Abstract
Objective
Smoking cessation self-efficacy and adaptive coping are posited as two important treatment targets in smoking cessation interventions, especially in the context of handling strong urges to smoke. Yet, less is known about whether intervention-related changes in these constructs predict long-term smoking outcomes. The current study aimed to examine changes in smoking urges, smoking cessation self-efficacy, and adaptive coping following a health-focused and cognitive-behavioral telephone-delivered smoking cessation treatment, and the association to smoking reduction during long-term, 12-month follow-up.
Methods
Participants (n = 61) were daily smokers enrolled in a 12-week pilot trial that tested the efficacy of two different health-focused interventions with an adjunct of traditional telephone-delivered cessation counseling. Smoking urges, smoking cessation self-efficacy, and adaptive coping were assessed as baseline and immediately post-treatment. Average of seven-day cigarettes use per day were assessed at post-treatment, and 6- and 12-months post-baseline follow-up timepoints.
Results
Smoking urges were significantly lower post-treatment, and smoking cessation self-efficacy and adaptive coping were significantly higher post-treatment, relative to baseline. After adjusting for baseline values, post-treatment smoking urges were significantly positively associated with cigarette use at post-treatment and 6-month follow-up. Post-treatment smoking cessation self-efficacy, but not adaptive coping, was significantly negatively predictive of cigarette use at post-treatment and 6- and 12-month follow-up timepoints. Post-treatment smoking cessation self-efficacy emerged as significant indirect predictor of the association between post-treatment smoking urges and post-treatment cigarette use.
Conclusions
Interventions that target smoking cessation self-efficacy may facilitate long-term reductions in smoking among daily smokers undergoing a quit attempt.
Keywords: tobacco, relapse prevention, coping skills
Cigarette smoking remains the leading cause of preventable death and illness in the United States1, as 16.8% smoke.2 The monetary cost of tobacco use is approximated to exceed $300 billion. 1 It is well-documented that multiple problematic health behaviors frequently co-occur with cigarette smoking, including other substance use and physical inactivity 3–6, which in part may compound risk for early mortality and morbidity. 7 Rates of relapse to cigarette use among individuals initiating a quit attempt are high, particularly within the first 5-10 days after quit date. 8 Thus, smoking cessation protocols that incorporate support leading up to and following quit date may be especially efficacious in reducing rates of relapse to smoking. Factors such as nicotine withdrawal, craving, and urge are predictors of early smoking relapse. 9 There is a need to examine factors that may increase long-term efficacy of smoking cessation treatments.
Theoretical models of relapse prevention 10,11 posit that a return (lapse, relapse) to substance use results from low perceived ability to abstain from use (abstinence self-efficacy) and limited adaptive coping strategies in the context of high-risk situations (e.g., strong urges to use). This theoretical model has been supported in a variety of studies with diverse populations and substances. 12–16 Specific to cigarette use, urge/craving is one of the most consistent predictors of relapse to re-initiation of smoking. 8 Various smoking cessation treatment programs have been developed to specifically bolster certain cognitive and behavioral skills to promote smoking cessation – including smoking cessation self-efficacy and coping skills, especially in the context of strong smoking urges/craving. 17,18
Data indicate that relapse prevention-based smoking cessation protocols increase self-efficacy for abstaining from cigarette use across a variety of situations, 19 and smoking cessation self-efficacy is among the strongest predictors of smoking abstinence, 20,21 particularly among those initiating a quit attempt. 22 Smoking cessation self-efficacy has also been found to explain (mediate) the link between craving and smoking outcomes. 23 Greater adaptive coping skills are also associated with positive smoking cessation treatment outcomes. 24,25 For example, the stress and coping model describes adaptive coping as a method to attenuate the impact of stress and decrease the potential for negative outcomes, such as a lapse or relapse. 26 Previous studies have examined the role of self-efficacy in smoking cessation, 21 but the current study adds to the literature by examining how smoking urges is related to smoking self-efficacy and adaptive coping to predict smoking cessation outcomes.
Utilizing intervention trial data from Abrantes et al., 27, the current study aimed to (a) examine changes in smoking urges, smoking cessation self-efficacy, and adaptive coping post-smoking cessation intervention, and (b) test whether these changes in smoking cessation self-efficacy and adaptive coping attenuate the relationship between urge and cigarette use post-treatment and at long-term follow-up. Specifically, the following hypotheses were proposed: (1) Smoking urges will be significantly lower post-treatment relative to baseline, and post-treatment smoking urges will be positively associated with greater cigarette use post-treatment and at follow-up; (2) Smoking cessation self-efficacy and adaptive coping will be significantly higher at post-treatment, relative to baseline, and post-treatment scores will be associated with lower cigarette use post-treatment and at follow-up; and (3) Post-treatment smoking urges will be negatively associated with post-treatment smoking cessation self-efficacy and adaptive coping, which in turn will be negatively associated with post-treatment cigarette use (i.e., test of mediation).
Method
Participants
Participants (n = 61; Mage = 47.3; SD = 9.6; 65.6% female) were recruited as part of a randomized controlled trial of two health-focused interventions for sedentary daily smokers. 27 Participants were eligible on the basis of smoking 10 or more cigarettes per day for the past year and exercising less than 60 minutes per week during the previous 6 months prior to enrollment. Participants were excluded if they had a current contraindicated psychiatric disorder (alcohol/drug use disorder, bipolar disorder, psychotic disorder, or eating disorder; defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)), current suicidal ideation or homicidal ideation, a physical or medical issue that would be prevent engagement in aerobic exercise (including pregnancy), and current use of pharmacotherapy for smoking cessation. Participants were primarily Caucasian (80.3%), smoked an average of 19.7 cigarettes per day at initial screening (SD = 8.6), and reported daily smoking for the past 27.5 years (SD = 10.1). For complete sample details, please see Abrantes et al.27
Procedures
Participants were randomized to receive either 12 weeks of an aerobic exercise intervention (AE; n = 30) or 12 weeks of a health education intervention (HEC; n = 31), as detailed in Abrantes et al.27 All participants received eight, 20-minute cognitive behavioral-based telephone sessions over the course of 8 weeks, modeled after cessation interventions delivered in prior work.28–31 These sessions were designed to prepare participants for their quit date (in week 4) via identifying high-risk situations for cigarette use, developing and utilizing coping strategies, increasing smoking cessation self-efficacy (through practiced reduction of 1-2 cigarettes/day prior to quitting), setting incremental goals, and relapse prevention (on quit day and 3 weeks following). All participants also received nicotine replacement therapy (via the transdermal nicotine patch) throughout the course of the study (see 27 for details on administration). Assessment of smoking behavior and urges, smoking cessation self-efficacy and adaptive coping were assessed via self-report at baseline (pre-treatment), immediately post-treatment, and at two follow-up time points (6 and 12-months post-baseline). All participants provided written informed consent prior to participants and all study procedures were approved by the institutional review board of Butler Hospital.
Measures
Cigarette Use
The Timeline Follow-back 32 is as calendar-based assessment of daily cigarette use, which was used to determine the average number of cigarettes smoked per day during the past-7 days prior to the assessment timepoint. Self-reported point prevalence abstinence (no smoking in the past 7 days) was verified using expired carbon monoxide breath sample (using a 10 ppm cutoff) and/or observer report (significant other), a method that has been validated in previous studies. 27
Smoking Cessation Self-Efficacy
The Smoking Self-Efficacy Scale 33 is a self-report assessment that requires participants to rate on a scale of 1 (not at all confident) to 5 (extremely confident) their perceived ability to avoid using cigarettes in a variety of situations. Cronbach’s alpha estimates suggested these items had excellent reliability (alpha = .90 at baseline, .95 at post-treatment).
Smoking Urges
The Questionnaire of Smoking Urges 34 is a 32-item self-report measure of the intensity of smoking urges (e.g., “Smoking would make me feel very good right now”). Items were rated on a scale of 1 (strongly disagree) to 7 (strongly agree). Reliability estimates were as follows: Cronbach’s alpha = .60 at baseline, .66 at post-treatment.
Adaptive Coping
The Brief COPE scale 35 is a 28-item measure designed to assess for a variety of coping strategies for stressful or difficult life situations. The measure asks participants how often they utilize various coping strategies during life situations on a scale of 1 (I usually don’t do this at all) to 4 (I usually do this a lot). Adaptive coping was assessed through the subscales of active coping, emotional support, instrumental support, planning, acceptance, and positive reframing. Estimates were suggestive of excellent reliability (Cronbach’s alpha = .91 at baseline, .92 at post-treatment).
Results
Changes in Smoking Urges, Smoking Cessation Self-Efficacy, and Coping
Regression analyses tested the effects of the intervention on smoking urges, smoking cessation self-efficacy, adaptive coping. Table 1 displays baseline and post-treatment means, across treatment conditions. Results indicated that smoking urges significantly decreased from baseline to post-treatment (beta = .36, p = .02), however there was no significant time × condition effect (beta = −.15, p = .33). Additionally, results indicated that there was a significant increase in smoking cessation self-efficacy from baseline to post-treatment (beta = .30, p = .05; see Table 1), although the time × condition interaction was again non-significant (beta = .14, p = .35). Similarly, analyses of adaptive coping indicated a significant increase in adaptive coping from baseline to post-treatment (beta = .57, p < .01); these results did not significantly differ by condition (beta = −.05, p = .69). Based on the non-significant treatment condition effects in the variables of interest, treatment conditions were collapsed in all subsequent analyses.
Table 1. Descriptive Summary of Baseline and Post-Treatment Scores.
|
|
|||||
|---|---|---|---|---|---|
| Baseline | Posttreatment | Sig. | |||
|
|
|||||
| Mean | SD | Mean | SD | ||
| Smoking Urges | 4.22 | 1.04 | 2.34 | 1.12 | * |
| Smoking Cessation Self-Efficacy | 2.46 | .97 | 3.89 | 1.05 | * |
| Adaptive Coping | 2.34 | .67 | 2.55 | .81 | ** |
p<.01;
p<.05
Predicting Reductions in Cigarette Use
Next, a series of regression models were constructed to test the role of smoking urges, smoking cessation self-efficacy, and adaptive coping as predictors of cigarette use at post-treatment, and follow-up time points (6- and 12-month post-baseline). In step one of each model, baseline number of cigarettes per day was entered as a covariate, in addition to the baseline score on the predictor variable. Step two included the post-treatment of the predictor variable. A total of 9 models were conducted (three predictors × three time points). Model results are presented in Table 2.
Table 2. Predicting reductions in cigarettes per day (CPD).
|
|
||||||
|---|---|---|---|---|---|---|
| CPD at Post-treatment |
CPD at 6 months |
CPD at 12 months |
||||
|
|
||||||
| Beta | Sig. | Beta | Sig. | Beta | Sig. | |
| Post-TX Smoking Urges | .52 | <.01 | .36 | =.03 | .06 | =.67 |
| Post-TX Self-Efficacy | −.64 | <.01 | −.51 | <.01 | −.36 | =.02 |
| Post-TX Adaptive Coping | −.10 | =.59 | −.10 | =.57 | −.01 | =.94 |
Note: CPD = Cigarettes Per Day in the past-7 days; Post-TX = Post-Treatment; All models conducted controlling for baseline cigarettes per day and baseline scores on the predictor measure.
Results from regression analyses revealed that post-treatment smoking urges were significantly (positively) associated with cigarette use at post-treatment and 6-month timepoints, but the effect was non-significant at 12-months. Regarding smoking cessation self-efficacy, post-treatment smoking cessation self-efficacy was significantly negatively predictive of cigarette use at post-treatment and at 6- and 12-month follow-up. All models with adaptive coping were non-significant.
Analyses of Indirect Effects
A model was constructed to test whether smoking cessation self-efficacy was an indirect predictor of the cross-sectional association between post-treatment smoking urges and cigarette use. Analyses were conducted using PROCESS, a conditional process modeling macro that tests for both direct and indirect effects using an ordinary least squares-based path analytical framework. 36 The 95-percentile confidence intervals (CI) for beta indices were obtained analytically while bootstrapping with 5,000 resamples was used to estimate CIs for the indirect effects. 37–39
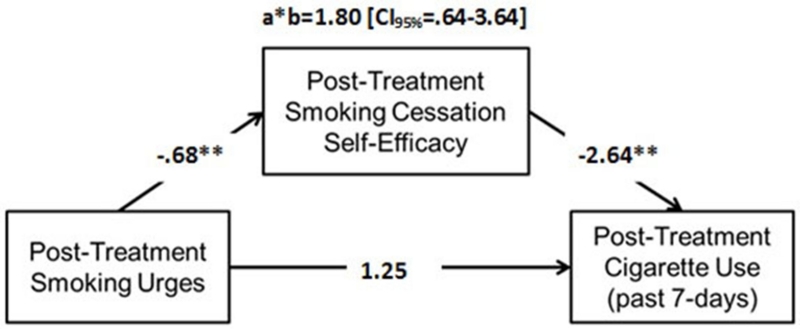
Figure 1 depicts results from indirect tests, utilizing baseline cigarette use, smoking cessation self-efficacy, and urge as covariates. Results indicated that there was a significant negative association between post-treatment smoking urges and smoking cessation self-efficacy (path a), and a significant negative association between smoking cessation self-efficacy and cigarette use (path b). There was a non-significant direct association between post-treatment smoking urges and cigarette use (path c). However, smoking urges were significantly associated with cigarette use indirectly via smoking cessation self-efficacy.
Figure 1.
Indirect Effects Model (n=39)
Figure 1 models the indirect effect of smoking urge to cigarette use through smoking cessation self-efficacy.
Note: a*b = indirect effect; Model covariates include baseline cigarette use, baseline smoking cessation self-efficacy, and baseline smoking urges
Discussion
The present study investigated the associations between smoking cessation self-efficacy, adaptive coping, smoking urge, and smoking outcomes among daily smokers undergoing a quit attempt who received a health-based intervention plus participated in eight, telephone-delivered cognitive-behavioral smoking cessation sessions. Results indicated that smoking cessation self-efficacy and adaptive coping significantly increased from baseline to post-treatment, while smoking urge decreased. In addition, increases in smoking cessation self-efficacy were associated with subsequent reductions in smoking at three follow-up periods, and reduction in urge predicted reductions in smoking at the post-treatment and 6-month timepoints. Indirect tests revealed that posttreatment urge was associated with use through smoking cessation self-efficacy. Overall, the findings support the importance of smoking cessation self-efficacy in predicting smoking outcomes and suggest avenues for future research.
Throughout the course of the smoking cessation treatment, participants in both conditions (i.e., exercise or health education) increased their self-efficacy for abstaining from smoking and adaptive coping strategies while decreasing smoking urge. Both conditions received the same smoking cessation treatment which was designed to help participants set incremental goals towards smoking abstinence and support participants through the course of their quit attempt. These sessions were individualized, focusing on the specific needs of the participant and included practicing coping strategies by reducing 1-2 cigarettes/day prior to the scheduled quit date. It is likely that participants developed a sense of mastery over their cigarette use, increasing their confidence in their ability to quit smoking. Results are consistent with theory 40 and are similar to other studies that have examined the role of abstinence self-efficacy in reducing rates of smoking. 21,22 Additionally, the present study found that the impact of smoking cessation self-efficacy was robust and persisted over the course of a long-term follow-up. Thus, initial gains in smoking cessation self-efficacy during the cessation treatment were related to beneficial smoking outcomes in the long-term.
The associations with adaptive coping and smoking outcomes were non-significant. Although adaptive coping increased from baseline to post-treatment, adaptive coping was not predictive of smoking outcomes. Specific coping strategies for high-risk smoking situations were not assessed: participants were asked broadly about the coping strategies that they utilize for stressful life situations. Thus, it is possible that, although participants were using these strategies, that they were not employing adaptive coping in smoking-specific situations. Future studies could examine this possibility by prompting participants to respond specifically regarding their coping strategies for smoking-related situations, such as urges and cravings to smoke. Further, it may be advantageous to study these constructs via ecological momentary assessment.
The relations between urges and smoking outcomes were significant via smoking cessation self-efficacy, even when controlling for baseline levels of the constructs. Previous studies have substantiated the role of smoking cessation self-efficacy as a mediator between urge and smoking outcomes, particularly among those with higher rates of negative affect. 23 In other work, postcessation self-efficacy has been shown to mediate the relationship between negative affect – which is commonly associated with urge to use – and outcomes. 20 Therefore, smoking cessation treatment may be especially efficacious by building smoking cessation self-efficacy for abstaining from use in times of high urge.
Several limitations should be noted. The study may have lacked power to detect significant findings due to the small sample size. Additionally, the sample was primarily Caucasian and female. All participants were sedentary smokers. Future studies should evaluate these relationships in a larger, more diverse sample.
Conclusions
Overall, this study provided promising support for the role of cognitive-behavioral smoking cessation treatment in increasing self-efficacy and adaptive coping while reducing urges to smoke. In particular, self-efficacy appears to be a robust factor that predicts long-term smoking outcomes in cessation treatment. Therefore, treatment initial treatment gains in self-efficacy can play an important role in sustained cessation effects.
Acknowledgments
This study utilizes data collected as part of a grant funded by the National Institute on Drug Abuse (K23 DA019950) awarded to Dr. Abrantes. Ms. Farris is supported by pre-doctoral National Research Service Award (F31-DA035564-03).
Footnotes
Declaration of Interests
The authors have no conflicts of interest to report.
References
- 1.US Department of Health and Human Services . The Health Consequences of Smoking - 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: 2014. [Google Scholar]
- 2.Centers for Disease Control and Prevention Current cigarette smoking among adults - United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(44):889–894. [PubMed] [Google Scholar]
- 3.Chiolero A, Wietlisbach V, Ruffieux C, Paccaud F, Cornuz J, doi:10.1016/j.ypmed.2006.01.011 Clustering of risk behaviors with cigarette consumption: A population-based survey. Prev Med (Baltim) 2006;42:348–353. doi: 10.1016/j.ypmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Fine LJ, Philogene GS, Gramling R, Coups EJ, Sinha S. Prevalence of multiple chronic disease risk factors: 2001 National Health Interview Survey. Am J Prev Med. 2004;27:18–24. doi: 10.1016/j.amepre.2004.04.017. doi:10.1016/j.amepre.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Lai S, Lai H, Page JB, McCoy CB. The association between cigarette smoking and drug abuse in the United States. J Addict Dis. 2000;19(4):11–24. doi: 10.1300/J069v19n04_02. doi:10.1300/J069v19n04_02. [DOI] [PubMed] [Google Scholar]
- 6.Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet. 1995;25(2):95–101. doi: 10.1007/BF02196920. doi:10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- 7.Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. doi: 10.1016/S0140-6736(09)60318-4. doi:10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piasecki TM. Relapse to smoking. Clin Psychol Rev. 2006;26(2):196–215. doi: 10.1016/j.cpr.2005.11.007. doi:10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Allen SS, Bade T, Hatsukami D, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine Tob Res. 2008;10(1):35–45. doi: 10.1080/14622200701705076. doi:10.1080/14622200701705076. [DOI] [PubMed] [Google Scholar]
- 10.Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. Guilford Press; New York: 1985. [Google Scholar]
- 11.Marlatt G, Donovan D. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. 2005. [Google Scholar]
- 12.Blevins CE, Stephens RS, Walker DD, Roffman RA. Situational determinants of use and treatment outcomes in marijuana dependent adults. Addict Behav. 2014;39:546–552. doi: 10.1016/j.addbeh.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larimer ME, Palmer RS, Marlatt GA. Relapse prevention: An overview of Marlatt’s Cognitive-Cehavioral Model. Alcohol Res Heal. 1999;23(2):151–160. doi:10.1186/1747-597X-6-17. [PMC free article] [PubMed] [Google Scholar]
- 14.Litt MD, Kadden RM, Cooney NL, Kabela E. Coping skills and treatment outcomes in cognitive-behavioral and interactional group therapy for alcoholism. J Consult Clin Psychol. 2003;71(1):118. doi: 10.1037//0022-006x.71.1.118. [DOI] [PubMed] [Google Scholar]
- 15.Litt MD, Kadden RM, Stephens RS. Coping and self-efficacy in marijuana treatment: Results from the Marijuana Treatment Project. J Consult Clin Psychol. 2005;73:1015–1025. doi: 10.1037/0022-006X.73.6.1015. [DOI] [PubMed] [Google Scholar]
- 16.Gwaltney CJ, Shiffman S, Balabanis MH, Paty J a. Dynamic Self-Efficacy and Outcome Expectancies: Prediction of Smoking Lapse and Relapse. J Abnorm Psychol. 2005;114(4):661–675. doi: 10.1037/0021-843X.114.4.661. doi:10.1037/0021-843X.114.4.661. [DOI] [PubMed] [Google Scholar]
- 17.Fiore MC, Jaén CR, Baker TB, et al. A Clinical Practice Guideline for Treating Tobacco Use and Dependence: 2008 Update. Am J Prev Med. 2008;35(May):158–176. doi: 10.1016/j.amepre.2008.04.009. doi:10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis J, Glaros A. Relapse Prevention and Smoking Cessation. Addict Behav. 1986;11:105–114. doi: 10.1016/0306-4603(86)90034-1. http://www.sciencedirect.com/science/article/pii/0306460386900341. [DOI] [PubMed] [Google Scholar]
- 19.Ratner P a., Johnson JL, Bottorff JL, Dahinten S, Hall W. Twelve-month follow-up of a smoking relapse prevention intervention for postpartum women. Addict Behav. 2000;25(1):81–92. doi: 10.1016/s0306-4603(99)00033-7. doi:10.1016/S0306-4603(99)00033-7. [DOI] [PubMed] [Google Scholar]
- 20.Cinciripini PM, Wetter DW, Fouladi RT, et al. The effects of depressed mood on smoking cessation: Mediation by postcessation self-efficacy. J Consult Clin Psychol. 2003;71(2):292–301. doi: 10.1037/0022-006x.71.2.292. doi:10.1037/0022-006X.71.2.292. [DOI] [PubMed] [Google Scholar]
- 21.Gwaltney CJ, Metrik J, Kahler CW, Shiffman S. Self-efficacy and smoking cessation: A meta-analysis. Psychol Addict Behav. 2009;23(1):56–66. doi: 10.1037/a0013529. doi:10.1037/a0013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldwin AS, Rothman AJ, Hertel AW, et al. Specifying the determinants of the initiation and maintenance of behavior change: An examination of self-efficacy, satisfaction, and smoking cessation. Heal Psychol. 2006;25(5):626–634. doi: 10.1037/0278-6133.25.5.626. doi:10.1037/0278-6133.25.5.626. [DOI] [PubMed] [Google Scholar]
- 23.Berndt NC, Hayes AF, Verboon P, Lechner L, Bolman C, De Vries H. Self-Efficacy Mediates the Impact of Craving on Smoking Abstinence in Low to Moderately Anxious Patients: Results of a Moderated Mediation Approach. Psychol Addict Behav. 2013;27(1):113–124. doi: 10.1037/a0028737. doi:10.1037/a0028737. [DOI] [PubMed] [Google Scholar]
- 24.Matheny KB, Weatherman KE. [Accessed January 11, 2016];Predictors of smoking cessation and maintenance. J Clin Psychol. 1998 54(2):223–235. doi: 10.1002/(sici)1097-4679(199802)54:2<223::aid-jclp12>3.0.co;2-l. http://www.ncbi.nlm.nih.gov/pubmed/9467767. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor RM, Stewart SH. Substance use disorders. In: McKay D, Abramowitz JS, Taylor S, editors. Cognitive-Behavioral Therapy for Refractory Cases: Turning Failure into Success. Washington DC: 2010. pp. 211–229. [Google Scholar]
- 26.Cohen S, Gottlieb BH, Underwood LG. Social relationships and health. In: Cohen S, Underwood LG, Gottlieb BH, editors. Social Support Measurement and Intervention. Oxford University Press; Oxford: 2000. pp. 3–25. [Google Scholar]
- 27.Abrantes AM, Bloom EL, Strong DR, et al. A Preliminary Randomized Controlled Trial of a Behavioral Exercise Intervention for Smoking Cessation. Nicotine Tob Res. 2014;16(8):1094–1103. doi: 10.1093/ntr/ntu036. doi:10.1093/ntr/ntu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown RA, Kahler CW, Niaura R, et al. [Accessed February 10, 2016];Cognitive-behavioral treatment for depression in smoking cessation. J Consult Clin Psychol. 2001 69(3):471–480. doi: 10.1037//0022-006x.69.3.471. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1832078&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown RA, Reed KMP, Bloom EL, et al. Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine Tob Res. 2013;15(12):2005–2015. doi: 10.1093/ntr/ntt093. doi:10.1093/ntr/ntt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown RA, Niaura R, Lloyd-Richardson EE, et al. Bupropion and cognitive-behavioral treatment for depression in smoking cessation. Nicotine Tob Res. 2007;9(7):721–730. doi: 10.1080/14622200701416955. doi:10.1080/14622200701416955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown RA, Abrantes AM, Strong DR, et al. Efficacy of sequential use of fluoxetine for smoking cessation in elevated depressive symptom smokers. Nicotine Tob Res. 2014;16(2):197–207. doi: 10.1093/ntr/ntt134. doi:10.1093/ntr/ntt134. [DOI] [PubMed] [Google Scholar]
- 32.Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12:101–112. [Google Scholar]
- 33.Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. [Accessed February 18, 2016];Relapse situations and self-efficacy: an integrative model. Addict Behav. 1990 15(3):271–283. doi: 10.1016/0306-4603(90)90070-e. http://www.ncbi.nlm.nih.gov/pubmed/2378287. [DOI] [PubMed] [Google Scholar]
- 34.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Addiction. 1991;86(11):1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. doi:10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 35.Carver CS. You want to Measure Coping But Your Protocol’s Too Long: Consider the Brief COPE. Int J Behav Med. 1997;4(1):92. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 36.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY, US: 2013. [Google Scholar]
- 37.Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Commun Monogr. 2009;76(4):408–420. doi:10.1080/03637750903310360. [Google Scholar]
- 38.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods, Instruments, Comput A J Psychon Soc Inc. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 39.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 40.Bandura . In: Self-Efficacy: The Exercise of Control. Company WHF, editor. New York: 1997. [Google Scholar]