Abstract
In patients with systemic sclerosis (SSc), gastrointestinal (GI) tract involvement is almost universal. Any segment of the GI tract from mouth to anus can be involved, and GI symptoms are a frequent cause of morbidity. In severe cases, GI tract involvement can progress to the point of malnutrition requiring parenteral nutrition. GI tract involvement in SSc contributes to disease-related mortality although mostly as a co-morbidity rather than direct cause of death. The review is intended to help address challenges in the assessment and treatment of GI tract involvement in SSc.
Keywords: systemic sclerosis, scleroderma, gastrointestinal, motility
Introduction
Systemic sclerosis (SSc) is a multisystem autoimmune disorder characterized by immune activation, vasculopathy, and abnormal collagen deposition in the skin and various internal organs. The gastrointestinal (GI) tract is involved in sine, limited cutaneous (lc) and diffuse cutaneous (dc) forms of the disease. In fact, the GI tract is one of the most commonly affected internal organ systems, involved in approximately 90% of patients with SSc (1-3). GI tract involvement is manifested primarily as dysmotility, though mucosal vascular malformations are also seen. The pathogenesis of dysmotility is related to a progression of myopathy, neuropathy and fibrosis leading to abnormalities in compliance and contractility of the GI tract wall. Any segment of the GI tract from the mouth to the anus may be affected although the esophagus and anorectum are most frequently involved. GI tract involvement is associated with a variety of morbid symptoms including dysphagia, heartburn, distention, bloating, abdominal pain, nausea, vomiting, diarrhea, constipation and fecal incontinence. Local complications can develop as a result of dysmotility including reflux esophagitis, small intestinal bacterial overgrowth and, rarely, megacolon. For a variety of reasons, malnutrition can develop, and this is the leading cause of mortality attributed to GI tract involvement. Acute GI complications such as mechanical or pseudo-obstruction can be life-threatening in the context of other organ based involvement, especially cardiorespiratory disease. Treatment of GI tract involvement is meant to lessen symptoms, prevent local complications, and maintain adequate nutrition (see Table I for common medications used to treat GI manifestations of GERD). Additionally, GI tract involvement is associated with depressed mood and lower quality of life, so a comprehensive treatment plan should also take this into account (4, 5).
Table I.
Medications for the treatment of gastrointestinal manifestations in systemic sclerosis
| Manifestation | Medication class | Medication example | Dosing information |
|---|---|---|---|
| GERD | proton-pump inhibitor | omeprazole | 20-40 mg 1-2 times daily 30-60 min before a meal |
| histamine type-2 receptor antagonist |
ranitidine | 150 mg 1-2 times or 300 mg once daily |
|
| GABA-B agonist | baclofen | 5-10 mg up to 3 times daily |
|
| Gastroparesis | dopamine receptor antagonist |
metoclopramide | 10 mg up to 4 times daily |
| domperidone | 10 mg up to 4 times daily |
||
| motilin receptor agonist | erythromycin | 250-500 mg 3 times daily up to 4 weeks |
|
| Small intestinal pseudo- obstruction |
somatostatin analogue | octreotide | 50-100 mcg SC nightly or 3 times daily |
| Small intestinal bacterial overgrowth |
antibiotics | rifaximin | 550 mg 2 times daily for 10-14 days |
| metronidazole | 500 mg 2 times daily for 10-14 days |
||
| Constipation | stool softener (hyperosmolar) |
polyethylene glycol | 17 gm daily, then titrated to effect |
| stimulant laxative | bisacodyl | 5-15 mg daily | |
| secretagogue | linaclotide | 145 mcg daily | |
| Colonic pseudo- obstruction |
cholinesterase inhibitor | neostigmine | 2 mg IV over 3-5 minutes |
Pathogenesis
SSc is a multisystem disease with shared pathobiological mechanisms that center on microvascular damage, structural vasculopathy with adventitial and intimal fibrosis and proliferative change and increase in interstitial extracellular matrix (6). In addition, there can be significant epithelial pathology that is especially relevant to the function and architecture of the GI tract. The innate and adaptive immune systems undoubtedly play a role in the development and progression of SSc in the skin and lung but the precise contribution of immunopathology in the GI tract is less clear. However, some reports suggest benefit from immunomodulatory strategies for GI manifestations including gastric antral vascular ectasia (GAVE) (7), and also histological examination has highlighted the presence of markers of immune activation and immuno-inflammation with lymphocytic infiltrates (8). Clinical benefit from long term intravenous immunoglobulin (IVIG) in some cases of GI scleroderma, especially those with overlap myositis also points toward immunopathogenesis (9). Animal models have begun to shed light on the pathogenesis of GI scleroderma and confirm that many of the structural or motility abnormalities may be the result of fibroblast dysfunction associated with overactivity of TGFbeta and related fibrogenic pathways (10). Finally, since the clinical impact of SSc centers on motility disturbance and SSc is associated with other features of autonomic dysfunction such as Raynaud’s phenomenon, it is plausible that intestinal neuropathology plays a part in pathogenesis and may occur as a primary event or secondary to vasculopathy and fibrosis as outlined above. Altered neural function appears to correlate with symptoms of lower bowel disease, especially anorectal incontinence (11).
Esophagus
Esophageal dysmotility
Esophageal dysmotility in SSc is characterized by decreased amplitude of esophageal peristalsis and decreased lower esophageal sphincter (LES) pressure (Figure 1). Patients typically report difficulty swallowing solids and liquids, and they may also complain of a sensation of something stuck in the throat, heartburn, reflux, chest discomfort or pain with swallowing.
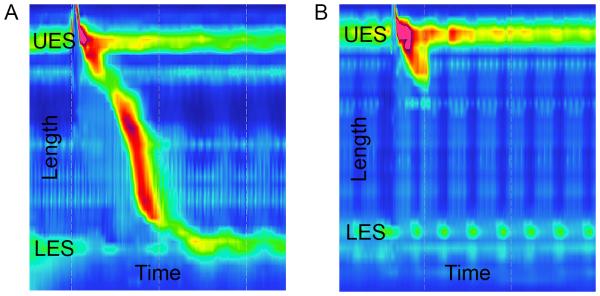
Figure 1.
Esophageal manometry images for a normal control patient (A) and a patient with systemic sclerosis (B) are shown. The y-axis shows the length measured in the esophagus, including the location of the upper esophageal sphincter (UES) and lower esophageal sphincter (LES). The x-axis shows the time of the recording that captured one swallow. In (A), the diagonal region with topographical color change shows a normal swallow with distal propagation of esophageal peristalsis with time and corresponding LES relaxation. In (B), esophageal peristalsis is notably absent from the distal two-thirds of the esophagus, and resting LES pressure is low.
Esophageal involvement is very common in SSc. In an autopsy case series of greater than 50 SSc patients published in 1969, atrophy or fibrosis was detected in the esophagus in 74% (12). When all cases of SSc seen at the Cleveland Clinic between 1952 and 1969 were reviewed, abnormalities were noted in 53% of the 233 patients who underwent barium esophagram (13). Only 3.2% had esophageal stricture on esophagram, and all of these cases also had esophagitis on endoscopy. While the vast majority of patients in this series with dysphagia had abnormal esophagrams, 25% of patients without esophageal symptoms also had abnormal studies. Prospective studies have reported abnormalities on esophageal manometry in about 70-75% of SSc patients (14-16).
Clinicians should have a high degree of suspicion for esophageal dysmotility due to the high prevalence of this condition in SSc. Upper endoscopy is the appropriate initial test to evaluate dysphagia, able to identify evidence of gastroesophageal reflux, other structural lesions, and various forms of esophagitis. The confirmatory test for esophageal dysmotility due to SSc is esophageal manometry that demonstrates diminished or absent peristalsis of the distal two-thirds of the esophagus along with a hypotensive lower esophageal sphincter (Figure 1). A barium esophagram is a relatively low risk test that is complementary to manometry and endoscopy in the identification of dysmotility and structural lesions, respectively, and it may also demonstrate reflux.
In general, the treatment of esophageal dysmotility is supportive, and patients are advised to take small bites, cut/chew food well, avoid dry or fibrous foods and take plenty of water with solid foods. Promotility agents are not particularly effective in this case, so they are not recommended for esophageal dysmotility. While cisapride did increase LES pressure and the frequency of gastric contractions compared to placebo in randomized, double-blinded, placebo-controlled, cross-over trial involving 20 patients (17), another small, randomized, double-blinded study, showed that cisapride compared to placebo was not effective in enhancing esophageal motility (18). Baclofen, a drug that reduces the incidence of transient LES relaxation, was shown to improve GERD in the short-term in the general population according to a recent meta-analysis (19), but there are no published reports in SSc patients., and in the context of a pathologically hypotensive LES, may not be helpful.
Complications related to esophageal dysmotility generally are attributed to gastroesophageal reflux as detailed below. There is a risk of aspiration with severe dysmotility and stasis of food and liquid in the esophagus.
Gastroesophageal reflux disease
Gastroesophageal reflux disease (GERD) refers to symptoms or complications related to the retrograde movement of acid and contents from the stomach into the esophagus. This is very common in SSc due to hypotensive LES, dysmotility of stomach and small intestine, and poor clearance of refluxed material due to esophageal dysmotility. Patients may complain of classic heartburn and reflux, and they may also report dysphagia, odynophagia, laryngitis, chronic cough, hoarseness, or asthma.
In a study of 90 patients from the German network for Systemic Sclerosis, about 70% reported heartburn during 1 year of follow-up.(3) In another study of more than 40 SSc patients, esophageal symptoms were present in 70%, abnormal reflux on 24-hour pH probe test was measured in 80%, and reflux esophagitis on endoscopy was seen in 33% (14).
In patients who complain only of heartburn or reflux without red flag symptoms, GERD can be diagnosed and treated empirically. Upper endoscopy is routinely employed to evaluate red flag symptoms or refractory GERD or to evaluate for alternative diagnoses. As noted above, ambulatory 24-hour pH probe test with or without impedance testing can clearly demonstrate gastroesophageal acid or non-acid reflux if present but this test is only required in resistant cases.
A systematic review from 2006 described the treatment of GERD in SSc patients with proton pump inhibitor (PPI) in 9 studies that each contained between 3 and 25 patients (20). Most studies were relatively short duration, and PPI benefit was uniformly noted though the rate of success dropped off in longer-term studies. In a small, randomized, double-blinded, placebo-controlled study lansoprazole 30 mg daily compared to placebo improved self-reported GI symptoms at 6 months, but the effect was lost by 12 months, and there was no difference in esophageal dysmotility (21). Promotility agents can be tried in addition to PPI in refractory cases, where the promotility agent presumably improves gastric emptying and may improve LES pressure or esophageal motility. In SSc patients partially responsive to twice daily omeprazole, the addition of domperidone or algycon was tested in a randomized, placebo-controlled trial with nearly 80 patients. Both medications led to improvements, and there was no difference in terms of the benefit provided by domperidone or algycon (22).
PPIs are cornerstone for management of GERD in SSc. Retrospective analyses of large claims databases or single center analyses have suggested a relationship between long-term PPI use and pneumonia (23), Clostridium difficile infection (24), cardiovascular events in those taking clopidogrel (25), osteoporosis (26), chronic kidney disease (27), and dementia (28). While these studies may support an effect in a large population, the effect on the individual level is unknown and no information is provided about SSc. The risks of unopposed acid reflux in SSc patients must be weighed also, such as heartburn, dysphagia, esophageal stricture and esophageal adenocarcinoma.
Complications of GERD are related primarily to inflammation, including esophagitis (Figure 2) and esophageal stricture, and to dysplasia, including Barrett’s esophagus and esophageal adenocarcinoma. Medical therapies are meant to limit acid-induced injury and prevent complications. Endoscopic dilation can be used to treat strictures. Barrett’s esophagus is monitored with endoscopy and biopsies in the earlier stages, and evolving endoscopic ablative and resection techniques mean the majority of dysplastic cases can be treated endoscopically. Also, some have noted a link between GERD and interstitial lung disease, possibly due to recurrent aspiration, and advocated for early and aggressive therapy for GERD, though a causal relationship has not been established (29-33).
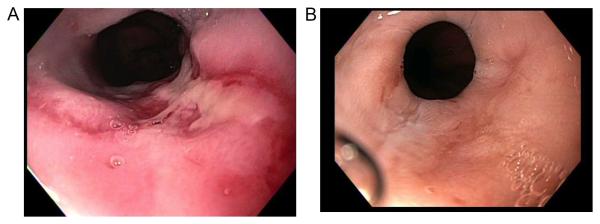
Figure 2.
Endoscopic images of the distal esophagus are shown from the same patient with systemic sclerosis depicting grade C esophagitis (A) and healed esophagitis (B) after several weeks of proton-pump inhibitor therapy.
Stomach
Gastroparesis
The most common form of gastric dysmotility encountered is gastroparesis, or delayed gastric emptying. Poor gastric accommodation and irregular gastric contractions, or dysrhythmias, are also reported. The symptoms of gastroparesis include early satiety, postprandial fullness, bloating, distention, abdominal pain, nausea and vomiting. Gastric dysrhythmias as measured by surface electrogastrography were positively correlated with the presence of patient-reported upper GI symptoms, including nausea, bloating and pain (34).
Gastroparesis was reported in 38-50% of SSc patients in 2 small studies (16, 35), and impairment of gastric myoelectric activity measured by electrogastrography was found in about 80% (35).
In patients with symptoms compatible with gastroparesis, upper endoscopy is used to rule out gastric outlet obstruction and alternative diagnoses, such as Helicobacter pylori infection. Other potential causes of delayed gastric emptying such as narcotic use or diabetes should be considered. Gastroparesis can be identified with a scintigraphic gastric emptying test, with some centers with expertise using other techniques such as ultrasound. Electrogastrography to measure myoelectric activity is not routinely used.
Dietary modifications and prokinetic agents are typical first-line therapies for gastroparesis. Diet modifications include eating smaller meals more frequently, not eating for several hours before lying down, and avoiding high fiber and high fat foods. The three agents most commonly employed can improve symptoms in the short term, but each has drawbacks as metoclopramide has a black box warning for tardive dyskinesia, domperidone is not approved for use in the US and has limited availability in the UK and has several significant side effects, and erythromycin is subject to tachyphylaxis that limits long-term use. In a small randomized, double-blinded, placebo-controlled trial, gastric emptying time was significantly reduced with administration of ghrelin compared to saline (36). Ghrelin is a natural ligand of the motilin family and still an investigational therapy. While cisapride improved gastric emptying and GI symptoms, but not esophageal motility, in a small study of SSc patients(37), it is not available due to cardiac toxicity.
Complications due to gastroparesis can arise due to severe symptoms. In the short term, intractable nausea and vomiting can lead to dehydration and electrolyte abnormalities requiring hospitalization. In the long term, a small intestinal feeding tube and a gastric decompression tube can be considered, but this option is more challenging in SSc where downstream dysmotility may limit tolerance of enteral feeding.
Gastric antral vascular ectasia
Gastric antral vascular ectasia (GAVE), also referred to as watermelon stomach based on its characteristic appearance, is a condition of arteriovenous malformations in the mucosa of that gastric antrum. These lesions can bleed and result in iron-deficiency anemia or overt GI bleeding. Punctate arteriovenous malformations can occur elsewhere in the intestinal tract as well.
In 50 patients with SSc, video capsule endoscopy found GAVE in 35%, gastrointestinal telangiectasia in 27%, and gastrointestinal angiodysplasia in 39% (38). In contrast, a case-control study using a European database estimated the prevalence of GAVE in SSc at only 1% (39). While most forms of GI involvement in SSc are evenly distributed between disease types, vascular mucosal lesions were significantly associated with lcSSc, digital ulcers, higher score of nailfold videocapillaroscopy, and anti-centromere antibody (38).
Upper endoscopy by experienced gastroenterologists should be pursued in SSc patients with unexplained iron-deficiency anemia or melena to evaluate for GAVE as mild cases may be frequently missed.
Treatment of GAVE relies on endoscopic techniques, most commonly argon plasma coagulation, to destroy the abnormal tissue (Figure 3). This is often successful, but may require multiple treatments. Blood or iron transfusions may be indicated during the course of management, and surgery is reserved for refractory cases. New endoscopic techniques such as radiofrequency ablation are currently undergoing assessment.
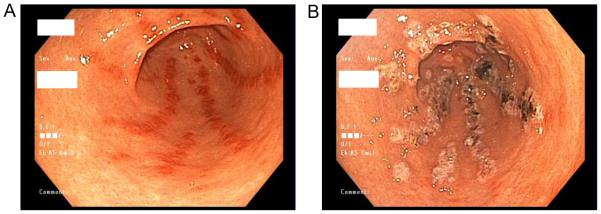
Figure 3.
Endoscopic images from a patient with systemic sclerosis depicting gastric antral vascular ectasia before (A) and after (B) argon plasma coagulation therapy.
Small intestine
Pseudo-obstruction
Small intestinal dysmotility leading to stasis of intestinal contents and dilation is referred to as pseudo-obstruction. A number of studies have reported decreased small intestinal motility with myopathic and neuropathic features in those with SSc (40-42), but serum peptides associated with motility were not different from controls as reported in one study (42). Patients typically complain of bloating, distention, abdominal pain, nausea, and/or vomiting. Some may develop decreased or absent passage of stool and flatus.
Small intestinal dysmotility seems to be quite common in patients with SSc. In an autopsy case series of greater than 50 patients published in 1969, atrophy or fibrosis was detected in the small intestine in 48% (12). Of the 364 cases of SSc seen in the Cleveland Clinic between 1952 and 1969 only 36 patients had small bowel imaging, but 42% of these examinations were abnormal, most commonly with dilated duodenum (13). Several studies have reported a significant increase in the orocecal transit time in SSc patients versus controls as measured by the lactulose breath test, essentially a surrogate for small intestinal transit (16, 43).
The diagnosis of small intestinal pseudo-obstruction is confirmed with cross-sectional imaging that shows small intestinal dilation in the absence of a transition point that would indicate a mechanical obstruction. In addition, cross-sectional imaging in SSc does have some notable findings, including the “hide-bound” sign (increased number and crowded jejunal folds despite dilation), sacculations (outpouchings similar to diverticula), and benign pneumatosis intestinalis (discussed further below) (44). Manometry or transit studies are typically not required.
Treatment of pseudo-obstruction depends on the acuity of the presentation and the severity of the symptoms. The acute onset of severe symptoms requires inpatient management, including bowel rest, intravenous hydration, correction of electrolyte abnormalities, and, oftentimes, nasogastric decompression. Prokinetics to stimulate motility and broad-spectrum antibiotics to decrease the intestinal bacterial load are also employed routinely. In a small study of SSc patients symptomatic with small intestinal involvement but without SIBO, ocreotide provided significant relief from GI symptoms (composite score based on abdominal pain, nausea, vomiting, and bloating) and disturbed defecation (45). In another small study, octreotide improved intestinal motility patterns and reduced the breath hydrogen scores, a marker of bacterial load (46). Daily octreotide at bedtime or a monthly depot form of octreotide can be used to treat chronic pseudo-obstruction in those responsive to prokinetics.
Small intestinal dysmotility may be complicated by bacterial overgrowth and malnutrition. Initially, this may be successfully treated with antibiotics as discussed below. In cases of refractory oral alimentation, SSc patients may benefit from a distal enteral feeding tube and a proximal decompression tube. If this approach is not effective, parenteral nutrition can be considered. Surgery should be avoided, but may be indicated in the event of bowel wall necrosis or perforation. It is important to distinguish true bowel wall necrosis from benign pneumatosis intestinalis, a finding of air within the bowel wall associated with a benign abdominal exam and stable vital signs. Benign pneumatosis intestinalis and benign pneumoperitoneum may be seen in SSc and should be treated conservatively (47, 48). Rarely, affected short segments of bowel, such as symptomatic megaduodenum refractory to conservative therapies, have been treated successfully with surgery (49).
Small intestinal bacterial overgrowth
The bacterial load of the small intestine is generally many magnitudes lower than that seen in the large intestine. This relative difference in bacterial abundance is maintained by multiple factors, but regular small intestinal motility is a key component. Thus, patients with SSc are at risk for small intestinal bacterial overgrowth (SIBO) due to dysmotility. Additionally, small bowel diverticula and, possibly, PPI use also may increase the risk in this population. SIBO likely causes symptoms in some because it interferes with the normal digestive and absorptive processes of the small intestine. Symptoms may include bloating, distention, discomfort, flatulence and diarrhea. Malnutrition and weight loss are severe complications of SIBO.
In several published studies that evaluated consecutive patients with SSc, the prevalence of SIBO was reported as 12-55% (16, 43, 50-52). In reviewing published studies that reported the results of testing for and treating SIBO in SSc, the prevalence was not higher among symptomatic patients (52, 53) than it was among consecutive patients who were asymptomatic (41, 43, 51). A significant challenge in this area is the lack of a true gold-standard test for the diagnosis of SIBO. The most long-standing test is quantitative in vitro growth of a jejunal aspirate collected during upper endoscopy, but this test is limited by the inability to culture all bacterial species and the risk of contamination during the collection procedure. Glucose or lactulose hydrogen breath tests (HBT) rely on bacterial metabolism of ingested substrates that are measured non-invasively in the exhalant. All of these tests require a threshold value for diagnosis to be designated along a spectrum of possible results. In a recent systematic review of the literature for published articles describing the diagnosis of SIBO in SSc, the glucose HBT was the only fully validated test, whereas jejunal culture, xylose and lactulose HBT, and 72 hour fecal fat test were partially validated (54). In practice, it seems reasonable to empirically treat SSc patients for SIBO if there is suspicion as the pre-test probability is quite high and the diagnostic accuracy of available tests is somewhat suspect.
Antibiotics are the mainstay of therapy for SIBO. There is little in the way of direct evidence to recommend one antibiotic over another, but a variety of antibiotics with activity against GI bacteria seem to be relatively effective. These antibiotics include rifaximin, ciprofloxacin, and metronidazole, among others. Rifaximin and metronidazole, in particular, are attractive options as they do not seem to confer risk for Clostridium difficile infection as much as other antibiotics. In 5 separate published reports on the treatment of SIBO with antibiotics in a total of 292 SSc patients, the combined mean rate of eradication was 55% (41, 43, 51-53). It should be noted that there was considerable heterogeneity among these studies in terms of the diagnostic tests and antibiotics employed. A general approach is to treat with one antibiotic for 10-14 days. If the initial antibiotic is ineffective, a second antibiotic can be tried. If patients exhibit a good response to antibiotics, then another course can be given in the future if symptoms return. If symptoms return soon after therapy, a rotating course of antibiotics can be used, where patients are treated for 10-14 days with one antibiotic in one month and then another antibiotic in the next month, and vice versa. In addition to antibiotics, small studies have reported some benefit from octreotide and probiotics for the treatment of SIBO in SSc (55, 56).
As discussed above, refractory SIBO is seen in conjunction with severe dysmotility, and this condition can then be complicated by malnutrition and intestinal failure. In these difficult cases, rotating antibiotics plus prokinetic agents with or without probiotics are often combined prior to considering parenteral nutrition. In select cases, slow and constant jejunal tube feeding with gastroduodenal venting as needed can prevent the need for the parenteral route.
Diarrhea
Diarrhea is a symptom of a number of etiologies in patients with SSc as discussed throughout this review, including SIBO, malabsorption, overflow diarrhea due to fecal impaction, and medications. In a study of 90 patients from the German network for systemic sclerosis, nearly 80% of patients had diarrhea during 1 year of follow-up (3). In a systematic review that commented on the adverse effects of methotrexate use in scleroderma, diarrhea was reported in 14% (57). Also, PPI and antibiotics are frequently reported to cause diarrhea.
Colon and anorectum
Slow transit constipation
In SSc, constipation is often the result of slow colonic transit due to dysmotility, functional anorectal outlet obstruction due to neuropathy and myopathy, or adverse effects of medications. In rare cases, symptoms of constipation actually may develop from mechanical obstruction. Patients may report a variety of symptoms that they attribute to constipation, including infrequent bowel movements, hard stools, straining, a sense of incomplete evacuation, bloating, distention, discomfort or a need for manual maneuvers.
In a study involving 402 consecutive SSc patients in the UK who completed questionnaires during outpatient visits, about 50% reported constipation (1). Constipation was reported by about 65% of the 90 SSc patients surveyed from the German network for Systemic Sclerosis (3). In an autopsy case series of greater than 50 SSc patients published in 1969, atrophy or fibrosis was detected in the large intestine in 39% (12). In two separate studies, abnormal colon transit was reported in about 40% of consecutive SSc patients (58) and in a little over 50% of SSc patients with GI symptoms (40). On imaging tests, particularly on barium enema, pathognomonic large-mouthed diverticula on the antimesenteric border of the colon may be seen, but this seems to be a relatively rare finding (13, 59).
Empiric treatment with stool softeners and stimulant laxatives is reasonable in the SSc patient with constipation as long as alarm features are absent. If colonoscopy is indicated for colorectal cancer screening based on age (50+) or family history, this should be pursued initially. Colonoscopy also is an appropriate test for those who are refractory to medical therapy or in the face of alarm features such as rectal bleeding. Abdominal radiographs or cross-sectional imaging are used in the evaluation of those with acute onset of symptoms, significant pain, or other signs or symptoms of obstruction, and barium enema can also be employed to assess for structural problems. As discussed below, anorectal manometry should be utilized in those suspected of having functional anorectal outlet problems. If questions linger regarding the etiology of symptoms, a stool transit study can be used in certain cases, although this is generally not required. Also, it is important to consider the possible contribution of medications to constipation. For instance, narcotics and calcium-channel blockers that are used in SSc patients are known to contribute to constipation (60, 61).
Initial treatments for constipation in SSc are similar to those recommended for the general population. Supplemental fiber is a reasonable first option in mild cases, though resulting bloating and flatulence may not be well tolerated. In more severe cases, supplemental fiber may be avoided due to concerns that it may cause impaction. Stool softeners, including polyethylene glycol and lactulose, are given to promote soft stool. Stimulant laxatives, including senna and bisacodyl, are used to promote a bowel movement and may be helpful particularly in the setting of early dysmotility. Oftentimes, a standing dose of a stool softener combined with an as needed stimulant laxative will be effective. Several newer enterokinetic agents and intestinal secretagogues are demonstrated to be effective in those with refractory constipation (62), but these medications have not been specifically tested in SSc. Several prokinetic agents have shown benefit in small, uncontrolled trials, but these medications are not routinely used in clinical practice. Cisapride, a 5HT-4 agonist, improved colonic transit as measured by radionuclide test in patients with scleroderma, and 8 of 12 reported improvement in clinical symptoms of constipation (63). Another 5HT-4 agonist, prucalopride, was reported to be effective for the treatment of slow transit constipation in 2 SSc patients who were refractory to laxatives and other prokinetic agents (64).
A severe manifestation of colonic dysmotility, acute colonic pseudo-obstruction is an urgent medical problem that requires in-hospital care. Imaging is required to rule-out mechanical obstruction given the risk for perforation. Patients are treated with bowel rest, intravenous fluids, and correction of electrolyte imbalances. If enemas or manual disimpaction are ineffective, then neostigmine, a cholinesterase inhibitor that promotes colonic motility, can be safe and effective for treatment (65). Rarely, colonic pseudo-obstruction can progress to megacolon requiring urgent surgical resection to prevent perforation (66). Elective segmental surgical resection may be a good option for colon inertia related to segmental involvement, particularly when small intestinal motility and anorectal function are relatively normal (67). Other severe complications reported in SSc patients include stenosis, volvulus, and stercoral ulceration (68-70).
Anorectal dysfunction
Coordinated neuromuscular control of the anorectum is critical for defecation and the maintenance of continence, and this region is commonly affected in SSc. Patients may complain of constipation, a sensation of incomplete emptying, the need to use manual pressure to aid in defecating, or fecal incontinence. Overflow diarrhea may also occur where liquid stool passes around a solid stool impacted in the rectum. It is important to note that 1) patients will often not volunteer a personal history of fecal incontinence, and 2) fecal incontinence is more strongly associated with depressed mood than any of the other GI symptoms commonly reported (5). So, the astute clinical will ask questions to uncover this condition in SSc patients.
As in other locations along the GI tract, neuropathy and myopathy combine to impact function. As discussed further below, SSc patients may show a characteristic pattern of diminished or absent rectoanal inhibitory reflex along with reduced anal sphincter resting and squeeze pressures associated with thinning of predominantly the internal anal sphincter muscles (71). Interestingly, a few studies have reported that SSc-related autoantibodies may be directly involved in this disease manifestation. IgG antibodies from symptomatic patients with SSc, but not controls, attenuated bethenacol-stimulated M(3)-muscarinic receptor-mediated activation of internal anal sphincter muscle tissue (72). Pooled IgG from healthy controls when mixed with IgG from SSc patients prevented the subsequent inhibition of M(3)-R activation mediated by IgG from SSc patients (73).
In a group of 90 patients with a diagnosis of SSc presenting for outpatient visits in Germany between 2005 and 2007, a little over 20% reported fecal incontinence on a GI symptoms questionnaire (3). In another study involving 83 consecutive patients with SSc, 18% regularly needed digital stimulation or evacuation of the rectum and 38% had fecal incontinence (74). In a study of 160 female patients with SSc, fecal incontinence was present in 40% and associated with a lower quality of life survey score (75). Physiological testing suggested that some degree of anorectal abnormality may be present in the majority of SSc patients who did not report incontinence as well (76).
In the appropriate setting, SSc patients with lower GI symptoms should be evaluated with colonoscopy, including those with a need for colorectal cancer screening based on age (50+) or family history, rectal bleeding, anemia, or, in some cases, pain and/or diarrhea. Imaging studies are employed when there is a need to evaluate for mechanical obstruction. In those SSc patients where functional anorectal problems are suspected, anorectal manometry with pudendal nerve latency testing is the confirmatory test, and characteristic findings in SSc include lower anal sphincter resting and squeezing pressures and diminished or absent rectoanal inhibitory reflex (76-80). Anorectal ultrasound or pelvic MRI can be used to further assess the soft tissue structures, but these tests are not routinely indicated. In a study comparing SSc patients with incontinence, SSc patients without incontinence, and control patients with incontinence, it is interesting that SSc patients with incontinence had higher anal pressures than control patients with incontinence, and SSc patients with incontinence were much more likely than controls or SSc patients without incontinence to exhibit diminished rectal sensation and abnormal rectoanal inhibitory reflex (11). These data suggest that neuropathic mechanisms contribute more to incontinence in SSc patients than in control patients where myopathy is primarily responsible, but it is noted that myopathic mechanisms contribute in SSc as well. Internal anal sphincter atrophy was present in SSc patients with incontinence but absent in control patients with incontinence, where the external anal sphincter predominantly was more affected (11, 81). In addition, imaging modalities have consistently found atrophy of the internal anal sphincter in SSc patients (11, 76, 77, 81).
The treatment of anorectal disorders can be quite challenging. Conservative measures to treat either liquid or hard stool constitute the first line of therapy, including the use of supplemental fiber or other stool softeners to promote soft stool. In refractory cases, sacral nerve stimulator or other surgical interventions can be considered. In one study where 4 of 5 women with SSC and incontinence improved with temporary sacral nerve stimulators, permanent stimulator placement reduced incontinence and urgency, improved quality of life, particularly social functioning, increased resting and squeezing anorectal pressures, and enhanced rectal sensitivity, all without any reported complications (82). However, another study reported that 9 of 10 female SSc patients with passive fecal incontinence, internal anal sphincter atrophy and reduced resting pressure did not see a reduction in symptoms after treatment with a sacral nerve stimulator (83). Surgical intervention also may be required in cases complicated by rectal prolapse (84).
Patient-reported symptoms tools
As the vast majority of patients with SSc have GI tract involvement and most have multiple affected GI organs often with overlapping symptoms, it is very challenging to quantify GI tract symptoms in SSc patients. Furthermore, the magnitude of GI tract symptoms may be poorly correlated with objective findings of disease involvement (35). So, there is a need for a validated patient-reported instrument to assess GI tract symptoms in SSc.
The University of California Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 (UCLA SCTC-GIT 2.0) was developed, refined and validated for the assessment of symptoms across 7 multi-item scales (reflux, distention/bloating, diarrhea, fecal soilage, constipation, emotional well-being and social functioning) in patients with SSc and is now available in multiple languages [contact the corresponding author Dinesh Khanna at khannad@umich.edu for further information] (85). It can aid in the clinical care with helping with diagnosing and assessing severity of GI symptoms. Subsequently, minimally important differences estimates for the UCLA SSC-GIT 2.0 were reported that can be used to interpret symptoms scores in clinical trials (86).
Acknowledgments
Dr. Khanna is supported by NIH//NIAMS K24 AR063120
Abbreviations
- SSc
systemic sclerosis
- GI
gastrointestinal
- lc
limited cutaneous
- dc
diffuse cutaneous
- UCLA SCTC-GIT 2.0
The University of California Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0
- LES
lower esophageal sphincter
- GERD
gastroesophageal reflux disease
- PPI
proton pump inhibitor
- GAVE
gastric antral vascular ectasia
- SIBO
small intestinal bacterial overgrowth
- HBT
hydrogen breath tests
References
- 1.Thoua NM, Bunce C, Brough G, Forbes A, Emmanuel AV, Denton CP. Assessment of gastrointestinal symptoms in patients with systemic sclerosis in a UK tertiary referral centre. Rheumatology (Oxford) 2010;49(9):1770–5. doi: 10.1093/rheumatology/keq147. [DOI] [PubMed] [Google Scholar]
- 2.Sandmeier B, Jager VK, Nagy G, Carreira PE, Tzankov A, Widuchowska M, et al. Autopsy versus clinical findings in patients with systemic sclerosis in a case series from patients of the EUSTAR database. Clin Exp Rheumatol. 2015;33(4 Suppl 91):S75–9. [PubMed] [Google Scholar]
- 3.Schmeiser T, Saar P, Jin D, Noethe M, Muller A, Soydan N, et al. Profile of gastrointestinal involvement in patients with systemic sclerosis. Rheumatol Int. 2012;32(8):2471–8. doi: 10.1007/s00296-011-1988-6. [DOI] [PubMed] [Google Scholar]
- 4.Bodukam V, Hays RD, Maranian P, Furst DE, Seibold JR, Impens A, et al. Association of gastrointestinal involvement and depressive symptoms in patients with systemic sclerosis. Rheumatology (Oxford) 2011;50(2):330–4. doi: 10.1093/rheumatology/keq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omair MA, Lee P. Effect of gastrointestinal manifestations on quality of life in 87 consecutive patients with systemic sclerosis. J Rheumatol. 2012;39(5):992–6. doi: 10.3899/jrheum.110826. [DOI] [PubMed] [Google Scholar]
- 6.Manetti M, Neumann E, Milia AF, Tarner IH, Bechi P, Matucci-Cerinic M, et al. Severe fibrosis and increased expression of fibrogenic cytokines in the gastric wall of systemic sclerosis patients. Arthritis Rheum. 2007;56(10):3442–7. doi: 10.1002/art.22940. [DOI] [PubMed] [Google Scholar]
- 7.Papachristos DA, Nikpour M, Hair C, Stevens WM. Intravenous cyclophosphamide as a therapeutic option for severe refractory gastric antral vascular ectasia in systemic sclerosis. Intern Med J. 2015;45(10):1077–81. doi: 10.1111/imj.12883. [DOI] [PubMed] [Google Scholar]
- 8.Manetti M, Neumann E, Muller A, Schmeiser T, Saar P, Milia AF, et al. Endothelial/lymphocyte activation leads to prominent CD4+ T cell infiltration in the gastric mucosa of patients with systemic sclerosis. Arthritis Rheum. 2008;58(9):2866–73. doi: 10.1002/art.23806. [DOI] [PubMed] [Google Scholar]
- 9.Raja J, Nihtyanova SI, Murray CD, Denton CP, Ong VH. Sustained benefit from intravenous immunoglobulin therapy for gastrointestinal involvement in systemic sclerosis. Rheumatology (Oxford) 2016;55(1):115–9. doi: 10.1093/rheumatology/kev318. [DOI] [PubMed] [Google Scholar]
- 10.Thoua NM, Derrett-Smith EC, Khan K, Dooley A, Shi-Wen X, Denton CP. Gut fibrosis with altered colonic contractility in a mouse model of scleroderma. Rheumatology (Oxford) 2012;51(11):1989–98. doi: 10.1093/rheumatology/kes191. [DOI] [PubMed] [Google Scholar]
- 11.Thoua NM, Abdel-Halim M, Forbes A, Denton CP, Emmanuel AV. Fecal incontinence in systemic sclerosis is secondary to neuropathy. Am J Gastroenterol. 2012;107(4):597–603. doi: 10.1038/ajg.2011.399. [DOI] [PubMed] [Google Scholar]
- 12.D'Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med. 1969;46(3):428–40. doi: 10.1016/0002-9343(69)90044-8. [DOI] [PubMed] [Google Scholar]
- 13.Poirier TJ, Rankin GB. Gastrointestinal manifestations of progressive systemic scleroderma based on a review of 364 cases. Am J Gastroenterol. 1972;58(1):30–44. [PubMed] [Google Scholar]
- 14.Arif T, Masood Q, Singh J, Hassan I. Assessment of esophageal involvement in systemic sclerosis and morphea (localized scleroderma) by clinical, endoscopic, manometric and pH metric features: a prospective comparative hospital based study. BMC Gastroenterol. 2015;15:24. doi: 10.1186/s12876-015-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepri G, Guiducci S, Bellando-Randone S, Giani I, Bruni C, Blagojevic J, et al. Evidence for oesophageal and anorectal involvement in very early systemic sclerosis (VEDOSS): report from a single VEDOSS/EUSTAR centre. Ann Rheum Dis. 2015;74(1):124–8. doi: 10.1136/annrheumdis-2013-203889. [DOI] [PubMed] [Google Scholar]
- 16.Savarino E, Mei F, Parodi A, Ghio M, Furnari M, Gentile A, et al. Gastrointestinal motility disorder assessment in systemic sclerosis. Rheumatology (Oxford) 2013;52(6):1095–100. doi: 10.1093/rheumatology/kes429. [DOI] [PubMed] [Google Scholar]
- 17.Kahan A, Chaussade S, Gaudric M, Freitag B, Amor B, Menkes CJ, et al. The effect of cisapride on gastro-oesophageal dysfunction in systemic sclerosis: a controlled manometric study. Br J Clin Pharmacol. 1991;31(6):683–7. doi: 10.1111/j.1365-2125.1991.tb05593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang SJ, La JL, Chen DY, Chen YH, Hsieh TY, Lin WY. Effects of cisapride on oesophageal transit of solids in patients with progressive systemic sclerosis. Clin Rheumatol. 2002;21(1):43–5. doi: 10.1007/s100670200010. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Shi S, Chen F, Lin J. The effects of baclofen for the treatment of gastroesophageal reflux disease: a meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2014;2014:307805. doi: 10.1155/2014/307805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallam H, McNearney TA, Chen JD. Systematic review: pathophysiology and management of gastrointestinal dysmotility in systemic sclerosis (scleroderma) Aliment Pharmacol Ther. 2006;23(6):691–712. doi: 10.1111/j.1365-2036.2006.02804.x. [DOI] [PubMed] [Google Scholar]
- 21.Pakozdi A, Wilson H, Black CM, Denton CP. Does long term therapy with lansoprazole slow progression of oesophageal involvement in systemic sclerosis? Clin Exp Rheumatol. 2009;27(3 Suppl 54):5–8. [PubMed] [Google Scholar]
- 22.Foocharoen C, Chunlertrith K, Mairiang P, Mahakkanukrauh A, Suwannaroj S, Namvijit S, et al. Effectiveness of add-on therapy with domperidone vs alginic acid in proton pump inhibitor partial response gastro-oesophageal reflux disease in systemic sclerosis: randomized placebo-controlled trial. Rheumatology (Oxford) 2016 doi: 10.1093/rheumatology/kew216. [DOI] [PubMed] [Google Scholar]
- 23.Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10(6):e0128004. doi: 10.1371/journal.pone.0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon D, Young LR, Reddy S, Bergman C, Young JD. Incidence of Clostridium difficile infection in patients receiving high-risk antibiotics with or without a proton pump inhibitor. J Hosp Infect. 2016;92(2):173–7. doi: 10.1016/j.jhin.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Sherwood MW, Melloni C, Jones WS, Washam JB, Hasselblad V, Dolor RJ. Individual Proton Pump Inhibitors and Outcomes in Patients With Coronary Artery Disease on Dual Antiplatelet Therapy: A Systematic Review. J Am Heart Assoc. 2015;4(11) doi: 10.1161/JAHA.115.002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CH, Lin CL, Kao CH. Gastroesophageal reflux disease with proton pump inhibitor use is associated with an increased risk of osteoporosis: a nationwide population-based analysis. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3510-1. [DOI] [PubMed] [Google Scholar]
- 27.Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, et al. Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease. JAMA Intern Med. 2016;176(2):238–46. doi: 10.1001/jamainternmed.2015.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomm W, von Holt K, Thome F, Broich K, Maier W, Fink A, et al. Association of Proton Pump Inhibitors With Risk of Dementia: A Pharmacoepidemiological Claims Data Analysis. JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2015.4791. [DOI] [PubMed] [Google Scholar]
- 29.Christmann RB, Wells AU, Capelozzi VL, Silver RM. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Semin Arthritis Rheum. 2010;40(3):241–9. doi: 10.1016/j.semarthrit.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Hershcovici T, Jha LK, Johnson T, Gerson L, Stave C, Malo J, et al. Systematic review: the relationship between interstitial lung diseases and gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34(11-12):1295–305. doi: 10.1111/j.1365-2036.2011.04870.x. [DOI] [PubMed] [Google Scholar]
- 31.Lock G, Pfeifer M, Straub RH, Zeuner M, Lang B, Scholmerich J, et al. Association of esophageal dysfunction and pulmonary function impairment in systemic sclerosis. Am J Gastroenterol. 1998;93(3):341–5. doi: 10.1111/j.1572-0241.1998.00341.x. [DOI] [PubMed] [Google Scholar]
- 32.Marie I, Dominique S, Levesque H, Ducrotte P, Denis P, Hellot MF, et al. Esophageal involvement and pulmonary manifestations in systemic sclerosis. Arthritis Rheum. 2001;45(4):346–54. doi: 10.1002/1529-0131(200108)45:4<346::AID-ART347>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 33.Troshinsky MB, Kane GC, Varga J, Cater JR, Fish JE, Jimenez SA, et al. Pulmonary function and gastroesophageal reflux in systemic sclerosis. Ann Intern Med. 1994;121(1):6–10. doi: 10.7326/0003-4819-121-1-199407010-00002. [DOI] [PubMed] [Google Scholar]
- 34.McNearney TA, Sallam HS, Hunnicutt SE, Doshi D, Wollaston DE, Mayes MD, et al. Gastric slow waves, gastrointestinal symptoms and peptides in systemic sclerosis patients. Neurogastroenterol Motil. 2009;21(12):1269–e120. doi: 10.1111/j.1365-2982.2009.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marie I, Levesque H, Ducrotte P, Denis P, Hellot MF, Benichou J, et al. Gastric involvement in systemic sclerosis: a prospective study. Am J Gastroenterol. 2001;96(1):77–83. doi: 10.1111/j.1572-0241.2001.03353.x. [DOI] [PubMed] [Google Scholar]
- 36.Ariyasu H, Iwakura H, Yukawa N, Murayama T, Yokode M, Tada H, et al. Clinical effects of ghrelin on gastrointestinal involvement in patients with systemic sclerosis. Endocr J. 2014;61(7):735–42. doi: 10.1507/endocrj.ej14-0088. [DOI] [PubMed] [Google Scholar]
- 37.Horowitz M, Maddern GJ, Maddox A, Wishart J, Chatterton BE, Shearman DJ. Effects of cisapride on gastric and esophageal emptying in progressive systemic sclerosis. Gastroenterology. 1987;93(2):311–5. doi: 10.1016/0016-5085(87)91020-1. [DOI] [PubMed] [Google Scholar]
- 38.Marie I, Antonietti M, Houivet E, Hachulla E, Maunoury V, Bienvenu B, et al. Gastrointestinal mucosal abnormalities using videocapsule endoscopy in systemic sclerosis. Aliment Pharmacol Ther. 2014;40(2):189–99. doi: 10.1111/apt.12818. [DOI] [PubMed] [Google Scholar]
- 39.Ghrenassia E, Avouac J, Khanna D, Derk CT, Distler O, Suliman YA, et al. Prevalence, correlates and outcomes of gastric antral vascular ectasia in systemic sclerosis: a EUSTAR case-control study. J Rheumatol. 2014;41(1):99–105. doi: 10.3899/jrheum.130386. [DOI] [PubMed] [Google Scholar]
- 40.Fynne L, Worsoe J, Gregersen T, Schlageter V, Laurberg S, Krogh K. Gastrointestinal transit in patients with systemic sclerosis. Scand J Gastroenterol. 2011;46(10):1187–93. doi: 10.3109/00365521.2011.603158. [DOI] [PubMed] [Google Scholar]
- 41.Marie I, Ducrotte P, Denis P, Hellot MF, Levesque H. Outcome of small-bowel motor impairment in systemic sclerosis--a prospective manometric 5-yr follow-up. Rheumatology (Oxford) 2007;46(1):150–3. doi: 10.1093/rheumatology/kel203. [DOI] [PubMed] [Google Scholar]
- 42.Sjolund K, Bartosik I, Lindberg G, Scheja A, Wildt M, Akesson A. Small intestinal manometry in patients with systemic sclerosis. Eur J Gastroenterol Hepatol. 2005;17(11):1205–12. doi: 10.1097/00042737-200511000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Parodi A, Sessarego M, Greco A, Bazzica M, Filaci G, Setti M, et al. Small intestinal bacterial overgrowth in patients suffering from scleroderma: clinical effectiveness of its eradication. Am J Gastroenterol. 2008;103(5):1257–62. doi: 10.1111/j.1572-0241.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 44.Amzallag-Bellenger E, Oudjit A, Ruiz A, Cadiot G, Soyer PA, Hoeffel CC. Effectiveness of MR enterography for the assessment of small-bowel diseases beyond Crohn disease. Radiographics. 2012;32(5):1423–44. doi: 10.1148/rg.325115088. [DOI] [PubMed] [Google Scholar]
- 45.Nikou GC, Toumpanakis C, Katsiari C, Charalambopoulos D, Sfikakis PP. Treatment of small intestinal disease in systemic sclerosis with octreotide: a prospective study in seven patients. J Clin Rheumatol. 2007;13(3):119–23. doi: 10.1097/RHU.0b013e3180645d2a. [DOI] [PubMed] [Google Scholar]
- 46.Owyang C. Octreotide in gastrointestinal motility disorders. Gut. 1994;35(3 Suppl):S11–4. doi: 10.1136/gut.35.3_suppl.s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ritchie M, Caravelli J, Shike M. Benign persistent pneumoperitoneum in scleroderma. Dig Dis Sci. 1986;31(5):552–5. doi: 10.1007/BF01320325. [DOI] [PubMed] [Google Scholar]
- 48.London NJ, Bailey RG, Hall AW. Spontaneous benign pneumoperitoneum complicating scleroderma in the absence of pneumatosis cystoides intestinalis. Postgrad Med J. 1990;66(771):61–2. doi: 10.1136/pgmj.66.771.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kudoh K, Shibata C, Funayama Y, Fukushima K, Takahashi K, Ogawa H, et al. Gastrojejunostomy and duodenojejunostomy for megaduodenum in systemic sclerosis sine scleroderma: report of a case. Dig Dis Sci. 2007;52(9):2257–60. doi: 10.1007/s10620-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 50.Marie I, Ducrotte P, Denis P, Menard JF, Levesque H. Small intestinal bacterial overgrowth in systemic sclerosis. Rheumatology (Oxford) 2009;48(10):1314–9. doi: 10.1093/rheumatology/kep226. [DOI] [PubMed] [Google Scholar]
- 51.Marie I, Leroi AM, Menard JF, Levesque H, Quillard M, Ducrotte P. Fecal calprotectin in systemic sclerosis and review of the literature. Autoimmun Rev. 2015;14(6):547–54. doi: 10.1016/j.autrev.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 52.Tauber M, Avouac J, Benahmed A, Barbot L, Coustet B, Kahan A, et al. Prevalence and predictors of small intestinal bacterial overgrowth in systemic sclerosis patients with gastrointestinal symptoms. Clin Exp Rheumatol. 2014;32(6 Suppl 86):S-82–7. [PubMed] [Google Scholar]
- 53.Kaye SA, Lim SG, Taylor M, Patel S, Gillespie S, Black CM. Small bowel bacterial overgrowth in systemic sclerosis: detection using direct and indirect methods and treatment outcome. Br J Rheumatol. 1995;34(3):265–9. doi: 10.1093/rheumatology/34.3.265. [DOI] [PubMed] [Google Scholar]
- 54.Braun-Moscovici Y, Braun M, Khanna D, Balbir-Gurman A, Furst DE. What tests should you use to assess small intestinal bacterial overgrowth in systemic sclerosis? Clin Exp Rheumatol. 2015;33(4 Suppl 91):S117–22. [PubMed] [Google Scholar]
- 55.Frech TM, Khanna D, Maranian P, Frech EJ, Sawitzke AD, Murtaugh MA. Probiotics for the treatment of systemic sclerosis-associated gastrointestinal bloating/ distention. Clin Exp Rheumatol. 2011;29(2 Suppl 65):S22–5. [PubMed] [Google Scholar]
- 56.Soudah HC, Hasler WL, Owyang C. Effect of octreotide on intestinal motility and bacterial overgrowth in scleroderma. N Engl J Med. 1991;325(21):1461–7. doi: 10.1056/NEJM199111213252102. [DOI] [PubMed] [Google Scholar]
- 57.Omair MA, Alahmadi A, Johnson SR. Safety and effectiveness of mycophenolate in systemic sclerosis. A systematic review. PLoS One. 2015;10(5):e0124205. doi: 10.1371/journal.pone.0124205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang SJ, Lan JL, Chen DY, Chen YH, Hsieh TY, Lin WY. Colonic transit disorders in systemic sclerosis. Clin Rheumatol. 2001;20(4):251–4. doi: 10.1007/s100670170038. [DOI] [PubMed] [Google Scholar]
- 59.Govoni M, Muccinelli M, Panicali P, La Corte R, Nuccio Scutellari P, Orzincolo C, et al. Colon involvement in systemic sclerosis: clinical-radiological correlations. Clin Rheumatol. 1996;15(3):271–6. doi: 10.1007/BF02229706. [DOI] [PubMed] [Google Scholar]
- 60.Ackerman Z, Lysy J, Meiner-Lavie V. The association of fecal impaction and verapamil in a patient with scleroderma. Am J Gastroenterol. 1989;84(8):981–2. [PubMed] [Google Scholar]
- 61.Giuggioli D, Manfredi A, Colaci M, Ferri C. Oxycodone in the long-term treatment of chronic pain related to scleroderma skin ulcers. Pain Med. 2010;11(10):1500–3. doi: 10.1111/j.1526-4637.2010.00849.x. [DOI] [PubMed] [Google Scholar]
- 62.Thayalasekeran S, Ali H, Tsai HH. Novel therapies for constipation. World J Gastroenterol. 2013;19(45):8247–51. doi: 10.3748/wjg.v19.i45.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang SJ, Lan JL, Lan JL, Chen DY, Chen YH, Hsieh TY, et al. Effects of cisapride on colonic transit in patients with progressive systemic sclerosis. Clin Rheumatol. 2002;21(4):271–4. doi: 10.1007/s100670200072. [DOI] [PubMed] [Google Scholar]
- 64.Boeckxstaens GE, Bartelsman JF, Lauwers L, Tytgat GN. Treatment of GI dysmotility in scleroderma with the new enterokinetic agent prucalopride. Am J Gastroenterol. 2002;97(1):194–7. doi: 10.1111/j.1572-0241.2002.05396.x. [DOI] [PubMed] [Google Scholar]
- 65.Valle RG, Godoy FL. Neostigmine for acute colonic pseudo-obstruction: A meta-analysis. Ann Med Surg (Lond) 2014;3(3):60–4. doi: 10.1016/j.amsu.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shamberger RC, Crawford JL, Kirkham SE. Progressive systemic sclerosis resulting in megacolon. A case report. JAMA. 1983;250(8):1063–5. [PubMed] [Google Scholar]
- 67.Lindsey I, Farmer CR, Cunningham IG. Subtotal colectomy and cecosigmoid anastomosis for colonic systemic sclerosis: report of a case and review of the literature. Dis Colon Rectum. 2003;46(12):1706–11. doi: 10.1007/BF02660780. [DOI] [PubMed] [Google Scholar]
- 68.Exadaktylos A, Papagrigoriadis S. Chronic constipation--a lethal danger in patients with systemic scleroderma. Eur J Emerg Med. 2001;8(4):333–5. doi: 10.1097/00063110-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 69.Sacher P, Buchmann P, Burger H. Stenosis of the large intestine complicating scleroderma and mimicking a sigmoid carcinoma. Dis Colon Rectum. 1983;26(5):347–8. doi: 10.1007/BF02561715. [DOI] [PubMed] [Google Scholar]
- 70.Budd DC, Nirdlinger EL, Sturtz DL, Fouty WJ., Jr. Transverse colon volvulus associated with scleroderma. Am J Surg. 1977;133(3):370–2. doi: 10.1016/0002-9610(77)90547-5. [DOI] [PubMed] [Google Scholar]
- 71.Thoua NM, Schizas A, Forbes A, Denton CP, Emmanuel AV. Internal anal sphincter atrophy in patients with systemic sclerosis. Rheumatology (Oxford) 2011;50(9):1596–602. doi: 10.1093/rheumatology/ker153. [DOI] [PubMed] [Google Scholar]
- 72.Singh J, Mehendiratta V, Del Galdo F, Jimenez SA, Cohen S, DiMarino AJ, et al. Immunoglobulins from scleroderma patients inhibit the muscarinic receptor activation in internal anal sphincter smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2009;297(6):G1206–13. doi: 10.1152/ajpgi.00286.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh J, Cohen S, Mehendiratta V, Mendoza F, Jimenez SA, Dimarino AJ, et al. Effects of scleroderma antibodies and pooled human immunoglobulin on anal sphincter and colonic smooth muscle function. Gastroenterology. 2012;143(5):1308–18. doi: 10.1053/j.gastro.2012.07.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trezza M, Krogh K, Egekvist H, Bjerring P, Laurberg S. Bowel problems in patients with systemic sclerosis. Scand J Gastroenterol. 1999;34(4):409–13. doi: 10.1080/003655299750026434. [DOI] [PubMed] [Google Scholar]
- 75.Umar SB, Griffing L, Garcia H, Foxx-Orenstein AE, DiBaise JK, Crowell MD. The Impact of Pelvic Floor and Lower Gastrointestinal Symptoms on Quality of Life in Women With Systemic Sclerosis. J Clin Gastroenterol. 2015 doi: 10.1097/MCG.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 76.Heyt GJ, Oh MK, Alemzadeh N, Rivera S, Jimenez SA, Rattan S, et al. Impaired rectoanal inhibitory response in scleroderma (systemic sclerosis): an association with fecal incontinence. Dig Dis Sci. 2004;49(6):1040–5. doi: 10.1023/b:ddas.0000034569.85066.69. [DOI] [PubMed] [Google Scholar]
- 77.Franck-Larsson K, Graf W, Eeg-Olofsson KE, Axelson HW, Ronnblom A. Physiological and structural anorectal abnormalities in patients with systemic sclerosis and fecal incontinence. Scand J Gastroenterol. 2014;49(9):1076–83. doi: 10.3109/00365521.2014.913188. [DOI] [PubMed] [Google Scholar]
- 78.Fynne L, Luft F, Gregersen H, Buntzen S, Lundby L, Lundager F, et al. Distensibility of the anal canal in patients with systemic sclerosis: a study with the functional lumen imaging probe. Colorectal Dis. 2013;15(1):e40–7. doi: 10.1111/codi.12063. [DOI] [PubMed] [Google Scholar]
- 79.Jaffin BW, Chang P, Spiera H. Fecal incontinence in scleroderma. Clinical features, anorectal manometric findings, and their therapeutic implications. J Clin Gastroenterol. 1997;25(3):513–7. doi: 10.1097/00004836-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 80.Sallam HS, McNearney TA, Chen JZ. Anorectal motility and sensation abnormalities and its correlation with anorectal symptoms in patients with systemic sclerosis: a preliminary study. ISRN Gastroenterol. 2011;2011:402583. doi: 10.5402/2011/402583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.deSouza NM, Williams AD, Gilderdale DJ. High-resolution magnetic resonance imaging of the anal sphincter using a dedicated endoanal receiver coil. Eur Radiol. 1999;9(3):436–43. doi: 10.1007/s003300050688. [DOI] [PubMed] [Google Scholar]
- 82.Kenefick NJ, Vaizey CJ, Nicholls RJ, Cohen R, Kamm MA. Sacral nerve stimulation for faecal incontinence due to systemic sclerosis. Gut. 2002;51(6):881–3. doi: 10.1136/gut.51.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Butt SK, Alam A, Cohen R, Krogh K, Buntzen S, Emmanuel A. Lack of effect of sacral nerve stimulation for incontinence in patients with systemic sclerosis. Colorectal Dis. 2015;17(10):903–7. doi: 10.1111/codi.12969. [DOI] [PubMed] [Google Scholar]
- 84.Leighton JA, Valdovinos MA, Pemberton JH, Rath DM, Camilleri M. Anorectal dysfunction and rectal prolapse in progressive systemic sclerosis. Dis Colon Rectum. 1993;36(2):182–5. doi: 10.1007/BF02051176. [DOI] [PubMed] [Google Scholar]
- 85.Khanna D, Hays RD, Maranian P, Seibold JR, Impens A, Mayes MD, et al. Reliability and validity of the University of California, Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument. Arthritis Rheum. 2009;61(9):1257–63. doi: 10.1002/art.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khanna D, Furst DE, Maranian P, Seibold JR, Impens A, Mayes MD, et al. Minimally important differences of the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument. J Rheumatol. 2011;38(9):1920–4. doi: 10.3899/jrheum.110225. [DOI] [PMC free article] [PubMed] [Google Scholar]