Abstract
Background: Body composition is an important indicator of nutritional status and health. How body composition changes during 12 mo of breastfeeding in HIV-infected women receiving antiretroviral therapy (ART) is unknown.
Objective: We assessed whether HIV or food insecurity was associated with adverse postpartum body-composition changes in Ugandan women.
Design: A cohort of 246 women [36.5% of whom were HIV positive (HIV+) and were receiving ART] were followed to 12 mo postpartum. Repeated measures included weight, fat mass, fat-free mass, midupper arm circumference, triceps skinfold thickness [which allowed for the derivation of arm muscle area (AMA) and arm fat area (AFA)], breastfeeding, and individual food insecurity. Longitudinal regression models were constructed to assess associations between HIV and food insecurity and changes in body composition over time.
Results: At baseline, HIV+ women compared with HIV-negative women had a higher mean ± SD food-insecurity score (11.3 ± 5.5 compared with 8.6 ± 5.5, respectively; P < 0.001) and lower AMA (40.6 ± 5.7 compared with 42.9 ± 6.9 cm3, respectively; P = 0.03). Participants were thin at 1 wk postpartum [body mass index (BMI; in kg/m2): 22.9 ± 2.9]. From 1 wk to 12 mo, the weight change was −1.4 ± 4.4 kg. In longitudinal models of body-composition outcomes, HIV was not associated with body composition (all P > 0.05), whereas food insecurity was inversely associated with body weight and BMI at 6, 9, and 12 mo and with AFA at 6 and 12 mo (all P < 0.05). At 6 mo, every 1-unit increase in the food-insecurity score was associated with a 0.13-kg lower body weight (P < 0.001) and a 0.26-cm3 lower AFA (P < 0.01).
Conclusions: Body-composition changes are minimal during lactation. HIV is not associated with body composition; however, food insecurity is associated with changes in body composition during lactation. This trial was registered at clinicaltrials.gov as NCT02922829 and NCT02925429.
Keywords: BMI, body composition, postpartum, pregnancy, Uganda
INTRODUCTION
To maximize HIV-free infant survival, the WHO recommends 6 mo of exclusive breastfeeding to be followed by continued breastfeeding for ≥12 mo (1, 2). It is also recommended that antiretroviral therapy (ART)11 should be initiated for anyone who is living with HIV and should be continued for life (3). However, some ART regimens may propagate weight loss and concomitant shifts in body composition (4, 5) and adversely affect micronutrient status (6–8). These deficits can be exacerbated during periods of higher nutritional needs such as lactation. Because HIV increases energy needs by ∼10% (9), lactating HIV-positive (HIV+) women who are receiving ART may be unable to simultaneously meet their own and their infant’s nutritional needs, which could lead to maternal nutritional depletion [(i.e., a negative change in nutritional status over one reproductive cycle (10)], suboptimal breast-milk quality or quantity, or less (exclusive) breastfeeding, among other sequelae (5).
Although it is plausible that lactating HIV+ mothers are at a greater risk of becoming nutritionally depleted, there are few data to support or refute this contention. Indeed, few studies have described changes in maternal body size or composition (fat and lean mass) beyond 6 mo in populations either with HIV (11–15) or without HIV (16–22). To our knowledge, only one study has examined body composition in HIV+ women from 8 to 24 wk (15), and to our knowledge, no studies have done so beyond 6 mo.
In terms of ARV exposure and weight change, 2 studies have described weight changes in women who initiated ART prenatally (12, 13), and weight changes in women who initiated ART in the postpartum period have been reported in 1 cohort (4, 5). However, these studies have not examined body composition (4, 5, 11–13).
Food insecurity (i.e., the uncertain or limited availability of nutritionally adequate, safe, or acceptable food) is common in individuals who are affected by HIV (23). In addition, in Uganda, as in many other countries, an annual hunger season of food scarcity can adversely affect nutritional status. During pregnancy and lactation, women may be vulnerable to adverse effects of food insecurity on health and nutritional status because of shifts in household resources, the ability to work, the ability to prepare food (24), and heightened energy and nutritional demands (24–26). However, we know of only one study that has examined the role of food insecurity on weight changes during pregnancy in HIV+ women (25), and to our knowledge, no study has examined this role during lactation.
Therefore, we examined body-composition changes and determinants of these changes, including HIV and food insecurity, from 1 wk to 12 mo postpartum in a cohort of postpartum Ugandan women of mixed HIV status. We hypothesized that HIV+ mothers would lose more weight, fat, and lean mass than would HIV-negative (HIV−) mothers. Further, we hypothesized that mothers with greater food insecurity would experience a greater loss of weight, fat mass, and lean mass.
METHODS
Data were collected in the context of the Prenatal Nutrition and Psychosocial Health Outcomes Study (PreNAPS) and the Postnatal Nutrition and Psychosocial Health Outcomes Study (PostNAPS) in Gulu, Uganda. Together, these composed a longitudinal observational cohort that was designed to examine the relations between food security, psychosocial health, and nutritional status during pregnancy and postpartum in postconflict Northern Uganda. Gulu was the epicenter of a protracted 20-y conflict between the Ugandan government and the Lord’s Resistance Army, which ended in 2006.
Data were collected between 10 October 2012 and 19 January 2015 at Gulu Regional Referral Hospital (GRRH). All women at GRRH receive antenatal care and medications free of charge, and HIV+ women receive free ART, as is consistent with national policy in Uganda, and sulfamethoxazole trimethoprim (Septrin; Aspen Pharmacare). HIV+ women who were not receiving highly active antiretroviral therapy at the first ANC visit were given the first-line option B+ (tenofovir, lamivudine, and efavirenz; Cipla Quality Chemicals Ltd.). HIV+ women who were receiving highly active antiretroviral therapy were given several options for continuing treatment as follows: 1) Duovir-N (zidovodine, lamivudine, and nevirapine; Cipla Quality Chemical Industries Ltd.), 2) Duomune (lamivudine and tenofovir; Cipla Quality Chemical Industries Ltd.) and nevirapine (Boehringer Ingleheim), or 3) Duomune and efavirenz (Bristol-Myers Squibb) (27).
Study procedures have been previously described (26, 28, 29). Briefly, women (n = 403) were invited to participate in PreNAPS if they met the following eligibility criteria: gestational age between 10 and 26 wk (assessed according to the last menstrual period), living ≤30 km of GRRH, and having a known HIV status. HIV+ women were oversampled to obtain a ratio of 2 HIV-uninfected to 1 HIV+ participants, which resulted in a higher HIV prevalence in the cohort than the age-adjusted HIV prevalence (∼10.3%) at antenatal care clinics in Northern Uganda (30). Mothers were enrolled and were followed monthly throughout pregnancy (mean ± SD prenatal visits per woman: 5.0 ± 1.1). The sample size for PreNAPS was designed to provide 80% power to detect a 50-g difference in weight gain between HIV+ and HIV− women at a 5% level of significance and accounting for a 10% loss to follow-up. The postnatal continuation of the study was exploratory; with the use of postpartum values from a South African cohort of HIV+ and HIV− women (15), with a sample size of 246, we had 98% power to detect a difference in the weight change between HIV+ and HIV− women with an α of 0.05. All PreNAPS participants who delivered after 9 May 2013 were invited to participate in PostNAPS after delivery if the pregnancy resulted in a live singleton birth, and all women accepted the invitation (n = 246).
Postnatal visits were conducted at 1 wk and 1, 3, 6, 9, and 12 mo postpartum. At enrollment and all follow-up visits, trained research staff obtained physical measures including weight (Seca 874; Seca North America), height (Seca 206; Seca North America), and midupper arm circumference (MUAC) with the use of a nonstretchable, retractable tape measure. Body composition was assessed at postnatal follow-up visits that occurred after 31 July 2013. At these visits, subscapular, triceps, suprailliac, and midthigh skinfold thickness measurements were obtained on the right side of the body with the use of Harpenden calipers (Baty International). Arm muscle area (AMA) and arm fat area (AFA) were calculated from triceps skinfold thickness and MUAC measurements as follows:
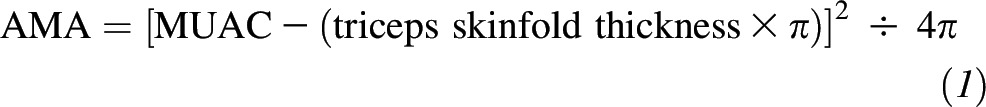 |
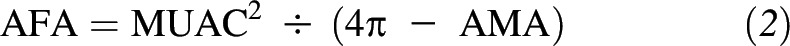 |
A bioelectrical impedance analysis (BIA 450; Biodynamics) was used to estimate fat-free mass and fat mass. Bioelectrical impedance analysis testing was completed after subjects drank ∼8 ounces (250 mL) H2O.
Other measures included an interviewer-administered, 10-item, individually focused food-insecurity access scale (26), the Center for Epidemiologic Studies Depression Scale (CES-D) (28), and the assessment of maternal dietary diversity from the previous day (31). At the enrollment visit during pregnancy, education, marital status, urban or rural residence, age, and report of previous displacement and living in an internally displaced person camp during the conflict in Gulu (ending in 2006) were obtained. A household-asset index was derived with the use of a principal components analysis from the self-report of household assets on the basis of the Ugandan National Panel Survey 2009/2010 (Supplemental Figure 1), whereby higher scores indicated greater wealth. Breastfeeding status was obtained by maternal report of any breastfeeding (yes or no) and any other dietary intake of the infant at each postpartum visit. At visits that occurred ≥1 mo postpartum, maternal experiences of diarrhea, vomiting, fever, and malaria in the previous month were ascertained. ART regimens were based on self-reports.
The Institutional Review Board (IRB) at Cornell University and the IRB at Gulu University approved the study procedures for the PreNAPS. These IRBs and the IRB at Weill Cornell Medical College approved the PostNAPS procedures. Permission to carry out the study in Uganda was granted by the Ugandan National Council for Science and Technology. All mothers provided written informed consent for both the PreNAPS and PostNAPS. These trials were registered at clinicaltrials.gov as NCT02922829 and NCT02925429
Statistical analyses were conducted with the use of Stata 12.0 software (StataCorp LP) with an α of 0.05 for statistical tests and an α of 0.1 for tests of effect modification. Baseline characteristics were compared between included and excluded dyads with the use of parametric tests for continuous, normally distributed variables and with the use of nonparametic tests for variables that were not normally distributed. These tests were also used to compare differences in baseline characteristics and body composition at 1 wk postpartum by HIV status.
Multivariable random-effects longitudinal models were used to assess the association between predictors and maternal body-composition changes from 1 wk to 12 mo accounting for the visit time as an indicator variable [1 wk (reference) and 1, 3, 6, 9, and 12 mo]. Models were built for the following body-composition outcomes: weight, BMI (in kg/m2), MUAC, AMA, AFA, fat mass, fat-free mass, and the sum of skinfold thickness. Hunger season was defined as dates inclusive of 1 April to 30 June. The primary covariates of interest were HIV status (yes or no) and time-varying lagged individual food insecurity [continuous score from previous study visit (e.g., the lagged value at 1 mo was assessed at 1 wk), whereby higher scores indicated greater food insecurity]. We chose to examine lagged individual food insecurity to strengthen the plausibility of associations (32). We evaluated whether the pattern of change over time varied by HIV status or food insecurity by including interaction terms between these factors and the visit; nonsignificant interaction terms were not retained in the final models.
Other covariates were included in the final model if the HIV or food-insecurity β coefficients appreciably changed (∼10%) in the base model with only these predictors or with strong literature justification. The time-independent covariates examined were as follows: household-asset index (score, continuous), maternal education beyond primary school (yes or no), maternal age (years, continuous), parity (number, continuous), urban residence (yes or no, comparison to rural), rate of pregnancy weight gain (kilograms per week, continuous, across all prenatal visits), and previous displacement (yes or no). Time-varying covariates were as follows: maternal dietary diversity (score, continuous), CES-D (score, continuous), hunger season occurring in previous month (yes or no), and currently exclusively breastfeeding (yes or no). Any breastfeeding was not included in the models because 8% of participants reported the complete cessation of breastfeeding by 12 mo. In a sensitivity analysis, a second set of longitudinal models were fit from 1 to 12 mo postpartum including the time-varying indicator variables for morbidities that were assessed at these visits (specifically, fever or malaria, vomiting, and diarrhea).
RESULTS
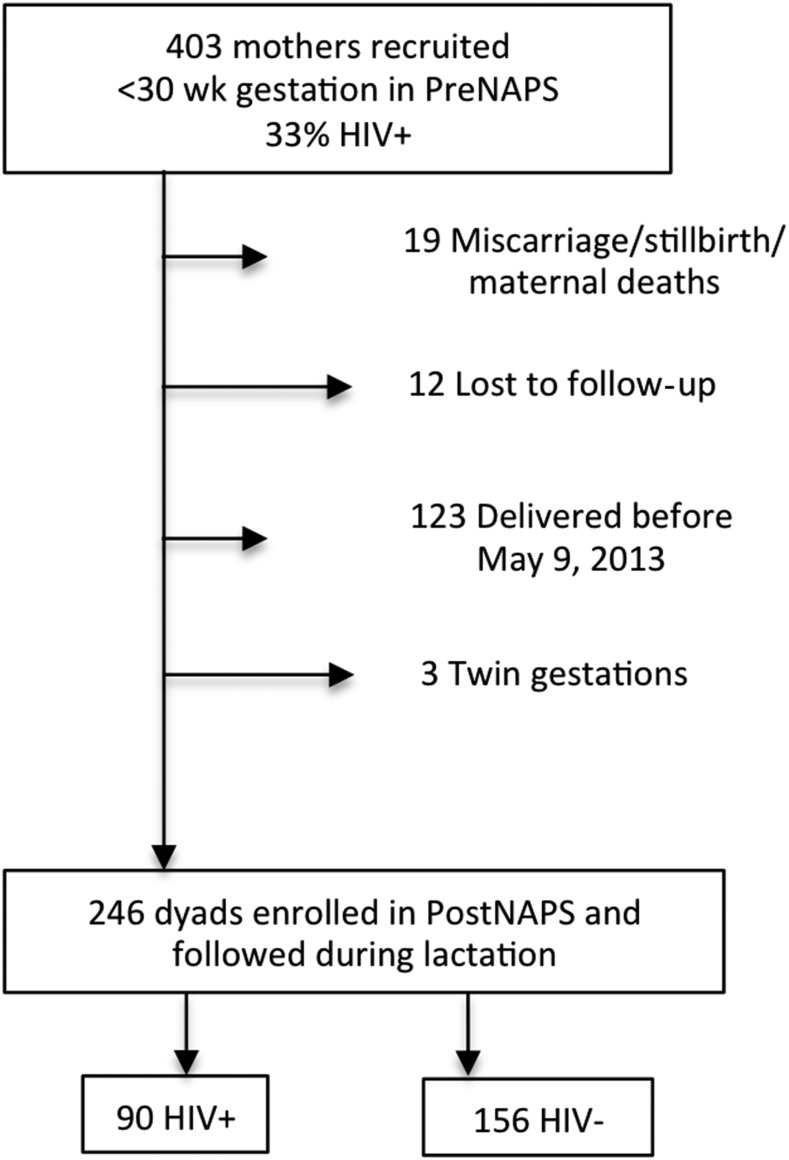
Data on body composition were available for 246 dyads (Figure 1). Characteristics of women included in this postnatal analysis (n = 246) were not different from those of women in the prenatal cohort who were not enrolled in the postnatal study (n = 157) and included household dietary diversity (P = 0.50), CES-D score (P = 0.07), the individual food-insecurity access score (P = 0.49), household assets (P = 0.83), parity (n = 0.23), maternal age (P = 0.21), BMI (n = 0.80), and prevalence of HIV (P = 0.69).
FIGURE 1.
Participant flow diagram from prenatal recruitment to postnatal follow-up of HIV+ and HIV− Ugandan women. HIV−, HIV negative; HIV+, HIV positive; IRB, institutional review board; PostNAPS, Postnatal Nutrition and Psychosocial Health Outcomes Study; PreNAPS, Prenatal Nutrition and Psychosocial Health Outcomes Study.
Compared with HIV+ women, HIV− women were more likely to be married, be educated beyond primary school, hold more household assets, and have higher rates of gestational weight gain (Table 1). Compared with HIV− women, HIV+ women had higher scores on the CES-D, higher individual food insecurity, and higher parity. Although the prevalence of breastfeeding initiation did not differ by HIV status, HIV+ women were more likely to be exclusively breastfeeding from 1 wk to 3 mo postpartum, whereas at 12 mo postpartum, the prevalence of any breastfeeding was lower in HIV+ women. Mean food insecurity remained relatively stable from 1 wk to 12 mo postpartum; mean ± SD food-insecurity scores were 5.8 ± 4.7 at 1 wk, 5.9 ± 4.7 at 1 mo, 6.4 ± 4.9 at 3 mo, 6.9 ± 5 at 6 mo, 6.3 ± 4.7 at 9 mo, and 5.8 ± 4.6 at 12 mo.
TABLE 1.
Prenatal characteristics and breastfeeding practices of HIV+ and HIV− Ugandan women who were participating in the PostNAPS (n = 246)1
| All (n = 246) | HIV+ (n = 90) | HIV− (n = 156) | P2 | |
| Prenatal | ||||
| Age, y | 25.2 ± 5.33 | 25.9 ± 5.5 | 24.8 ± 5.2 | 0.14 |
| Parity, n | 3.4 ± 3.1 | 4.1 ± 3.3 | 3.0 ± 3.0 | 0.006 |
| Married, n (%) | 215 (87.4) | 73 (81.1) | 142 (91.0) | 0.02 |
| Education beyond primary school, n (%) | 114 (46.3) | 33 (36.7) | 81 (51.9) | 0.02 |
| Asset index,4 score | 0.01 ± 1.6 | −0.32 ± 1.4 | 0.21 ± 1.6 | 0.009 |
| Employed,5 n (%) | 132 (53.7) | 46 (51.1) | 86 (55.1) | 0.54 |
| Prenatal dietary diversity,6 score | 6.6 ± 1.8 | 6.9 ± 1.5 | 7.4 ± 1.1 | 0.004 |
| Prenatal IFIAS,6 score | 9.6 ± 5.6 | 11.3 ± 5.5 | 8.6 ± 5.5 | <0.001 |
| Prenatal CES-D,6 score | 20.9 ± 12.7 | 20.2 ± 9.8 | 17.4 ± 8.9 | 0.02 |
| Rate of gestational weight gain, kg/wk | 0.28 ± 0.18 | 0.24 ± 0.20 | 0.31 ± 0.16 | 0.002 |
| Height, cm | 163.1 ± 6.0 | 163.2 ± 5.7 | 163.0 ± 6.2 | 0.74 |
| Previously displaced,7 n (%) | 186 (75.6) | 67 (74.4) | 119 (76.3) | 0.75 |
| Previously displaced to an IDP camp,7 n (%) | 131 (53.3) | 48 (53.3) | 83 (53.2) | 0.99 |
| Breastfeeding, n (%) | ||||
| Initiated8 | 237 (98.3) | 87 (98.9) | 150 (98.0) | 0.63 |
| Exclusive | ||||
| 1 wk (n = 236) | 185 (78.4) | 74 (86.1) | 111 (74.0) | 0.03 |
| 1 mo (n = 236) | 151 (64.0) | 63 (73.3) | 88 (58.7) | 0.03 |
| 3 mo (n = 237) | 118 (49.8) | 53 (60.2) | 65 (43.6) | 0.01 |
| 6 mo (n = 232) | 28 (12.1) | 15 (17.2) | 13 (9.0) | 0.06 |
| At 12 mo (n = 228) | 210 (92.1) | 70 (84.3) | 140 (96.6) | 0.001 |
CES-D, Center for Epidemiologic Studies Depression Scale; HIV−, HIV negative; HIV+, HIV positive; IDP, internally displaced person; IFIAS, individual food-insecurity access scale; PostNAPS, Postnatal Nutrition and Psychosocial Health Outcomes Study.
Comparisons between HIV+ and HIV− women were conducted with the use of parametric tests for continuous, normally distributed variables and with the use of nonparametric tests for variables that were not normally distributed.
Mean ± SD (all such values).
Derived from a principal components analysis of the possession of 20 different household assets, whereby households with higher values represented greater household wealth relative to that of others in the sample (Supplemental Figure 1).
Includes formal, self, informal, and other self-reported employment.
Values across all prenatal visits.
Participants reported previous displacement during the conflict in Northern Uganda area that ended in 2006 (participant enrollment started in October 2012).
Data were available for 241 women.
Overall, mean ± SD BMI at 1 wk postdelivery was 22.9 ± 2.9, which indicated that women were thin during pregnancy. Participants lost a small amount of weight in the first year postpartum with a mean weight change of −1.4 ± 4.4 kg (range of weight Δ: −13.0 to 10.6 kg) from 1 wk to 12 mo postpartum across the entire study population (Figure 2A). The majority of women (67%) lost weight (Δ <0 kg) from 1 wk to 12 mo postpartum with a mean weight loss of 3.8 ± 2.7 kg in women who lost weight. Only 3 women (∼1%) remained weight stable, and 32% of women gained weight with a mean weight gain of 3.6 ± 2.8 kg. The mean weight change between each follow-up period was a weight loss of −0.83 ± 1.9 kg from 1 wk to 1 mo, and from 1 to 3 mo, women gained weight (0.11 ± 2.1 kg). Weight loss was again observed from 3 to 6 mo (−0.24 ± 2.3 kg), 6 to 9 mo (−0.32 ± 2.2 kg), and 9 to 12 mo (−0.1 ± 2.2 kg).
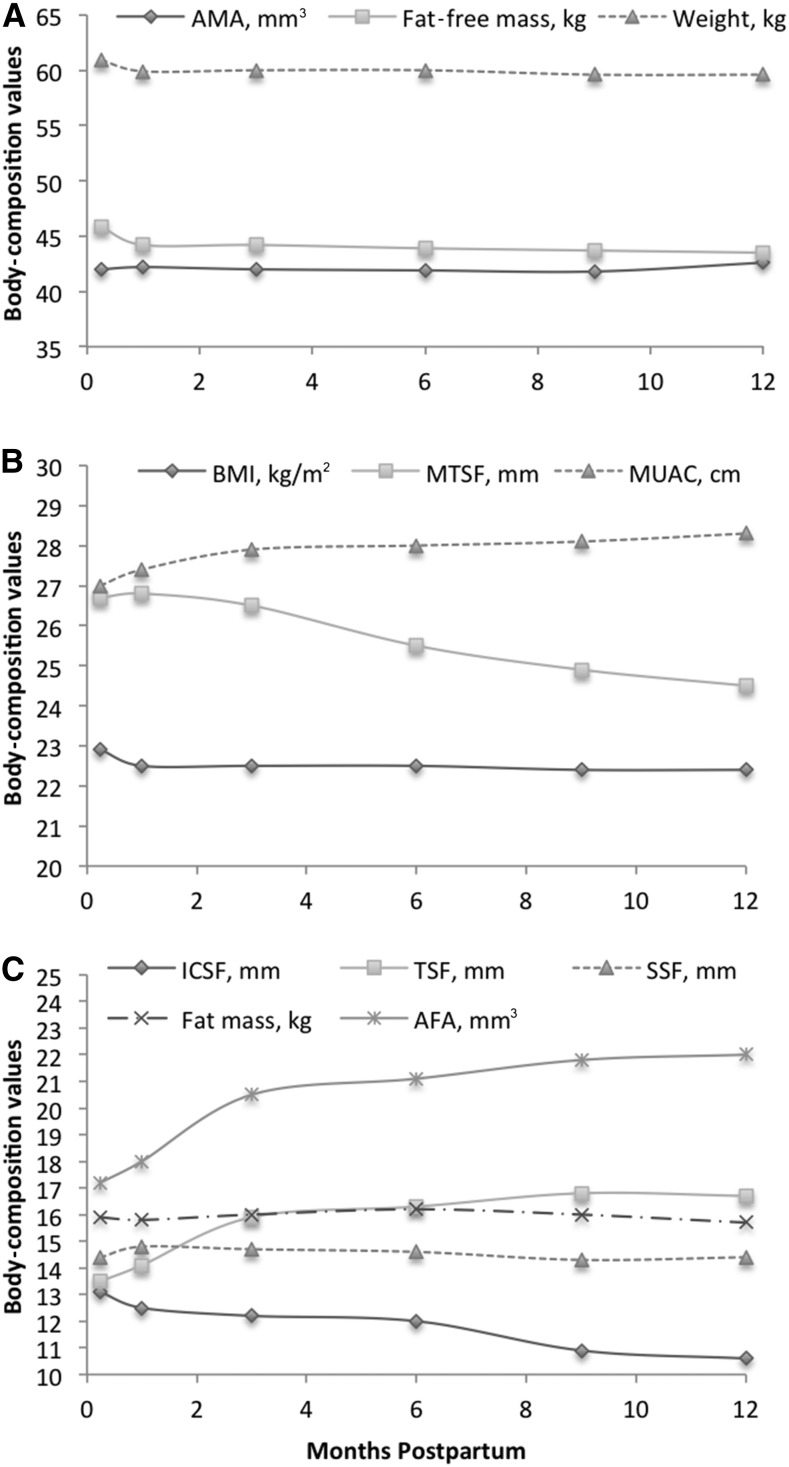
FIGURE 2.
Mean body-composition values from 1 wk to 12 mo in HIV-positive and HIV-negative Ugandan women (n = 246). Values are for AMA, fat-free mass, and weight (A); BMI, MTSF, and MUAC (B); and ICSF, TDF, SSF, fat mass, and AFA (C). AFA, arm fat area; AMA, arm muscle area; ICSF, iliac crest skinfold thickness; MTSF, midthigh skinfold thickness; MUAC, midupper arm circumference; SSF, subscapular skinfold thickness; TSF, triceps skinfold thickness.
Overall, changes in other body-composition measures were also relatively minimal from 1 wk to 12 mo postpartum. The most notable changes were small increases in the average triceps skinfold thickness and AFA from 1 wk to 3 mo and decreases in the midthigh skinfold thickness and iliac crest skinfold thickness from 1 wk to 12 mo (Figure 2B, C).
In terms of HIV, at 1 wk postpartum, body composition did not differ significantly by HIV status with the exception of AMA (Table 2). Weight changes did not differ by maternal HIV status (HIV+ 1.1 ± 4.4 compared with HIV− 1.6 ± 4.4 kg, P = 0.4). The proportion of women with weight loss or weight gain was not different by HIV status (P = 0.28).
TABLE 2.
Body composition of HIV+ and HIV− Ugandan women at 1 wk postpartum by HIV status (n = 246)1
| HIV+ | HIV− | P2 | |
| Weight, kg | 60.4 ± 7.9 [90] | 61.1 ± 9.0 [156] | 0.52 |
| BMI, kg/m2 | 22.7 ± 2.9 [90] | 23.0 ± 2.8 [156] | 0.43 |
| MUAC, cm | 26.9 ± 2.6 [89] | 27.1 ± 2.7 [155] | 0.46 |
| TSF, mm | 13.7 ± 5.7 [62] | 13.4 ± 5.2 [99] | 0.74 |
| SSF, mm | 13.7 ± 4.8 [61] | 14.8 ± 5.6 [99] | 0.21 |
| ICSF, mm | 12.3 ± 5.6 [60] | 13.6 ± 6.0 [99] | 0.19 |
| MTSF, mm | 26.3 ± 11.1 [61] | 27.0 ± 11.1 [98] | 0.70 |
| Sum-SFs, mm | 66.2 ± 23.8 [60] | 68.6 ± 23.9 [98] | 0.54 |
| AMA, cm3 | 40.6 ± 5.7 [61] | 42.9 ± 6.9 [98] | 0.03 |
| AFA, cm3 | 17.1 ± 8.0 [61] | 17.3 ± 7.8 [98] | 0.89 |
| Fat mass, kg | 15.7 ± 4.3 [62] | 16.0 ± 4.7 [96] | 0.61 |
| Fat-free mass, kg | 45.4 ± 5.9 [62] | 46.0 ± 5.7 [96] | 0.56 |
All values are means ± SDs [n]. AFA, arm fat area; AMA, arm muscle area; HIV−, HIV negative; HIV+, HIV positive; ICSF, iliac crest skinfold thickness; MTSF, midthigh skinfold thickness; MUAC, midupper arm circumference; SSF, subscapular skinfold thickness; Sum-SF, sum of skinfold thickness; TSF, triceps skinfold thickness.
Comparisons between HIV+ and HIV− women were conducted with the use of parametric tests for continuous, normally distributed variables and with the use of nonparametric tests for variables that were not normally distributed.
To test the hypotheses that HIV and food insecurity might be associated with adverse changes in body composition, we built longitudinal random-effects models of maternal body composition over time (Supplemental Tables 1 and 2). We observed no significant interactions between the study visit and HIV status (all P > 0.01); thus, HIV was included as a covariate.
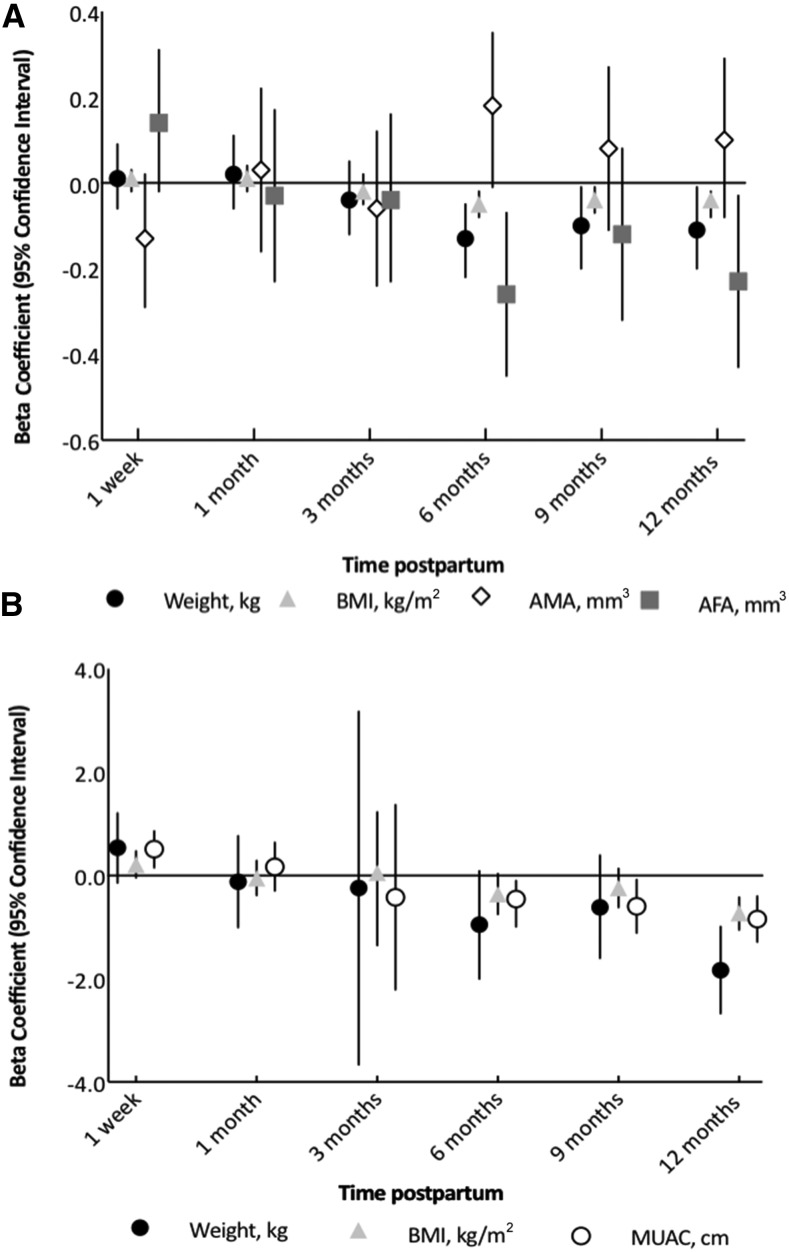
HIV status was not associated with body composition over time for any outcome (all P > 0.05). For food insecurity, significant interactions were observed between lagged individual food insecurity and study visits for weight, BMI, AMA, and AFA, which suggested that the effects of food insecurity varied over time for these outcomes. Specifically, at 6, 9, and 12 mo postpartum, greater food insecurity was associated with lower weight, BMI, and AFA (Figure 3A). The strongest associations were observed at 6 mo postpartum, whereby a 1-unit increase in the lagged food insecurity score was associated with 0.13-kg lower weight, 0.5-units lower BMI, and 0.26-cm3 lower AFA. The sum of skinfold thickness, fat mass, fat-free mass, or MUAC was not associated with the lagged food-insecurity score (all P > 0.05).
FIGURE 3.
Predicted β (95% CI) effects of food insecurity (A) and hunger season (B) on maternal body composition over time. Results shown are estimates from multivariate random-effects longitudinal models that were adjusting for maternal height and covariates and included interaction terms between the study visit with food insecurity and hunger exposure in the previous month. (A) Predicted effects for a 1-unit increase in individual food-insecurity access score on body composition at each observation. (B) Predicted effects of exposure to hunger season in the month before the study visit on body composition at each observation. The hunger season was defined as dates that were inclusive of 1 April to 30 June. Sample sizes for each outcome were as follows: weight, BMI, and MUAC (n = 246); AMA and AFA (n = 244); and fat-free mass and fat mass (n = 243). AFA, arm fat area; AMA, arm muscle area; MUAC, midupper arm circumference.
Generally, the hunger season (from April to June when food stores were running low) was associated with lower body-composition values. Specifically, if the month before the observation occurred during the hunger season, lower fat mass (β = −0.37 kg; 95% CI: −0.67, −0.06 kg) and AMA (β = −0.76 cm3; 95% CI: −1.41, −0.10 cm3) were observed (Supplemental Tables 1 and 2). For weight, BMI, and MUAC, effects varied by the timing of the hunger season relative to the month of lactation (all P-interaction over time < 0.1) (Figure 3B). In the early postpartum period (1 wk), the hunger season was associated with a higher MUAC, but at 9 mo, the hunger season was associated with lower MUAC, and by 12 mo postpartum, the hunger season was associated with lower MUAC, BMI, and weight.
Other factors that were associated with body-composition changes postpartum included the rate of pregnancy weight gain, maternal age, locale of residence (i.e., urban or rural), parity, depression, and maternal education (Supplemental Tables 1 and 2). In these models, the strongest predictor of body-composition change was the rate of pregnancy weight gain (kilograms per week). We showed that the rate of pregnancy weight gain was predictive of greater weight, BMI, MUAC, fat-free mass, and AMA. Older maternal age was positively associated with all body-composition outcomes, whereas urban residence was associated with higher weight, BMI, AFA, fat mass, and sum of skinfold thickness. Parity was inversely associated with weight, BMI, fat mass, and fat-free mass, whereas maternal education beyond primary school was associated with lower MUAC and AMA. A greater maternal depression score was associated with lower weight, BMI, and fat-free mass.
Because morbidity may be closely related to dietary intake and energy expenditure, we conducted a sensitivity analysis to determine whether further adjustment for morbidity would influence the effects of food insecurity and HIV on maternal body-composition changes. We observed that the β coefficients for HIV and food insecurity were not appreciably altered (Supplemental Tables 3 and 4).
DISCUSSION
In this first study, to our knowledge, of body composition in women of mixed HIV status across the first year postpartum, we observed minimal changes in body composition from 1 wk to 12 mo, which was a result that was contrary to our first hypothesis that HIV status would affect body composition. Our second hypothesis, that food insecurity would lead to significant adverse effects on body composition, was supported by our findings that at 6, 9, and 12 mo postpartum, individual food insecurity was inversely associated with body weight, BMI, and AFA.
It is important to provide some context regarding our study participants and Northern Uganda. A majority of the population around Gulu township, including a majority of our study participants (76%), had been forcibly displaced during the protracted internal conflict that ended in 2006, with 53% of participants having reported being relocated to internally displaced person camps. Although we followed our participants for >6 y after this conflict, their experiences during this period may have affected their long-term physical and psychosocial health, which should be considered when considering the generalizability of these results.
In our cohort, weight was remarkably stable from 1 wk to 12 mo postpartum. In general, lactating women experienced mild and gradual weight loss, but there is substantial heterogeneity (17, 33). Weight loss, weight maintenance, and even moderate weight gains have been reported in HIV+ (4, 5, 11, 15) and HIV− (17, 34, 35) postpartum populations. For example, in HIV+ women in the Kesho Bora Study, weight changes from 2 to 6 mo were generally stable, ranging from +1.5 to −0.9 kg (11). In the Safe Milk for African Children study, a weight loss of 1.1 ± 3.5 kg was observed from 1 to 6 mo postpartum (13).
We observed no effect of HIV on weight change or body composition. This result was slightly different from that in a cohort of ART-naive South African women in whom HIV+ women lost weight (−1.4 ± 3.1 kg) and HIV− women gained weight (+0.4 ± 3.3 kg) (P = 0.004) (15) from 2 to 6 mo postpartum; longer-term weight changes were not examined. However, in the same study, there was no association between HIV status and body composition, as was the case in our study.
We showed that exclusive breastfeeding was not associated with body-composition changes, which to our knowledge has not been previously examined. One trial in HIV+ Zambian women (n = 958) randomly assigned participants to different periods of breastfeeding and reported postnatal weight changes (14). Short-duration breastfeeding was associated with mean weight gain from 4 to 24 mo (2.3 kg; 95% CI: 1.6, 2.9 kg), whereas longer breastfeeding was associated with less mean weight gain (1.0 kg; 95% CI: 0.3, 1.7 kg; P = 0.01) (14). Because the majority of our study participants continued to breastfeed at 12 mo postpartum, as was comparable with the longer breastfeeding group in the Zambian cohort, our results of relative weight stability were similar to these results.
Our findings of exposure to the hunger season being associated with lower MUAC, weight, and BMI were also similar to the results of the Zambian cohort, for whom the dry season (May to September) (β = 0.05 kg, P < 0.01) was associated with weight gain, and a food shortage (January 2002 to April 2003) (β = −1.0 kg, P < 0.01) was associated with lower weight (14).
The maintenance of weight in this rather thin population was surprising and may have been attributable to several adaptations, some of which were behavioral and others of which were physiologic. Potentially intersecting mechanisms may include 1) increases in dietary intake, 2) nutrient partitioning to support maternal nutrient stores, and 3) adaptive mechanisms to promote long-term energy balance (35) such as increases in metabolic efficiency (i.e., decreased basal metabolic rate) or lower activity (i.e., reduced energy expenditure) (22, 35). These adaptations could be better elucidated through a more precise measurement of macronutrient intake, breast-milk composition, frequency and duration of breastfeeding episodes, energy expenditure, and physical activity in future studies.
Perhaps related to adaptations to nutrient demands, food insecurity was only associated with greater losses of maternal weight, BMI, and AFA at 6 mo postpartum and onward when energy demands for breast milk could have been highest (36). Specifically, at 6 mo, a 1-unit increase in the individual food-insecurity access score was associated with a 0.13-kg lower weight, 0.5-units lower BMI, and 0.26-cm3 lower AFA. These results suggest that subcutaneous body fat depots (e.g., upper arm) were mobilized to support continued breastfeeding and maternal nutritional status, whereas arm-muscle depots were conserved. The association between food insecurity and loss of weight and BMI, but not with loss of body fat or lean mass, suggested that greater food insecurity was associated with a proportional loss of lean and adipose tissue over time. To our knowledge, there have been no previous studies on food insecurity and maternal body composition during lactation to compare these results with.
We observed associations between body composition and several modifiable factors that could be leveraged in future interventions and have programmatic implications (Supplemental Tables 1 and 2). The finding that higher rates of pregnancy weight gain were associated with gains in all body-composition outcomes during lactation suggests that supporting adequate gestational weight gain can support maternal nutrition and health postpartum. We observed that depression was associated with lower weight, BMI, and fat-free mass, thereby indicating that lactating women with poor mental health may be vulnerable to poor nutritional status and loss of lean mass. Several possible pathways could link depression to these outcomes including poor dietary intake or suppressed appetite. Last, screening for food insecurity during pregnancy, regardless of HIV-infection status, could be useful for identifying women in need of nutritional support to maintain the maternal energy balance and possibly support breastfeeding.
Our study has a number of strengths including a rigorous, prospective, longitudinal assessment of food insecurity and numerous other predictors of body composition over the course of 12 mo. Notably, to our knowledge, this trial is the longest and most comprehensive study of body composition during lactation in HIV+ and HIV− women. Limitations included our inability to estimate the effects of breastfeeding compared with exclusive breastfeeding because of their collinearity, the absence of clinical information that may have affected breastfeeding or health status during breastfeeding (i.e., viral load and breast health), self-reported ART adherence, and the collection of more-precise data that would have allowed for the testing of behavioral and physiologic adaptations as previously described. Finally, Gulu receives a myriad of humanitarian aid interventions, and participants in this study may have benefited from development and reconstruction-driven aid, but we did not collect data on this topic.
In conclusion, HIV infection is not associated with postpartum body-composition changes from 1 wk to 12 mo, and food insecurity is associated with greater weight loss and regional subcutaneous fat loss in the arm after 6 mo postpartum. Lactating women who experience food insecurity may need nutritional support to ensure the maintenance of maternal nutritional reserves and breast-milk quality.
Acknowledgments
We thank the GRRH antenatal care clinic for providing space for the research team within the hospital. We thank Sophie Becky Ajok, who is the coordinator for the prevention of mother-to-child transmission of HIV services at the GRRH antenatal clinic, for helping to recruit pregnant women to the study; Angela Arbach for assisting with the coordination of the PreNAPS; and Nicole Sirotin for her involvement with the PostNAPS. We also thank the following PreNAPS and PostNAPS Uganda staff for their dedication and hard work on this study: Stella Adoch, Gladys Acayo, Hillary Kilama, Joe Cord, Daniel Onen, and Geoffrey Abwola.
The authors’ responsibilities were as follows—EMW: designed and performed the data analysis, wrote the manuscript, and had primary responsibility for the final content of the manuscript; EMW, SMC, and DA: conducted data cleaning and management; EMW, SMC, SG, JKG, and SLY: provided a critical review of the manuscript; EMW and SLY: conceptualized the research question and conceived and designed the PostNAPS study; SMC: supervised the data collection; HK: assisted with the literature reviews and tables; CB, WA, and HA: conducted the participant interviews and measurements; DA: managed the study databases; SG, JKG, and SLY: conceived and designed the PreNAPS study; and all authors: reviewed and contributed to the intellectual content of the manuscript and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AFA, arm fat area; AMA, arm muscle area; ART, antiretroviral therapy; CES-D, Center for Epidemiologic Studies Depression Scale; GRRH, Gulu Regional Referral Hospital; HIV−, HIV negative; HIV+, HIV positive; IRB, Institutional Review Board; MUAC, midupper arm circumference; PostNAPS, Postnatal Nutrition and Psychosocial Health Outcomes Study; PreNAPS, Prenatal Nutrition and Psychosocial Health Outcomes Study.
REFERENCES
- 1.World Health Organization. Guidelines on HIV and infant feeding: principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva (Switzerland): World Health Organization Press; 2010. [PubMed] [Google Scholar]
- 2.World Health Organization, United Nations Children’s Fund. Guideline: updates on HIV and infant feeding: the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. Geneva (Switzerland): World Health Organization; 2016. [PubMed] [Google Scholar]
- 3.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva (Switzerland): WHO Press; 2015. [PubMed] [Google Scholar]
- 4.Kayira D, Bentley ME, Wiener J, Mkhomawanthu C, King CC, Chitsulo P, Chigwenembe M, Ellington S, Hosseinipour MC, Kourtis AP, et al. A lipid-based nutrient supplement mitigates weight loss among HIV-infected women in a factorial randomized trial to prevent mother-to-child transmission during exclusive breastfeeding. Am J Clin Nutr 2012;95:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widen EM, Bentley ME, Kayira D, Chasela CS, Jamieson DJ, Tembo M, Soko A, Kourtis AP, Flax VL, Ellington SR, et al. Maternal weight loss during exclusive breastfeeding is associated with reduced weight and length gain in daughters of HIV-infected Malawian women. J Nutr 2013;143:1168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen LH, Hampel D, Shahab-Ferdows S, York ER, Adair LS, Flax VL, Tegha G, Chasela CS, Kamwendo D, Jamieson DJ, et al. Antiretroviral therapy provided to HIV-infected Malawian women in a randomized trial diminishes the positive effects of lipid-based nutrient supplements on breast-milk B vitamins. Am J Clin Nutr 2015;102:1468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widen EM, Bentley ME, Chasela CS, Kayira D, Flax VL, Kourtis AP, Ellington SR, Kacheche Z, Tegha G, Jamieson DJ, et al. Antiretroviral treatment is associated with iron deficiency in HIV-infected malawian women that is mitigated with supplementation, but is not associated with infant iron deficiency during 24 weeks of exclusive breastfeeding. J Acquir Immune Defic Syndr 2015;69:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flax VL, Adair LS, Allen LH, Shahab-Ferdows S, Hampel D, Chasela CS, Tegha G, Daza EJ, Corbett A, Davis NL, et al. Plasma micronutrient concentrations are altered by antiretroviral therapy and lipid-based nutrient supplements in lactating HIV-infected malawian women. J Nutr 2015;145:1950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Nutrient requirements for people living with HIV/AIDS: report of a technical consultation. Geneva (Switzerland): WHO Press; 2003. [Google Scholar]
- 10.Winkvist A, Rasmussen KM, Habicht JP. A new definition of maternal depletion syndrome. Am J Public Health 1992;82:691–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cames C, Cournil A, de Vincenzi I, Gaillard P, Meda N, Luchters S, Nduati R, Naidu K, Newell ML, Read JS, et al. Postpartum weight change among HIV-infected mothers by antiretroviral prophylaxis and infant feeding modality in a research setting. AIDS 2014;28:85–94. [DOI] [PubMed] [Google Scholar]
- 12.Chetty T, Carter RJ, Bland RM, Newell ML. HIV status, breastfeeding modality at 5 months and postpartum maternal weight changes over 24 months in rural South Africa. Trop Med Int Health 2014;19:852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giuliano M, Guidotti G, Andreotti M, Scarcella P, Amici R, Jere H, Sagno JB, Buonomo E, Mancinelli S, Marazzi MC, et al. Weight changes during and after 6 months of breastfeeding in HIV-infected mothers receiving antiretroviral therapy in Malawi. AIDS Res Hum Retroviruses 2014;30:1155–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murnane PM, Arpadi SM, Sinkala M, Kankasa C, Mwiya M, Kasonde P, Thea DM, Aldrovandi GM, Kuhn L. Lactation-associated postpartum weight changes among HIV-infected women in Zambia. Int J Epidemiol 2010;39:1299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papathakis PC, Van Loan MD, Rollins NC, Chantry CJ, Bennish ML, Brown KH. Body composition changes during lactation in HIV-infected and HIV-uninfected South African women. J Acquir Immune Defic Syndr 2006;43:467–74. [DOI] [PubMed] [Google Scholar]
- 16.Adair LS, Pollitt E, Mueller WH. Maternal anthropometric changes during pregnancy and lactation in a rural Taiwanese population. Hum Biol 1983;55:771–87. [PubMed] [Google Scholar]
- 17.Butte NF, Hopkinson JM. Body composition changes during lactation are highly variable among women. J Nutr 1998;128(2 Suppl):381S–5S. [DOI] [PubMed] [Google Scholar]
- 18.AbuSabha R, Greene G. Body weight, body composition, and energy intake changes in breastfeeding mothers. J Hum Lact 1998;14:119–24. [DOI] [PubMed] [Google Scholar]
- 19.Dugdale AE, Eaton-Evans J. The effect of lactation and other factors on post-partum changes in body-weight and triceps skinfold thickness. Br J Nutr 1989;61:149–53. [DOI] [PubMed] [Google Scholar]
- 20.Forsum E, Sadurskis A, Wager J. Estimation of body fat in healthy Swedish women during pregnancy and lactation. Am J Clin Nutr 1989;50:465–73. [DOI] [PubMed] [Google Scholar]
- 21.Martínez H, Allen LH, Lung’aho M, Chavez A, Pelto GH. Maternal fatness in Mexican women predicts body composition changes in pregnancy and lactation. Adv Exp Med Biol 1994;352:99–107. [DOI] [PubMed] [Google Scholar]
- 22.Adair LS, Popkin BM. Prolonged lactation contributes to depletion of maternal energy reserves in Filipino women. J Nutr 1992;122:1643–55. [DOI] [PubMed] [Google Scholar]
- 23.Weiser SD, Young SL, Cohen CR, Kushel MB, Tsai AC, Tien PC, Hatcher AM, Frongillo EA, Bangsberg DR. Conceptual framework for understanding the bidirectional links between food insecurity and HIV/AIDS. Am J Clin Nutr 2011;94:1729S–39S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laraia BA, Siega-Riz AM, Gundersen C, Dole N. Psychosocial factors and socioeconomic indicators are associated with household food insecurity among pregnant women. J Nutr 2006;136:177–82. [DOI] [PubMed] [Google Scholar]
- 25.Young SL, Plenty AH, Luwedde FA, Natamba BK, Natureeba P, Achan J, Mwesigwa J, Ruel TD, Ades V, Osterbauer B, et al. Household food insecurity, maternal nutritional status, and infant feeding practices among HIV-infected Ugandan women receiving combination antiretroviral therapy. Matern Child Health J 2014;18:2044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natamba BK, Kilama H, Arbach A, Achan J, Griffiths JK, Young SL. Reliability and validity of an individually focused food insecurity access scale for assessing inadequate access to food among pregnant Ugandan women of mixed HIV status. Public Health Nutr 2015;18:2895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Republic of Uganda Ministry of Health. National antiretrovial treatment and care guidelines for adults adolescents, and children. Kampala (Uganda): Ministry of Health, Government of the Republic of Uganda; 2008. [Google Scholar]
- 28.Natamba BK, Achan J, Arbach A, Oyok TO, Ghosh S, Mehta S, Stoltzfus RJ, Griffiths JK, Young SL. Reliability and validity of the center for epidemiologic studies-depression scale in screening for depression among HIV-infected and -uninfected pregnant women attending antenatal services in northern Uganda: a cross-sectional study. BMC Psychiatry 2014;14:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natamba BMS, Achan J, Stoltzfus R, Griffiths J, Young S. The association between food insecurity and depressive symptoms severity among pregnant women differs by social support category: a cross-sectional study. Matern Child Nutr 2016. Aug 9 (Epub ahead of print; DOI: 10.1111/mcn.12351). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabiani M, Nattabi B, Pierotti C, Ciantia F, Opio AA, Musinguzi J, Ayella EO, Declich S. HIV-1 prevalence and factors associated with infection in the conflict-affected region of North Uganda. Confl Health 2007;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Food and Agriculture Organization. Guidelines for measuring household and individual dietary diversity. Rome (Italy): Food and Agriculture Organization; 2010. [Google Scholar]
- 32.Weiser SD, Tsai AC, Gupta R, Frongillo EA, Kawuma A, Senkungu J, Hunt PW, Emenyonu NI, Mattson JE, Martin JN, et al. Food insecurity is associated with morbidity and patterns of healthcare utilization among HIV-infected individuals in a resource-poor setting. AIDS 2012;26:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkvist A, Rasmussen KM. Impact of lactation on maternal body weight and body composition. J Mammary Gland Biol Neoplasia 1999;4:309–18. [DOI] [PubMed] [Google Scholar]
- 34.Winkvist A, Habicht JP, Rasmussen KM, Frongillo ED Jr. Malnourished mothers maintain their weight throughout pregnancy and lactation. Adv Exp Med Biol 2000;478:415–6. [DOI] [PubMed] [Google Scholar]
- 35.Winkvist A, Jalil F, Habicht JP, Rasmussen KM. Maternal energy depletion is buffered among malnourished women in Punjab, Pakistan. J Nutr 1994;124:2376–85. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen KM. The influence of maternal nutrition on lactation. Annu Rev Nutr 1992;12:103–17. [DOI] [PubMed] [Google Scholar]