Abstract
Background: Multivitamin/mineral products (MVMs) are the dietary supplements most commonly used by US adults. During manufacturing, some ingredients are added in amounts exceeding the label claims to compensate for expected losses during the shelf life. Establishing the health benefits and harms of MVMs requires accurate estimates of nutrient intake from MVMs based on measures of actual rather than labeled ingredient amounts.
Objectives: Our goals were to determine relations between analytically measured and labeled ingredient content and to compare adult MVM composition with Recommended Dietary Allowances (RDAs) and Tolerable Upper Intake Levels.
Design: Adult MVMs were purchased while following a national sampling plan and chemically analyzed for vitamin and mineral content with certified reference materials in qualified laboratories. For each ingredient, predicted mean percentage differences between analytically obtained and labeled amounts were calculated with the use of regression equations.
Results: For 12 of 18 nutrients, most products had labeled amounts at or above RDAs. The mean measured content of all ingredients (except thiamin) exceeded labeled amounts (overages). Predicted mean percentage differences exceeded labeled amounts by 1.5–13% for copper, manganese, magnesium, niacin, phosphorus, potassium, folic acid, riboflavin, and vitamins B-12, C, and E, and by ∼25% for selenium and iodine, regardless of labeled amount. In contrast, thiamin, vitamin B-6, calcium, iron, and zinc had linear or quadratic relations between the labeled and percentage differences, with ranges from −6.5% to 8.6%, −3.5% to 21%, 7.1% to 29.3%, −0.5% to 16.4%, and −1.9% to 8.1%, respectively. Analytically adjusted ingredient amounts are linked to adult MVMs reported in the NHANES 2003–2008 via the Dietary Supplement Ingredient Database (http://dsid.usda.nih.gov) to facilitate more accurate intake quantification.
Conclusions: Vitamin and mineral overages were measured in adult MVMs, most of which already meet RDAs. Therefore, nutrient overexposures from supplements combined with typical food intake may have unintended health consequences, although this would require further examination.
Keywords: dietary supplement, reference material, multivitamins, sampling plan, NHANES, quality control, overage, Recommended Dietary Allowance (RDA), upper limit, US Pharmacopeia
INTRODUCTION
In the United States, dietary supplements (DSs)7 containing vitamins and minerals contribute significantly to the total intake of many micronutrients (1, 2). In the 2007–2010 NHANES (3), 49% of adults reported the use of ≥1 DS product within the previous 30 d, and multivitamin/mineral products (MVMs) were the most common type of DSs reported (31.9% of adults) (4). DS consumption significantly reduces the number of adults and children with an intake below the Estimated Average Requirements for all analyzed nutrients (1, 5).
Despite the popularity of MVMs, observational studies and randomized controlled trials with the use of MVMs in populations that are mostly nutrient replete provide mixed results on the efficacy of MVMs for health maintenance and for disease prevention and management (6–10). Studies of MVM safety and effectiveness conducted to date have relied on nutrient content that is based exclusively on the label claims. However, the nutrient values on MVM labels may differ significantly from these products’ actual analytic contents (11). The concept of overages (i.e., analytically determined content of the nutrient exceeding the amounts claimed on the label) is consistent with Current Good Manufacturing Practices, which recognize their use in the food and DS industry as a means to ensure that claimed ingredient amounts are valid at the end of the product shelf life (12). However, the ingredient amounts added above those claimed by the labels are unknown or publicly unavailable. The 2010 Dietary Guidelines Advisory Committee stated that knowledge of analytic DS composition is critical for more precise dietary intake assessment and for understanding the associations of DSs with health and disease risk in surveys and epidemiologic nutritional studies (10, 13). The analytically determined nutrient content of DSs is also important for evaluating risk of exposure to doses in excess of labeled amounts and Recommended Dietary Allowances (RDAs), and, particularly, the Tolerable Upper Intake Levels (ULs) (14).
To provide analytically derived estimates of ingredient content in nationally representative DSs, the NIH Office of Dietary Supplements, the USDA Nutrient Data Laboratory (NDL), and other federal agencies collaborated in launching the Dietary Supplement Ingredient Database (DSID) project in 2004 (15–18). The DSID-3 contains estimates of vitamin and mineral content in adult, children’s and nonprescription prenatal MVMs, and tables linking DSID data to specific cycles of the NHANES DS files (19).
Our goals were to establish relations between measured and labeled ingredient content, and to provide research tools to improve the assessment of nutrient intake from DSs in the US. We also compared adult MVM composition with RDAs and ULs to assess whether risk of nutrient overexposures should be investigated.
METHODS
Overview
We purchased a sample of products representative of the US adult MVM market, and chemically analyzed them for their ingredient content. We determined the percentage differences in nutrient content between claimed and analytically measured ingredient amounts. We then applied regression analysis to produce an equation for each nutrient that best describes the relation between the percentage differences and label values. These equations were used to analytically predict ingredient content based on label claims and to decipher the sources of content variability.
Sampling plan
A sampling plan was developed with the use of approaches similar to the food sampling plans designed for the National Food and Nutrient Analysis Program (20) for USDA food composition databases (21). The goal of the sampling plan was to provide nationally representative samples for measuring the analytic content of vitamins and minerals in adult MVMs. Adult MVMs frequently consumed in the United States [the top market share (TMS)] and products representative of the lower market share (LMS) were identified for purchase. The terms “supplement product,” “supplement,” and “product” are used interchangeably throughout the text, except for the description of variance component analysis, in which only the term “supplement” is used for simplicity and clarity. Detailed sampling plan and statistical methods can be found in the Supplemental Materials and Methods.
Frequency of intake information for reported supplements was derived from the 2001–2002 NHANES DS data files that are population weighted to indicate reported usage trends (22). Adult MVMs were defined as those containing ≥3 vitamins with or without minerals and other bioactive components that were “standard formulations” (for adults) or “mature formulations” (for seniors). In the NHANES DS files, population weights for all NHANES respondents reporting adult MVM use were summed to obtain a total population weight for all products reported by adult MVM consumers. A product’s weighted frequency of use was calculated by dividing the summed weights of respondents who reported this product by the total population weight for all adult MVMs. The NHANES most commonly reported adult MVMs were identified by ranking according to the product weighted frequency of use.
To update the NHANES 2001–2002 data on commonly used adult MVMs, the NDL commissioned a nationally representative market survey from an independent marketing firm in 2006. This firm surveyed ∼5050 respondents who had been preidentified as MVM users from an existing panel of 55,000 adults. The survey included the respondents’ reported use of adult MVM by brand name and type (e.g., for ages ≥50 or for women), along with the market channel of purchase. The results of the nationally representative sample were weighted to US census data (±1.4% at P < 0.05). We also reviewed data from the Multiethnic Cohort Study of supplements reported by adults in Hawaii and California (23) and from the Nutrition Business Journal’s Supplement Business Report (24). The lists of reported supplements from these 4 sources were combined, and frequency data for the same products were averaged so that newer products on the market would be included along with products commonly reported in national surveys. These integrated weightings, referred to as relative market share estimates (RMSEs), were used to identify 35 products that represented >55% of the summed RMSEs as TMS products. Adult MVM LMS products were identified with the use of NHANES 2003–2004 DS data [the most recent available at that time (25)]. The RMSEs for these products were based solely on the product-weighted frequency of use from NHANES. A total of 148 products with a summed RMSE of 25.4% were divided into 3 strata of equally summed RMSEs (8.4% each). Products within each stratum were designated for purchase statistically, based on probability proportional to size (RMSE, in this case).
Products were obtained from retail sales market channels in 2006 and 2007, including mass-market stores (e.g., grocery and drug stores, warehouse stores, and Wal-Mart) and natural and specialty stores (e.g., Whole Foods, organic markets, and Vitamin Shoppe) in 6 US states (California, Georgia, Maryland, Minnesota, Texas, and Virginia) to attain diverse marketing and geographic sample sources. Products not sold in retail stores were purchased by the NDL from direct marketing sales channels, including multilevel marketers (e.g., Herbalife and Amway), the Internet, and medical practitioners.
On average, 5 lots/TMS product and 2 lots/LMS product were purchased for a total of ∼400 samples and 109 products. For each lot, 240–300 capsules or tablets were purchased with ≥1 y to expiration date. The analytic content of ≤8 vitamins and ≤10 minerals in each sample was measured in 2007–2009.
Laboratory selection
For the analysis of MVM samples for the DSID, the NDL identified and selected qualified laboratories based on pilot study results and proposals submitted through the Federal Business Opportunities process [https://www.fbo.gov/ (26); see also reference 27].
Quality control
Samples were stored at room temperature in their original containers. For laboratory analysis, products were assigned to batches that also contained quality control (QC) materials. The QC materials included a set of product duplicates (2 sets of 20 tablets of the same MVM with different test sample identification numbers) and 2 in-house control materials. To create each in-house control material, a case of a single lot of an MVM with a matrix similar to that of the study samples was purchased. These control materials were added to each batch to evaluate the precision of laboratory methods over time. National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 3280 (28), an MVM matrix with certified values for vitamins and minerals, was also sent in most batches to evaluate laboratory accuracy.
Sample batches were sent to the selected laboratories for analysis of specific vitamins and minerals declared on the label [if the amount was ≥2% of the Daily Value (DV)]. Sample weighing and homogenization procedures were documented previously (29, 30). Laboratories were instructed to weigh and then homogenize ≥20 tablets or capsules before subsampling them for ingredient analyses while following US Pharmacopeia (USP) recommendations for DS sample preparation (31, 32)
Methods of analysis for vitamins and minerals
A previous pilot study identified methods and laboratories for the analysis of vitamins and minerals in MVMs and acceptable intralaboratory relative SDs (calculated as SD/mean × 100) based on results for NIST SRM 3280 and a commercial MVM (16). References for the methods used to measure each ingredient are provided and summarized in Table 1, and details for modifications to published methods are provided in Supplemental Table 1.
TABLE 1.
Analytic methods1
| Ingredient | Method summary | Method reference |
| Minerals | ||
| Calcium, copper, iron, manganese, magnesium, phosphorus, potassium, and zinc | Multielement inductively coupled plasma spectrometry after wet-ashing | AOAC OMA 984.27 and 985.01 (33) |
| Selenium | Hydride generation and atomic absorption spectroscopy | AOAC OMA 986.15 (33) |
| Iodine | Thiosulfate titration | AOAC OMA 935.14 and 932.21 (33), modified2 |
| Vitamins | ||
| Folic acid | Microbiological method with the use of Enterococcus hirae | AOAC OMA 944.12 (33) |
| Niacin, riboflavin, thiamin, vitamin B-6 (pyridoxine) | HPLC with ultraviolet detection at 210 nm and 270 nm | (34), modified3 |
| Vitamin B-12 (cyanocobalamin) | Microbiological method with the use of Lactobacillus delbrueckii | AOAC OMA 952.20, 960.46 (33) |
| Vitamin C (ascorbic acid) | HPLC with ultraviolet detection at 245 nm4 | AOAC OMA 967.22 (33), modified,5 (35) |
| Vitamin E (α-tocopherol and mixed tocopherols) | HPLC with fluorescence detection at 294 nm6 | (36), modified7 |
Vitamin names in parentheses are the chemicals that are measured. For in-house method modifications, see Supplemental Table 1. AOAC, Association of Official Analytic Chemists International; OMA, Official Methods of Analysis.
Updated procedure for solution preparation.
Added details about preparation of working standards and chromatographic gradient.
An oxidation step converts any dehydroascorbic acid to ascorbic acid.
Added heating of extracting acid solution in the sample preparation (if necessary); modified sample extraction procedure.
For mixed tocopherols, the sample is first treated with an enzyme (pancreatin).
Added phase extraction markers; updated standard preparation, HPLC conditions, and pump program. For in-house method modifications, see Supplemental Table 1.
Eight minerals (calcium, copper, iron, magnesium, manganese, phosphorus, potassium, and zinc) were analyzed by inductively coupled plasma spectrometry after wet-ashing. Selenium was analyzed by hydride generation and atomic absorption spectroscopy, and iodine content was measured by thiosulfate titration. Five vitamins (vitamins C and B-6, thiamin, riboflavin, and niacin) were analyzed with the use of HPLC with ultraviolet detection. Two vitamins (folic acid and vitamin B-12) were analyzed with the use of microbiological methods, and vitamin E was measured with the use of HPLC with fluorescence detection. Some product data were received for vitamin A (β-carotene and retinol), vitamin D, and chromium, but unacceptable QC results revealed methodology issues that needed to be addressed before analyzing more products.
Review of QC and DS product results
Laboratory results reported in milligrams per gram or micrograms per gram were compared with label claims. The percentage difference from the label claim was calculated for each analyzed ingredient in each sample: [(analytic value − label value)/label value] × 100%. Laboratory data were reviewed, and samples with unusually large percentage differences from label claims, high variability among lots of the same product, and/or QC results showing biased results for the batch were retested. Laboratory data for all water-soluble vitamins with test dates past the expiration date were excluded. The final laboratory data were sent to the NDL’s consulting statistician for statistical review.
DS information on labels is reported per serving and calculated as a percentage of the FDA DVs. DVs are based on the RDAs, but DVs do not address ULs (37). Labeled and measured product ingredient content in the products was adjusted to per-day amounts, based on label instructions. These were compared with RDAs, ULs, and market shares to estimate the potential impact of adult MVM use on attaining recommended daily nutrient intake.
Statistical methods
Data for each nutrient were identified by supplement, lot, sample, and repeated laboratory analysis (Supplemental Figure 1).
Regression analysis
Relations between the label and percentage difference of analytically determined amounts from label were estimated by regression with an SAS mixed-model procedure (SAS software, version 9.3, SAS Institute Inc, Cary, NC). For each supplement ingredient, the label value was the independent variable and the percentage difference from the label value (based on the laboratory analysis) was the dependent variable. Ingredient data from laboratory analysis were prepared for weighted regression analysis by applying RMSEs as product weights. Multiple laboratory observations for the same sample were averaged, and sample means were used for the statistical regression analysis and plots. For each ingredient, influence tests for sample, lot, and supplement were conducted sequentially. Laboratory data were identified as overly influential and excluded from the final data set when a predicted value, tested with the use of a jackknife technique while deleting one supplement at a time, changed by >2 percentage points (see details in the Supplemental Materials and Methods).
Three models were used to fit the data for each ingredient as a function of the label values. Those models were a mean-only model (constant across all label values; had only an intercept and no other regression coefficients) and linear and quadratic models. The most complex and statistically significant model was selected. The SEM and the SE of an individual observation were calculated. Because the regression equation was used to predict ingredient values of individual supplement samples, SEs were adjusted with the use of sums of squares to reflect this expected greater prediction variability. The random portion of the model fit the variances in supplements within label claim, lots within supplement, and samples (residual) within lot. The selected regression equations were used to predict mean analytic concentrations for each ingredient in adult MVMs: label value × (1 + predicted percentage difference/100) (Supplemental Materials and Methods).
Validation
The accuracy of the models was assessed with validation techniques that evaluated the prediction success of each regression equation compared with label claims. For the regression equations, predictions were classified as “successful” if the predicted value was within 10% of its analytic result and “unsuccessful” if it was not within 10% of the analytic result. Predictions were repeated with the use of a jackknife technique while removing one supplement at a time, and the percentage success and 95% CIs were computed. For the equation predictions to be considered a successful approach, the equation predictions would have to perform better, i.e., have a greater success rate, than when simply using the labeled value as a predictor of the analytic value (within 10%).
Variability assessment
The final regression data sets and equations were used for variance component analysis to estimate the variability between supplements, between lots within supplements, and between samples within lots.
The supplement variance component is an estimate of how the supplements vary around the regression line, excluding the lot and sample variances. The lot variance component is an estimate of how lots vary around the supplement mean averaged over all supplements, excluding sample variance. The sample variance component is an estimate of how samples vary around the lot mean averaged over all lots. The sample variance component includes the random variance associated with within-lot variability, e.g., sampling of tablets from lots, preparation of samples for laboratory analysis, and laboratory technique variability.
Exploratory analyses: effect of sales channel, market share, and expiration date
Three sales channels, RMSEs, and the number of days from analytic testing to expiration date were added to the final regression models for each ingredient to test their association with the percentage differences from labels. Manufacture dates are unavailable on DS labels; therefore, the expiration date was used to estimate the stage of shelf life for products. This, however, is not an ideal measure for determining the age of a manufactured product, because different manufacturers choose different time frames for expiration dates. The results for these exploratory analyses were inconclusive and so are only reported in the Supplemental Results.
RESULTS
QC analyses: SRM, in-house control, and duplicate results
To monitor laboratory accuracy, analytic measurements for ingredients in the NIST SRM were compared with the certified values to detect any potential bias in laboratory performance. For 9 ingredients, mean analytic results were within 2% of the NIST certified or reference values. For another 7 ingredients, the mean results were within 2–6% of the NIST values. For the other 2 ingredients, mean analytic results were 10–11% of the NIST values (Table 2). The acceptability of the mean and variability for SRM results in this study was also tested and confirmed (except for niacin because of its narrow certified range) with the use of the method in Application Note 1 from the Joint Research Centre of the European Commission’s Institute for Reference Materials and Measurements (38).
TABLE 2.
Quality-control results: laboratory accuracy and precision evaluated by ingredient measurements for NIST SRM 3280 included in batches for the adult MVM study1
| Ingredient | NIST certified or reference value2 | DSID laboratory, mean value | NIST value, uncertainty range, calculated as % | DSID laboratory,3 mean % difference from NIST value | DSID laboratory,4 mean % RSD | DSID analyses, n |
| Minerals | ||||||
| Calcium, mg/g | 110.7 | 114 | 4.79 | 3.17 | 2.68 | 19 |
| Copper, mg/g | 1.4 | 1.4 | 12.14 | 1.35 | 4.63 | 19 |
| Iodine, mg/g | 132.7 | 132 | 4.97 | −0.85 | 6.14 | 21 |
| Iron, mg/g | 12.35 | 12.2 | 7.37 | −1.09 | 3.08 | 20 |
| Magnesium, mg/g | 67.8 | 66.8 | 5.90 | −1.43 | 2.87 | 19 |
| Manganese, mg/g | 1.44 | 1.44 | 7.64 | 0.33 | 3.52 | 19 |
| Phosphorus, mg/g | 75.7 | 78.6 | 4.23 | 3.83 | 3.06 | 19 |
| Potassium, mg/g | 53.1 | 56 | 13.18 | 5.38 | 3.21 | 19 |
| Selenium,5 μg/g | 17.6 | 17.8 | 4.55 | 1.28 | 9.05 | 20 |
| Zinc, mg/g | 10.15 | 10.5 | 7.98 | 3.79 | 2.38 | 19 |
| Vitamins | ||||||
| Folic acid, μg/g | 394 | 379 | 5.58 | −3.8 | 9.52 | 21 |
| Niacin, mg/g | 14.106 | 14.5 | 1.63 | 2.50 | 2.14 | 21 |
| Riboflavin, mg/g | 1.32 | 1.34 | 12.88 | 1.19 | 5.04 | 21 |
| Thiamin, mg/g | 0.8346 | 0.923 | 11.3 | 10.7 | 9.57 | 21 |
| Vitamin B-12,7 μg/g | 4.8 | 5.41 | 38.78 | 10.4 | 5.06 | 21 |
| Vitamin B-6,7 mg/g | 1.496 | 1.49 | 9.40 | 0.27 | 5.2 | 21 |
| Vitamin C,7 mg/g | 42.2 | 42.8 | 8.77 | 1.51 | 10.9 | 18 |
| Vitamin E,7 mg/g | 21.4 | 20.5 | 16.36 | −4.43 | 8.06 | 21 |
DSID, Dietary Supplement Ingredient Database; MVM, multivitamin/mineral product; NIST, National Institute of Standards and Technology; RSD, relative SD; SRM, Standard Reference Material.
SRM 3280 multivitamin and multielement tablets, available at https://www-s.nist.gov/m-srmors/certificates/3280.pdf (28). NIST values reported as published NIST SRM 3280 certificate in 2009.
Mean percentage difference from NIST certified values were outside the reference range for thiamin and niacin.
RSD is calculated as (SD/mean) × 100.
Only reference values are available for this ingredient.
NIST values were converted to free vitamin forms (for niacin, vitamin B-6, and thiamin) by application of molecular weight ratios.
Vitamin B-12 was measured as cyanocobalamin, vitamin B-6 as pyridoxine, vitamin C as ascorbic acid, and vitamin E as α-tocopherol.
Laboratory data for in-house control materials and duplicate products were monitored to evaluate laboratory precision (between and within days). Measurements outside of 10% of the in-house control and duplicate means were flagged for further review and possible retest. The summary QC results for all ingredients are presented in Supplemental Tables 2 and 3. The vast majority of SRM and in-house control results met the acceptable limits established by the NDL in a previous study for laboratory between- and within-day precision (16).
Product label and analytic content in comparison with RDAs, ULs, and market shares
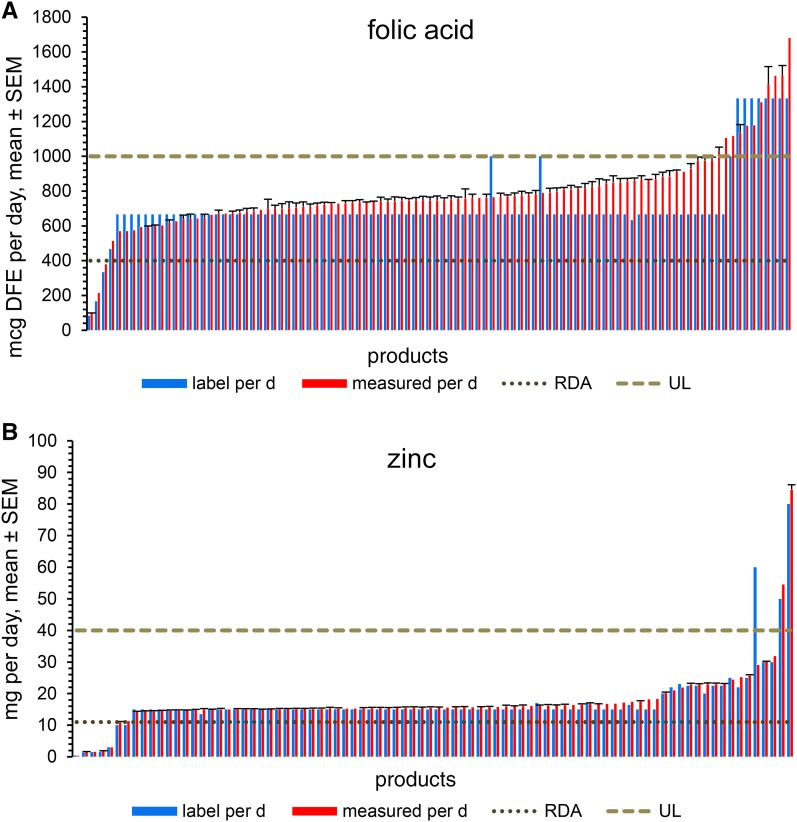
Product distributions for folic acid and zinc in comparison with their RDAs and ULs are shown in Figure 1, and product groupings for each nutrient are summarized in Table 3. Products with nutrients labeled below their RDAs were included in group I. All products for calcium, phosphorus, potassium and the vast majority of products for magnesium were included in group I; for the rest of the ingredients, 2–54% of products were labeled below the RDAs. Products labeled between the RDAs and ULs were in group II, with 40–99% of products for 12 ingredients (copper; iodine; iron; zinc; folic acid; niacin; riboflavin; thiamin; and vitamins B-12, B-6, C, and E) included. Except for niacin and folic acid (with 31% and 8% of products, respectively, labeled above the ULs), group III products were rare, with 2–3.5% for magnesium, manganese, zinc, vitamin B-6, and vitamin C.
FIGURE 1.
Adult multivitamin/mineral products were ordered by measured content of folic acid (A) and zinc (B) for intake per day (as recommended on product labels). A majority of products had overages in folic acid content and provided a daily intake above the RDA, but below the folic acid UL [as DFEs; 1 DFE = 1 μg food folate = 0.6 μg of folate from dietary supplements consumed with food (14)]. One-half of the products labeled above the folic acid UL also had measured overages, whereas the rest had measured folic acid below the label amount. Note that products with the same labeled amount of folic acid differ greatly in their actual content. A majority of products were labeled above the zinc RDA but significantly below the zinc UL, and the measured overages were very small. DFE, dietary folate equivalent; RDA, Recommended Dietary Allowance; UL, Tolerable Upper Intake Level.
TABLE 3.
Product distribution by ingredient content compared with RDAs, with analytically measured mean percentage difference from label values and market shares1
| Group I: products labeled below maximum RDA |
Group II: products labeled within range from maximum RDA to maximum UL |
Group III: products labeled above UL |
|||||||
| Nutrient | Overall mean percentage difference from label | RMSE | Products,2 n | Overall mean percentage difference from label | RMSE | Products,2 n | Overall mean percentage difference from label | RMSE | Products,2 n |
| Calcium | 19.6 (−28 to 431) | 0.73 ± 1.65 | 81 | — | — | 0 | — | — | 0 |
| Copper | 55.9 (−15.4 to 319.3) | 0.20 ± 0.23 | 7 | 8.57 (−27.7 to 114.3) | 0.78 ± 1.74 | 72 | — | — | 0 |
| Iodine | 66.6 (10.6–207.4) | 0.25 ± 0.25 | 6 | 25.7 (−2 to 104) | 0.76 ± 1.82 | 63 | — | — | 0 |
| Iron | 4.3 (−21.6 to 17.1) | 0.29 ± 0.25 | 13 | 1.1 (−14.3 to 17.6) | 0.96 ± 2.03 | 37 | — | — | 0 |
| Magnesium3 | 3.6 (−6.5 to 32.7) | 0.79 ± 1.73 | 73 | — | — | 0 | −3.4 (−3.6 to −3.1) | 0.09 ± 0.01 | 2 |
| Manganese | 5.5 (−7.9 to 31.7) | 0.92 ± 2.13 | 46 | 7.18 (−5.7; 39.4) | 0.46 ± 0.56 | 31 | 18.2 | 0.12 | 1 |
| Phosphorus | 15.4 (−2.6 to 349) | 0.94 ± 2.11 | 46 | — | — | 0 | — | — | 0 |
| Potassium4 | 8.6 (−3.5 to 51.1) | 0.99 ± 2.1 | 46 | — | — | 0 | — | — | 0 |
| Selenium | 23 (−0.8 to 74) | 1.01 ± 2.23 | 42 | 25.2 (−41.2 to 64.2) | 0.42 ± 0.44 | 36 | — | — | 0 |
| Zinc | 10.4 (3.5 to 13.7) | 0.16 ± 0.16 | 7 | 4 (−4.7 to 22.8) | 0.76 ± 1.7 | 76 | −12.1 (−51.4 to 9.2) | 0.29 ± 0.31 | 3 |
| Folic acid | 20.1 (14.1 to 29.8) | 0.16 ± 0.04 | 3 | 13 (−23.4 to 66.8) | 0.69 ± 1.58 | 89 | 1.54 (−14.8 to 26.1) | 0.21 ± 0.22 | 8 |
| Niacin | 6.5 (−4.1 to 22.1) | 0.95 ± 1.24 | 6 | 2.3 (−39 to 21.2) | 0.8 ± 1.84 | 62 | −1 (−77.6 to 23.7) | 0.25 ± 0.25 | 30 |
| Riboflavin4 | 80 (−6.4 to 166.3) | 0.28 ± 0.11 | 2 | 11.6 (−90.8 to 108.9) | 0.64 ± 1.52 | 97 | — | — | 0 |
| Thiamin4 | −27.9 (−31.2 to −24.7) | 0.28 ± 0.11 | 2 | −3.9 (−66.4 to 48.8) | 0.64 ± 1.52 | 97 | — | — | 0 |
| Vitamin B-124 | 65.1 | 0.06 | 1 | 8.4 (−49.4 to 64.3) | 0.63 ± 1.52 | 98 | — | — | 0 |
| Vitamin B-6 | 20.8 (−5.5 to 58.6) | 0.21 ± 0.15 | 3 | 2.6 (−67.4 to 22.8) | 0.64 ± 1.52 | 98 | 2 (−9.2 to 13.1) | 0.14 ± 0.08 | 2 |
| Vitamin C | 7.6 (−13.1 to 36.8) | 1.04 ± 2.18 | 43 | 8 (−96.7 to 25.5) | 0.37 ± 0.45 | 51 | 12.9 (11.1 to 13.8) | 0.09 ± 0.08 | 3 |
| Vitamin E | 2.9 (−7.7 to 8.6) | 0.15 ± 0.18 | 3 | 4.7 (−47.6 to 37.2) | 0.71 ± 1.6 | 87 | — | — | 0 |
Values are percentages (ranges) or means ± SDs. For every nutrient, we selected maximum RDA and UL values found for men (≥19 y) or women (nonpregnant, nonlactating) (14). RDA, Recommended Dietary Allowance; RMSE, relative market share estimate; UL, Tolerable Upper Intake Level.
Median n values differed between groups I and III and between groups II and III, but not between groups I and II (7, 62.5, and 0 for group I, II, and III, respectively; Kruskal-Wallis ANOVA on ranks, P < 0.001; pairwise comparison by Tukey test; P < 0.05).
The UL is determined only for magnesium intake from pharmacologic agents. This amount is below the RDA that was determined for magnesium intake from natural sources.
Nutrients for which ULs were not established.
In summary, for 12 of 18 nutrients, most products were labeled at or above the maximum RDA and also had high market shares. Products with ingredients above the ULs had the lowest market shares. Overages for nutrients varied somewhat by proximity of labeled amount to RDAs and ULs: the median overage was smaller in group III than in group I, although median overages did not differ in group I compared with group II, and in group II compared with group III. For most products, however, applying the analytically determined overages did not change their categorization relative to RDAs and ULs. For detailed results and statistical evaluations for Table 3, see the Supplemental Results. Also, the product distributions by percentage differences from label for each ingredient are summarized in Supplemental Table 4.
Variability in ingredient content before influence testing
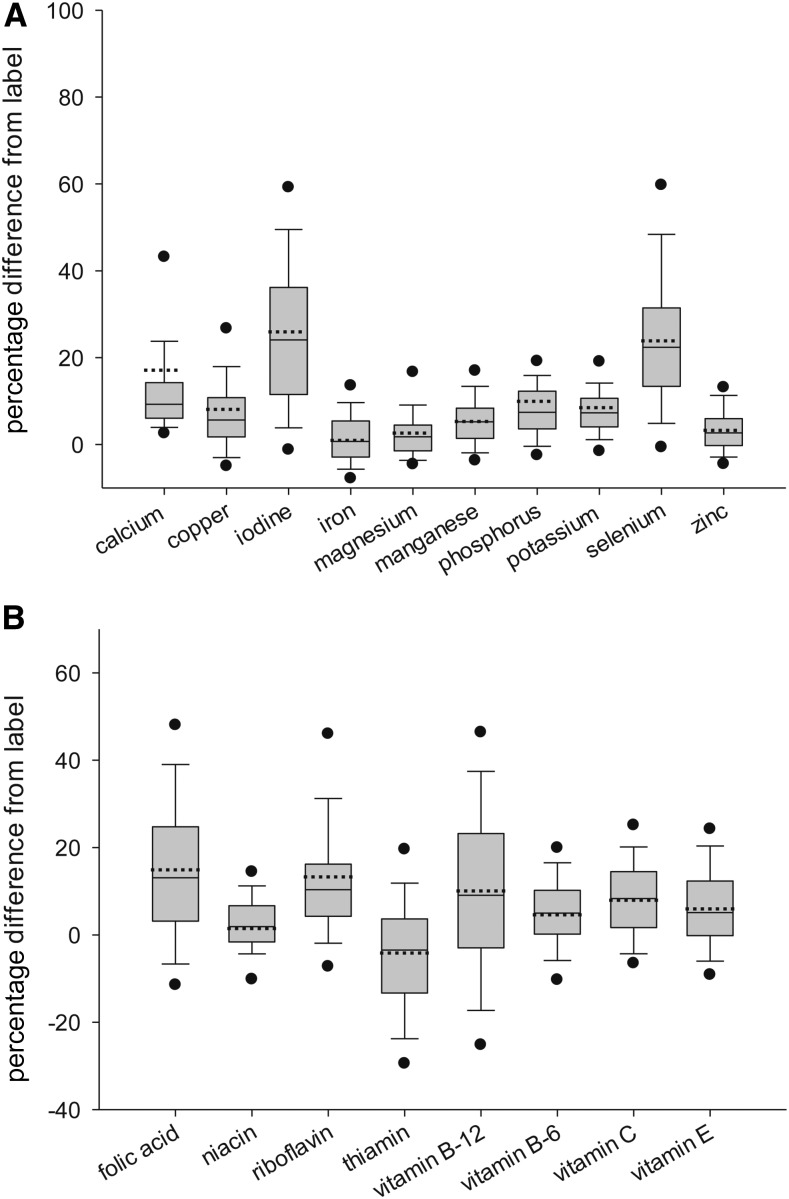
The complete set of final laboratory data are graphed in box plots (Figure 2). For the minerals, the mean percentage differences from label distributions for copper, iron, magnesium, manganese, phosphorus, potassium, and zinc were within 1–10% of label. The IQRs (measured as the range between the upper boundaries of the first and third quartiles) were all within 6–9% of label. Iodine and selenium had the highest mean values (25.9% and 23.9%, respectively) and the highest variability (IQR: 24.7% and 18%, respectively), whereas calcium had a 17.3% mean and an IQR of 8%. For the vitamins, the mean percentage difference from label distributions were within 1–15% of the label, with the exception of thiamin (−4.2%). However, the IQRs were more variable, with relatively narrow IQRs (within 8–13%) for niacin, riboflavin, and vitamins B-6, C and E, and wider IQRs (within ∼17–26%) for folic acid, thiamin, and vitamin B-12.
FIGURE 2.
Distributions of percentage differences between analytic and label ingredient amounts for minerals (A) and vitamins (B). The box plots show the 5th (lower circle), 10th (lower whisker), 25th (box bottom boundary), 50th (median, solid line), mean (dashed line), 75th (box top boundary), 90th (upper whisker), and 95th (upper circle) percentiles. Observations: calcium, n = 295; copper, n = 284; iodine, n = 250; iron, n = 200; magnesium, n = 280; manganese, n = 281; phosphorus, n = 188; potassium, n = 192; selenium, n = 282; zinc, n = 300; folic acid, n = 342; niacin, n = 338; riboflavin, n = 339; thiamin, n = 339; vitamin B-12, n = 337; vitamin B-6, n = 347; vitamin C, n = 329; and vitamin E, n = 320.
Regression analysis for 18 vitamins and minerals
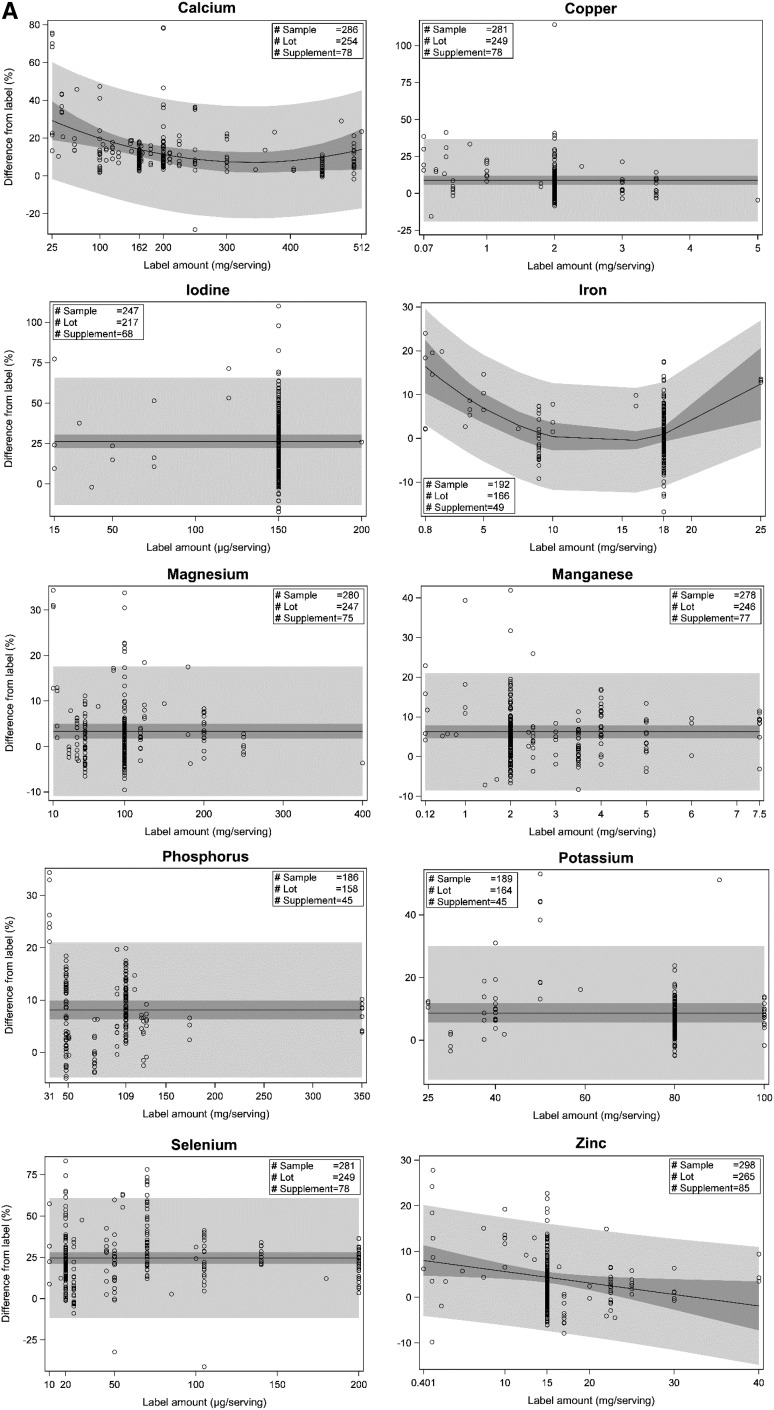
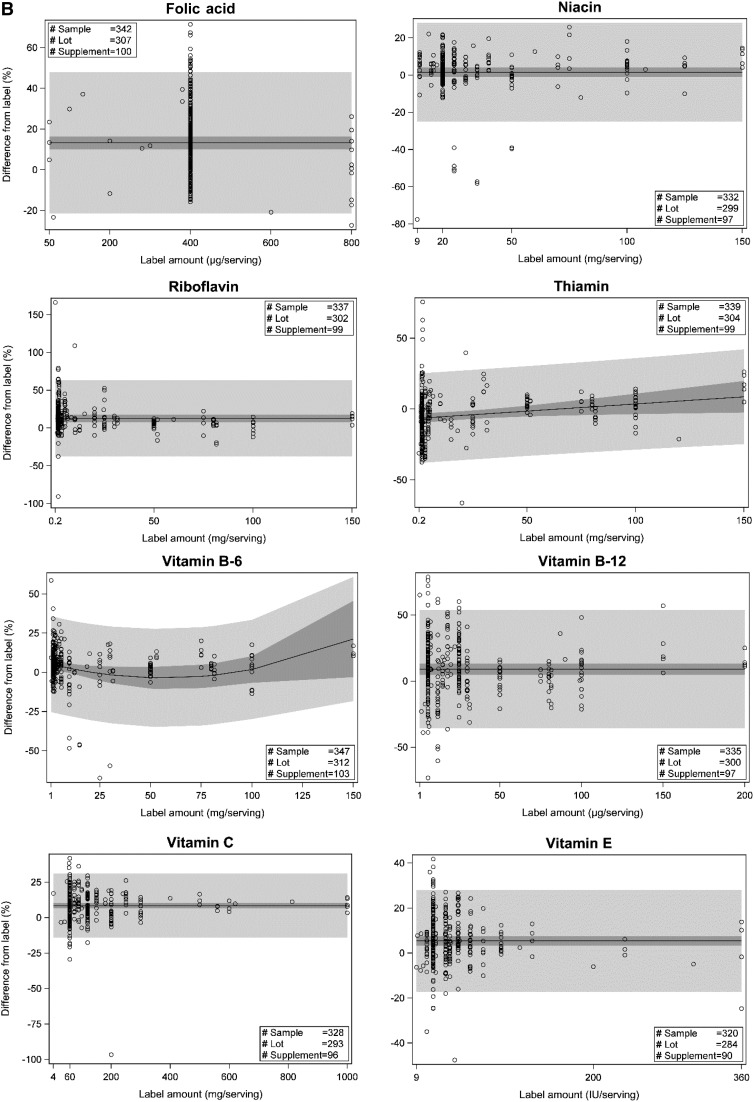
Regression results are reported for 8 vitamins (folic acid, niacin, riboflavin, thiamin, vitamin B-12, vitamin B-6, vitamin C, and vitamin E) and 10 minerals (calcium, copper, iodine, iron, magnesium, manganese, phosphorus, potassium, selenium, and zinc). After influence testing, the number of overly influential observations removed ranged from 1 to 7/ingredient. Regression results for mean predicted percentage differences from label and the associated SEs varied by ingredient and, in some cases, by ingredient labeled amount.
Plots representing best fit regression models for each ingredient are shown in Figure 3, including 95% CIs for the predicted mean and for individual product content. Estimates for parameters in the regression equations are listed in Table 4. The predicted mean percentage differences from label for the most common label amount and predicted ranges (variability) for vitamin and mineral content for adult MVMs are summarized in Table 5. If a linear or quadratic regression model was selected, the predicted mean percentage difference was dependent on the labeled amount, and a range of percentage differences from label was predicted. If a mean-only model was selected, the predicted mean percentage difference was not dependent on the label concentration.
FIGURE 3.
Regression plots showing relations between label ingredient amounts and percentage differences from label for analytically measured mineral(A) and vitamin (B) content. Solid lines indicate the mean predicted percentage differences. Dark-gray belts represent 95% CIs for the predicted meanpercentage differences. Light-gray belts represent 95% CIs for the predicted individual observation percentage differences from label. Relations between thelabel and percentage difference from label were estimated by regression with the SAS mixed-model procedure (SAS software version 9.3, SAS Institute, Inc.Cary, NC). The selected regression equations were used to predict mean analytic concentrations for each ingredient in adult multivitamin/mineral products:label value 3 (1 + predicted percentage difference/100). See detailed statistical methods in the Supplemental Methods.
TABLE 4.
Parameter estimates for regression equations describing relations between label ingredient claims and percentage difference from the claims for analytic measurements in nationally representative adult multivitamin/mineral products
| Ingredient | Model | Regression coefficient1 | Regression coefficient estimate ± SE | P |
| Minerals | ||||
| Calcium | Quadratic | β0 | 33.0 ± 6.0 | |
| β1 | −0.153 ± 0.052 | |||
| β2 | 0.000227 ± 0.000091 | ≤0.014 | ||
| Copper | Mean | μ | 9.0 ± 1.5 | ≤0.001 |
| Iodine | Mean | μ | 26.2 ± 2.1 | ≤0.001 |
| Iron | Quadratic | β0 | 18.6 ± 3.3 | |
| β1 | −2.87 ± 0.63 | |||
| β2 | 0.105 ± 0.027 | ≤0.001 | ||
| Magnesium | Mean | μ | 3.35 ± 0.79 | ≤0.001 |
| Manganese | Mean | μ | 6.25 ± 0.79 | ≤0.001 |
| Phosphorus | Mean | μ | 8.13 ± 0.88 | ≤0.001 |
| Potassium | Mean | μ | 8.7 ± 1.5 | ≤0.001 |
| Selenium | Mean | μ | 24.58 ± 1.76 | ≤0.001 |
| Zinc | Linear | β0 | 8.2 ± 1.7 | |
| β1 | −0.25 ± 0.10 | ≤0.017 | ||
| Vitamins | ||||
| Folic acid | Mean | μ | 13.2 ± 1.6 | ≤0.001 |
| Niacin | Mean | μ | 1.5 ± 1.3 | ≤0.234 |
| Riboflavin | Mean | μ | 12.6 ± 2.5 | ≤0.001 |
| Thiamin | Linear | β0 | −6.5 ± 1.7 | |
| β1 | 0.101 ± 0.041 | ≤0.017 | ||
| Vitamin B-12 | Mean | μ | 9.0 ± 2.2 | ≤0.001 |
| Vitamin B-6 | Quadratic | β0 | 5.6 ± 1.9 | |
| β1 | −0.32 ± 0.14 | |||
| β2 | 0.0028 ± 0.0013 | ≤0.032 | ||
| Vitamin C | Mean | μ | 8.4 ± 0.95 | ≤0.001 |
| Vitamin E | Mean | μ | 5.4 ± 1.1 | ≤0.001 |
Relations between the label and percentage difference from the label were estimated by regression with the SAS mixed-model procedure. P values indicate statistical significance for hypothesis that highest order model coefficient equals zero. See detailed statistical methods and model description in the Online Supplemental Materials, pages 11–12.
TABLE 5.
Predicted mean percentage difference from label claim based on regression analysis for ingredients measured in adult multivitamin/mineral products1
| Ingredient | Range of predicted mean % differences from label | Most common label value per serving | Predicted mean % differences at most common label claim | SE for predicted mean % difference at most common label | SE for individual predicted % difference at most common label |
| Minerals | |||||
| Calcium | 7.1 to 29.3 | 162 mg | 14.1 | 1.8 | 14.7 |
| Copper | 9.0 | 2 mg | 9.0 | 1.6 | 13.9 |
| Iodine | 26.2 | 150 μg | 26.2 | 2.1 | 19.7 |
| Iron | −0.5 to 16.4 | 18 mg | 0.9 | 0.85 | 5.9 |
| Magnesium | 3.4 | 100 mg | 3.4 | 0.80 | 7.1 |
| Manganese | 6.3 | 2 mg | 6.3 | 0.80 | 7.4 |
| Phosphorus | 8.1 | 109 mg | 8.1 | 0.89 | 6.4 |
| Potassium | 8.7 | 80 mg | 8.7 | 1.5 | 10.5 |
| Selenium | 24.6 | 20 μg | 24.6 | 1.8 | 18.1 |
| Zinc | −1.9 to 8.1 | 15 mg | 4.4 | 0.60 | 5.9 |
| Vitamins | |||||
| Folic acid | 13.2 | 400 μg | 13.2 | 1.6 | 17.5 |
| Niacin | 1.5* | 20 mg | 1.5* | 1.3 | 13.4 |
| Riboflavin | 12.6 | 1.7 mg | 12.6 | 2.5 | 25.4 |
| Thiamin | −6.5 to 8.6 | 1.5 mg | −6.4 | 1.7 | 15.9 |
| Vitamin B-12 | 9.0 | 6 μg | 9.0 | 2.2 | 22.5 |
| Vitamin B-6 | −3.5 to 21 | 2 mg | 4.9 | 1.8 | 15.4 |
| Vitamin C | 8.4 | 60 mg | 8.4 | 0.96 | 11.3 |
| Vitamin E | 5.4 | 27 IU | 5.4 | 1.1 | 11.4 |
The selected regression equations were used to predict mean analytic concentrations for each ingredient in adult multivitamin/mineral products: label value × (1 + predicted percentage difference/100). *Not significantly different from the label amount.
For 8 ingredients (vitamin B-12, vitamin C, vitamin E, copper, magnesium, manganese, phosphorus, and potassium), the predicted mean percentage differences from label were slightly above label (3.4–10%) at the most common label amounts and across all label values. For 4 ingredients (thiamin, vitamin B-6, zinc, and iron), predicted percentage differences from label were more variable, ranging from slightly below (−6.5% to −0.5%) to 8.1–21% above label across the regression ranges (linear and quadratic models). For riboflavin and folic acid, predicted percentage differences were ∼13% higher than label for all label amounts. For calcium, the predicted percentage differences from label ranged from 7.1% to 27% (quadratic model). For iodine and selenium, the predicted percentage differences from label were well above label (26% and 25%, respectively), regardless of amount claimed. Niacin (1.5% above label) was the only ingredient with mean predicted values not significantly different from the label claims. One ingredient, thiamin, had predicted means slightly below label for a majority of the amounts claimed. At the most common label amount, 1.5 mg/serving, the mean analytic content was predicted to be 6.4% below label. Some ingredients had much higher variability than others, indicated by differences in both 95% CIs for individual product content predictions and in the 95% CIs for the mean value for many products.
Model validation results indicate that the regression equations are better predictors of analytic content than the label claims for most vitamins and minerals, even for those with analytic results close to label values (see the Supplemental Results for detailed results of model validation, Supplemental Table 5).
Analysis weighted by RMSE compared with unweighted analysis
The effects of RMSE on regression results were examined. For all minerals and vitamins, the predicted percentage differences from label claims changed by <2% when unweighted regression analyses were applied compared with the results from weighted regression analyses (data not shown).
Variance component analysis
This study included 3164 observations for vitamins and 2545 observations for minerals. Of these observations, ∼84% were single analytic values, and ∼16% of the values were replicated with ≥2 analytic values. The total variability in the statistical models includes components representing variance between supplements, variance between lots within supplements, and variance between samples within lots (Tables 6 and 7).
TABLE 6.
Dietary supplement ingredient variability when separating supplement, lot, and sample variance components for minerals1
| Variance component | Calcium | Copper | Iodine | Iron | Magnesium | Manganese | Phosphorus | Potassium | Selenium | Zinc | Mean |
| Total pooled variance | 188.2 | 183.5 | 375.3 | 31.6 | 48.6 | 52.5 | 38.8 | 105.2 | 319.1 | 32.2 | 137.5 |
| Total pooled SD2 | 13.7 | 13.5 | 19.4 | 5.6 | 7 | 7.2 | 6.2 | 10.3 | 17.9 | 5.7 | 11.7 |
| Between supplements, % | 95.6 | 92.9 | 56.9 | 64.7 | 86.3 | 73.2 | 75.6 | 92.4 | 56.4 | 70.3 | 76.4 |
| Between lots within supplements, % | 3.1 | 3.5 | 39.9 | 21.8 | 8.5 | 18.5 | 18.5 | 4.4 | 41.6 | 22.4 | 18.2 |
| Between samples within lots, % | 1.4 | 3.7 | 3.1 | 13.5 | 5.2 | 8.2 | 5.9 | 3.2 | 2 | 7.3 | 5.4 |
The final regression equations were used for variance component analysis to estimate the variability between supplements, between lots within supplements, and between samples within lots. The supplement variance is an estimate of how the supplements vary around the regression line, excluding the lot and sample variances. The lot variance component is an estimate of how lots vary around the supplement mean averaged over all supplements, excluding sample variance. The sample variance component is an estimate of how samples vary around the lot mean averaged over all lots.
Square root of variance.
TABLE 7.
Dietary supplement ingredient variability when separating supplement, lot, and sample variance components for vitamins1
| Variance component | Folic acid | Niacin | Riboflavin | Thiamin | Vitamin B-12 | Vitamin B-6 | Vitamin C | Vitamin E | Mean |
| Total pooled variance | 295 | 174.7 | 628.2 | 243.7 | 490.4 | 214.4 | 125.9 | 126.1 | 287.3 |
| Total pooled SD2 | 17.2 | 13.2 | 25.1 | 15.6 | 22.1 | 14.6 | 11.2 | 11.2 | 16.9 |
| Between supplements, % | 60.1 | 79.7 | 93.9 | 76.9 | 80.6 | 87.3 | 37.4 | 53.4 | 71.2 |
| Between lots within supplements, % | 32 | 19.8 | 4.5 | 20.9 | 15.3 | 10.2 | 46.9 | 33.7 | 22.9 |
| Between samples within lots, % | 7.9 | 0.5 | 1.6 | 2.3 | 4 | 2.5 | 15.7 | 13 | 5.9 |
The final regression equations were used for variance component analysis to estimate the variability between supplements, between lots within supplements, and between samples within lots. The supplement variance is an estimate of how the supplements vary around the regression line, excluding the lot and sample variances. The lot variance component is an estimate of how lots vary around the supplement mean averaged over all supplements, excluding sample variance. The sample variance component is an estimate of how samples vary around the lot mean averaged over all lots.
Square root of variance.
For minerals, the supplement variance accounted for 56% (selenium) to 96% (calcium) of the total variability (Table 6). For most ingredients, the lot variance component accounted for ≤25% of the total variability. Exceptions were iodine and selenium, with lot variances at ∼40% of total variability. Sample variability was the smallest component of variability (except for copper, for which sample and lot variability were similar) and accounted for ≤13.5% of the variability for iron and as little as 1.4% of the variability for calcium.
Among the vitamins, supplement variance accounted for 37% (vitamin C) to 94% (riboflavin) of the total variability (Table 7). Except for vitamin C, the supplement component accounted for >50% of the total variability. The lot variance components accounted for up to one-third of total variability, except for vitamin C, for which the lot variance was 47% of total variability. Sample variability was the smallest component of variability, accounting for ≤16% of the variability for vitamin C and as little as 0.5% of the variability for niacin.
In summary, between-supplement variability was the largest source of variability for all ingredients, except for vitamin C. On average, in both vitamins and minerals, the variance associated with the supplement plus lot variability was ∼94% of the total variance. The remaining variability was due to sample variability. The overall mean total variance for vitamins was twice as high as for minerals. However there were both vitamins and minerals with high variability compared with the mean (folic acid, riboflavin, vitamin B-12, calcium, iodine, and selenium).
DISCUSSION
This study of adult MVMs estimated the relation between label and analytic values for 18 vitamins and minerals in products representative of the US market. Reliable analytic methods are now available for many ingredients in DSs. Methods for the analysis of vitamins and minerals that were originally developed to analyze nutrients in foods have been modified to accommodate the concentrations and matrices in DSs (16). In addition, methods continue to be optimized as manufacturers acquire new ingredient forms (to increase stability or bioavailability) and microencapsulation materials (coatings used, for example, to increase shelf life or mask taste). DS products are sold in different forms (e.g., powders, liquids, tablets, capsules, chewables, and gummy candies), which add unique challenges to homogenization, complete extraction, and reproducible quantification (39). The results for SRMs, in-house control materials, and product duplicates in this study’s QC program ensure confidence in the adult MVM results reported.
The percentage differences from label measured in this study were ingredient-specific and largely within the USP limits applicable in 2005–2007 (product purchase dates) (34, 40). These limits are recommended standards for the analytic content of MVM tablets and capsules: not more than 10% below labeled amounts for all nutrients, and not more than 25% above labeled amounts for minerals, 65% for oil-soluble vitamins, and 50% for water-soluble vitamins. Accordingly, in our study, the IQRs for percentage differences from label for vitamins were much wider than for minerals, excluding highly variable iodine and selenium. For these minerals, the USP has recently expanded the limits to 60% above label (31, 32). Also, for most products, applying the analytically determined ingredient overages did not change their categorization relative to the RDAs and ULs.
During MVM manufacturing, many companies intentionally add higher than claimed ingredient concentrations to compensate for losses during the shelf life, and this practice probably contributed to the overages measured in adult MVMs. We found that overall nutrient overages did not differ between products labeled below and at or above RDAs. In products labeled at or above ULs, overages were also measured, indicating that manufacturers appeared to apply overages even to high amounts of labeled ingredients. This conclusion about industry practice was supported by the results of our systematic regression analyses. Independent of the proximity of labeled amounts to RDAs and ULs, predicted mean percentage differences from label exceeded labeled amounts by 3–13% for 10 nutrients (copper; manganese; magnesium; phosphorus; potassium; folic acid; riboflavin; and vitamins B-12, C, and E); and by ∼25% for selenium and iodine. Predicted values for niacin were not significantly different from the label claims. In contrast, thiamin, vitamin B-6, calcium, iron, and zinc had linear or quadratic relations between the labels and the percentage differences from label, with ranges from −6.5% to 8.6%, −3.5% to 21%, 7.1% to 29.3%, −0.5% to 16.4%, and −1.9 to 8.1%, respectively. Only zinc had a negative slope, indicating smaller predicted overages for higher labeled content.
Another aspect of DS manufacturing is the apparent nonstandardized application of overages between different manufacturers, which may be a major factor in the variability seen in these products. For all minerals and vitamins (except for vitamin C), the between-supplement variability in percentage difference from labels was the largest source of variability, followed by the between-lot and a substantially smaller between-sample variability. The between-supplement and between-lot variability for selenium and vitamin E in our study largely resembles findings on selenium and vitamin E amounts in nutritional supplements purchased and analyzed in 2000–2003 (41, 42).
Products labeled at or above the RDAs (but not exceeding the ULs) for 12 nutrients (copper; iodine; iron; zinc; folic acid; niacin; riboflavin; thiamin; and vitamins B-12, B-6, C, and E) also had high market shares. If we add the measured overages, these 12 nutrients might be the most probable candidates for population nutrient overexposure (43). Many consumers take MVMs over years, and some combine MVM intake with the intake of higher dosage single vitamin or mineral DSs and highly fortified foods, and this raises safety concerns if the ULs are exceeded (4, 43, 44). NHANES 2003–2006 data indicated that although DS use reduced the percentage of adults (≥19 y) below the Estimated Average Requirement for calcium; magnesium; and vitamins A, D, C, and E, it also increased the percentage of participants whose intake exceeded ULs for niacin, vitamin A, folate, iron, magnesium, and zinc (1). In our study, the products with the 6 nutrients that were labeled above their ULs had low market shares. Thus, such products are less likely to lead to overexposure at the population level, although they still may be a concern for individual consumers (45) and increase their risk of adverse interactions [e.g., between MVM and prescription medicines (46, 47)].
Monitoring total nutrient intake with the use of DSID analytically verified ingredient content is necessary to adequately evaluate the population’s nutritional status and adherence to dietary recommendations, and to ensure consumer safety (2). The DSID provides research tools to more accurately assess nutrient intake from DSs. DSID estimates complement the USDA Agricultural Research Service National Nutrient Database for Standard Reference data for foods (21, 48). The regression equations are implemented in an online interactive “Adult MVM Calculator” released with DSID-3 (49). Analytically adjusted amounts of 18 ingredients are linked to the adult MVMs reported in the NHANES 2003–2008 (19).
A second set of adult MVMs was purchased in 2011 and analyzed with similar methods to assess the dynamics of DSID estimates and their applicability to various cycles of NHANES. Currently, DSID adjustments are linked to the NHANES cycles preceding, coinciding with, and after the products’ purchase date for each study. The results of the follow-up adult MVM study will be released in DSID-4, with estimates for vitamin D, vitamin A, and chromium.
In conclusion, we found that many ingredients in adult MVMs had mean percentage differences that were above label claims and were highly variable between individual products in a representative sampling of the US market. For the first time, to our knowledge, we provide unique information on overage statistics and sources of ingredient content variability across the wide landscape of adult MVMs. DSID adjustments can be applied to DS label information for more precise assessments of nutrient intake from DSs. The risk of excessive intake should be investigated when nutrient intake from foods, especially fortified foods, is combined with nutrient overages in MVMs and a prevalence of products labeled at or above the RDAs.
Acknowledgments
We thank Joanne M Holden (deceased) for her vision and expertise. We thank Jaime J Gahche for providing essential materials and advice regularly via the Dietary Supplement Working Group; Charles Perry, who provided statistical advice for the sampling plan; and National Institute of Standards and Technology, for providing Standard Reference Material 3280 for the study.
The authors’ responsibilities were as follows—KWA, JMR, PRP, and JTD: designed the research (project conception, development of overall research plan, and study oversight); KWA and JMR: conducted the research (hands-on conduct of experiments and data collection); KWA, JMR, PAG, JP, PTD, SS, FH, and LWD: analyzed the data or performed statistical analysis; KWA, PAG, and LWD: wrote the manuscript; KWA, PAG, LWD, and JTD: had primary responsibility for the final content; KWA, JMR, and PRP: supervised the study; JMB: provided essential materials; JTD, JMB, LGS, and RLB: provided advice regularly via the Dietary Supplement Working Group; and all authors: provided critical revision of the manuscript for important intellectual content and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: DS, dietary supplement; DSID, Dietary Supplement Ingredient Database; DV, Daily Value; LMS, lower market share; MVM, multivitamin/mineral product; NDL, Nutrient Data Laboratory; NIST, National Institute of Standards and Technology; QC, quality control; RDA, Recommended Dietary Allowance; RMSE, relative market share estimate; SRM, Standard Reference Material; TMS, top market share; UL, Tolerable Upper Intake Level; USP, US Pharmacopeia.
REFERENCES
- 1.Fulgoni VL III, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: where do Americans get their nutrients? J Nutr 2011;141:1847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr 2010;140:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. Centers for disease control and prevention. About the National Health and Nutrition Examination Survey, National Center for Health Statistics. 2014 [cited 2015 Dec 21]. Available from: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
- 4.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med 2013;173:355–61. [DOI] [PubMed] [Google Scholar]
- 5.Bailey RL, Fulgoni VL III, Keast DR, Dwyer JT. Examination of vitamin intakes among US adults by dietary supplement use. J Acad Nutr Diet 2012;112:657-63.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macpherson H, Pipingas A, Pase MP. Multivitamin-multimineral supplementation and mortality: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2013;97:437–44. [DOI] [PubMed] [Google Scholar]
- 7.Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2013;159:824–34. [DOI] [PubMed] [Google Scholar]
- 8.Angelo G, Drake VJ, Frei B. Efficacy of multivitamin/mineral supplementation to reduce chronic disease risk: a critical review of the evidence from observational studies and randomized controlled trials. Crit Rev Food Sci Nutr 2015;55:1968–91. [DOI] [PubMed] [Google Scholar]
- 9.Bailey RL, Fakhouri TH, Park Y, Dwyer JT, Thomas PR, Gahche JJ, Miller PE, Dodd KW, Sempos CT, Murray DM. Multivitamin-mineral use is associated with reduced risk of cardiovascular disease mortality among women in the United States. J Nutr 2015;145:572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comerford KB. Recent developments in multivitamin/mineral research. Adv Nutr 2013;4:644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ConsumerLab.com. Multivitamin and multimineral supplements review [cited 2015 Dec 21]. Available from: https://www.consumerlab.com/reviews/multivitamin_review_comparisons/multivitamins/.
- 12.LeDoux MA, Appelhans KR, Braun LA, Dziedziczak D, Jennings S, Liu L, Osiecki H, Wyszumiala E, Griffiths JC. A quality dietary supplement: before you start and after it’s marketed—a conference report. Eur J Nutr 2015;54(Suppl 1):S1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Department of Agriculture and US Department of Health and Human Services. Report of the dietary guidelines advisory committee on the dietary guidelines for Americans, 2010 [cited 2015 Dec 21]. Available from: http://www.cnpp.usda.gov/sites/default/files/dietary_guidelines_for_americans/2010DGACReport-camera-ready-Jan11-11.pdf.
- 14.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes tables and application [cited 2015 Dec 21]. Available from: http://iom.nationalacademies.org/Activities/Nutrition/SummaryDRIs/DRI-Tables.aspx.
- 15.Coates PM. Dietary supplements and health: the research agenda. Novartis Found Symp 2007;282:202–7, discussion 207–18. [DOI] [PubMed] [Google Scholar]
- 16.Roseland JM, Holden J, Andrews K, Zhao C, Schweitzer A, Harnly J, Wolf W, Perry C, Dwyer J, Picciano M, et al. Dietary supplement ingredient database (DSID): preliminary USDA studies on composition of adult multivitamin-mineral supplements. J Food Compost Anal 2008;21:S69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dwyer J, Picciano MF, Raiten DJ. Estimation of usual intakes: what we eat in America—NHANES. J Nutr 2003;133:609S–23S. [DOI] [PubMed] [Google Scholar]
- 18.Dwyer J, Picciano MF, Raiten DJ. Food and dietary supplement databases for what we eat in America—NHANES. J Nutr 2003;133:624S–34S. [DOI] [PubMed] [Google Scholar]
- 19.US Department of Agriculture. Agricultural Research Service, US Department of Health and Human Services, National Institutes of Health, Office of Dietary Supplements. Dietary supplement ingredient database (DSID); release 3.0 [cited 2015 Mar 25]. Available from: http://dsid.usda.nih.gov/.
- 20.Pehrsson PR, Haytowitz DB, Holden JM, Perry CR, Beckler DG. USDA’s national food and nutrient analysis program: food sampling. J Food Comp Anal 2000;13:379–89. [Google Scholar]
- 21.US Department of Agriculture. Agricultural Research Service. USDA national nutrient database for standard reference (SR), release 28, 2015 [cited 2015 Dec 21]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=8964.
- 22.National Center for Health Statistics. Centers for Disease Control and Prevention. National health and nutrition examination survey. NHANES 2001-2002 dietary data [cited 2015 Dec 21]. Available from: http://wwwn.cdc.gov/nchs/nhanes/search/DataPage.aspx?Component=Dietary&CycleBeginYear=2001.
- 23.Murphy SP, Wilkens LR, Monroe KR, Steffen AD, Yonemori KM, Morimoto Y, Albright CL. Dietary supplement use within a multiethnic population as measured by a unique inventory method. J Am Diet Assoc 2011;111:1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nutrition Business Journal. NBJ’s global supplement & nutrition industry report 2007. New York: Penton Media Inc.; 2007. [Google Scholar]
- 25.National Center for Health Statistics. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. NHANES 2003-2004 dietary data [cited 2015 Dec 21]. Available from: http://wwwn.cdc.gov/nchs/nhanes/search/DataPage.aspx?Component=Dietary&CycleBeginYear=2003.
- 26.Fed.Biz.Opps.Gov [cited 2015 Dec 21]. Available from: https://www.fbo.gov/.
- 27.Dwyer JT, Holden J, Andrews K, Roseland J, Zhao C, Schweitzer A, Perry CR, Harnly J, Wolf WR, Picciano MF, et al. Measuring vitamins and minerals in dietary supplements for nutrition studies in the USA. Anal Bioanal Chem 2007;389:37–46. [DOI] [PubMed] [Google Scholar]
- 28.US Department of Commerce. National Institute of Standards & Technology, certificate of analysis, standard reference material 3280, multivitamin/multielement tablets [cited 2015 Dec 21]. Available from: https://www-s.nist.gov/m-srmors/certificates/3280.pdf.
- 29. United States Pharmacopeia. National formulary, USP28-NF23. Rockville (MD): United States Pharmacopeial Convention; 2004.
- 30.Andrews KW, Schweitzer A, Zhao C, Holden JM, Roseland JM, Brandt M, Dwyer JT, Picciano MF, Saldanha LG, Fisher KD, et al. The caffeine contents of dietary supplements commonly purchased in the US: analysis of 53 products with caffeine-containing ingredients. Anal Bioanal Chem 2007;389:231–9. [DOI] [PubMed] [Google Scholar]
- 31. The United States Pharmacopeial Convention. Oil- and water-soluble vitamins with minerals tablets United States Pharmacopeia National Formulary, USP39-NF34. Rockville (MD): The United States Pharmacopeial Convention; 2016. p. 7014–40.
- 32. The United States Pharmacopeial Convention. Oil- and water-soluble vitamins with minerals capsules. United States Pharmacopeia National formulary, USP39-NF34. Rockville (MD): The United States Pharmacopeial Convention; 2016. p. 6974–7001.
- 33.Association of Official Analytical Chemists International. Official methods of analysis, 18th ed. Gaithersburg (MD): Association of Official Analytical Chemists International; 2005. [Google Scholar]
- 34. The United States Pharmacopeial Convention. Oil- and water-soluble vitamins with minerals tablets. United States Pharmacopeia National formulary, USP28-NF23. Rockville (MD): The United States Pharmacopeial Convention; 2004. p. 2155–73.
- 35.Asami DK, Hong YJ, Barrett DM, Mitchell AE. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J Agric Food Chem 2003;51:1237–41. [DOI] [PubMed] [Google Scholar]
- 36.Cort WM, Vicente TS, Waysek EH, Williams BD. Vitamin E content of feedstuffs determined by high-performance liquid chromatographic fluorescence. J Agric Food Chem 1983;31:1330–3. [DOI] [PubMed] [Google Scholar]
- 37.US Food and Drug Administration. Guidance for industry: a food labeling guide (14. Appendix F: calculate the percent daily value for the appropriate nutrients) [cited 2016 Jul 6]. Available from: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm064928.htm.
- 38.Linsinger T. Comparison of measurement result with the certified value. Geel (Belgium): Joint Research Centre, Institute for Reference Materials and Measurements; 2005. [Google Scholar]
- 39.Szpylka J, DeVries JW. Determination of beta-carotene in supplements and raw materials by reversed-phase high pressure liquid chromatography: collaborative study. J AOAC Int 2005;88:1279–91. [PMC free article] [PubMed] [Google Scholar]
- 40. The Unites States Pharmacopeial Convention. Oil and water-soluble vitamins with minerals capsules. Unites States Pharmacopeia, National formulary, USP 28-NF23. Rockville, MD: The United States Pharmacopeial Convention; 2004. p. 2145–49.
- 41.Feifer AH, Fleshner NE, Klotz L. Analytical accuracy and reliability of commonly used nutritional supplements in prostate disease. J Urol 2002;168:150–4, discussion 154. [PubMed] [Google Scholar]
- 42.Veatch AE, Brockman JD, Spate VL, Robertson JD, Morris JS. Selenium and nutrition: the accuracy and variability of the selenium content in commercial supplements. J Radioanal Nucl Chem 2005;264:33–8. [Google Scholar]
- 43.NIH State-of-the-Science Panel. National Institutes of Health state-of-the-science conference statement: multivitamin/mineral supplements and chronic disease prevention. Ann Intern Med 2006;145:364–71. [DOI] [PubMed] [Google Scholar]
- 44.Dickinson A, MacKay D. Health habits and other characteristics of dietary supplement users: a review. Nutr J 2014;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulholland CA, Benford DJ. What is known about the safety of multivitamin-multimineral supplements for the generally healthy population? Theoretical basis for harm. Am J Clin Nutr 2007;85:318S–22S. [DOI] [PubMed] [Google Scholar]
- 46.Yetley EA. Multivitamin and multimineral dietary supplements: definitions, characterization, bioavailability, and drug interactions. Am J Clin Nutr 2007;85:269S–76S. [DOI] [PubMed] [Google Scholar]
- 47.Seely D, Kanji S, Yazdi F, Tetzlaff J, Singh K, Tsertsvadze A, Sears ME, Tricco A, Ooi TC, Turek M, et al. Dietary supplements in adults taking cardiovascular drugs comparative effectiveness reviews, 2012th ed US: Agency for Healthcare Research and Quality; 2012. [PubMed] [Google Scholar]
- 48.Ahuja JK, Moshfegh AJ, Holden JM, Harris E. USDA food and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice. J Nutr 2013;143:241S–9S. [DOI] [PubMed] [Google Scholar]
- 49.US Department of Agriculture. Agricultural Research Service, US Department of Health and Human Services, National Institutes of Health, Office of Dietary Supplements. Dietary supplement ingredient database (DSID); release 3.0. Adult MVM calculator [cited 2015 Dec 21]. Available from: http://dsid.usda.nih.gov/ingredient_calculator/calc_adult.php.