three different aspects of depressive-like behavior to be tested: controllability over a stressor, loss of control over a stressor, and first exposure to a stressor. The ability of animals to escape a shock on the testing day is used as a dependent measure of depressive-like behavior.
Methods
Subjects
Pregnant female multiparous Sprague-Dawley rats (250–275 g) were obtained from Charles River Laboratories (Wilmington, Mass., USA) on day 13 of gestation. The day of birth was designated as postnatal day 0 (P0). At P2, litters were culled to 10 pups (5 males and 5 females) and litters were randomly assigned to either a maternal separation group (MS group) or animal facility-reared control group (CON group). Pups in the MS group were isolated for 4 h per day between P2 and P20, and kept at thermo-neutral temperature. This procedure is identical to procedures used previously by this laboratory [19, 20] and similar to others [10]. Pups in the CON group were not disturbed after P2, except for routine weekly changes in cage bedding, during which all pups were weighed. Rats were housed with food and water available ad libitum in constant temperature and humidity conditions on a 12-hour light/12-hour dark cycle (light period 07.00–19.00). Rats were weaned on P21–22, and group-housed in same-sex caging with 3–4 rats/cage until experimentation. Only 1 rat per litter was used per age per condition to avoid litter effects. Subjects were tested during early adolescence (P36–38) and the sample size was n = 5–7/condition. These experiments were conducted in accordance with the 1996 Guide for the Care and Use of Laboratory Animals (NIH) and were approved by the Institutional Animal Care and Use Committee at McLean Hospital.
Learned Helplessness
Rats were tested in the LH paradigm between P36 and P38. The triadic LH protocol was used to assess the role of controllability of stress on behavior [18]. On day 1, rats are assigned to a triad consisting of (1) an inescapable shock (IS) subject; (2) an escapable shock (ES) subject, and (3) a no shock (NS) control subject. Subjects from the MS or CON conditions were counterbalanced within the triad to avoid possible differences in shock exposure on day 1 as a result of condition [21]. Rats were given 100 trials of a tail shock (1.0 mA for trials 1–30, 1.3 mA for trials 31–60, and 1.6 mA for trials 61–100) in a wheel-turn box condition [21]. Unsignaled shocks were delivered on a variable-time 45-second schedule (range 30–60 s). While the ES subject could turn a wheel to terminate the shock, the IS subject was yoked to the ES subject and thus received the same amount of shock as the ES subject. The IS subject could not terminate the shock, which continued until the ES rat completed the wheel-turn response or a maximum of 30 s. The NS rat was restrained in a wheel-turn box but received no shock and is used to assess generalized deficits in learning or motivation on day 2. On day 2, each subject was placed into a shuttle box (Med Associates Inc., St Albans, Vt., USA). Rats from all three conditions could terminate a 1-mA footshock by shuttling to the other side for trials 1–5, or by shuttling to the other side and back again for trials 6–30. This response was cued by a tone that preceded the shock by 2 s. The shock remained on for 30 s, or until terminated by the appropriate behavioral response. Consistent with previous studies [21], data from the first 5 trials were not used in the analyses as subjects were learning the appropriate behavioral response. The number of escape failures and the mean latency to escape was reported for trials 6–30. Groups were counterbalanced on day 1 to account for any learning differences in escape responding.
Western Blotting
Western immunoblots of posterior lateral prefrontal cortex (plPFC) tissue was analyzed from adolescent and adult male and female rats from each group (n = 6–7), using methods described previously [22]. Briefly, sonicated tissue was processed for protein and 30 µg were loaded into a 15% Tris-HCl polyacrylamide gel and subjected to SDS-PAGE. Samples were transferred to a nitro-cellulose membrane and probed for PVB protein using mouse monoclonal anti-PVB IgG (1: 500, Sigma) and actin (to control for loading protein) using mouse polyclonal anti-actin IgG (1: 10,000, MP Pharmaceuticals, Aurora, Ohio, USA). Anti-mouse secondary antibodies conjugated with horseradish peroxidase were visualized with chemiluminescence (West Pico Kit; Pierce, Rockford, Ill., USA). Optical densities of the bands were measured using ImageJ software and normalized with actin. Group differences were determined in each region with 2-way (age × treatment × sex group) ANCOVAs, with Western blot run as a covariate. Bonferroni’s post hoc t tests compared group means after interactions were found.
Immunohistochemistry
We used immunohistochemistry to confirm whether PVB protein content changed or PVB-immunoreactive (IR) cells were lost (or less able to be visualized) in adolescent or adult MS and CON rats (n = 6/age of both sexes). At 40 or 100 days of age, rats were deeply anesthetized and intracardially perfused. Full methodology is described in our previous paper [22], using standard immunohistochemical methods [23]. Forty-micrometer sections were labeled with primary and then secondary antibodies (PVB monoclonal mouse antibody 1: 10,000, Sigma, St. Louis, Mo., USA; biotinylated anti-mouse secondary serum, 1: 500, Sigma) and streptavidin (1: 4,000; Invitrogen, Camarillo, Calif., USA). The signal was visualized with DAB, mounted on gelatin-coated slides, dehydrated, clarified, and coverslipped with Permount (Thermo Fisher Scientific Inc., Waltham, Mass., USA). Four serial coronal sections (intersection interval 240 µm) per animal were analyzed with Stereo Investigator Image Analysis System (MBF Bioscience, Williston, Vt., USA). The entire plPFC was outlined at ×4 magnification and the number of PVB-IR cells was measured at ×20 exclusively within the outlined area. Investigators were strictly blinded to the conditions for all analyses. Tracings of the plPFC boundaries were used for calculation of the surface area (a) in each section. The density of PVB-IR cells/mm2 was based on the total number of PVB-IR cells divided by Σa for each subject (the sum of areas obtained from all outlined regions). Volume of the plPFC was calculated according to the Cavalieri principle [24] as v = z × i × Σa, where z is the thickness of the section (40 µm) and i is the section interval (24; i.e., number of serial sections between each section and the following one within a compartment). Group differences were determined by 2-way (age × stress group) ANOVA. Bonferroni’s post hoc t tests compared group means after interactions were found.
Data Analysis
Day 2 LH data were analyzed by 3-way ANOVA with treatment (MS, CON), sex, and LH condition (IS, ES, NS) as variables, followed by Bonferroni post hoc corrections on subsequent ANOVAs.
Results
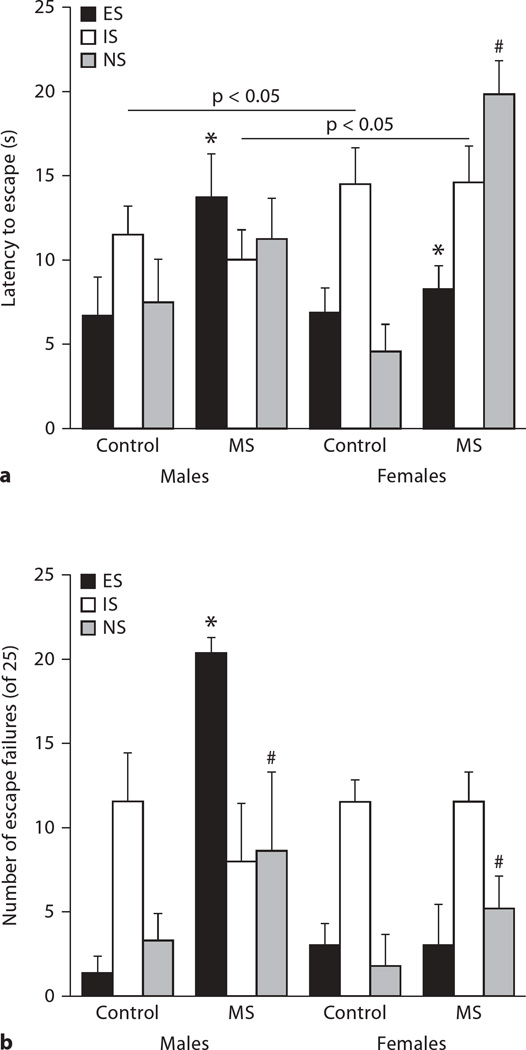
ANOVA demonstrated a significant treatment × LH condition × sex interaction for latency to escape (F2, 65 = 6.03, p < 0.005). As shown in figure 1, MS increased the latency to escape differentially across the three groups, as expected. The effects of MS were greater in male subjects than females.
Fig. 1.
Escape latencies (a) or escape failures (b) for males and females following LH conditioning 24 h prior to testing. MS produced differential effects on helplessness relative to control conditions. *, #p < 0.05 within each LH condition and the line indicates significant difference as a function of sex. Means + SEM for n = 5–7 subjects/condition are presented.
In order to better describe this complex interaction, we dissected out the triads into the three groupings and statistically corrected the treatment × sex post hoc ANOVAs with a Bonferroni correction factor (fig. 1a). Hence, we will discuss the results for the ES, IS, and NS groups individually by examining the treatment × sex interaction. In subjects that were able to control the stressor (e.g. the ES group), MS significantly increased the latency to escape in both males and females (treatment: F1, 22 = 4.91, p < 0.05), with no significant interaction or main effect of sex. In contrast, no such effects were observed for the IS group (treatment × sex interaction p = 0.92), although females had longer latencies to escape (females 14.6 ± 1.3 vs. males 10.2 ± 1.5; F1, 22 = 4.59, p < 0.05). The most interesting result, in our opinion, was found in the NS group. Here, MS significantly interacted with sex (F1, 22 = 12.34, p < 0.005). This significant interaction was largely driven by the female MS group, who took 3.4 times longer to escape than the female CON group.
Escape failures demonstrated a similar profile of results, including a significant 3-way interaction (F2, 65 = 3.56, p < 0.05; fig. 1b). Again, 2-way ANOVAs were conducted and 2 of the 3 interactions reached significance (treatment × LH: F2, 65 = 5.45, p = 0.007; sex × LH: F2, 65 = 3.56, p = 0.04), with the third interaction attaining trend level differences (treatment × sex: F2, 65 = 3.5, p = 0.067). Again, all the following post hoc analyses of these interactions are Bonferroni-corrected. MS subjects in the ES condition had more escape failures than CON (F1, 21 = 8.3801, p = 0.01), an effect driven by the males (effect of sex: p = 0.04). In the IS condition, escape failures were not affected by treatment or sex. Finally, MS subjects demonstrated significantly more failures than CON subjects in the NS condition (F1, 22 = 4.95, p = 0.04).
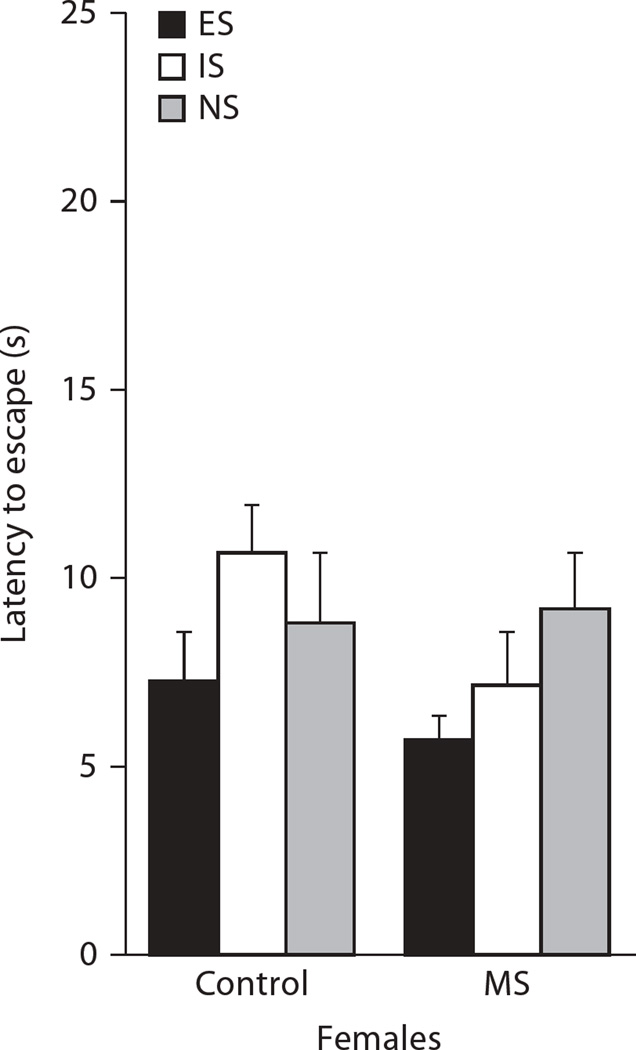
Figure 2 shows that the effects of MS on LH were not sustained into adulthood. Females were tested at P100 and no significant differences emerged in the latency to escape between treatment, LH condition, and their interaction (p = 0.41). Escape failures were all approximately 25 out of 25 trials across all treatment and LH conditions.
Fig. 2.
Escape latencies for P100 females following LH conditioning 24 h prior to testing. MS failed to produce differential effects on helplessness relative to control conditions. Means + SEM for n = 5–7 subjects/condition are presented.
GABA PVB
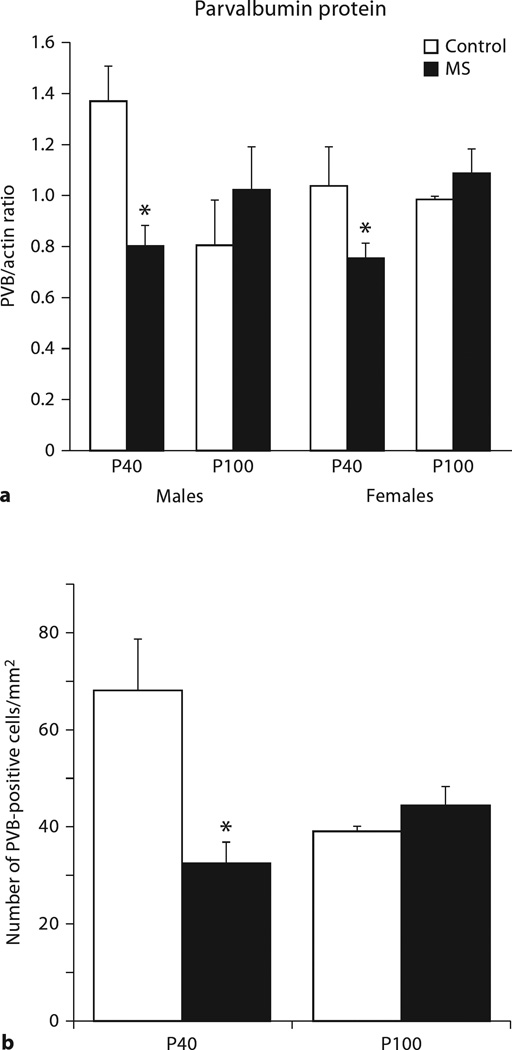
Results from Western immunoblotting and immunohistochemistry both show parallel findings of reduced PVB levels following MS in males at P40, with no significant difference at P100 (fig. 3a). Data from Western immunoblots demonstrate a treatment × age interaction, with no effect of sex (F1, 37 = 11.9, p = 0.001). The blot data further suggest a trend difference in an age × sex interaction (p = 0.07), males show pruning of PVB levels between P40 and P100, but females do not. To determine whether PVB protein data were reflected in PVB-IR cell counts, a second set of subjects (male only) was used.
Fig. 3.
Western immunoblotting (a) or cell counts (b) of PVB immunoreactivity in animals with a history of MS relative to control conditions. * p < 0.05 comparison between MS and controls. Means + SEM for n = 5–7 subjects/condition are presented.
Immunohistochemistry in males corroborated results from Western blotting (fig. 3b). A treatment × age interaction (F1, 19 = 2.887, p = 0.034) revealed that PVB was significantly lower in MS animals at P40 (p = 0.041), but not at P100. Similar to Western blot data, PVB levels trended towards lower levels at P100 when control males were compared with P40 control counterparts (p = 0.077).
Discussion
The results demonstrate that MS increases depressive-like behaviors during adolescence, similar to clinical findings in humans [2]. The nature of these depressive-like behaviors differed as a function of treatment, sex, and LH condition. The triadic model of LH illuminated the impact of controllability on responses to adverse conditions after MS. MS significantly impaired adolescent males in the ES (controllable) situation, as they took longer and had more failures to escape than any other group. MS females in the ES condition had less difficulty learning to escape, reflecting the influence of sex as well as the importance of parsing depressive behaviors based on responses to discrete situations.
In contrast to the ES condition, the IS condition of the triadic model is most akin to the induced helplessness that is produced by the forced swim paradigm. Our results show that MS does not differentially affect escape latencies or failures in the IS condition. While some forced swim studies show a lack of MS effect that is consistent with our data [25, 26], other studies [27–29] report decreased swim times or increased immobility in MS animals. Our data on adult females are consistent with these observations. No studies, to our knowledge, have tested the effect of MS on LH during adolescence. Furthermore, methodological differences between the triadic model and forced swim also make it difficult to explain potentially divergent results. For example, the training and testing in the triadic model take place in two different environments and involve different responses for differing conditions. Therefore, the forced swim paradigm assesses LH in a conditioned context, while the IS condition of the triadic model strictly assesses LH in the absence of a conditioned association [18].
The NS group, also referred to as a ‘naïve to aversive stimuli group’ [17], undergoes the training phase without the shock. In other words, the NS group has its first encounter with an aversive stimulus and the ability to escape on day 2. MS females in this group took significantly longer to escape than control females, indicating a motivational deficit after MS. Males exposed to MS did not display the same impairment in the NS condition. As discussed by Pryce et al. [17], helplessness behavior can also reflect differences in incentive-motivational processes. The inability to escape likely reflects errors in goal-directed behavior [30]. In the NS condition, the tone and the shock signal the impending tone, yet the animal fails to escape. The identification that MS animals show impairment in escape behavior is consistent with work by Der-Avakian and Markou [31] that suggest that adult male rats exposed to MS show anhedonia in adulthood as measured by intracranial self-stimulation. In our study, MS affects motivational responding in adolescent females, but not males. This observation is consistent with findings by others who used a chronic social instability stress paradigm [12]. It is therefore important to note that depressive behavior in adolescence may manifest differently from adults, just as is true in human depression. Depression in teenagers is often marked by increased irritability rather than sadness [5]. Similarly, a lack of motivation to escape in female adolescents upon first encounter with a shock might reflect a lack of responding to negative reinforcement that parallels an increased threshold for positive reinforcement seen in MS-exposed adults [31].
We believe that the use of the triadic model enabled a more fine-tuned analysis of the types of depression that is produced by early adverse experience. In a recent review by Pryce et al. [17], the LH phenomenon as it relates to the clinic is discussed as a valuable assessment of emotional-cognitive psychological concepts. Seminal papers by Maier and Watkins [18] have laid an important foundation for the use of the triadic model to study depressive-like behaviors as greatly advantageous over other, simple models. These unique aspects of the triadic model over the forced swim test (where both train and test occur in the same tank of water) permit an understanding and dissection of whether the training can carry over to different environments and hence is generalizable [17]. This distinction may be especially important when examining adolescent depressive-like behaviors, since adolescent depression is much less characterized in preclinical research [11] and therefore requires careful attention to its additive components.
GABA PVB Differences
Our data replicate and extend our previous findings of reduced GABA PVB protein in the plPFC of animals with a history of MS compared with controls. In our previous study [22], we found a 56.6% decrease of PVB expression in male MS animals. Here, we confirm this finding in males (an average 42.3 ± 6.3% decrease in this sample). In addition, we now show that a significant, but diminished PVB protein loss is observed in MS females (27.5 ± 6.3%) and is absent (or slightly higher relative to controls) by P100. Quantitative cell counts of PVB-IR neurons further raise the plausibility of cell loss, although decreased PVB expression that reduces their quantification cannot be ruled out conclusively. The finding of reduced GABA in the PFC is consistent with a number of human studies that show reduced GABA in the occipital and frontal cortex region of individuals with depression [3, 32]. Magnetic resonance spectroscopy placed this GABA attenuation at 52% in humans – remarkably similar to what we observed in our MS rats. In a single animal study to examine GABA levels with magnetic resonance spectroscopy, Kumar et al. [33] found a decrease in GABA levels following exposure to chronic variable stress in male adult rats.
Sex Differences
As discussed throughout the text, sex differences exist in the outcome of MS that need to be taken into consideration. There is a growing body of literature that illustrates sex differences in stress circuitry [34]; therefore, early stress exposure may differentially impair males and females when tested in adolescence. Males are more vulnerable than females in the ES condition. The ES condition requires a generalization between two contexts and a shift in escape strategy on day 2. We have previously observed that adolescent males with a history of MS commit more errors than controls in a win-shift working memory task, which directly assesses recall and strategy-shifting [35]. Together, it appears that the ES condition revealed a cognitive impairment in adolescent MS males. In contrast, adult females exposed to MS display reduced performance in an object location task performance, whereas adult males exposed to MS did not [16].
The inability to escape after being trained with controllable shock in adolescent MS males is indicative of PFC dysfunction [36] and is consistent with other reports that periadolescent males, but not females, are impaired in other cognitive tasks after MS [37, 38] or social isolation stress [11]. The greater loss of PVB in MS adolescent males may provide a plausible mechanism that merits further investigation. The relative loss of PVB is seemingly proportional to the level of impairment in the ES condition at P40. In contrast, MS females show more impairment in the NS condition, which may contribute to the acceleration of depression in the female abused population [8]. Additionally, the observation that MS females were impaired upon the first encounter with an aversive stimulus may help explain why abused females are significantly more likely than males to develop depression [39]. Depressed female teens experience more sadness, moodiness, guilt-related self-image and feelings of failure, and difficulty concentrating, while depressed boys are more anhedonic [2]. These differential symptoms in human depression further highlight the importance of the discrete responses observed here. Notably, our observations in males are similar to the LH changes reported after adolescent social stress [11], while females appear to display impairments after MS exposure, but not after adolescent social stress [11]. Therefore, sex may have an important influence on sensitive periods to stress and depression.
As discussed above, the inability to escape upon the first encounter with a stressor reflects a motivational impairment in MS females. This observation is consistent with the sex differences found in fear conditioning studies. It has been proposed that male rats exposed to MS show greater conditioned fear, and female rats show greater unconditioned fear as well as enhanced responses to aversive and appetitive stimuli [40]. These sex-specific effects of MS may reflect neuronal reorganization in stress responsive areas such as the PFC and hippocampus that occurs during typical development [41] or possible sex differences in the noradrenergic response [42]. Females also trended toward longer escape latencies in the IS condition, as previously shown in adolescents [11]. In contrast, adult females have been shown to display less LH after either ES or IS [43], again reflecting the difference between adolescent and adult manifestation of depression. Together, our results with others suggest that MS alters sexually dimorphic aspects of depressive-like behavior that has received relatively little attention.
Treatment Implications
The impairment that we observed here in MS-exposed rats suggests that depressed females with an abuse history may benefit from behavioral therapy that introduces ‘safety signals’, which can mitigate the effects of an inescapable stressor [44]. The inability of the MS males to either learn to control initially (day 1) or fail to generalize this response to the different environment on testing (day 2) may suggest that treatment strategies such as cognitive behavior therapy may have limited utility. Our earlier findings have shown that treatment with the cyclooxygenase-2 inhibitor, NSD-398, prevented PVB cell loss [22] and may possibly be able to reduce depressive effects in MS animals. Certainly, more research with MS rats and the triadic model will help to determine whether these predictions are valid.
Conclusions
The work highlighted here illustrates a number of important points regarding the animal modeling of human depression. First, exposure to early adverse experiences increases the likelihood of depression in both humans [2] and animals (this study). Second, depressive behaviors are evident in adolescence. Third, important sex differences exist in the qualitative aspects of depression that may have important implications for early intervention approaches.
Acknowledgments
The authors wish to acknowledge NARSAD (to S.L.A. and H.C.B.) and the Shine Foundation for their support of this project. We further recognize the Rosenberg Family for their support.
References
- 1.Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J. Childhood and adolescent depression: a review of the past 10 years. Part 2. J Am Acad Child Adolesc Psychiatry. 1996;35:1575–1583. doi: 10.1097/00004583-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Sanacora G, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 4.Klempan TA, et al. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. 2009;14:175–189. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- 5.Teicher MH, et al. Locomotor activity in depressed children and adolescents. 1. Circadian dysregulation. J Am Acad Child Adolesc Psychiatry. 1993;32:760–769. doi: 10.1097/00004583-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Uher R. The implications of gene-environment interactions in depression: will cause inform cure? Mol Psychiatry. 2008;13:1070–1078. doi: 10.1038/mp.2008.92. [DOI] [PubMed] [Google Scholar]
- 7.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 8.Teicher MH, Samson JA, Polcari A, Andersen SL. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. J Clin Psychiatry. 2009;70:684–691. doi: 10.4088/jcp.08m04235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 11.Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- 12.Herzog CJ, et al. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience. 2009;159:982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 13.George ED, Bordner KA, Elwafi HM, Simen AA. Maternal separation with early weaning: a novel mouse model of early life neglect. BMC Neurosci. 2010;11:123. doi: 10.1186/1471-2202-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidal J, et al. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiol Behav. 2007;92:824–830. doi: 10.1016/j.physbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Reus GZ, et al. Maternal deprivation induces depressive-like behaviour and alters neurotrophin levels in the rat brain. Neurochem Res. 2011;36:460–466. doi: 10.1007/s11064-010-0364-3. [DOI] [PubMed] [Google Scholar]
- 16.Mourlon V, et al. Maternal deprivation induces depressive-like behaviours only in female rats. Behav Brain Res. 2010;213:278–287. doi: 10.1016/j.bbr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Pryce CR, et al. Helplessness: a systematic translational review of theory and evidence for its relevance to understanding and treating depression. Pharmacol Ther. 2011;132:242–267. doi: 10.1016/j.pharmthera.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Andersen SL, Lyss PJ, Dumont NL, Teicher MH. Enduring neurochemical effects of early maternal separation on limbic structures. Ann NY Acad Sci. 1999;877:756–759. doi: 10.1111/j.1749-6632.1999.tb09317.x. [DOI] [PubMed] [Google Scholar]
- 20.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- 21.Drugan RC, Basile AS, Ha JH, Healy D, Ferland RJ. Analysis of the importance of controllable versus uncontrollable stress on subsequent behavioral and physiological functioning. Brain Res Brain Res Protoc. 1997;2:69–74. doi: 10.1016/s1385-299x(97)00031-7. [DOI] [PubMed] [Google Scholar]
- 22.Brenhouse HC, Andersen SL. Non-steroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol Psychiatry. 2011;70:434–440. doi: 10.1016/j.biopsych.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berretta S, et al. Long-term effects of amygdala GABA receptor blockade on specific subpopulations of hippocampal interneurons. Hippocampus. 2004;14:876–894. doi: 10.1002/hipo.20002. [DOI] [PubMed] [Google Scholar]
- 24.Cavalieri B. Unione Tipografico-Editrice Torinese. Torino: Typis Clementis Feronji; 1966. Geometria degli Indivisibili. [Google Scholar]
- 25.Marais L, van Rensburg SJ, van Zyl JM, Stein DJ, Daniels WM. Maternal separation of rat pups increases the risk of developing depressive- like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neurosci Res. 2008;61:106–112. doi: 10.1016/j.neures.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, et al. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci Res. 2007;58:32–39. doi: 10.1016/j.neures.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Desbonnet L, et al. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226. doi: 10.1016/j.neuroscience.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 29.MacQueen GM, Ramakrishnan K, Ratnasingan R, Chen B, Young LT. Desipramine treatment reduces the long-term behavioural and neurochemical sequelae of early-life maternal separation. Int J Neuropsychopharmacol. 2003;6:391–396. doi: 10.1017/S1461145703003729. [DOI] [PubMed] [Google Scholar]
- 30.Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 31.Der-Avakian A, Markou A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience. 2011;170:1189–1198. doi: 10.1016/j.neuroscience.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhagwagar Z, et al. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–812. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 33.Kumar BS, Mishra SK, Rana P, Singh S, Khushu S. Neurodegenerative evidences during early onset of depression in CMS rats as detected by proton magnetic resonance spectroscopy at 7T. Behav Brain Res. 2012;232:53–59. doi: 10.1016/j.bbr.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Park MK, Hoang TA, Belluzzi JD, Leslie FM. Gender specific effect of neonatal handling on stress reactivity of adolescent rats. J Neuroendocrinol. 2003;15:289–295. doi: 10.1046/j.1365-2826.2003.01010.x. [DOI] [PubMed] [Google Scholar]
- 35.Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frankola KA, et al. Effects of early rearing conditions on cognitive performance in prepubescent male and female rats. Neurobiol Learn Mem. 2010;94:91–99. doi: 10.1016/j.nlm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Jiao J, Dulawa SC. Infant maternal separation impairs adult cognitive performance in BALB/cJ mice. Psychopharmacology (Berl) 2011;216:207–218. doi: 10.1007/s00213-011-2209-4. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher JM. Childhood mistreatment and adolescent and young adult depression. Soc Sci Med. 2009;68:799–806. doi: 10.1016/j.socscimed.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Kosten TA, Lee HJ, Kim JJ. Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006;1087:142–150. doi: 10.1016/j.brainres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 42.Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- 43.Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2008;33:1559–1569. doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- 44.Christianson JP, et al. Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol Psychiatry. 2011;70:458–464. doi: 10.1016/j.biopsych.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]