Abstract
Background: Postprandial dysmetabolism—an exaggerated spike in triglycerides, glucose, and insulin—increases cardiovascular disease risk by inducing oxidative stress, inflammation, and endothelial dysfunction. Polyphenol-rich foods may blunt these effects when they are incorporated into a high-fat, calorie-dense meal. Strawberries are a rich source of polyphenols, but there is little research on their postprandial effects.
Objective: This study was designed to investigate the effect of adding 40 g freeze-dried strawberry powder (∼1 lb. or 0.45 kg fresh strawberries) to a high-fat (50 g total fat) meal on postprandial vascular function, as well as triglyceride, glucose, and insulin responses.
Design: Healthy, overweight or obese [mean ± SEM body mass index (in kg/m2): 31 ± 0.5] adults (mean ± SEM age: 28 ± 2 y; 17 men and 13 women) consumed a control meal and a strawberry meal in a randomized crossover design. Testing sessions were separated by ≥1 wk for men and ∼1 mo for women to control for hormonal variations. Blood samples were obtained before the meal and 0.5, 1, 2, and 4 h after the meal. Central blood pressure and arterial stiffness indexes were measured at baseline and 2 and 4 h postmeal with the use of pulse waveform analysis.
Results: There were no significant differences between the strawberry and control meals for any outcomes. Consumption of either meal significantly decreased the augmentation index at 2 and 4 h (P < 0.002) and significantly increased triglycerides, insulin, and glucose at all time points (P < 0.001) relative to baseline.
Conclusions: The strawberry intervention did not alter vascular function or attenuate postprandial metabolic derangements in triglycerides, glucose, or insulin relative to the control meal. Additional research is needed to clarify whether strawberries or other polyphenol-rich interventions improve postprandial responses, and future studies should take into account the acute meal-induced improvements in measures of vascular function. This trial was registered at clinicaltrials.gov as NCT01989637.
Keywords: augmentation index, cardiovascular disease, hyperlipidemia, phytochemicals, postprandial dysmetabolism
INTRODUCTION
Postprandial dysmetabolism is characterized by large spikes in and delayed clearance of triglycerides, glucose, and insulin. This causes oxidative stress and transiently impairs vascular function (1, 2). Over time, these repeated insults to the endothelium promote atherogenesis (3, 4). Plant-based whole foods are recommended for reducing cardiovascular disease (CVD)9 risk (5) and provide bioactive polyphenols. Effects on postprandial metabolism may be one mechanism by which polyphenols improve health (6). However, it has yet to be established whether incorporating polyphenol-rich plant-based foods and/or beverages into a high-fat meal can attenuate postprandial dysmetabolism and/or favorably alter acute changes in vascular function.
Previous studies have demonstrated promising results for certain interventions (e.g., spices, berries, tea, and red wine), but evidence remains limited. For instance, in overweight men, incorporating 14 g antioxidant-rich spices into a high-fat meal significantly attenuated postprandial triglyceride responses (7, 8), potentially via inhibition of lipase enzymes. However, this effect has yet to be conclusively demonstrated with other interventions. Berries have been found to improve the postprandial glycemic profile (9–11), but previous studies have been limited primarily to carbohydrate-based meals. In addition, although there is evidence that polyphenol-rich interventions attenuate transient postprandial impairments in endothelium-dependent vascular function (12), few studies have evaluated whether central blood pressure and indexes of arterial stiffness can be modified in this context.
Strawberries are a rich source of numerous polyphenols and other bioactive compounds (13, 14), and are one of the most commonly consumed fruits in the United States (15). Longer-term strawberry supplementation has been shown to improve multiple CVD risk factors (16–25), but less is known about the acute effects of strawberries in the context of a fat-containing meal. A modest attenuation of the 6-h postprandial triglyceride, insulin, and oxidized LDL response was found in overweight hyperlipidemic adults when 10 g freeze-dried strawberry powder was incorporated into a meal with 30 g total fat (26, 27). In a subsequent study, incorporating 40 g freeze-dried strawberry powder into a high-carbohydrate meal with 25 g total fat significantly reduced the 6-h insulin response in individuals with insulin resistance, but had no effect on postprandial glucose, triglycerides, or oxidized LDL (28). Effects on postprandial central blood pressure and arterial stiffness have yet to be tested, and additional research is needed to evaluate the potential of strawberries to mitigate the metabolic consequences of consuming a high-fat meal in different study populations.
The present study (NCT01989637) was designed to investigate whether incorporating 40 g freeze-dried strawberry powder into a high-fat (50 g total fat) meal could improve postprandial responses relative to a macro- and micronutrient-matched control meal in overweight or obese but otherwise healthy adults. We hypothesized that this 40-g dose of freeze-dried strawberry powder—which is equivalent to ∼1 pound or 0.45 kg fresh strawberries and provides a substantial amount of polyphenols—would improve vascular function by attenuating the exaggerated spikes in triglycerides, glucose, insulin, and markers of oxidative stress caused by the high-fat meal.
METHODS
Study population
Men and women 20–50 y of age and free of any serious illness were recruited for the study. Other inclusion criteria consisted of a BMI (in kg/m2) of 28–39, resting blood pressure <160/100 mm Hg, fasting triglycerides <350 mg/dL, and fasting LDL <160 mg/dL. Exclusion criteria were acute or chronic inflammatory conditions; liver or kidney dysfunction; a history of heart disease; use of tobacco products; intolerance for high-fat meals; allergies or sensitivities to wheat or strawberries; and use of nonsteroidal anti-inflammatories, immunosuppressants, or medications or supplements for elevated lipids, blood pressure, or glucose.
Participant recruitment
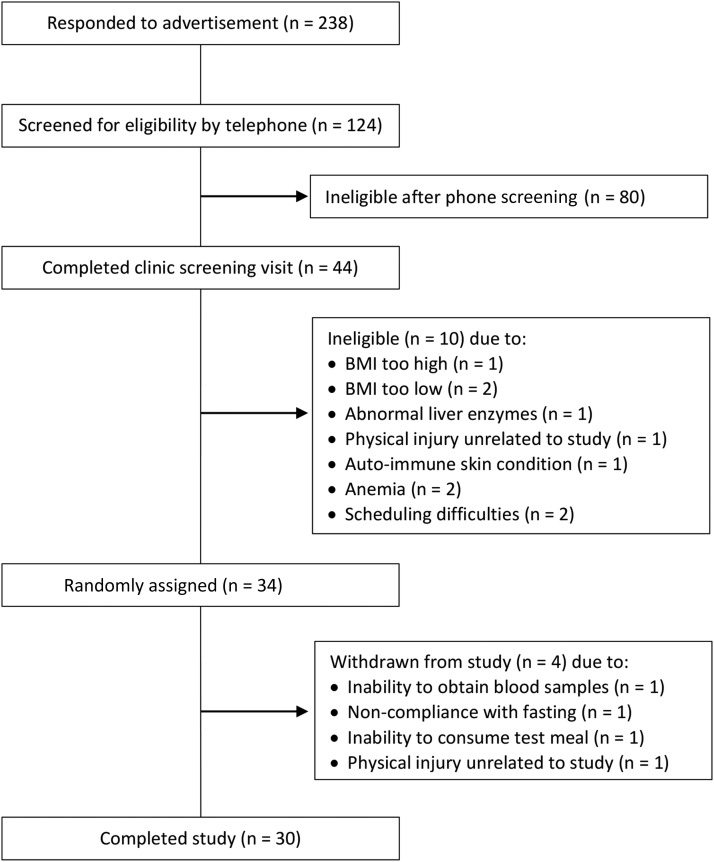
Participants were recruited from July 2013 to March 2014 via fliers in the community, campus e-mail lists, and a university research website. Potential subjects sent an e-mail or called to indicate interest in participating in the study, and were then given additional information about the study. If interested, they were asked a series of medical history and lifestyle questions to screen for eligibility. A schematic of participant recruitment for the study is provided in Figure 1. Of the 238 initial respondents who provided contact information, 124 elected to complete the initial screening questions by telephone. Forty-four of these volunteers met study criteria and completed a screening appointment at the Pennsylvania State University Clinical Research Center (CRC) to verify eligibility. After written informed consent was obtained, a urine pregnancy test was performed for premenopausal women, and a blood sample was drawn for a complete blood count and standard chemistry profile (lipid panel, glucose, and liver and kidney function) to rule out the presence of illness (autoimmune disease, cancer, and immunodeficiency). Blood pressure was measured according to the guidelines of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (29). Briefly, after a 5-min seated rest, 3 readings were taken by nurses in a controlled environment with the use of a calibrated mercury sphygmomanometer. The mean of the last 2 readings was used to determine eligibility. Body weight and height were measured (without shoes and in light clothing) to calculate BMI. Of the 44 individuals who were screened, 34 met eligibility criteria and were enrolled in the study. Four participants withdrew during the study. Thus, data are reported for 30 participants (n = 17 men and n = 13 women). A balanced randomization scheme with a block size of 6 was developed in advance (by ACS-R) with the use of an online randomization generator, and subjects were assigned to a treatment sequence at enrollment (by CKR). Sample size for this preliminary study was based on earlier studies of postprandial metabolic responses that reported a significant reduction in triglycerides with a similar polyphenol-rich intervention (8, 26). No power calculation was performed for our primary outcome of postprandial vascular function because of the scarcity of published studies with the postprandial use of brachial pulse wave analysis to assess the effects of incorporating a polyphenol-rich intervention into a meal. The study was conducted in accordance with the Helsinki Declaration, and was approved by the Institutional Review Board of the Pennsylvania State University.
FIGURE 1.
Schematic of participant recruitment and reasons for exclusion.
Study design and intervention
This was a randomized, placebo-controlled, 2-period crossover study in which participants completed 2 postprandial challenges that consisted of single-day visits that lasted ∼5 h each. The 2 test conditions were as follows: 1) a high-fat control meal with a strawberry-flavored placebo powder that was devoid of strawberry bioactives, and 2) the same high-fat meal with 40 g freeze-dried strawberry powder. The control and strawberry meals were matched for macro- and micronutrients (Table 1), and differed only in polyphenol and vitamin C content. The meals consisted of 2 cheese blintzes (248 g) with heavy whipped cream (12 g) and strawberry-flavored syrup (50 g), a hard-boiled egg, and bacon (24 g cooked). The placebo and strawberry powders were mixed into the cheese filling of the blintzes. All meal components were weighed in advance and prepared fresh before each day of testing. No heat was applied to the powders during food preparation to avoid any potential degradation of polyphenols. The control and strawberry meals were matched for taste and appearance to maintain the blinding of participants and researchers to treatment sequence. Both the freeze-dried strawberry powder and the control powder were provided and analyzed by the California Strawberry Commission. The detailed nutrient profile and polyphenol content of the strawberry and control powders are available in Supplemental Tables 1 and 2.
TABLE 1.
Nutrient composition of test meals
| Control meal | Strawberry meal | |
| Calories, kcal | 1000 | 1000 |
| Calories from fat, kcal | 450 | 450 |
| Total fat, g | 50 | 50 |
| Saturated fat, g | 25 | 25 |
| trans Fat, g | 0.5 | 0.5 |
| Cholesterol, mg | 435 | 435 |
| Carbohydrate, g | 106 | 105 |
| Fiber, g | 7 | 7 |
| Protein, g | 30 | 32 |
| Sodium, mg | 1390 | 1320 |
| Vitamin C, % daily value | 2 | 380 |
Postprandial challenges were separated by a minimum of 1 wk for men. Visits for female participants were scheduled during the first 7 d of the menstrual cycle to avoid hormonal effects on vascular measures, and were therefore separated by ∼1 mo. All postprandial challenges were conducted at the CRC according to standardized protocols. During the 48 h before testing visits, participants were instructed to avoid high-antioxidant foods (berries, cocoa and chocolate), strenuous exercise, and alcohol; refrain from taking pain relievers, vitamins, or minerals; and limit their intake of coffee and tea to ≤1 cup/d. Testing visits were conducted after an overnight fast (no food or drink other than water for 12 h).
At each visit, baseline vascular function testing was performed before an intravenous catheter was established for blood sampling. A topical skin anesthetic was offered to numb the area before catheter insertion. After baseline blood sample collection, participants were asked to consume the control or strawberry meal within ∼15 min. No food or drinks (other than water) were allowed for the remainder of the testing period. Additional blood samples and vascular function measures were taken at prespecified time intervals relative to when the meal was finished; blood samples were collected at 0.5, 1, 2, and 4 h postmeal, and vascular function measures were performed at 2 and 4 h after the meal. At the end of the 4-h period, the catheter was removed and participants were briefly evaluated for safety before leaving the CRC. Study procedures began in August 2013 and were completed by May 2014.
Vascular function measures
Vascular function, in terms of central blood pressure and arterial stiffness indexes, was assessed with the use of the SphygmoCor System pulse waveform analysis (AtCor Medical). All measurements were performed in a temperature-controlled, quiet, dimly lit room.
Pulse wave analysis: central (aortic) blood pressure and augmentation index
After a 5 min seated rest, central pressures and wave reflection characteristics [i.e., augmentation pressure (AP) and the augmentation index (AI)] were derived from brachial pressure waveforms with the use of a generalized transfer function that is considered by the US FDA to be substantially equivalent to generalized transfer functions for radial tonometry that have been validated against an indwelling catheter (30–32). At each time point, 3 pulse wave analysis measurements were taken while following the guidelines of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (29), with 1 min between each reading. The last 2 pulse wave analysis results were averaged and used for analysis. An augmentation index normalized to a heart rate of 75 beats/min (AI@75) was used to correct for the independent inverse effect of heart rate on augmentation of the pulse wave form (33).
Pulse wave velocity
Aortic stiffness was assessed by carotid-femoral pulse wave velocity (PWV). Carotid and femoral arterial pressure waveforms were measured simultaneously via an applanation tonometry sensor manually held in place above the right common carotid artery and a blood pressure cuff placed on the right femoral artery. Distance measurements were taken from the sternal notch to the carotid artery, from the sternal notch to the top of the femoral cuff, and from the femoral artery to the top of the femoral cuff. Based on these measurements, the SphygmoCor System automatically calculates the distance traveled by the pulse wave from the carotid artery to the femoral artery. Transit time between the carotid and femoral pressure waves is determined by the SphygmoCor System with the use of the foot-to-foot method (34). PWV is then calculated as distance over transit time. At each time point, 2 PWV measurements were obtained in the supine position, with 1 min between readings, and averaged for analysis.
Blood sample collection and assay methods
Blood drawn into anticoagulant-coated tubes containing lithium heparin and EDTA was immediately centrifuged for 15 min at 1500 × g at room temperature. Blood drawn into serum separator tubes was allowed to clot for 30 min before centrifugation. Total cholesterol and triglycerides were measured by enzymatic procedures (Quest Diagnostics; CV <2% for both). HDL was estimated according to the modified heparin-manganese procedure (CV <2%). Fasting LDL was calculated with the use of the Friedewald equation [LDL = TC − (HDL + TG/5), where TC is total cholesterol and TG is triglyceride]. Postprandial LDL was not determined, because calculated values are not accurate during nonfasting conditions. Insulin was measured by radioimmunoassay (Quest Diagnostics). Glucose was determined by spectrophotometry procedures (Quest Diagnostics). Serum high-sensitivity C-reactive protein (CRP) was measured by latex-enhanced immunonephelometry (Quest Diagnostics; assay CV <8%). For other endpoints, aliquots of serum and plasma were stored at −80°C for batch analysis.
Biomarkers of oxidative stress
Malondialdehyde was measured in plasma from EDTA-coated tubes with the use of the thiobarbituric acid–reactive substances assay (Cayman Chemical Co.; assay CV <6%). Oxidized LDL was measured in plasma containing EDTA by ELISA (Mercodia; assay CV <8%).
Pancreatic lipase inhibition
Extracts of the freeze-dried strawberry powder were prepared with 10 volumes of acetone:water:acetic acid (80%:20%:0.1%, vol:vol:vol) overnight. The organic solvent was removed under vacuum and the remaining aqueous solution was freeze-dried. Porcine type II pancreatic lipase (PL) and 98% 4-nitrophenyl butyrate (4-NPB) were purchased from Sigma-Aldrich. Stock solutions were prepared in dimethyl-sulfoxide (EMD Chemicals) and stored at −20°C.
Inhibition of PL by the freeze-dried strawberry powder was tested by monitoring the cleavage of 4-NPB to release 4-nitrophenol. PL was suspended in water (10 mg/mL) and incubated at 37°C for 5 min. The solution was centrifuged for 5 min at 664 × g at room temperature, and the supernatant was then used as the enzyme source for subsequent experiments. For each experiment, the PL supernatant was diluted 1:50 in buffer solution (20 mmol/L Tris-HCl and 1.3 mmol/L CaCl2, pH = 8.0). Freeze-dried strawberry powder extracts (0–200 μg/mL) were combined with PL, and 4-NPB (0.2 mmol/L) was added to start the reaction. After 10 min of incubation at 37°C, absorbance was read at 400 nm. Analyses were performed in triplicate. Assay CV was <10%.
Statistical analyses
All statistical analyses were performed with the use of SAS version 9.3. Independent 2-sample t tests were used to assess sex differences at screening (PROC TTEST). Paired t tests (PROC TTEST) were used to compare the premeal baseline characteristics of participants at the strawberry and control meal visits. Postprandial change scores were calculated by subtracting fasting baseline values for each visit from postmeal values. AUC was calculated for postprandial insulin, glucose, and triglyceride responses with the use of the trapezoidal rule. The Matsuda insulin sensitivity index was calculated with the use of the formula developed by Matsuda and DeFronzo (35). Participants with any missing postprandial insulin values because of hemolysis (n = 9) were removed before the calculation of insulin AUC and the Matsuda index. Outcomes were assessed for normality (PROC UNIVARIATE), and positively skewed variables (skew >1; triglycerides, glucose, insulin, glucose AUC, insulin AUC, Matsuda index, central systolic blood pressure, and PWV) were log transformed for analysis. Two participants experienced symptoms of a vasovagal reaction (acute blood pressure decrease and lightheadedness) in response to blood sampling before the postprandial SphygmoCor measurements and were excluded from analyses of vascular outcomes. Two participants with acute inflammation (i.e., CRP >9.5 mg/L) were excluded from all analyses to ensure that the analysis was performed on the target population of healthy overweight adults. One participant with fasting triglycerides >350 mg/dL before consuming the test meal was excluded from analyses of blood outcomes because he did not meet specified screening criteria (i.e., triglycerides <350 mg/dL). The mixed-models procedure (PROC MIXED) was used to test the effects of meal, time point, and their interaction on outcome measures. Baseline values were included as covariates. Period effects were also assessed to evaluate any habituation to testing and were found to be universally nonsignificant. Selection of model covariance structures was based on optimizing fit statistics (evaluated as lowest Bayesian Information Criterion). Models for postprandial metabolic effects (triglycerides, glucose, and insulin) used a doubly repeated covariance structure that was unstructured for time point and used compound symmetry for period effects. Compound symmetry was used to model both period and time point for all vascular endpoints other than AP and PWV. Models for AP and PWV used a doubly repeated covariance structure that was unstructured for time point and used compound symmetry for period. Compound symmetry was used to model treatment effects for changes in markers of oxidative stress (oxidized LDL and malondialdehyde), which were measured at a single postprandial time point, as well as the Matsuda index and AUC values for insulin, glucose, and triglycerides. Means are reported as least squares means ± SEMs. For all tests, α was set at 0.05.
Unblinded exploratory analyses were also conducted to identify potential predictors (i.e., BMI, sex, and age) of participants’ general responsiveness to the strawberry intervention. Participants were designated as “nonresponders” or “responders” based on their response to the strawberry intervention. Individuals who exhibited the hypothesized beneficial effects of the strawberry intervention for glucose, insulin, triglyceride, AP, or AI@75 responses were considered to be responders. These continuous variables were dichotomized by calculating the maximum percentage increase after each meal. For triglycerides, this was calculated as the percentage increase from baseline to the 4-h time point. Because there tends to be more variation in the timing of the maximal glucose and insulin response between individuals, the percentage increase in insulin was calculated based on the change from baseline to the maximum response in each participant, and the percentage increase in glucose was calculated as the change from the minimum value to the maximum response in each participant. If the increase after the control meal was larger than the increase after the strawberry meal, the participant was deemed to be a responder for glucose or insulin. For triglycerides, participants were designated as responders if a ≥10% increase occurred after the control meal compared with the strawberry meal. For AP and AI@75, which tended to decrease postprandially, the percentage change was calculated with the use of the change from baseline to the lowest postprandial value, and participants who experienced a greater reduction after the strawberry meal were deemed to be responders. Logistic regression was performed in SAS with the use of BMI, sex, and age as main effects.
RESULTS
The screening characteristics of participants who completed the study are presented in Table 2. There were no significant differences between male and female participants at screening. With regard to premeal baseline characteristics of participants at the strawberry compared with control meal visits, small but statistically significant differences were found for CRP (mean difference: 0.3 mg/L; P = 0.01) and BMI (mean difference: 0.1; P = 0.04) (Supplemental Table 3).
TABLE 2.
Screening characteristics of participants who completed the study1
| Women (n = 13) | Men (n = 17) | |
| Age, y | 28.1 ± 2.7 (20–47) | 28.2 ± 2.0 (20–49) |
| Weight, kg | 85.0 ± 3.0 (74.0–111) | 98.5 ± 2.2 (83.6–114) |
| BMI, kg/m2 | 31.4 ± 0.8 (28.0–37.9) | 31.3 ± 0.6 (28.5–36.5) |
| SBP, mm Hg | 113.8 ± 2.7 (97–135) | 123.1 ± 2.4 (109–145) |
| DBP, mm Hg | 77.4 ± 2.0 (68–94) | 81.5 ± 1.4 (68–89) |
| Glucose, mg/dL | 85.9 ± 1.5 (77–91) | 91.6 ± 0.9 (83–98) |
| TC, mg/dL | 161.1 ± 9.7 (117–228) | 164.6 ± 7.2 (110–222) |
| HDL, mg/dL | 54.8 ± 1.9 (44–69) | 41.6 ± 1.8 (28–57) |
| LDL, mg/dL | 87.8 ± 9.0 (39–158) | 96.8 ± 6.2 (48–147) |
| TGs, mg/dL | 92.8 ± 15.6 (33–186) | 131.0 ± 16.7 (35–339) |
Values are means ± SEMs (ranges), n = 30. Values were calculated and compared with the use of an independent 2-sample t test (PROC TTEST; SAS version 9.3). There were no significant differences between male and female participants for any variables. DBP, diastolic blood pressure; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Postprandial vascular function
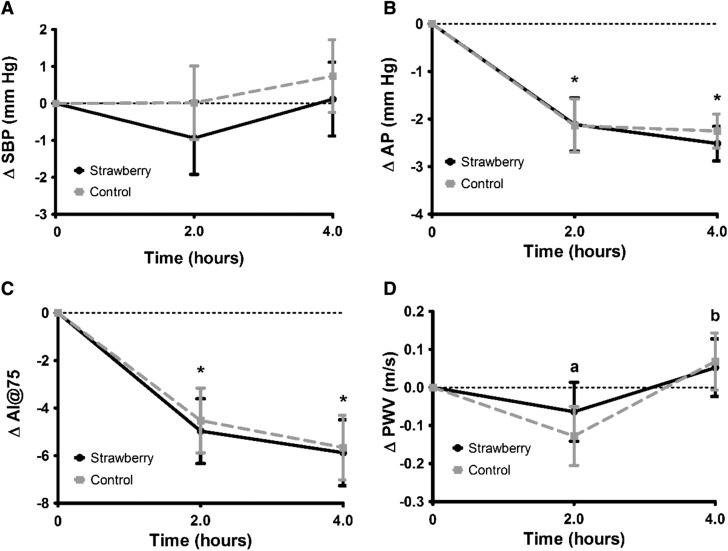
Meal consumption significantly altered several measures of vascular function, but these effects were not significantly different between the strawberry and control meals (Figure 2). AP and the AI@75 were both significantly lower at 2 and 4 h after meal consumption than they were at baseline (Figure 2). There was a significant time point effect for PWV, with values at the 4-h time point being 3% higher than were values at 2 h, although these values were not significantly different from baseline (Figure 2). There was no significant treatment effect for the change in central systolic blood pressure after the strawberry meal (mean 4-h postprandial change: −0.4 ± 0.9 mm Hg) compared with the control meal (mean 4-h postprandial change: 0.4 ± 0.9 mm Hg). Central systolic blood pressure also did not significantly change from baseline at any time point (mean postprandial change at 2 h: −0.5 ± 0.9 mm Hg; mean postprandial change at 4 h: 0.4 ± 0.9 mm Hg).
FIGURE 2.
Mean ± SEM changes in central SBP (A), AP (B), AI@75 (C), and PWV (D) after consumption of the control and freeze-dried strawberry test meals. Data are presented as unadjusted means ± SEMs, n = 26. Values represent untransformed change scores that were calculated by subtracting the fasting baseline values for each visit from postmeal values, and were compared with the use of the MIXED procedure in SAS version 9.3. For all outcomes, there were no significant interaction terms in the model, and no significant differences between the responses to the strawberry and control meals. Times annotated with different letters were significantly different from one another. *Significantly different from baseline, P < 0.05. AI@75, augmentation index normalized to a heart rate of 75 beats/min; AP, augmentation pressure; PWV, pulse wave velocity; SBP, systolic blood pressure.
Postprandial metabolism
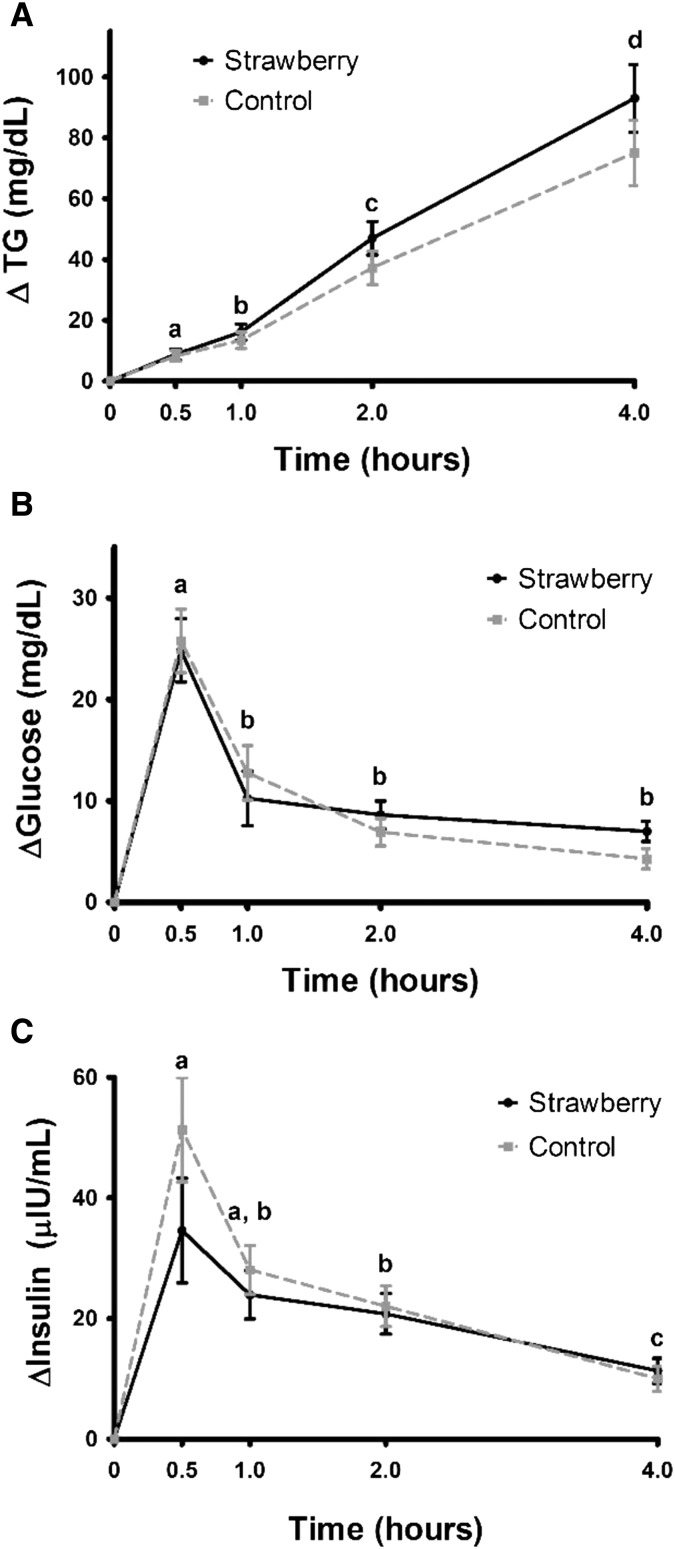
Serum triglycerides, insulin, and glucose significantly increased from baseline at all time points after the consumption of both meals (P < 0.0001 compared with baseline) (Figure 3). There were no significant differences between the 2 meals. The triglyceride response was highest at 4 h postmeal (+86.7 mg/dL relative to baseline), whereas insulin and glucose responses peaked 30 min postmeal (+42.9 μIU/mL and +25.3 mg/dL relative to baseline, respectively), and decreased thereafter. There was no significant treatment effect for the Matsuda insulin sensitivity index [geometric mean (95% CI) after the strawberry meal: 10.8 (8.1, 14.4) compared with after the control meal: 10.5 (8.1, 13.5); P = 0.7]. AUC values for postprandial insulin, glucose, and triglyceride responses also were not significantly different after the strawberry meal compared with the control meal (P = 0.8, 0.6, and 0.8, respectively; data not shown).
FIGURE 3.
Mean ± SEM changes in TGs (A), glucose (B), and insulin (C) after consumption of the control and freeze-dried strawberry test meals. Data are presented as unadjusted means ± SEMs, n = 27. Values represent untransformed change scores that were calculated by subtracting the fasting baseline values for each visit from postmeal values, and were compared with the use of the MIXED procedure in SAS version 9.3. All time points were significantly different from baseline. For all outcomes, there were no significant interaction terms in the model, and no significant differences between the responses to the strawberry and control meals. Times annotated with different letters were significantly different from one another, P < 0.05. TG, triglyceride.
Predictors of postprandial metabolic and vascular function responses
Hypothesis-generating subset analyses identified age and sex as potential predictors of the effect of the freeze-dried strawberry powder intervention on metabolic (triglycerides, glucose, and insulin) responses. Each additional year of age increased the odds of an individual having an attenuated triglyceride response with the strawberry intervention (OR: 1.2; 95% CI: 1.0, 1.4; P < 0.05). However, for glucose responses, each additional year of age was associated with individuals being marginally less likely to respond to the strawberry intervention (OR: 0.9; 95% CI: 0.7, 1.0; P = 0.06). A trend for a negative effect of age was also found for insulin responses (OR: 0.9; 95% CI: 0.7, 1.0; P = 0.1). Moreover, men were significantly more likely to respond to the strawberry intervention with attenuated glucose responses (OR: 22.2; 95% CI: 2.2, 644.6; P = 0.03), but sex had no effect on the triglyceride or insulin response to strawberries. Age, BMI, and sex were not significant predictors of vascular function (AP and AI@75) responses (data not shown).
Markers of oxidative stress and PL activity
There was no significant postprandial change from baseline in malondialdehyde or oxidized LDL, and no significant difference between the response to the strawberry meal and that for the control meal (Supplemental Table 4). There was no inhibitory effect on PL activity in vitro at any concentration of strawberry powder extract ≤200 μg/mL (Supplemental Table 5).
DISCUSSION
This study investigated the postprandial effects of incorporating freeze-dried strawberry powder into a high-fat meal. We observed marked elevations in triglycerides, glucose, and insulin, whereas the AI@75 was significantly reduced after meal consumption. However, there was no change in malondialdehyde and oxidized LDL postprandially, and no significant treatment effect of the strawberry intervention for any outcomes.
In contrast to the detrimental effect of postprandial lipemia on nitric oxide–dependent vasodilation (36), the AI and other indexes of arterial stiffness are reduced by meal consumption (37–40), potentially because of the vasodilatory effect of insulin (38). However, this may be a paradoxical effect whereby meal-induced elevations in insulin are acutely beneficial, but sustained hyperinsulinemia (resulting in insulin resistance and related conditions) is associated with arterial stiffness (41–45). Given that these postprandial reductions can be maintained hours after a meal, they also may be due in part to increased blood flow to digestive organs, with compensatory reductions in flow to other organs (46). Measurement of vascular function beyond the 4-h time point in future studies would help to clarify how long these reductions are maintained.
Postprandial insulin-induced reductions in indexes of arterial stiffness also have important implications for study design. For instance, a higher-carbohydrate test meal that produces a greater insulin response may falsely appear to have beneficial vascular effects compared with a higher-fat meal with a less robust insulin response. Few studies investigating postprandial changes in AI and/or PWV after a mixed meal have assessed potential associations with metabolic responses (40). We timed vascular function measurements to coincide with peak polyphenol and triglyceride concentrations (2 and 4 h postmeal, respectively), but insulin peaks much earlier and has largely declined by 2 h. We found no significant relation between peak insulin (at 30 min) and peak AI@75 (at 4 h), or the 2-h change in AI@75 and insulin, when excluding several outlier responses (data not shown). Vascular function measurements at earlier time points are needed to further explore this relation.
To our knowledge, few studies have investigated whether administering a polyphenol-rich food or beverage with a meal alters measures of vascular function derived from pulse waveform analysis (47–49). In healthy adults, a high-nitrate spinach meal (∼90 kcal and <1 g fat) produced a greater reduction in the AI@75 at 3 h than did a matched low-nitrate meal (48), but the AI did not change in older individuals after a test meal (∼475 kcal) with less nitrate (220 compared with 845 mg inorganic nitrate) (47). Conversely, adding oats to a high-fat meal abolished the 3-h postprandial reduction in the AI@75 (49). This may have been due to a blunted postprandial insulin response from the β-glucan in oats (50), but insulin was not measured. Strawberries do not provide the β-glucan found in oats and contain much less nitrate than spinach contains (51). Thus, divergent postprandial effects on the AI may depend on the unique bioactive profile of the intervention, and additional research is needed. These studies also did not measure postprandial changes in triglycerides, glucose, and insulin. Assessment of these metabolic outcomes, as well as vascular function measurements later in the postprandial period (i.e., after 4 h), would help clarify potential effects on the AI and indexes of arterial stiffness. Given the vasodilatory effect of insulin, future studies should also use test meals with similar insulin-inducing properties.
Other polyphenol-rich interventions also have not attenuated postprandial lipemia and/or glycemic responses to a meal (52–55). It is unclear why our results differ from the significant reductions in postprandial insulin (27, 28), as well as triglycerides and oxidized LDL (26), found in the 2 previous postprandial strawberry studies. Dairy proteins and the timing of meal intake may diminish the effects of polyphenols (56, 57); however, significant benefits have been achieved with the use of a milk-based beverage consumed with a test meal (26, 28). Although the amount of strawberries required to optimize effects on clinical outcomes is unknown, our 40-g dose of freeze-dried powder was equivalent to ∼3 cups of fresh strawberries [13 g freeze-dried powder is equivalent to 1 cup fresh (C Christian California Strawberry Commission, personal communication, 2014)] and provided a substantial amount of polyphenols (Supplemental Table 2). A similar amount of total anthocyanins was provided in the postprandial study by Park et al. (28), although the freeze-dried strawberry powder used by Burton-Freeman and colleagues (26, 27) had a higher oxygen radical absorbance capacity value and a higher concentration of select polyphenolic compounds, including quercetin and pelargonidin-3-glucoside. These differences may account, in part, for the discrepancies between our results and those of Burton-Freeman et al. (26). Based on our exploratory analyses, characteristics of our study population such as age and sex also may have influenced responses. Moreover, compared with the 2 previous postprandial strawberry studies (26, 28), our participants tended to be younger, did not have elevated total or LDL cholesterol, and were not insulin resistant. Thus, the potential benefits of strawberries may be more likely in individuals at greater CVD risk, and our hypothesis-generating findings should be investigated in future studies.
We further speculate that the unique polyphenol profile of an intervention and its subsequent ability to inhibit specific digestive enzymes may also determine outcomes. For instance, spices, in which phenolic acids, flavones, and flavonols tend to predominate (58), potently inhibit PL in vitro (7). Alternatively, strawberry polyphenols, which are largely anthocyanins, ellagitannins, and ellagic acid (59), may be more effective at altering carbohydrate metabolism, because they consistently inhibit carbohydrate digestive enzymes (60–62), and improved the insulin response to white bread in healthy women (11). Although an extract of strawberry polyphenols has been shown to inhibit PL in vitro (63), our analysis of the freeze-dried strawberry powder showed no inhibitory effect. Future studies are needed to clarify whether effects on postprandial metabolism depend on the phenolic profile of the intervention and/or the macronutrient composition of the test meal.
Longer-term strawberry intake may be required to alter CVD risk factors. Although we found no effect within 4 h of meal consumption, multiple studies have found that 3–12 wk of strawberry supplementation improved oxidative stress and the lipid profile of individuals with elevated CVD risk. Therefore, although it remains unclear whether strawberries alter postprandial dysmetabolism, there is promising evidence that strawberries improve other CVD risk factors.
Our participants were recruited specifically on the basis of BMI (28–39) and were representative of the general population; however, they were otherwise relatively healthy, and effects may be more apparent in individuals at greater CVD risk. Providing the strawberry intervention as part of a meal is reflective of real-world dietary habits, and the strawberry and control meals were well-matched. Hormonal effects on vascular function were minimized by testing female participants in the first week of their menstrual cycle. Postprandial metabolism and vascular function were also measured concurrently. The study meals induced a robust postprandial triglyceride, glucose, and insulin response, but it is possible that significant attenuation of the glycemic response might have been achieved with a carbohydrate-based lower-fat test meal. Our study was limited to a 4-h postprandial period, and it is possible that changes may have occurred at later time points. Malondialdehyde also has limited reliability as a marker of lipid peroxidation (64, 65), and more specific and sensitive biomarkers should be used in future studies. Our sample size was consistent with previous studies; however, as is typically the case with postprandial studies, our study may have been underpowered, given the inherent inter- and intraindividual variability in postprandial responses.
In conclusion, the consumption of a high-fat meal significantly reduced the AI@75, indicating an acute improvement in vascular function, despite marked elevations in postprandial triglycerides, glucose, and insulin. Incorporating 40 g freeze-dried strawberry powder did not further improve vascular function or attenuate postprandial dysmetabolism. Additional research is needed to determine whether polyphenol-rich foods and beverages reduce CVD risk by modifying postprandial vascular function and dysmetabolism. Future postprandial studies should consider the influence of meal-induced insulin elevations on vascular outcomes.
Acknowledgments
The authors’ responsibilities were as follows—CKR, ACS-R, DNP, and PMK-E: designed the research; ACS-R: coordinated the implementation of the study procedures and provided assistance with data collection; CKR: conducted the research; JDL: selected and performed the oxidative stress and pancreatic lipase inhibition assays; CKR, ACS-R, and TLG: performed the statistical analyses; CKR, ACS-R, and PMK-E: wrote the manuscript; and all authors: took responsibility for the manuscript’s final content and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AI, augmentation index; AI@75, augmentation index normalized to a heart rate of 75 beats/min; AP, augmentation pressure; CRC, clinical research center; CRP, C-reactive protein; CVD, cardiovascular disease; PL, pancreatic lipase; PWV, pulse wave velocity; 4-NPB, 4-nitrophenyl butyrate.
REFERENCES
- 1.Ceriello A. The post-prandial state and cardiovascular disease: relevance to diabetes mellitus. Diabetes Metab Res Rev 2000;16:125–32. [DOI] [PubMed] [Google Scholar]
- 2.Wallace JP, Johnson B, Padilla J, Mather K. Postprandial lipaemia, oxidative stress and endothelial function: a review. Int J Clin Pract 2010;64:389–403. [DOI] [PubMed] [Google Scholar]
- 3.Burdge GC, Calder PC. Plasma cytokine response during the postprandial period: a potential causal process in vascular disease? Br J Nutr 2005;93:3–9. [DOI] [PubMed] [Google Scholar]
- 4.Jackson KG, Poppitt SD, Minihane AM. Postprandial lipemia and cardiovascular disease risk: interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 2012;220:22–33. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th ed. Washington (DC): US Department of Health and Human Services and US Department of Agriculture; 2015. [cited 2016 Aug 24]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 6.O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol 2007;100:899–904. [DOI] [PubMed] [Google Scholar]
- 7.McCrea CE, West SG, Kris-Etherton PM, Lambert JD, Gaugler TL, Teeter DL, Sauder KA, Gu Y, Glisan SL, Skulas-Ray AC. Effects of culinary spices and psychological stress on postprandial lipemia and lipase activity: results of a randomized crossover study and in vitro experiments. J Transl Med 2015;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skulas-Ray AC, Kris-Etherton PM, Teeter DL, Chen CY, Vanden Heuvel JP, West SG. A high antioxidant spice blend attenuates postprandial insulin and triglyceride responses and increases some plasma measures of antioxidant activity in healthy, overweight men. J Nutr 2011;141:1451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Törrönen R, Sarkkinen E, Tapola N, Hautaniemi E, Kilpi K, Niskanen L. Berries modify the postprandial plasma glucose response to sucrose in healthy subjects. Br J Nutr 2010;103:1094–7. [DOI] [PubMed] [Google Scholar]
- 10.Törrönen R, Kolehmainen M, Sarkkinen E, Mykkanen H, Niskanen L. Postprandial glucose, insulin, and free fatty acid responses to sucrose consumed with blackcurrants and lingonberries in healthy women. Am J Clin Nutr 2012;96:527–33. [DOI] [PubMed] [Google Scholar]
- 11.Törrönen R, Kolehmainen M, Sarkkinen E, Poutanen K, Mykkanen H, Niskanen L. Berries reduce postprandial insulin responses to wheat and rye breads in healthy women. J Nutr 2013;143:430–6. [DOI] [PubMed] [Google Scholar]
- 12.Kay CD, Kris-Etherton PM, West SG. Effects of antioxidant-rich foods on vascular reactivity: review of the clinical evidence. Curr Atheroscler Rep 2006;8:510–22. [DOI] [PubMed] [Google Scholar]
- 13.Carlsen MH, Halvorsen BL, Holte K, Bohn SK, Dragland S, Sampson L, Willey C, Senoo H, Umezono Y, Sanada C, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J 2010;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy MM, Barraj LM, Herman D, Bi X, Cheatham R, Randolph RK. Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. J Acad Nutr Diet 2012;112:222–9. [DOI] [PubMed] [Google Scholar]
- 15.Bachman JL, Reedy J, Subar AF, Krebs-Smith SM. Sources of food group intakes among the US population, 2001-2002. J Am Diet Assoc 2008;108:804–14. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Suarez JM, Giampieri F, Tulipani S, Casoli T, Di Stefano G, Gonzalez-Paramas AM, Santos-Buelga C, Busco F, Quiles JL, Cordero MD, et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J Nutr Biochem 2014;25:289–94. [DOI] [PubMed] [Google Scholar]
- 17.Basu A, Betts NM, Nguyen A, Newman ED, Fu D, Lyons TJ. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J Nutr 2014;144:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zunino SJ, Parelman MA, Freytag TL, Stephensen CB, Kelley DS, Mackey BE, Woodhouse LR, Bonnel EL. Effects of dietary strawberry powder on blood lipids and inflammatory markers in obese human subjects. Br J Nutr 2012;108:900–9. [DOI] [PubMed] [Google Scholar]
- 19.Basu A, Fu DX, Wilkinson M, Simmons B, Wu M, Betts NM, Du M, Lyons TJ. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr Res 2010;30:462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basu A, Wilkinson M, Penugonda K, Simmons B, Betts NM, Lyons TJ. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: baseline and post intervention effects. Nutr J 2009;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moazen S, Amani R, Homayouni Rad A, Shahbazian H, Ahmadi K, Taha Jalali M. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: a randomized double-blind controlled trial. Ann Nutr Metab 2013;63:256–64. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins DJ, Nguyen TH, Kendall CW, Faulkner DA, Bashyam B, Kim IJ, Ireland C, Patel D, Vidgen E, Josse AR, et al. The effect of strawberries in a cholesterol-lowering dietary portfolio. Metabolism 2008;57:1636–44. [DOI] [PubMed] [Google Scholar]
- 23.Giampieri F, Forbes-Hernandez TY, Gasparrini M, Alvarez-Suarez JM, Afrin S, Bompadre S, Quiles JL, Mezzetti B, Battino M. Strawberry as a health promoter: an evidence based review. Food Funct 2015;6:1386–98. [DOI] [PubMed] [Google Scholar]
- 24.Forbes-Hernandez TY, Gasparrini M, Afrin S, Bompadre S, Mezzetti B, Quiles JL, Giampieri F, Battino M. The healthy effects of strawberry polyphenols: which strategy behind antioxidant capacity? Crit Rev Food Sci Nutr 2016;56 Suppl 1:S46–59. [DOI] [PubMed] [Google Scholar]
- 25.Afrin S, Gasparrini M, Forbes-Hernandez TY, Reboredo-Rodriguez P, Mezzetti B, Varela-Lopez A, Giampieri F, Battino M. Promising health benefits of the strawberry: a focus on clinical studies. J Agric Food Chem 2016;64:4435–49. [DOI] [PubMed] [Google Scholar]
- 26.Burton-Freeman B, Linares A, Hyson D, Kappagoda T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J Am Coll Nutr 2010;29:46–54. [DOI] [PubMed] [Google Scholar]
- 27.Edirisinghe I, Banaszewski K, Cappozzo J, Sandhya K, Ellis CL, Tadapaneni R, Kappagoda CT, Burton-Freeman BM. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br J Nutr 2011;106:913–22. [DOI] [PubMed] [Google Scholar]
- 28.Park E, Edirisinghe I, Wei H, Vijayakumar LP, Banaszewski K, Cappozzo JC, Burton-Freeman B. A dose-response evaluation of freeze-dried strawberries independent of fiber content on metabolic indices in abdominally obese individuals with insulin resistance in a randomized, single-blinded, diet-controlled crossover trial. Mol Nutr Food Res 2016;60:1099–109. [DOI] [PubMed] [Google Scholar]
- 29.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr., et al. The seventh report of the joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- 30.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 1997;95:1827–36. [DOI] [PubMed] [Google Scholar]
- 31.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001;38:932–7. [DOI] [PubMed] [Google Scholar]
- 32.Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, Garrahy P, Wilkinson IB, Marwick TH. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension 2006;47:1203–8. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 2000;525:263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–605. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–70. [DOI] [PubMed] [Google Scholar]
- 36.West SG. Effect of diet on vascular reactivity: an emerging marker for vascular risk. Curr Atheroscler Rep 2001;3:446–55. [DOI] [PubMed] [Google Scholar]
- 37.Ahuja KD, Robertson IK, Ball MJ. Acute effects of food on postprandial blood pressure and measures of arterial stiffness in healthy humans. Am J Clin Nutr 2009;90:298–303. [DOI] [PubMed] [Google Scholar]
- 38.Greenfield JR, Samaras K, Chisholm DJ, Campbell LV. Effect of postprandial insulinemia and insulin resistance on measurement of arterial stiffness (augmentation index). Int J Cardiol 2007;114:50–6. [DOI] [PubMed] [Google Scholar]
- 39.Berry SE, Tucker S, Banerji R, Jiang B, Chowienczyk PJ, Charles SM, Sanders TA. Impaired postprandial endothelial function depends on the type of fat consumed by healthy men. J Nutr 2008;138:1910–4. [DOI] [PubMed] [Google Scholar]
- 40.Phillips LK, Peake JM, Zhang X, Hickman IJ, Kolade O, Sacre JW, Huang BE, Simpson P, Li SH, Whitehead JP, et al. The effect of a high-fat meal on postprandial arterial stiffness in men with obesity and type 2 diabetes. J Clin Endocrinol Metab 2010;95:4455–9. [DOI] [PubMed] [Google Scholar]
- 41.Webb DR, Khunti K, Silverman R, Gray LJ, Srinivasan B, Lacy PS, Williams B, Davies MJ. Impact of metabolic indices on central artery stiffness: independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia 2010;53:1190–8. [DOI] [PubMed] [Google Scholar]
- 42.Agnoletti D, Lieber A, Zhang Y, Protogerou AD, Borghi C, Blacher J, Safar ME. Central hemodynamic modifications in diabetes mellitus. Atherosclerosis 2013;230:315–21. [DOI] [PubMed] [Google Scholar]
- 43.Schram MT, Henry RM, van Dijk RA, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Westerhof N, Stehouwer CD. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension 2004;43:176–81. [DOI] [PubMed] [Google Scholar]
- 44.Aoun S, Blacher J, Safar ME, Mourad JJ. Diabetes mellitus and renal failure: effects on large artery stiffness. J Hum Hypertens 2001;15:693–700. [DOI] [PubMed] [Google Scholar]
- 45.Safar ME, Thomas F, Blacher J, Nzietchueng R, Bureau JM, Pannier B, Benetos A. Metabolic syndrome and age-related progression of aortic stiffness. J Am Coll Cardiol 2006;47:72–5. [DOI] [PubMed] [Google Scholar]
- 46.Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J Surg Res 2000;93:182–96. [DOI] [PubMed] [Google Scholar]
- 47.Liu AH, Bondonno CP, Croft KD, Puddey IB, Woodman RJ, Rich L, Ward NC, Vita JA, Hodgson JM. Effects of a nitrate-rich meal on arterial stiffness and blood pressure in healthy volunteers. Nitric Oxide 2013;35:123–30. [DOI] [PubMed] [Google Scholar]
- 48.Jovanovski E, Bosco L, Khan K, Au-Yeung F, Ho H, Zurbau A, Jenkins AL, Vuksan V. Effect of spinach, a high dietary nitrate source, on arterial stiffness and related hemodynamic measures: a randomized, controlled trial in healthy adults. Clin Nutr Res 2015;4:160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devlin N, McKenzie J, Gow IF. Effect of oatmeal on postprandial vascular compliance following a high fat meal. J Clin Nutr Diet 2016;2:4. [Google Scholar]
- 50.Wang Q, Ellis PR. Oat beta-glucan: physico-chemical characteristics in relation to its blood-glucose and cholesterol-lowering properties. Br J Nutr 2014;112 Suppl 2:S4–13. [DOI] [PubMed] [Google Scholar]
- 51.White JW., Jr Relative significance of dietary sources of nitrate and nitrite. J Agric Food Chem 1975;23:886–91. [DOI] [PubMed] [Google Scholar]
- 52.Mathew AS, Capel-Williams GM, Berry SE, Hall WL. Acute effects of pomegranate extract on postprandial lipaemia, vascular function and blood pressure. Plant Foods Hum Nutr 2012;67:351–7. [DOI] [PubMed] [Google Scholar]
- 53.Burton-Freeman B, Talbot J, Park E, Krishnankutty S, Edirisinghe I. Protective activity of processed tomato products on postprandial oxidation and inflammation: a clinical trial in healthy weight men and women. Mol Nutr Food Res 2012;56:622–31. [DOI] [PubMed] [Google Scholar]
- 54.Joris PJ, Mensink RP. Beetroot juice improves in overweight and slightly obese men postprandial endothelial function after consumption of a mixed meal. Atherosclerosis 2013;231:78–83. [DOI] [PubMed] [Google Scholar]
- 55.Clegg ME, Pratt M, Meade CM, Henry CJ. The addition of raspberries and blueberries to a starch-based food does not alter the glycaemic response. Br J Nutr 2011;106:335–8. [DOI] [PubMed] [Google Scholar]
- 56.Tadapaneni RK, Banaszewski K, Patazca E, Edirisinghe I, Cappozzo J, Jackson L, Burton-Freeman B. Effect of high-pressure processing and milk on the anthocyanin composition and antioxidant capacity of strawberry-based beverages. J Agric Food Chem 2012;60:5795–802. [DOI] [PubMed] [Google Scholar]
- 57.Sandhu AK, Huang Y, Xiao D, Park E, Edirisinghe I, Burton-Freeman B. Pharmacokinetic characterization and bioavailability of strawberry anthocyanins relative to meal intake. J Agric Food Chem 2016;64:4891–9. [DOI] [PubMed] [Google Scholar]
- 58.Opara EI, Chohan M. Culinary herbs and spices: their bioactive properties, the contribution of polyphenols and the challenges in deducing their true health benefits. Int J Mol Sci 2014;15:19183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basu A, Nguyen A, Betts NM, Lyons TJ. Strawberry as a functional food: an evidence-based review. Crit Rev Food Sci Nutr 2014;54:790–806. [DOI] [PubMed] [Google Scholar]
- 60.McDougall GJ, Shpiro F, Dobson P, Smith P, Blake A, Stewart D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J Agric Food Chem 2005;53:2760–6. [DOI] [PubMed] [Google Scholar]
- 61.da Silva Pinto M, Kwon YI, Apostolidis E, Lajolo FM, Genovese MI, Shetty K. Functionality of bioactive compounds in Brazilian strawberry (Fragaria x ananassa Duch.) cultivars: evaluation of hyperglycemia and hypertension potential using in vitro models. J Agric Food Chem 2008;56:4386–92. [DOI] [PubMed] [Google Scholar]
- 62.Cheplick S, Kwon Y-I, Bhowmik P, Shetty K. Phenolic-linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertension. Bioresour Technol 2010;101:404–13. [DOI] [PubMed] [Google Scholar]
- 63.McDougall GJ, Kulkarni NN, Stewart D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem 2009;115:193–9. [Google Scholar]
- 64.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 1990;9:515–40. [DOI] [PubMed] [Google Scholar]
- 65.Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, Knight AR, Taylor EL, Oettrich J, Ruskovska T, et al. Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal 2015;23:1144–70. [DOI] [PMC free article] [PubMed] [Google Scholar]