Abstract
The transcription factor Nrf2 has been shown to play an important role in many different kidney diseases from AKI to CKD, and there have been preliminary Nrf2 based therapeutic trials in humans. In the current issue of Kidney Int, Shelton and colleagues present an integrated transcriptomic and proteomic analysis of mouse kidney to reveal Nrf2 targets with potentially important roles in kidney homeostasis and pathophysiology. These results can help further our understanding of Nrf2 based mechanisms and develop therapeutics for a wide range of kidney diseases.
Keywords: Nrf2 Pathway, CDDO-Me, Kidney, oxidative stress
Commentary
The nuclear factor erythroid-derived 2-like 2 (Nrf2) is a well-characterized transcription factor that controls primary defense mechanisms against reactive oxygen species (ROS)-induced tissue injury. Experimental studies in kidney cells and animal models, as well as limited data in humans, have implicated an increase in Nrf2 as beneficial in a range of kidney diseases including acute kidney injury (AKI), chronic kidney disease (CKD), diabetic nephropathy (DN), polycystic kidney disease and hypertension (1, 2). The protective effects of Nrf2 regulated antioxidant responses are largely attributed to its transcriptional control over hundreds of antioxidant and xenobiotic genes that are actively transcribed following an insult. Some of these key genes include heme oxygenase 1 (HO-1), Nqo1, catalase, and NADPH. Under stress-free conditions, Nrf2 is bound to its canonical inhibitor kelch-like ECH-associated protein 1 (Keap1) that promotes its ubiquitination and subsequent proteasomal degradation which maintains homeostatic levels of Nrf2 in the cytoplasm. However, in the presence of ROS and other potentially harmful stimuli, Keap1 dissociates from Nrf2 allowing it to translocate into the nucleus, bind to antioxidant response elements (AREs) along with other binding partners, and actively transcribe antioxidant and anti-inflammatory genes (Figure) (1,2,3). Pharmacologic and genetic approaches have been designed to disengage Keap1 from Nrf2 to increase cellular antioxidant responses. There are also naturally occurring agents that augment Nrf2, including derivatives from Brussels sprouts (sulforaphane) and grapes (resveratrol).
Figure. Working model of Nrf2-Keap1 pathway in kidney diseases.
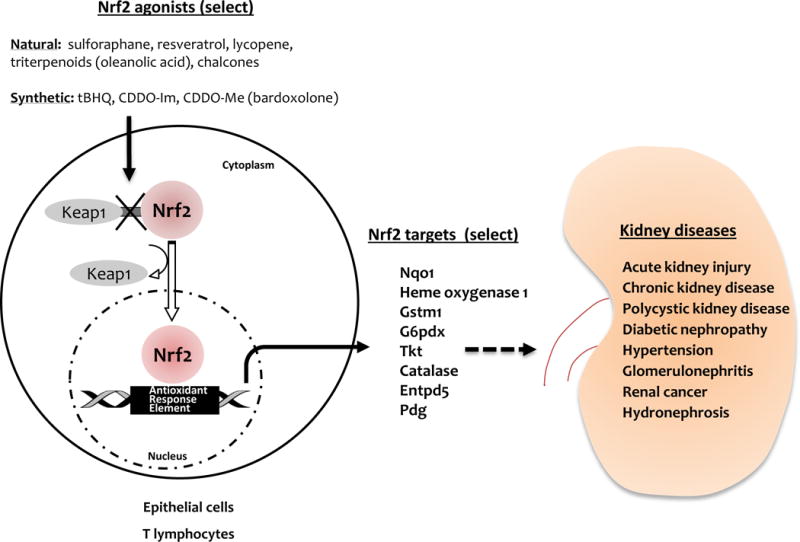
Natural and synthetic Nrf2 activators induce structural changes in Keap1 protein that compromises its ability to retain Nrf2 in the cytoplasm. This allows nuclear translocation of Nrf2 where it binds to antioxidant response elements (AREs) to initiate transcription of multiple target genes (Gstm1, HO-1, Catalase, Entpd5, G6pdx, Pdg, Tkt, Nqo1 etc.) important for antioxidant, xenobiotic and homeostatic responses. Targeting these Nrf2 responses could provide significant protection and therapy in kidney diseases including acute kidney injury, chronic kidney disease, polycystic kidney disease, diabetic nephropathy, hypertension, glomerulonephritis, renal cancer and hydronephrosis.
Broad discovery based microarray analysis of postischemic mouse kidney initially revealed that Nrf2 was a master regulator of AKI stress responses (3). Subsequent studies in mice with genetic deficiency of Nrf2 led to worse renal function after IRI or cisplatin (1), while pharmacologic enhancement of Nrf2 function was protective in AKI (4,5). There were initially promising results in human DN using a synthetic triterpenoid derivative (CDDO-Me, also known as bardoxolone methyl) which dissociated Keap1 from Nrf2 to enhance its activity (6). However, a multinational, double blinded, placebo controlled phase III clinical trial of CDDO-Me in CKD patients with type 2 diabetes was terminated due to an increase in heart failure, possibly due to increases in salt and water retention (7). Despite the initial clinical setback with bardoxolone for DN, given the wide range of kidney diseases that Nrf2 can mediate and studies on both natural and synthetic Nrf2 agonists, there has been a revival of interest in Nrf2 in the renal and pharmacology community.
In the current issue of Kidney Int, Shelton and colleagues (8) have undertaken an integrated transcriptomic and proteomic analyses of mouse kidney to reveal many potential regulatory functions of Nrf2 following CDDO-Me treatment. The authors validated pharmacologic effects of CDDO-Me activation of Nrf2 pathways in human primary kidney epithelial cells before carrying out detailed in vivo studies. A single intraperitoneal injection of CDDO-Me (3mg/kg) in mice, when studied 24 hrs later, upregulated Nrf2 activity and altered 2561 transcripts and 240 proteins in the kidneys of Nrf2 deficient mice in comparison to WT mice. Additionally, 3122 transcripts and 68 proteins were differentially expressed in kidneys from WT mice that received CDDO-Me treatment in comparison to the vehicle treated mice. Detailed bioinformatics analysis of these differentially expressed genes/proteins predicted their involvement in coordinating the synthesis and conjugation of glutathione (Gstm1, Catalase and Entpd5), maintaining cellular redox balance, controlling the metabolism and disposition of a wide range of xenobiotics (G6pdx, Pgd, Tkt), regulating the supply of NADPH (Nqo1) and other cellular fuels and influencing the provision of NADPH and glutathione in the kidney in vivo. The global regulatory analysis of Nrf2 in mouse kidney by Shelton and colleagues is an important and timely addition to our existing knowledge of the functions and mechanism by which Nrf2 influences normal and diseased kidney.
Despite this study being exciting and valuable, there are many important aspects that were not addressed. The authors studied Nrf2 regulated genes/proteins in normal mouse kidney, but not in the context of any kidney disease model. Another opportunity that awaits to be addressed is the exploration of the roles Nrf2 of the different cell types in the kidney. In this regard, conditional deletion of Keap1 in T cells has recently been demonstrated to play a key role in protection from experimental AKI, revealing the importance of Nrf2 regulated antioxidant response in immune cells (9). The impact of Nrf2 function on different parts of the nephron are also relatively unknown, and we do not know if Nrf2 functions differently in the glomerulus, cortex, medulla and collecting system. A selective Keap1 deletion using Ksp Cre in renal epithelial cells led to congenital hydronephrosis in murine kidney (ASN abstract, J Am Soc Nephrol, 2014; 25, 538A). Another important unknown for the renal patient is the optimal and safe dosing of Nrf2 agonists. In the current study by Shelton et al, only a single dose of CDDO-Me was administered. Previous and ongoing studies for DN in humans have used chronic dosing. The route of Nrf2 agonist administration is also important. Most experimental and human studies administered Nrf2 activators via oral route, however Shelton and colleagues examined these molecular changes following an intraperitoneal dose of CDDO-Me. Other potential side effects of Nrf2 agents need to be evaluated. Studies on Nrf2 protection to prevent cisplatin nephrotoxicity should also evaluate the effects of Nrf2 on cancer growth, since the same cytoprotection afforded to normal tissues by Nrf2 could make it more difficult to kill tumor cells that have enhanced Nrf2 (10).
In summary, the transcriptomic and proteomic signatures described by Shelton and colleagues provide a clearer insight into regulatory roles of Nrf2 in murine kidney. Future studies in experimental kidney disease models and humans are required to assign relevant biological functions and molecular mechanism to these genes/proteins in kidney. Strategies to “Keap” Nrf2 activated could prove beneficial for our kidney disease patients.
Footnotes
Disclosure: The authors have nothing to disclose.
References
- 1.Liu M, Grigoryev DN, Crow MT, et al. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009;76:277–85. doi: 10.1038/ki.2009.157. [DOI] [PubMed] [Google Scholar]
- 2.Pedruzzi LM, Cardozo LF, Daleprane JB, et al. Systemic inflammation and oxidative stress in hemodialysis patients are associated with down-regulation of Nrf2. J Nephrol. 2015;28:495–501. doi: 10.1007/s40620-014-0162-0. [DOI] [PubMed] [Google Scholar]
- 3.Leonard MO, Kieran NE, Howell K, et al. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20:2624–6. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- 4.Liu M, Reddy NM, Higbee EM, et al. The Nrf2 triterpenoid activator, CDDO-imidazolide, protects kidneys from ischemia-reperfusion injury in mice. Kidney Int. 2014;85:134–41. doi: 10.1038/ki.2013.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu QQ, Wang Y, Senitko M, et al. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARγ, and HO-1. Am J Physiol Renal Physiol. 2011;300:F1180–92. doi: 10.1152/ajprenal.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–36. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 7.Chin MP, Reisman SA, Bakris GL, et al. Mechanisms contributing to adverse cardiovascular events in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. Am J Nephrol. 2014;39:499–508. doi: 10.1159/000362906. [DOI] [PubMed] [Google Scholar]
- 8.Shelton LM, Lister A, Walsh J, et al. Integrated Transcriptomic and Proteomic Analyses Reveal the Regulatory Roles of Nrf2 in the Kidney. Kidney Int. 2015;xx:xxx–xxx. doi: 10.1038/ki.2015.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noel S, Martina MN, Bandapalle S, et al. T lymphocyte-specific activation of Nrf2 protects from AKI. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinch L, Grishin NV, Brugarolas J. Succination of Keap1 and activation of Nrf2-dependent antioxidant pathways in FH-deficient papillary renal cell carcinoma type 2. Cancer Cell. 2011;20:418–20. doi: 10.1016/j.ccr.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


