Abstract
Background: Large portions of food promote intake, but the mechanisms that drive this effect are unclear. Previous neuroimaging studies have identified the brain-reward and decision-making systems that are involved in the response to the energy density (ED) (kilocalories per gram) of foods, but few studies have examined the brain response to the food portion size (PS).
Objective: We used functional MRI (fMRI) to determine the brain response to food images that differed in PSs (large and small) and ED (high and low).
Design: Block-design fMRI was used to assess the blood oxygen level–dependent (BOLD) response to images in 36 children (7–10 y old; girls: 50%), which was tested after a 2-h fast. Pre-fMRI fullness and liking were rated on visual analog scales. A whole-brain cluster-corrected analysis was used to compare BOLD activation for main effects of the PS, ED, and their interaction. Secondary analyses were used to associate BOLD contrast values with appetitive traits and laboratory intake from meals for which the portions of all foods were increased.
Results: Compared with small-PS cues, large-PS cues were associated with decreased activation in the inferior frontal gyrus (P < 0.01). Compared with low-ED cues, high-ED cues were associated with increased activation in multiple regions (e.g., in the caudate, cingulate, and precentral gyrus) and decreased activation in the insula and superior temporal gyrus (P < 0.01 for all). A PS × ED interaction was shown in the superior temporal gyrus (P < 0.01). BOLD contrast values for high-ED cues compared with low-ED cues in the insula, declive, and precentral gyrus were negatively related to appetitive traits (P < 0.05). There were no associations between the brain response to the PS and either appetitive traits or intake.
Conclusions: Cues regarding food PS may be processed in the lateral prefrontal cortex, which is a region that is implicated in cognitive control, whereas ED activates multiple areas involved in sensory and reward processing. Possible implications include the development of interventions that target decision-making and reward systems differently to moderate overeating.
Keywords: energy density, neuroimaging, obesity, pediatric, portion size
See corresponding editorial on page 289.
INTRODUCTION
Obesogenic environments promote intake from large portions of high energy–dense foods (1). The food portion size (PS)7 and energy density (ED) (kilocalories per gram) positively influence energy intake (2, 3), but the mechanisms that derive their effects are unclear (4). Food intake is controlled by both metabolic and hedonic signals that originate from neuroregulatory systems (5, 6). Images of food elicit the activation of brain systems that regulate appetite, reward, and inhibitory control (7–13). Therefore, food images at different PSs and EDs may differentially engage brain systems.
Research that has used fMRI has advanced our understanding of the way in which the brain processes food cues and how this procedure affects eating behavior. Brain regions that are involved in reward (11, 14), executive function (7, 11, 15), and recognition (16, 17) are more responsive to food stimuli with higher appetitive values than with lower appetitive values. These effects are also modulated by hunger (8, 10, 13) and satiety (18). However, the interpretation of previous fMRI studies has been limited because 1) the PS of food stimuli has rarely been controlled, and 2) children <10 y old have rarely been included (7, 9, 15–17, 19). The determination of how children’s brains respond to PS and ED cues could shed light on how these cues interact to influence eating patterns and food intakes.
Previous studies have investigated the impact of visual food characteristics, such as the PS, on eating behaviors (20–24). For example, physical features such as food shape influence children’s food preferences (22). Food PS cues may influence consumption by providing 1) information about the amount available to consume (20) or 2) a reference point for an amount that is socially appropriate to consume (21). Food ED also influences the amount of energy consumed (25). For example, children increased energy intakes >30% from a high-ED entrée than from a low-ED entrée despite similar levels of palatability (3). Disentangling the effects that PS and ED have on the brain’s cognitive and reward-processing systems will provide insight into why these food cues promote intake.
To gather insight into the neurobiological mechanisms that are involved with the processing of food PS and ED cues in children, this study used a whole-brain fMRI analysis to test competing hypotheses. On the basis of the cognitive influences that food PSs may have in anticipation of a meal (20, 21, 23, 24, 26), we hypothesized that large-PS cues compared with small-PS cues would activate regions that are responsible for cognitive control. Because the food energy content is positively associated with the activation of appetitive brain networks (14, 27), we hypothesized that high-ED cues compared with low-ED cues would activate regions that are involved in sensory and reward processing. Because of the evidence that food PSs and EDs interact to promote excess energy intake (28), we anticipated that regions that are important for reward valuation would show a PS × ED interaction. A secondary aim was to determine associations between the brain response and measures of eating behavior.
METHODS
Participants
Participants were children between the ages of 7 and 10 y (mean ± SD age: 8.9 ± 1.2; non-Hispanic whites: 97%; girls: 50%). Potential participants were recruited through advertisements that were posted in the local community and by word of mouth. Inclusion criteria were as follows: righthandedness to reduce variance on the basis of brain hemisphere dominance (29); native English speakers who were reading at or above their grade levels; a lack of metal in or on the body (e.g., braces) to avoid fMRI-scanner artifacts; not currently taking medications that may influence brain activity; and being healthy with no food allergies (Supplemental Figure 1). We excluded children with diagnosed psychological conditions (as indicated by parent report) that might have affected the children’s comfort in the scanner (e.g., claustrophobia and attention-deficit hyperactivity disorder). The sample size was determined on the basis of Desmond and Glover (30), who recommended a sample size of 48 participants on the basis of a 50% success rate in the scanner to achieve 80% power with the expectation of moderate effect sizes and correction for multiple comparisons. In addition, previous fMRI studies that have used comparable designs were consulted to determine expected effect sizes (9, 11, 17). Our success rate with 36 of 38 participants was 94.7%, which suggested that we were well powered for whole-brain analyses. Parental consent and child assent were obtained at the first visit. Participants received a modest monetary incentive at the completion of each visit. The study was approved by the Pennsylvania State University Institutional Review Board.
Study design
We previously tested a limited number of a priori–defined brain regions that have been implicated in reward processing and decision making in this same cohort with the use of a different analytic approach (31). However, there may be other brain regions that have not been previously tested that are responsive to food PS and ED cues. The current article used an exploratory whole-brain analysis approach to address that gap in knowledge. We used a within-subject experimental design to evaluate whole-brain responses to food images that varied in PSs (large PS compared with small PS) and ED (high ED compared with low ED). An additional aim of this study was to assess brain mechanisms of children’s laboratory intakes in response to variations in food PSs (Supplemental Table 1). These methods have been described previously (4). The entire study took place across 5 visits with the test meals occurring at visits 1–4, and the fMRI scan occurring at visit 5. Children were tested at the same time for all visits (either lunch or dinner time according to availability). The children arrived at each visit after a 2-h fast. At the first visit, parents and children completed anthropometric measurements and assessments of appetitive traits on the Child Eating Behavior Questionnaire (32). Test meals at visits 1–4 included commonly consumed, age-appropriate foods that have been used in similar laboratory paradigms (33) (Supplemental Table 2). Foods with high EDs (≥1.5 kcal/g) that were served included macaroni and cheese, garlic bread, and cake, and foods with low EDs (<1.5 kcal/g) included broccoli, tomatoes, and grapes. Portions of all foods were increased simultaneously to reach the following 4 PS conditions of test meals: 100% (reference; 824 kcal), 133%, 167%, and 200%. Children received the meals in a randomized order and could eat as much as they liked at each meal. Mock fMRI training was completed with children after test-meal visits 3 and 4 (4). At the fifth visit, BOLD fMRI was conducted to assess the whole-brain response as children viewed food images that were presented at 2 PSs (large PS and small PS) and 2 EDs (high ED and low ED).
Stimuli
The Continuing Survey of Food Intake by Individuals was used to select 60 foods that are commonly eaten by children, which were split into a high-ED category (≥1.5 kcal/g) and a low-ED category (<1.5 kcal/g) (34). The amount of food shown for the small-PS and large-PS conditions was photographed at approximately the 10th and 90th percentiles, respectively, of amounts that are commonly consumed by children in this age group according to the Continuing Survey of Food Intake by Individuals. Examples of high-ED foods included chicken nuggets, French fries, and cookies. Examples of low-ED foods included grilled chicken, green beans, and blueberries (Supplemental Figure 2). All pictures were taken with a Canon PowerShot SX260 HS camera (Canon USA Inc.) at an angle (52.8°) to approximate a child’s view if seated at a dining table. Foods were photographed with white dishware [18-oz. bowls, 10.25- in (26-cm) plates; Corelle Livingware Winter Frost White) on a background of blue linen tablecloth. Inconsistencies in food color, size, and depth were manually adjusted with image-manipulation software (GIMP v. 2.8).
Anthropometric measurements
Height was measured to the nearest centimeter and weight was measured to the nearest hundredth of a kilogram by trained researchers with the use of a stadiometer (Seca model 202 Chino) and a standard scale (Detecto model 437). The percentage of body fat was collected via a bioelectric impedance analysis (Tanita model BF-350). BMI (in kg/m2) was calculated as weight divided by the square of height. The CDC growth reference data were used to convert BMI to age- and sex-specific BMI z scores and BMI percentiles (35).
Visual analog scales
Perceived fullness levels were rated by children immediately before and after fMRI with the use of a 150-mm visual analog scale (VAS), which depicted a stick figure with a rectangular stomach (36). Because of the known influence of the appetitive state on the brain’s response to food stimuli (7), children were scanned after a 2-h fast. A neutral, pre-fMRI fullness rating was defined between 38 and 112 mm (i.e., roughly the middle 50% of the scale). The pre-fMRI fullness rating was used as a covariate in analyses and is referred to as fullness. Children rated their subjective liking of images that were viewed during the fMRI immediately after the scan. By pointing at a computerized 1500-mm VAS that was anchored by “not at all” and “very much”, children responded to the following question: “How much do you like this food/item?” The difference in the mean rated liking of food images within a condition (e.g., the mean liking of high ED minus the mean liking of low ED) was used as a covariate in analyses and is referred to as liking.
Appetitive traits
Child appetitive traits were measured with the use of the Child Eating Behavior Questionnaire (32), which is a 35-item instrument that can be reduced to 8 subscales that are commonly referred to as appetitive traits. Parents reported how often their child exhibited behaviors on a scale from 1 (never) to 5 (always). The study focused on the following traits that are most-commonly associated with overeating (37): satiety responsiveness (e.g., “my child gets full up easily”), slow eating (e.g., “my child eats slowly as a meal progresses”), food responsiveness (e.g., “given the choice, my child would eat most of the time”), and enjoyment of food (e.g., “my child enjoys eating”). Internal consistency was acceptable at a Chronbach’s α > 0.70 for each of the subscales.
fMRI paradigm
Before the fMRI scan, children received training in a simulated scanning environment (i.e., the mock scanner) that mimicked the appearance and sounds of the actual scanner. This 2-session training was developed for this study and has been detailed elsewhere (4). Briefly, the first session allowed researchers to observe the child comfort level in the mock scanner. The second session was focused on training the children to remain still and answer questions without moving as they completed several minutes of a simulated fMRI paradigm.
A total of 180 images were presented in a block design with four food conditions with varying PSs (i.e., large PS and small PS) and EDs (i.e., high ED and low ED) and 2 control conditions of nonfood images (i.e., furniture and scrambled images) for a total of 6 conditions. The fMRI battery included one structural scan to determine the brain anatomy and 6 functional runs to obtain the BOLD activity of the brain in response to the 6 conditions. Each run contained 30 images that included 5 pictures from each of the 6 conditions that were shown in a pseudorandomized order so that the child would not see more than 2 food conditions in a row before seeing a control condition. An example of this functional paradigm has been published elsewhere (4). The stimuli duration was set at 2000 ms with a 500-ms interstimulus interval of a fixation cross and a randomly generated time for the intertrial interval that ranged from 2000 to 11,000 ms. Control conditions were scrambled images and furniture; however, these data are not presented in this article because the objective was to present the contrasts between PS and ED food conditions. Therefore, the main comparisons of interest were the BOLD activation in response to food images that were shown with large PSs compared with small PSs (mean energy contents of 227 compared with 46 kcal, respectively) and high ED compared with low ED (mean energy contents of 222 compared with 51 kcal, respectively) and their interactions.
fMRI data acquisition
Data were acquired from a Siemens MAGNETOM Trio 3T MRI scanner (Siemens Medical Solutions) with a standard coil (12 channels). Pillows and padding around the head, arms, and body of participants were used to restrict motion. The stimulus presentation was controlled by a computer with the use of Matlab v. 8.0 software. Functional scans used a T2*-weighted gradient, single-shot echo, planar-imaging sequence (echo time: 25 ms; repetition time: 2000 ms; flip angle: 90°; matrix: 64 × 64) with an in-plane resolution of 3 × 3 mm (field of view: 220 mm) to acquire thirty-three 3-mm (interleaved) slices along the anterior commissure–posterior commissure plane. In-scan prospective movement correction was used to assist with the motion-induced effects during acquisition by adjusting the slice positioning (38). Structural scans were collected with the use of a T1-weighted magnetization-prepared rapid gradient-echo sequence (repetition time: 1650 ms; echo time: 2.03 ms; flip angle: 9°; field of view: 256 mm; slice thickness: 1 mm; sagittal plane, voxel size: 1 × 1 × 1 mm3). Researchers verbally checked participant comfort and alertness in between functional runs. The entire fMRI battery (structural scan and 6 functional runs) was designed for completion in ∼30 min but varied between 21 and 35 min depending on participant performance.
Data analysis
Participant characteristics were analyzed with the use of SPSS 22.0 software (IBM Corp.) and are reported as means ± SDs. Differences in pre- and post-fMRI measures for perceived fullness and liking were analyzed with the use of paired t tests. A P-value cutoff of 0.05 was used to determine significance.
Exploratory statistical analyses for whole-brain activation during fMRI were conducted with BrainVoyager QX software (v. 2.8.2; Brain Innovation). Functional data preprocessing steps included the trilinear 3-dimensional motion correction of 6 vectors (3 translations and 3 rotations), temporal filtering (high-pass filtering with the use of a general linear model (GLM)–Fourier basis set with 6 cycles/time course), and 3-dimensional spatial smoothing with a Gaussian filter with a full width at half maximum of 8 mm3. Preprocessed functional data from each run were co-registered to each participant’s anatomical data. Anatomical data were normalized to Talairach space (39) by manually fitting 6 variables (anterior, posterior, inferior, superior, left, and right) with the use of the anterior commissure–posterior commissure landmark and spatially realigning within the BrainVoyager program. To be included in the final analyses, subjects had to have a successful anatomical scan and ≥1 functional run below the excessive motion cutoff of 3 mm or 3° in any direction. Of the final 36 participants analyzed, 23 of 228 possible functional runs were excluded due to excessive motion. Participants in the final sample had an average of 5.36 (range of 3–6) successful functional runs that were entered into analyses.
Following standard preprocessing steps, fMRI data were analyzed with the use of a random-effects GLM with task-related regressors. Regressors for each condition were convolved with a standard 2-gamma hemodynamic response function and entered into a GLM to obtain variable estimates (i.e., beta weights) for each participant. Variable estimates obtained for each participant were then entered into a random-effects group analysis at the second level and analyzed across subjects with the use of contrast coding to obtain statistical t maps and repeated measures ANOVA to obtain F statistics. A gray-matter mask was applied to reduce the search area. Correction for multiple comparisons included a familywise approach (α < 0.05; P < 0.01, k > 10 voxels), determined by Monte Carlo simulation (40, 41) with the BrainVoyager QX Cluster-Level Statistical Threshold Estimator plug-in (Brain Innovation). Cluster-extent thresholds of 6 (corresponding to the main effect of PS) and 7 (corresponding to the main effect of ED and the PS × ED–interaction effects) contiguous voxels (and each in combination with a per-voxel threshold of P < 0.01) were applied to statistical maps. The estimated full width at half maximum (i.e., smoothness) of each map was 1 mm3 after correction for PS, ED, and PS × ED. Peak coordinates within each brain region that exhibited significant effects are presented in the results tables. These tests were followed up by post hoc analyses to correct for multiple, pairwise comparisons. Beta weights within all significant regions from all conditions were extracted to the SPSS program to conduct secondary analyses to control for relevant covariates (i.e., fullness, BMI z score, liking, and sex). Similar analysis strategies have been reported in related fMRI studies of high-calorie food-image viewing (16, 42).
Brain regions that showed activation in response to PS, ED, and their interaction were associated with child appetitive traits in follow-up analyses with the use of SPSS software. For these analyses, differences in the BOLD signal were calculated by subtracting β weights to one factor from another to create contrast values. The BOLD contrast values for ED—i.e., high ED − low ED—were created by taking the mean β weights from high ED minus low ED. Similarly, the BOLD contrast values for PS—i.e., large PS − small PS— were created by taking the mean β weights from large PS minus small PS. Pearson correlations were conducted to examine the relation between those BOLD contrast values and appetitive trait scores. Mixed linear model analyses determined whether BOLD contrast values for PS and ED interacted with test-meal PS conditions (i.e., 100%, 133%, 167%, and 200%) to influence energy intake (Supplemental Table 2). Because food intake shows a curvilinear relation (33) in response to increased PS, fixed factors included PS conditions treated as linear and quadratic functions, whereas subjects were treated as a random factor. BOLD contrast values and their interaction with the PS condition at the test meal were included as covariates.
RESULTS
Participant characteristics and behavioral data
Descriptive data are shown in Table 1. The majority of children were of healthy weight with a mean BMI z score −0.20 ± 0.8. There were no differences between prescan and postscan fullness ratings (P > 0.10). Mean liking ratings were significantly higher for images of high-ED foods than for images of low-ED foods [t(35) = 6.6, P < 0.01].
TABLE 1.
Descriptive statistics for participants (n = 36; girls: 50%)1
| Characteristic | Mean ± SD | Range |
| Age, y | 8.9 ± 1.2 | 7–10 |
| Body fat, % | 16.4 ± 6.5 | 5.3–37 |
| BMI z score | −0.2 ± 0.8 | −1.5 to 2.0 |
| CEBQ appetitive traits (α > 0.70) | ||
| Satiety responsiveness | 2.9 ± 0.7 | 1.4–4.2 |
| Slow eating (lower = faster) | 2.7 ± 0.8 | 1.3–4.3 |
| Enjoyment of food | 3.8 ± 0.6 | 3.0–5.0 |
| Food responsiveness | 2.5 ± 0.6 | 1.2–4.0 |
| Visual analog scale ratings, mm | ||
| Liking of large PS | 101.3 ± 23.2 | 62–150 |
| Liking of small PS | 100.7 ± 23.8 | 40–140 |
| Liking of high ED2 | 112.7 ± 24.7 | 57–150 |
| Liking of low ED | 89 ± 26.4 | 39–140 |
| Fullness pre-fMRI | 40.8 ± 39.4 | 0–136 |
| Fullness post-fMRI | 36.3 ± 39.0 | 0–142 |
Data (n = 36) were analyzed with the use of paired t tests when appropriate. Pre- and post-fMRI fullness levels did not significantly differ. The liking of images of large-PS foods compared with small-PS foods did not significantly differ. CEBQ, Child Eating Behavior Questionnaire; ED, energy density; PS, portion size.
Liking ratings of high ED compared with low ED were significantly different [t(35) = 6.6, P < 0.01].
fMRI data
Main effects for PS
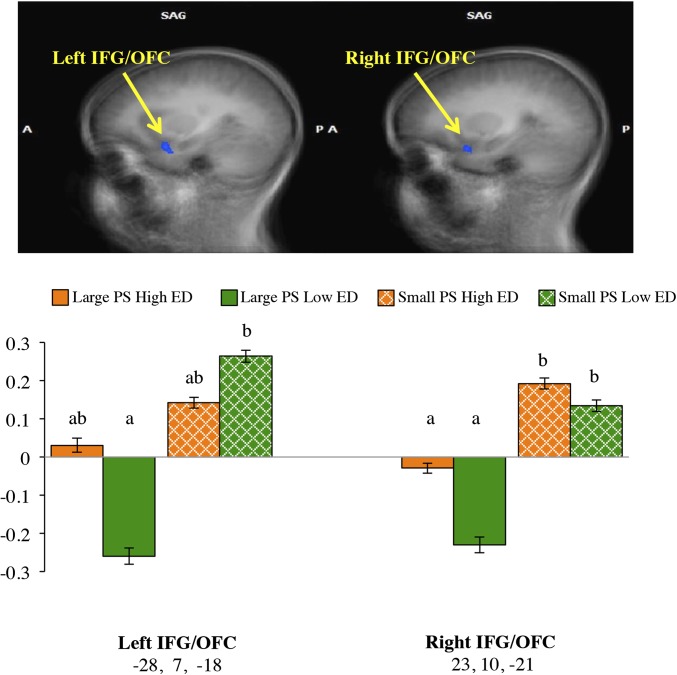
Table 2 details the significant functional activations with peak statistical values. As shown in Figure 1, exposure to images of large-portion foods compared with images of small-portion foods revealed decreased activation in the bilateral inferior frontal gyrus (IFG), which corresponded to Brodmann area (BA) 47. The effects in the IFG remained significant after adjustment for BMI z score and sex (P < 0.05) but were no longer significant after adjustment for either fullness, (left, P = 0.15; right, P = 0.08) or liking (left, P = 0.55; right, P = 0.36) (Table 3). To aid in the interpretation of this effect, Figure 1 shows that post hoc comparisons revealed reduced activation to images of large-PS, low-ED foods than to images of small-PS, low-ED foods in the left IFG (P < 0.01).
TABLE 2.
Peak coordinates of functional areas with main effects for PS, ED, and their interaction1
| Brain region | Hemisphere | BA | k | x | y | z | F | P |
| Main effects for PS | ||||||||
| IFG/OFC | Left | 47 | 14 | −28 | 7 | −18 | 5.61 | 0.023 |
| IFG/OFC | Right | 47 | 14 | 23 | 10 | −21 | 7.18 | 0.011 |
| Main effects for ED | ||||||||
| Fusiform gyrus | Right | 37 | 39 | 47 | −56 | −12 | 5.70 | 0.022 |
| Insula (superior/posterior) | Left | 13 | 52 | −40 | −20 | 12 | 5.00 | 0.031 |
| Superior temporal gyrus | Left | 38 | 15 | −52 | 13 | −15 | −9.90 | 0.003 |
| Insula (inferior/anterior) | Right | 13 | 17 | 41 | −2 | −6 | −7.50 | 0.010 |
| Caudate | Left | — | 151 | −1 | 4 | 15 | 9.91 | 0.003 |
| Parahippocampal gyrus | Right | 34 | 62 | 11 | 1 | −21 | 12.50 | 0.001 |
| Posterior cingulate gyrus | Right | 31 | 43 | 2 | −29 | 36 | 5.00 | 0.026 |
| Anterior cingulate gyrus | Right | 32 | 78 | 5 | 19 | 33 | 5.40 | 0.031 |
| Cerebellar tonsil | Right | — | 44 | 23 | −41 | −38 | 11.50 | 0.002 |
| Declive | Left | — | 27 | −4 | −77 | −21 | 4.50 | 0.041 |
| Declive | Left | — | 48 | −43 | −74 | −18 | 4.40 | 0.042 |
| Precentral gyrus | Left | 6 | 46 | −67 | 7 | 7 | 4.90 | 0.033 |
| Interaction for PS × ED | ||||||||
| Superior temporal gyrus | Left | 38 | 14 | −34 | 3 | −15 | 12.48 | 0.001 |
Whole-brain activation (n = 36) at the second level from a random-effects analysis. Results represent the brain region in the right or left hemisphere and respective BA in which either main effects (PS or ED) or an interaction (PS × ED) were shown with the use of a repeated-measures ANOVA, the k (number of contiguous voxels in the cluster), and coordinates of the peak statistical voxel for each region (x, y, and z in Talairach space). A primary voxel threshold of P < 0.01 and a minimum k of 10 were applied before correction for multiple comparisons to provide a corrected cluster threshold of 6 for PS and 7 for both ED and PS × ED at P < 0.05. BA, Brodmann area; ED, energy density; IFG, inferior frontal gyrus; k, cluster size; OFC, orbitofrontal cortex; PS, portion size.
FIGURE 1.
fMRI statistical maps in the SAG view showing activation to large PSs compared with activation to small PSs and co-registered with average structural MRI data from participants. Results are from whole-brain analyses (n = 36) that tested the main effects of PS and were co-registered with averaged structural MRI data from participants. (Top) Representative t maps in the SAG view showing reduced activation (blue colors) in the bilateral IFG (P < 0.01, corrected). (Bottom) Mean ± SEM BOLD magnitude for each condition of food images (large PS, high ED; large PS, low ED; small PS, high ED; and small PS, low ED) within peak coordinates (x, y, and z in Talairach space) of each functional cluster. Bars that do not share a common letter (a, b, or c) are significantly different at P < 0.05. A, anterior; ED, energy density; IFG, inferior frontal gyrus, OFC, orbitofrontal cortex; P, posterior; PS, portion size; SAG, sagittal.
TABLE 3.
Influence of covariates on regions with main effects or interactions between PS and ED1
| Fullness |
BMI z score |
Liking |
Sex |
|||||
| Brain region | F | P | F | P | F | P | F | P |
| Main effects for PS | ||||||||
| IFG/OFC, left | 3.4 | 0.076 | 6.2 | 0.018* | 1.5 | 0.224 | 4.3 | 0.046* |
| IFG/OFC, right | 2.2 | 0.151 | 7.0 | 0.012* | 2.8 | 0.103 | 4.3 | 0.046* |
| Main effects for ED | ||||||||
| Fusiform gyrus, right | 1.7 | 0.201 | 4.6 | 0.038* | 3.5 | 0.071 | 2.0 | 0.208 |
| Insula (superior/posterior), left | 1.3 | 0.257 | 4.8 | 0.036* | 6.2 | 0.018* | 3.2 | 0.084 |
| Superior temporal gyrus, left | 2.8 | 0.105 | 9.1 | 0.005** | 4.4 | 0.044* | 2.3 | 0.140 |
| Insula (inferior/anterior), right | 6.9 | 0.013* | 7.2 | 0.011* | 1.1 | 0.296 | 3.1 | 0.089 |
| Caudate, left | 1.2 | 0.290 | 7.7 | 0.009** | 6.6 | 0.015* | 6.7 | 0.014* |
| Parahippocampal gyrus, right | 6.5 | 0.016* | 12.2 | 0.010* | 1.9 | 0.178 | 5.3 | 0.028* |
| Posterior cingulate gyrus, right | 1.5 | 0.225 | 4.3 | 0.046* | 1.2 | 0.273 | 9.4 | 0.004** |
| Anterior cingulate gyrus, right | 0.9 | 0.429 | 4.1 | 0.050 | 8.2 | 0.007** | 7.2 | 0.011* |
| Cerebellar tonsil, right | 8.9 | 0.005** | 11.3 | 0.002** | 7.0 | 0.120 | 5.2 | 0.029* |
| Declive, left | 0.8 | 0.371 | 3.7 | 0.062 | 0.6 | 0.444 | 1.8 | 0.189 |
| Declive, left | 0.3 | 0.587 | 3.3 | 0.078 | 0.9 | 0.342 | 0.3 | 0.589 |
| Precentral gyrus, left | 3.9 | 0.055 | 6.5 | 0.015* | 2.7 | 0.110 | 2.9 | 0.082 |
| Interaction for PS × ED | ||||||||
| Superior temporal gyrus, left | 2.2 | 0.145 | 9.4 | 0.004** | 5.6 | 0.024* | 0.8 | 0.382 |
Whole-brain activation (n = 36) at the second level. A random-effects analysis revealed brain regions in the right or left hemisphere with either significant main effects or an interaction for PS and ED in primary analyses. An ANCOVA with post hoc least significant differences was used to assess large PS compared with small PS, high ED compared with low ED, and the PS × ED interaction adjusted for covariates of either pre-fMRI fullness rating, liking of food images, or BMI z score. *,**Main effects and interactions that remained significant after adjustment for respective covariates: *P < 0.05, **P < 0.01. ED, energy density; IFG, inferior frontal gyrus; OFC, Orbitofrontal cortex; PS, portion size.
Main effects for ED
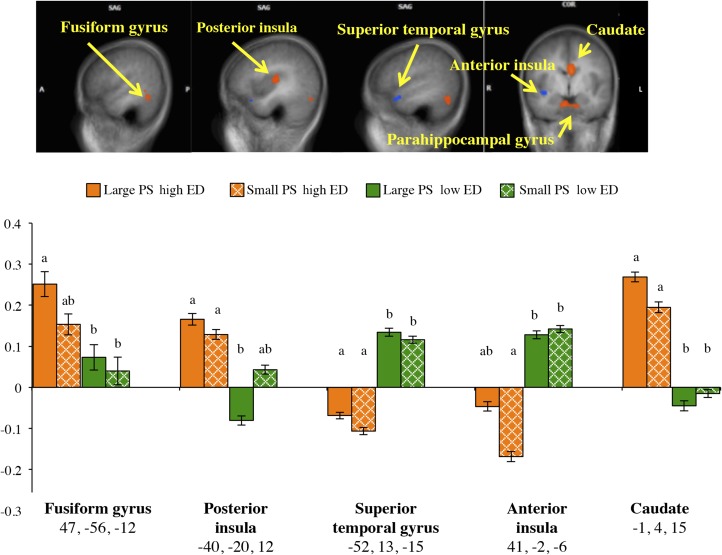
As reported in Table 2 and depicted in Figure 2, exposure to images of high-ED foods compared with images of low-ED foods increased the activation in the fusiform gyrus, superior and posterior insula, and several other regions. In contrast, the response to low-ED food cues was associated with increased activation compared with the response to high-ED food cues (Figure 2) in the superior temporal gyrus and inferior and anterior insula. After adding fullness as a covariate, effects for ED in the inferior insula (P < 0.05), parahippocampal gyrus (P < 0.05), and cerebellum (P < 0.01) remained significant (Table 3). The effects in all regions remained significant after controlling for the BMI z score with the exception of both clusters in the declive (P = 0.06, P = 0.08). Table 3 also shows that the effects for ED in the posterior insula, superior temporal, gyrus, caudate, and anterior cingulate remained significant after controlling for liking (P < 0.05). Also, the effects in several regions remained significant after adjustment for sex (Table 3).
FIGURE 2.
fMRI statistical maps in SAG and COR views showing activation to high ED compared with low ED and co-registered with average structural MRI data from participants. Results are from whole-brain analyses (n = 36) that tested the main effects of ED and were co-registered with averaged structural MRI data from participants. (Top) Representative t maps in SAG and COR views showing increased BOLD activation (red colors) in the caudate, fusiform gyrus, and posterior insula (P < 0.01, corrected). Two areas with decreased BOLD activation (blue colors) to images of high-ED foods compared with low-ED foods are shown in the superior temporal gyrus and inferior insula (P < 0.01, corrected). The L side of each SAG view is A, and the L side of the COR view is the R hemisphere. (Bottom) Mean ± SEM BOLD magnitude for each condition of food images (large PS, high ED; small PS, high ED; large PS, low ED; small PS, low ED) within peak coordinates (x, y, and z in Talairach space) of each functional cluster. Bars that do not share a common letter (a, b, or c) are significantly different at P < 0.05. A, anterior; COR, coronal; ED, energy density; L, left; P, posterior; PS, portion size; R, right; SAG, sagittal.
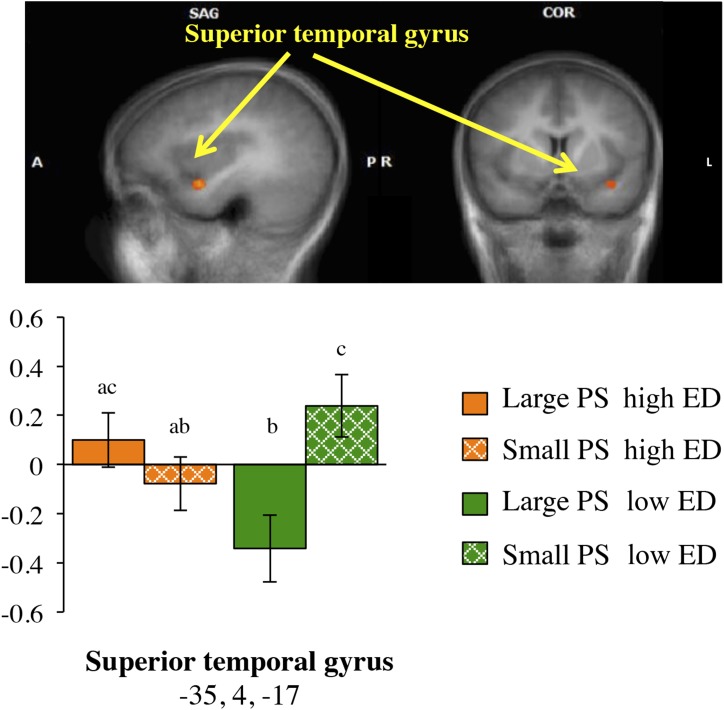
Interactions between PS and ED
At the whole-brain level, one cluster was identified with significant activation in the superior temporal gyrus (P < 0.005) as described in Table 2 and Figure 3. Table 3 shows that this interaction remained significant after adjustment for the BMI z score (P < 0.005) and liking (P < 0.05) but not for fullness (P = 0.15) or sex (P = 0.38). No other significant interactions were identified.
FIGURE 3.
fMRI statistical maps in SAG and COR views showing activation clusters in the superior temporal gyrus for the interaction of PS and ED and co-registered with average structural MRI data from participants. Results are from whole-brain analyses (n = 36) that tested the interactions between PS and ED and were co-registered with averaged structural MRI data from participants. (Top) Representative t maps in SAG and COR views showing increased BOLD activation (red colors) in the superior temporal gyrus (P < 0.01, corrected). The L side of the SAG view is A. The L side of the COR view is the R hemisphere. (Bottom) Mean ± SEM BOLD magnitude for each condition of food images (large PS, high ED; small PS, high ED; large PS, low ED; small PS, low ED) within peak coordinates (x, y, and z in Talairach space) of the superior temporal gyrus. Bars that do not share a common letter (a, b, or c) are significantly different at P < 0.05. A, anterior; COR, coronal; ED, energy density; L, left; P, posterior; PS, portion size; R, right; SAG, sagittal.
Associations between brain response to food cues and child eating behavior
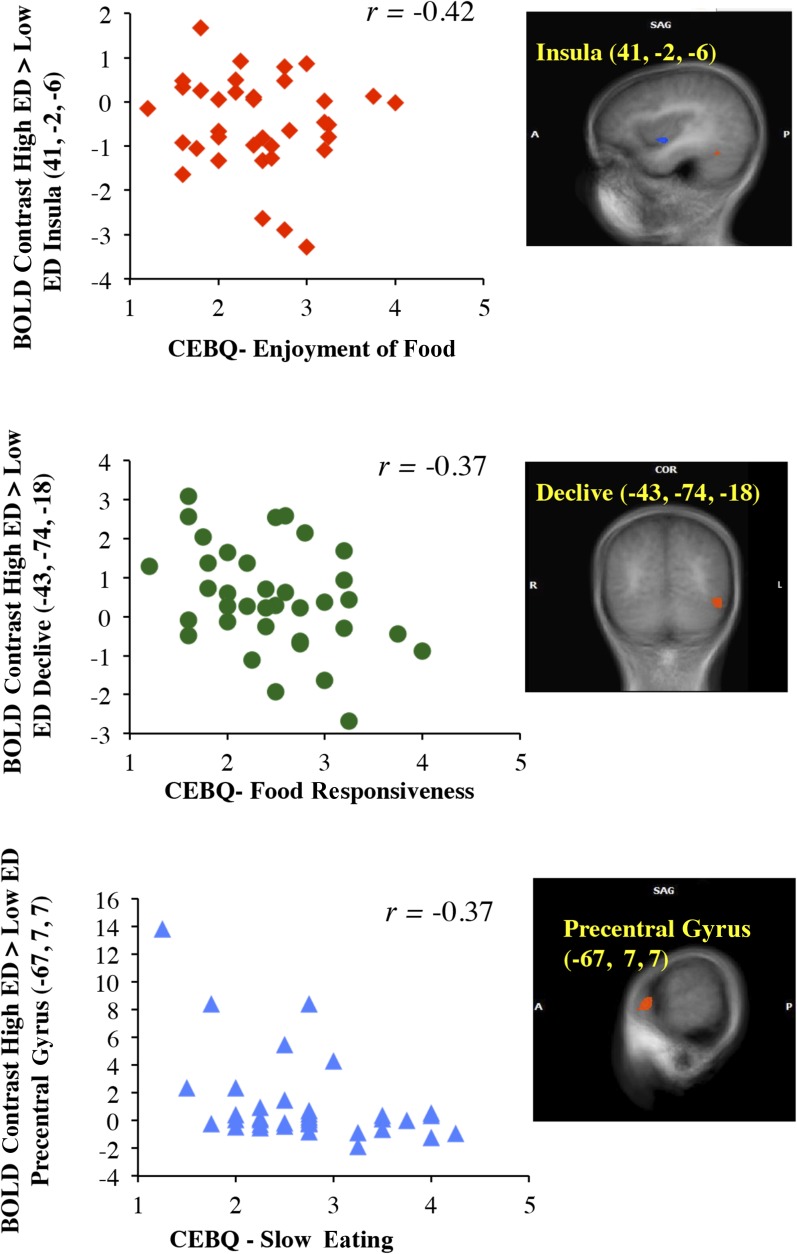
fMRI data and appetitive traits
Figure 4 shows that activation to high ED – low ED was negatively correlated with scores on the enjoyment of food subscale in the anterior insula, food-responsiveness scores in the declive (P < 0.05), and slow-eating scores in the precentral gyrus (P < 0.05). However, an outlier influenced the correlation in the precentral gyrus, and this association was no longer significant when this outlier was removed (r = −0.29, P = 0.09). No significant correlations were identified between activation in the regions with main effects for PS or PS × ED interactions and child appetitive traits.
FIGURE 4.
Significant correlation plots between appetitive trait scores and BOLD contrast values. (Left) Plots show significant negative correlations between BOLD contrast values of ED (i.e., high ED − low ED) and appetitive trait scores (n = 36) on the enjoyment of food, food responsiveness, and slow eating subscales. None of the regions identified in whole-brain analyses with main effects for portion size showed significant correlations with CEBQ subscales. (Right) Representative t maps in SAG and COR views showing the regions which BOLD contrast values were from (P < 0.01, corrected). The L side of the SAG view is A (insula and precentral gyrus). The L side of the COR view (declive) is the R hemisphere. A, anterior; CEBQ, Child Eating Behavior Questionnaire; COR, coronal; ED, energy density; L, left; P, posterior; R, right; SAG, sagittal.
fMRI data and food intake
There was a main effect of the test-meal condition on children’s energy intakes [F(35) = 20.1, P < 0.001], but there was no interaction between the portion condition (either linear or quadratic functions) and brain response to PS cues in the IFG (left P = 0.18; right P = 0.54). Activation to high ED – low ED cues in the declive interacted with PS to influence meal energy intake[F(35) = 5.7, P < 0.05]. There were no other associations between brain activation and meal-energy intake.
DISCUSSION
To our knowledge, our whole-brain analyses yielded new evidence of distinct patterns of brain activation to PS- and ED-food cues in children. Results showed a reduced response in a brain region that is important for inhibition and information processing (i.e., the IFG) when children viewed large portions of food compared with small portions of food. Greater activation was observed in several areas of the brain that are involved with reward and taste processing, including the insula, caudate, and cingulate, when children viewed foods with high EDs than when they viewed foods with low EDs. Therefore, the food PS may be processed in a region that has been previously implicated in motivation and cognitive control, whereas ED may be processed in regions that are involved in reward and emotion processing.
Large-portion cues were associated with decreased activation bilaterally in the IFG, which is a region that is located in the anterior prefrontal cortex (BA 47) that contains the lateral orbitofrontal cortex (OFC) (43). This region is important for semantic selection (44) and behavioral control of emotion and motivation (45, 46). We previously reported increased activation to large-portion cues (31) in the IFG that were from BA 44, which is in proximity to a region that has been implicated in the taste response to cues (47) and is posterior to BA 47. We speculate that differences between results from that analysis and the current study may have been due to the different locations and functions within the IFG. Although hyperactivity to food cues is typically seen in the IFG and OFC (7, 11, 48, 49), larger portions were associated with reduced activation in the current study. Post hoc tests showed that this association was driven by the low-ED food condition. We speculate that large portions of low-ED foods compared with small portions of low-ED foods may be less motivating for children. An alternative interpretation is that greater activation of attention-based networks is engaged when viewing small portions than with large portions because of the involvement of the IFG in attentional control (50). The main effects were independent of BMI z score and sex but not of the pre-fMRI fullness level or liking. Therefore, how children’s brains process information about food PSs in this region may be dependent not only on the food’s ED but also on food liking and appetitive state. The whole-brain response to food PS in the bilateral IFG and OFC was not associated with appetitive traits or laboratory intake when portions of all foods varied. Thus, additional studies are needed to determine the extent to which the IFG and OFC are implicated in eating behaviors.
Compared with images of low-ED foods, exposure to images of high-ED foods was associated with increased activation in brain areas that are commonly responsive to food cues including the insula, caudate, and cingulate (7, 15, 16) as well as the superior temporal and precentral gyri (16, 51). These results support the hypothesis that higher-energy foods activate brain centers that function in appetite regulation, reward, and somatosensory processing (11, 48, 52). The effects in the posterior insula, caudate, and anterior cingulate remained significant after adjustment for liking but not for fullness. Thus, areas that are associated with both reward-based decisions and interoception (53) are modulated by food cues that vary by ED, but this response may not be governed solely by how much a child likes the food. By contrast, the effects in the anterior insula and cerebellum remained significant after adjustment for fullness but not for liking. Thus, these connected areas that are involved in saliency detection (53) could be modulated by the ED of food cues and not governed solely by appetitive state. Previous fMRI studies in healthy-weight adolescents compared with obese adolescents have shown greater activation in the insula, caudate, and cingulate in response to food images than to nonfood images (7, 15), and our research extends these findings in children by showing that these brain regions are differentially responsive to food energy contents.
Trait-like dimensions of appetite were related to the contrast in brain activation from cues of high-ED foods – low-ED foods. Enjoyment-of-food scores were negatively related to activation in the anterior insula [i.e., the primary taste cortex (47)], which is a brain region that has been implicated in cognition (53) and is commonly responsive to both food tastes and images (54). Similarly, food-responsiveness scores were negatively related to activation in the declive, which is part of the posterior cerebellum that is involved in cognitive processing (55) with previous associations to food cues in adults (42) and adolescents (56). This finding supports an inverse association between children’s brain responses to cues of higher energy–dense food and parental ratings of child food responsiveness and enjoyment, which was unanticipated because of previous associations between obesity and these traits (37). The fact that our cohort was primarily of healthy weight may help to explain these unexpected findings, but further investigation is needed.
Our hypothesis that PS and ED cues would show an interaction was partially supported by findings in the superior temporal gyrus, which is a region that is thought to be involved in multimodal semantic processing (57) and functionally related to the primary gustatory cortex (9). Both reduced activation (17) and greater activation (16) in the superior temporal gyrus to cues of high–energy content foods have been reported previously in adolescents. Inconsistencies in the responsiveness of this and other brain regions to food cues may be related to the fMRI paradigm used (combining all foods into one category), the appetitive state during testing (premeal or postmeal), and the type of analyses conducted (whole brain compared with region of interest) (52). Although food PSs and EDs have independent and additive effects on energy intake (2, 3, 25, 28), there was no association between activation in the superior temporal gyrus and eating behaviors, but this result may have been due to a lack of power.
The whole-brain analysis used in this study is a comprehensive approach to characterizing the brain's involvement in the processing of visual food stimuli compared with region-of-interest analyses (11, 12), which are limited to only predefined brain regions. Additional strengths of this study included the high fMRI success rate in 7–10-y-old children, the novel presentation of food images that allow for the disentangling of the effects of PS and ED, and the incorporation of eating-behavior measures that aided in the interpretation of the brain response to food cues. These results extend previous research in adolescents (7, 15, 16, 19, 52), but the homogeneity of our sample in ethnicity and socioeconomic status may have reduced the generalizability of the findings. In addition, our results could have been influenced by differences in low-level visual features (e.g., complexity of images and detection of food edges) or the overall energy content presented in the food stimuli. Future fMRI paradigms should include nonfood objects at different sizes to rule out variations that might be due to size perception more generally. In addition, we were underpowered to determine the effects of brain response on laboratory intake, and thus, the lack of associations between brain response and intake at the test meals should be interpreted with caution. These exploratory analyses used a liberal threshold (P < 0.01) similar to the approaches used in previous studies (58, 59). Because of recent arguments for the use of higher thresholds (i.e., P < 0.001) (60), we advocate for future investigations to apply more-stringent thresholds to potentially improve the cluster inference and control over false positives. Finally, there may be subtle anatomical differences in the functional clusters that we reported on the basis of our spatial normalization into stereotaxic coordinates. Some researchers have advocated for the use of child-specific templates (61, 62), but the impact of these differences appears to be minimal (63).
In conclusion, functionally distinct brain regions respond to food PSs compared with food EDs. Because food PS and ED are robust promoters of intake, the results of this study fill an important gap in the literature on neurobiological correlates of eating behaviors in children. In addition, these findings may contribute to the development of evidence-based cognitive strategies to help children control intake from large portions of high-energy-dense foods.
Acknowledgments
Scans were conducted at the Penn State Social, Life, and Engineering Sciences Imaging Center 3T MRI Facility.
The authors’ responsibilities were as follows—LKE: wrote the manuscript; LKE and SNF: conducted the research (hands-on conduct of the experiments, data collection, and preprocessing); LKE and SJW: analyzed the data and performed the statistical analysis; KLK: oversaw the writing of the manuscript and had primary responsibility for the final content of the manuscript; and all authors: designed the research (project conception, development of overall research plan, and study oversight), contributed feedback, and read and approved the final manuscript before submission. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: BA, Brodmann area; ED, energy density; GLM, general linear model; IFG, inferior frontal gyrus; OFC, orbitofrontal cortex; PS, portion size; VAS, visual analog scale.
REFERENCES
- 1.Lake A, Townshend T. Obesogenic environments: exploring the built and food environments. J R Soc Promot Health 2006;126:262–7. [DOI] [PubMed] [Google Scholar]
- 2.Rolls BJ, Roe LS, Meengs JS. The effect of large portion sizes on energy intake is sustained for 11 days. Obesity (Silver Spring) 2007;15:1535–43. [DOI] [PubMed] [Google Scholar]
- 3.Fisher JO, Liu Y, Birch LL, Rolls BJ. Effects of portion size and energy density on young children’s intake at a meal. Am J Clin Nutr 2007;86:174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.English L, Lasschuijt M, Keller KL. Mechanisms of the portion size effect. What is known and where do we go from here? Appetite 2015;88:39–49. [DOI] [PubMed] [Google Scholar]
- 5.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron 2011;69:664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron 2002;36:199–211. [DOI] [PubMed] [Google Scholar]
- 7.Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes (Lond) 2010;34:1494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstone AP, Prechtl CG, Scholtz S, Miras AD, Chhina N, Durighel G, Deliran SS, Beckmann C, Ghatei MA, Ashby DR, et al. Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr 2014;99:1319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Killgore WDS, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage 2003;19:1381–94. [DOI] [PubMed] [Google Scholar]
- 10.Mehta S, Melhorn SJ, Smeraglio A, Tyagi V, Grabowski T, Schwartz MW, Schur EA. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr 2012;96:989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoeckel LE, Weller RE, Cook EW III, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008;41:636–47. [DOI] [PubMed] [Google Scholar]
- 13.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res 2006;169:111–9. [DOI] [PubMed] [Google Scholar]
- 14.Carnell S, Benson L, Pantazatos SP, Hirsch J, Geliebter A. Amodal brain activation and functional connectivity in response to high-energy-density food cues in obesity. Obesity (Silver Spring) 2014;22:2370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davids S, Lauffer H, Thoms K, Jagdhuhn M, Hirschfeld H, Domin M, Hamm A, Lotze M. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J Obes (Lond) 2010;34:94–104. [DOI] [PubMed] [Google Scholar]
- 16.Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, Nollen NL, Savage CR. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage 2005;27:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killgore WDS, Yurgelun-Todd DA. Developmental changes in the functional brain responses of adolescents to images of high and low-calorie foods. Dev Psychobiol 2005;47:377–97. [DOI] [PubMed] [Google Scholar]
- 18.Thomas JM, Higgs S, Dourish CT, Hansen PC, Harmer CJ, McCabe C. Satiation attenuates BOLD activity in brain regions involved in reward and increases activity in dorsolateral prefrontal cortex: an fMRI study in healthy volunteers. Am J Clin Nutr 2015;101:697–704. [DOI] [PubMed] [Google Scholar]
- 19.Cascio CJ, Foss-Feig JH, Heacock JL, Newsom CR, Cowan RL, Benningfield MM, Rogers BP, Cao A. Response of neural reward regions to food cues in autism spectrum disorders. J Neurodev Disord 2012;4:9 DOI: 10.1186/1866-1955-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burger KS, Fisher JO, Johnson SL. Mechanisms behind the portion size effect: visibility and bite size. Obesity (Silver Spring) 2011;19:546–51. [DOI] [PubMed] [Google Scholar]
- 21.Marchiori D, Papies EK, Klein O. The portion size effect on food intake. An anchoring and adjustment process? Appetite 2014;81:108–15. DOI: 10.1016/j.appet.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Olsen A, Ritz C, Kramer L, Møller P. Serving styles of raw snack vegetables. What do children want? Appetite 2012;59:556–62. [DOI] [PubMed] [Google Scholar]
- 23.Raghubir P, Krishna A. Vital dimensions in volume perception: can the eye fool the stomach? J Mark Res 1999;30:313–26. [Google Scholar]
- 24.Rolls BJ, Rowe EA, Rolls ET. How flavour and appearance affect human feeding. Proc Nutr Soc 1982;41:109–17. [DOI] [PubMed] [Google Scholar]
- 25.Kral TV, Rolls BJ. Energy density and portion size: their independent and combined effects on energy intake. Physiol Behav 2004;82:131–8. [DOI] [PubMed] [Google Scholar]
- 26.Brunstrom JM. The control of meal size in human subjects: a role for expected satiety, expected satiation and premeal planning. Proc Nutr Soc 2011;70:155–61. [DOI] [PubMed] [Google Scholar]
- 27.Dagher A. The neurobiology of appetite: hunger as addiction. Int J Obes (Lond) 2009;33(Suppl 2):S30–3. [DOI] [PubMed] [Google Scholar]
- 28.Kral TV, Roe LS, Rolls BJ. Combined effects of energy density and portion size on energy intake in women. Am J Clin Nutr 2004;79:962–8. [DOI] [PubMed] [Google Scholar]
- 29.Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein E-B, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain 2000;123:2512–8. [DOI] [PubMed] [Google Scholar]
- 30.Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods 2002;118:115–28. [DOI] [PubMed] [Google Scholar]
- 31.English LK, Fearnbach SN, Lasschuijt M, Schlegel A, Anderson K, Harris S, Wilson SJ, Fisher JO, Savage JS, Rolls BJ, et al. Brain regions implicated in inhibitory control and appetite regulation are activated in response to food portion size and energy density in children. Int J Obes (Lond) 2016;40:1515–22. [DOI] [PubMed] [Google Scholar]
- 32.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the children’s eating behaviour questionnaire. J Child Psychol Psychiatry 2001;42:963–70. [DOI] [PubMed] [Google Scholar]
- 33.Roe LS, Kling SM, Rolls BJ. What is eaten when all of the foods at a meal are served in large portions? Appetite 2016;99:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smiciklas-Wright H, Mitchell DC, Mickle SJ, Goldman JD, Cook A. Foods commonly eaten in the United States, 1989-1991 and 1994-1996: are portion sizes changing? J Am Diet Assoc 2003;103:41–7. [DOI] [PubMed] [Google Scholar]
- 35.Cole TJ. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller KL, Assur SA, Torres M, Lofink HE, Thornton JC, Faith MS, Kissileff HR. Potential of an analog scaling device for measuring fullness in children: development and preliminary testing. Appetite 2006;47:233–43. [DOI] [PubMed] [Google Scholar]
- 37.Carnell S, Wardle J. Appetitive traits and child obesity: measurement, origins and implications for intervention. Proc Nutr Soc 2008;67:343–55. [DOI] [PubMed] [Google Scholar]
- 38.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med 2000;44:457–65. [DOI] [PubMed] [Google Scholar]
- 39.Talairach J, Tournoux P. Co‐planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 40.Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp 2006;27:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995;33:636–47. [DOI] [PubMed] [Google Scholar]
- 42.Nock NL, Dimitropolous A, Tkach J, Frasure H, von Gruenigen V. Reduction in neural activation to high-calorie food cues in obese endometrial cancer survivors after a behavioral lifestyle intervention: a pilot study. BMC Neurosci 2012;13:74 DOI: 10.1186/1471-2202-13-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 2007;45:2883–901. [DOI] [PubMed] [Google Scholar]
- 44.Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron 2001;31:329–38. [DOI] [PubMed] [Google Scholar]
- 45.Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist 2007;13:214–28. [DOI] [PubMed] [Google Scholar]
- 46.Roberts AC, Wallis JD. Inhibitory control and affective processing in the prefrontal cortex: neuropsychological studies in the common marmoset. Cereb Cortex 2000;10:252–62. [DOI] [PubMed] [Google Scholar]
- 47.Rolls ET. Taste, olfactory, and food reward value processing in the brain. Prog Neurobiol 2015;127–128:64–90. [DOI] [PubMed] [Google Scholar]
- 48.Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite 2012;58:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Killgore WDS, Yurgelun-Todd DA. Body mass predicts orbitofrontal activity during visual presentations of high-calorie foods. Neuroreport 2005;16:859–63. [DOI] [PubMed] [Google Scholar]
- 50.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 2010;50:1313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr 2006;83:1297–305. [DOI] [PubMed] [Google Scholar]
- 52.van Meer F, van der Laan LN, Adan RA, Viergever MA, Smeets PA. What you see is what you eat: an ALE meta-analysis of the neural correlates of food viewing in children and adolescents. Neuroimage 2015;104:35–43. [DOI] [PubMed] [Google Scholar]
- 53.Cauda F, Costa T, Torta DM, Sacco K, D’Agata F, Duca S, Geminiani G, Fox PT, Vercelli A. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage 2012;62:343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huerta CI, Sarkar PR, Duong TQ, Laird AR, Fox PT. Neural bases of food perception: coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obesity (Silver Spring) 2014;22:1439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 2012;59:1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gearhardt AN, Yokum S, Stice E, Harris JL, Brownell KD. Relation of obesity to neural activation in response to food commercials. Soc Cogn Affect Neurosci 2014;9:932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Visser M, Lambon Ralph MA. Differential contributions of bilateral ventral anterior temporal lobe and left anterior superior temporal gyrus to semantic processes. J Cogn Neurosci 2011;23:3121–31. [DOI] [PubMed] [Google Scholar]
- 58.Bruce AS, Bruce JM, Black WR, Lepping RJ, Henry JM, Cherry JB, Martin LE, Papa VB, Davis AM, Brooks WM, et al. Branding and a child’s brain: an fMRI study of neural responses to logos. Soc Cogn Affect Neurosci 2014;9:118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruce AS, Lepping RJ, Bruce JM, Cherry JB, Martin LE, Davis AM, Brooks WM, Savage CR. Brain responses to food logos in obese and healthy weight children. J Pediatr 2013;162:759–64.e2. [DOI] [PubMed] [Google Scholar]
- 60.Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016;113:7900–5. DOI: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaillard WD, Grandin CB, Xu B. Developmental aspects of pediatric fMRI: considerations for image acquisition, analysis, and interpretation. Neuroimage 2001;13:239–49. [DOI] [PubMed] [Google Scholar]
- 62.Yoon U, Fonov VS, Perusse D, Evans AC. The effect of template choice on morphometric analysis of pediatric brain data. Neuroimage 2009;45:769–77. [DOI] [PubMed] [Google Scholar]
- 63.Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage 2002;17:184–200. [DOI] [PubMed] [Google Scholar]