Abstract
The small (16,569 base pair) human mitochondrial genome plays a significant role in cell metabolism and homeostasis. Mitochondrial DNA (mtDNA) contributes to the generation of complexes which are essential to oxidative phosphorylation (OXPHOS). As such, mtDNA is directly integrated into mitochondrial biogenesis and signaling and regulates mitochondrial metabolism in concert with nuclear-encoded mitochondrial factors. Mitochondria are a highly dynamic, pleiomorphic network that undergoes fission and fusion events. Within this network, mtDNAs are packaged into structures called nucleoids which are actively distributed in discrete foci within the network. This sensitive organelle is frequently disrupted by insults such as oxidants and inflammatory cytokines, and undergoes genomic damage with double- and single-strand breaks that impair its function. Collectively, mtDNA is emerging as a highly sensitive indicator of cellular stress, which is directly integrated into the mitochondrial network as a contributor of a wide range of critical signaling pathways.
Keywords: Mitochondria, Mitochondrial DNA, Fusion, Fission, Review
2. mtDNA IN MITOCHONDRIAL BIOENERGETICS AND BIOGENESIS
Our understanding of mitochondria has undergone profound revision over recent years. While these endosymbiont-derived organelles had previously been thought of as a collection of battery-like organelles, current evidence reveals that mitochondria are an incredibly dynamic organellar network, of dual genetic origin, that is crucial to bioenergetics and metabolism, as well as a range of vital cellular processes.
As endosymbiotic organelles, mitochondria originated two billion years ago from the engulfment of a α-proteobacterium by an ancestor of a modern eukaryotic cell (1, 2). As such, human mitochondria closely resemble bacteria in both their membrane structure and maintenance of DNA. Mitochondria are membrane-bound organelles containing both an outer and inner mitochondrial membrane. The inner membrane contains the five complexes of oxidative phosphorylation (OXPHOS), required for mitochondrial ATP production. Unique among human organelles, mitochondria contain their own genetic material, mitochondrial DNA (mtDNA). MtDNA is circular, similar to bacterial plasmid DNA, and encodes factors that combine with those produced from nuclear DNA to comprise the OXPHOS complexes of the inner membrane, providing the cell with the necessary ATP. The two strands of mtDNA are differentiated into heavy (H-strand) and light strands (L-strand), with the H-strand being primarily composed of guanine and the light strand rich in cytosine (3). The mitochondrial genome in humans contains 16,569 base pairs that code for 37 genes: 13 polypeptides, 22 tRNAs, and the small and large rRNA subunits (4). MtDNA is maternally inherited through the oocyte during conception (5–7). During early embryonic development, paternal mtDNA in the mitochondria of spermatocytes is selected for destruction. Paternal mtDNA inheritance is exceedingly rare in humans (8).
Mitochondria also contain the nuclear-encoded factors necessary for the replication and transcription of this extra-nuclear genetic material. MtDNA replication requires proteins such as DNA polymerase γ (POLG), the mitochondrial single-stranded DNA binding protein (mtSSB), mitochondrial DNA helicase (Twinkle), and a number of accessory proteins and transcription factors. The role of mtSSB in replication is to stimulate the activity of the mitochondrial replicative helicase Twinkle and polymerase (9). Recent studies have shown Twinkle helicase to be essential for mtDNA replication, as depletion of Twinkle causes severe mtDNA depletion (10). Pif1 is another helicase that has also been shown to cause mitochondrial instability: depletion of Pif1 causes deficiency in repairing oxidative stress-induced mtDNA damage and mitochondrial myopathy (11). POLG is crucial as it is the only replicative, highly-processive polymerase in the mitochondria, and also is necessary in the repair of mtDNA (12). The initiation of mtDNA transcription requires the binding of mitochondria transcription factor (mtTFB) to the mtRNA polymerase (POLRMT). MtDNA typically is present at ~1,000 copies per cell, varying between cell and tissue type (13). Transcription factor A, mitochondrial (TFAM) was originally identified for its key role in the activation of mtDNA transcription (14), but more recently has been shown to play a critical role in modulating mtDNA content within mammalian cells: exogenous expression TFAM is able to increase mtDNA content, independent of changes in transcription (15–17). The level of TFAM within the cell is directly modulated by the Lon protease, allowing for Lon-dependent changes in mtDNA copy number (18), using phosphorylation of TFAM in the HMG-1 domain to promote proteolytic degradation by Lon (19).
Effective mitochondrial ATP production requires the transcription and translation of mtDNA-encoded genes, along with the import of nuclear-encoded polypeptides and the coordinate assembly of proteins from both genomes into the multisubunit OXPHOS complexes. The biogenesis of OXPHOS complexes and mitochondrial content as a whole is controlled by cell signaling pathways that can upregulate mitochondrial content and gene expression in response to cellular metabolic demand. The OXPHOS complexes are large, multisubunit protein complexes located in the mitochondrial inner membrane. Complexes I-IV catalyze electron transfer from NADH and FADH2, providing the energy for the proton pumping that generates the transmembrane potential across the inner membrane (Δψm). This Δψm drives ATP synthesis by the F1F0 ATP synthase, in which the rotation of the gamma and epsilon subunits causes ADP and Pi to coalesce to form ATP (20). With the exception of Complex II (succinate dehydrogenase), each of the complexes contains one or more mtDNA-encoded polypeptides that are required for assembly and function. Complex I (NADH dehydrogenase), the largest of the five complexes, is composed of more than 40 proteins, as well as iron-sulfur clusters and flavin prosthetic groups, and is the entry point for electrons from NADH. Of the numerous polypeptides that make up Complex I, seven (ND1, ND2, ND3, ND4, ND4L, ND5, and ND6) are encoded by mtDNA. Complex III (coenzyme Q: cytochrome c oxidoreductase) is comprised of 11 subunits, including 1 mtDNA-encoded subunit (cytochrome b), while Complex IV (cytochrome c oxidase) contains 14 polypeptide subunits, of which CO1, CO2, and CO3 are mtDNA-encoded. The F1F0 ATP synthase contains two mtDNA-encoded proteins, ATP6 and ATP8, as part of its complement of 16 subunits (21). Assembly of these complexes requires the transcription and translation of mtDNA-encoded factors and the import of nuclear-encoded proteins, followed by assembly and insertion of the finished complex into the mitochondrial inner membrane. Loss or mutation of the genes for any of these mtDNA-encoded subunits can disrupt the assembly and function of the complex affected: a host of point mutations, partial deletions (Δ-mtDNAs), and depletion of mtDNA cause loss of specific complexes or OXPHOS activity as a whole (22), illustrating the crucial role of mtDNA in cellular bioenergetics, in spite of the small handful of gene products derived from mtDNA.
The mitochondrial proteome is composed of over 1,000 proteins, varying across species (23–26). The cell’s mitochondrial content, expression of OXPHOS components and other mitochondrial proteins, and mtDNA copy number varies dramatically between cell and tissue types, depending on metabolic demand and cellular signaling cues. For example, cardiac muscle has the highest mitochondrial content of human tissues, with 20–30% of cellular volume composed of mitochondria, consistent with the extreme bioenergetic demands of the heart (27). To respond to energetic demand, mtDNA copy number, OXPHOS capacity, and mitochondrial content are regulated by mitochondrial biogenesis factors controlling the coordinated expression of mtDNA- and nuclear-derived factors. The best-characterized of these is PPAR-gamma related cofactor-1 alpha (PGC-1α), which was first identified in brown fat cells, and was found to induce dramatic increases in mitochondrial content when expressed in white adipose tissue (28). PGC-1α directly activates the expression of nuclear respiratory factors-1 and −2 (NRF-1, NRF-2) (29). These transcription factors directly activate the expression of nuclear-encoded mitochondrial proteins including OXPHOS complex subunits and TFAM, the key mtDNA-packaging protein that modulates mtDNA copy number. Similar to nuclear-encoded OXPHOS components, TFAM expression is controlled by NRF-1 (30) and NRF-2 (31), placing mtDNA copy number control under PGC-1α-mediated mitochondrial biogenesis signaling. MtDNA copy number may also be regulated by DNA methylation of the nuclear encoded DNA polymerase gamma (32). MtDNA content is thus coordinately regulated along with overall mitochondrial content to provide bioenergetic capacity in response to cellular demand. PGC-1α has since emerged as a master regulator of mitochondrial biogenesis, and interacts with numerous signaling factors such as AMPK and SIRT3 that have dramatic impacts on cellular metabolism and energetics (33, 34). In addition, several nuclear transcription factors have been found to localize to the mitochondria, with the ability to activate transcription of mtDNA-encoded factors: cyclic AMP response binding element-binding protein (CREB) binds to the D-loop of mtDNA (35) and produces mtDNA-derived mRNA (36), while myocyte enhancer factor-2D (MEF2D) localizes to the mitochondria and activates expression of Complex I subunits (37). Conversely, nuclear factor kappa B (NFκB), a key cellular stress response factor, also localizes to the mitochondria, but appears to repress the expression of mtDNA-encoded genes (38, 39). Collectively, it is clear that multiple signaling pathways converge to modulate mitochondrial biogenesis, mtDNA copy number, and the expression of both nuclear- and mtDNA-encoded OXPHOS subunits.
3. INTEGRATION OF mtDNA INTO THE ORGANELLAR NETWORK
To maintain bioenergetic homeostasis, mtDNA nucleoids are distributed at regular intervals throughout the highly dynamic mitochondrial network. This organellar network continuously balances fusion and fission events to maintain structural homeostasis in response to cellular cues. To unite separate organelles into an interconnected reticulum, mitochondria employ distinct GTPase factors to establish continuity of both the outer and inner mitochondrial membranes. Fusion of the mitochondrial outer membrane is mediated by the mitofusins 1 and 2 (MFN1 and MFN2), which act both individually and in concert with each other to accomplish fusion (40). Inner membrane fusion is carried out by optic atrophy-1 (OPA1). As an inner membrane protein, OPA1 is present in multiple isoforms (41). Long isoforms of OPA1 (L-OPA1) are fusion-active (42), which are cleaved to short, fusion-inactive forms (S-OPA1) by several proteases including Yme1 (43) and OMA1 (44, 45). OPA1 interacts with MFN1 and 2, permitting coordination of both outer and inner membrane fusion (43). The opposing process, fission, is mediated by the cytosolic GTPase dynamin-related protein-1 (DRP1). The outer membrane proteins Fis1 and MFF1 act to recruit DRP1 to the organelle (46, 47), where they bind DRP1 and promote the formation of DRP1 multimers that wrap around the mitochondrial tubule, using GTP hydrolysis to constrict the organelle and carry out membrane scission (48, 49). Thus, mitochondrial dynamics are mediated by two opposing sets of factors, balancing these complex interactions to establish structural homeostasis.
These organellar dynamics are mechanistically tied to bioenergetic function. Based on their experiments using photobleaching and recovery of mitochondrial fluorescence, Skulachev and co-workers proposed that the fused, reticular organization of mitochondria mediates energetic connectivity (50), while subsequent work found that loss of bioenergetic function resulted in an inability to maintain mitochondrial interconnection (51). More recently, it has become clear that mitochondrial fusion requires an intact Δψm across the inner membrane (52). OPA1-mediated fusion of the inner membrane is Δψm - dependent: loss of Δψm causes cleavage of L-OPA1 to fusion-inactive S-OPA1 isoforms (42). The groups of Langer and van der Bliek concurrently found that this Δψm - sensitive cleavage of OPA1 is mediated by the OMA1 metalloprotease (44, 45). In addition, dissipation of Δψm activates fission (53), engaging calcium-dependent DRP1 dephosphorylation signaling mechanisms (54). Thus, loss of Δψm impacts both mitochondrial fusion and fission, causing the collapse of mitochondrial structure to the completely fragmented organization observed in genetic and pharmacological models of bioenergetic dysfunction.
In addition to their integration with bioenergetics and metabolism, fission/fusion dynamics are critical to mitochondrial participation in key cellular signaling pathways including apoptosis (53), mitosis (55), autophagy (56), and stemness (57). For example, during nutrient starvation, functional mitochondria undergo fusion and elongation, protecting them from degradation (58), while mitochondria with low Δψm are selectively targeted for autophagy via PINK1/Parkin signaling (59). Similarly, DRP1-mediated mitochondrial fission is an integral component of apoptotic pathways (60), while OPA1 interacts with SIRT3 as part of cellular stress response signaling (61). Collectively, mitochondrial structural dynamics are critical to the organelle’s role in both bioenergetics and crucial cell-wide signaling events, directly impacting the survival or death of the cell.
To ensure effective distribution of mtDNA within this pleiomorphic, dynamic organellar network, nucleoids are distributed at regular intervals throughout the mitochondrial reticulum. This distribution, when visualized microscopically, has a striking ‘beads on a string’ appearance (62, 63), allowing mtDNA-derived transcripts and gene products to diffuse efficiently throughout the mitochondrial network. Measurements of nucleoid foci using multiple visualization methods reveal that nucleoids are found at a frequency of one nucleoid every 0.8. µM of mitochondrial length (64). While nucleoids are somewhat constrained in their ability to diffuse via their association with the inner membrane (65), they are nevertheless sufficiently mobile to repopulate mitochondria lacking mtDNA and restore bioenergetic function, as shown through cell fusion experiments (13). Mitochondrial nucleoids do not appear to exchange genetic material (66), but they have been shown to divide, likely as a result of replication events followed by the partitioning of mtDNAs into daughter nucleoids (67). While a fused mitochondrial network allows for increased bioenergetic function (68) and an electrically-connected mitochondrial continuum (50), the spatial distribution of nucleoids also allows for efficient mtDNA partitioning during fission of the network. A variety of imaging methods, including in situ hybridization (63), ethidium bromide, and anti-DNA immunolabeling (64), reveal that when the mitochondrial network undergoes fission to exist as a population of individual organelles, each mitochondrion will contain at least one nucleoid. Moreover, nucleoids appear to be protected from fission events: DRP1-mediated fission occurs to either side, but not at, the site of nucleoids (Iborra et al., 2004); the MFF and FIS1 factors that recruit DRP1 appear to prevent fission at nucleoid sites. This is similar to prokaryotic mechanisms, in which bacterial nucleoids are protected from cell division by specific factors such as Noc to prevent loss of nucleoid genetic material (69, 70).
The arrangement of nucleoids throughout this highly dynamic network plays a major role in determining the bioenergetic status of the cell, and allows for both complementation between individual organelles and elimination of mitochondria carrying deleterious mtDNA mutations. Mitochondrial genetics are best thought of as population genetics on a cellular scale: the ~1,000 copies of mtDNA within a human cell frequently contain mixed populations of wildtype (WT) and mutant mtDNAs. These competing populations of WT and mutant mtDNAs, referred to as heteroplasmy (71), lead to heterogeneity of function within the mitochondrial network. Following the sequencing of the mitochondrial genome (4), it was shown that mutations of mtDNA cause loss of mitochondrial bioenergetics, via maternally-inherited base-change mutations in polypeptides and tRNAs, as well as through large-scale somatic Δ-mtDNAs (22). In evaluating mtDNA mutations and their phenotypic impact, it became quickly apparent that heteroplasmy was a crucial determinant of bioenergetic capacity: patients with pathogenic mtDNA mutations showed a sharp threshold effect of mtDNA mutation load (72), while complementation experiments by Attardi’s group found that just 10% of WT mtDNA was sufficient to restore full mitochondrial function (73). Thus, the presence of WT and mutant mtDNAs within the cell creates a heterogeneous population of both functional and dysfunctional mitochondria.
This heterogeneity reveals the role of fission/fusion dynamics in determining mitochondrial phenotypes. Hayashi’s group demonstrated that two respiration-deficient cell lines, each carrying a different mtDNA point mutation, were able to transcomplement and restore mitochondrial function upon cell fusion (74). Similarly, mitochondria in mice carrying both WT and Δ-mtDNAs showed robust ability to fuse and maintain mitochondrial function unless the overall mutation load exceeded threshold, at which point bioenergetic function was lost (75). While nucleoids themselves do not appear to exchange mtDNAs, as discussed above, this complementation results from diffusion of gene products between individual organelles upon fusion of the outer and inner membranes (52, 66), as even mitochondria in cells lacking mtDNA have fusion machinery and can fuse with ‘healthy’ mtDNA-containing mitochondria to permit complementation to occur (76). Conversely, fission permits the isolation and degradation of dysfunctional mitochondria. Autophagy has emerged as a major cellular mechanism mediating the selective degradation of dysfunctional mitochondria. Collapse of Δψm causes recruitment of the Parkin E3 ubiquitin ligase and subsequent targeting of individual organelles to autophagosomes (59). This selective degradation requires both the loss of Δψm (59) and DRP1-mediated fission to isolate the dysfunctional organelle (58). Mitochondria carrying pathogenic mtDNA mutations that impact Δψm can thus be targeted for autophagic degradation (77).
4. MITOCHONDRIAL AND mtDNA DAMAGE
As a network that is directly integrated into crucial cell signaling pathways, with multiple Δψm -dependent factors that sense cellular cues to alter organellar dynamics, mitochondria are a highly responsive indicator of cellular stress. While mitochondria have long been thought to be a major cellular target of oxidative damage, recent findings indicate that a wide range of cellular stresses cause damage to both the mitochondrial network and mtDNA.
In consuming oxygen as the final electron acceptor of the redox reactions catalyzed by Complexes I–IV, mitochondria frequently produce reactive oxygen species (ROS), such as superoxide and hydroxyl radical, as byproducts during oxidative phosphorylation. Denham Harman first proposed that these free radicals produced by the ETC accumulate to damage mitochondria, contributing to the free radical theory of aging (78). Subsequent examination of oxidative damage to mtDNA showed that oxidative lesions to mtDNA occur more frequently, and persist longer, than nuclear DNA damage (79, 80). While it had been postulated that mitochondria lack histone packaging, making its DNA more susceptible to damage (81), the extensive packaging of mtDNA by TFAM makes this much less plausible (82, 83). Moreover, while mitochondria contain less overall DNA repair machinery than the nucleus, they are not devoid of repair mechanisms, instead maintaining both base-excision and putative mismatch repair mechanisms (BER and MMR, respectively). The BER pathway has a robust ability to repair oxidative lesions in DNA (84, 85). As the major replicative DNA polymerase in mitochondria, POLG is a critical player in the mitochondrial BER pathway. When a lesion occurs, DNA glycosylases cleave the N-glycosidic bond between the damaged base and its deoxyribose. An abasic site is left allowing recognition by apurinic/apyrimidinic endonuclease 1 (APE1) which then removes the ribose (86). When ribose is removed final repair can occur by POLG and ligase III. POLG fills in the gaps while ligase III seals the backbone (87). In MMR, base pairs that are inserted or deleted during DNA replication are repaired (86). Bohr’s group showed mismatch-binding activity in mitochondria involving the YB-1 repair factor (88), while Lightowlers’ group also found low-level mismatch repair activity, but did not find the nuclear MMR factor Msh2 in mitochondria (89). Mlh1 overexpression may allow for MMR in the D-loop region to protect mitochondrial integrity (90). It is worth noting, however, that robust mismatch-specific repair has not been demonstrated in purified mitochondria.
Mitochondria thus have both active packaging of mtDNA by TFAM and functional base-excision repair machinery. In addition, the mitochondrial genetic threshold effect further predicts that oxidative base-change mutations are not a likely source of widespread mitochondrial dysfunction. For a single base-change mutation of mtDNA to cause a metabolic defect, even in a tRNA or similar highly-deleterious position, the mutated mtDNA variant must first accumulate to become a majority of the overall mtDNA content of the cell (typically 60–90%, depending on the type of mutation (22). This would seem to suggest that oxidative mutation of mtDNA is not a likely form of critical mitochondrial damage.
Alternately, however, oxidants are found to cause single- and double-strand breaks in mtDNA as the predominant form of mtDNA damage, directly causing loss of mtDNA content through degradation, with the potential for devastating impacts on mitochondrial function and the viability of the cell as a whole. Experiments treating cultured human cells with oxidants such as hydrogen peroxide reveal that while ROS do not elicit any significant increase in base-change mutations of mtDNA, the same treatment elicits a tenfold increase in strand breakage (91). Subsequently, this oxidative strand breakage of mtDNA was shown to decrease OXPHOS activity (92). This is consistent with the loss of mitochondrial metabolism in cells depleted of mtDNA (52), and demonstrates a clear link between oxidative of mtDNA and decreased mitochondrial bioenergetic function.
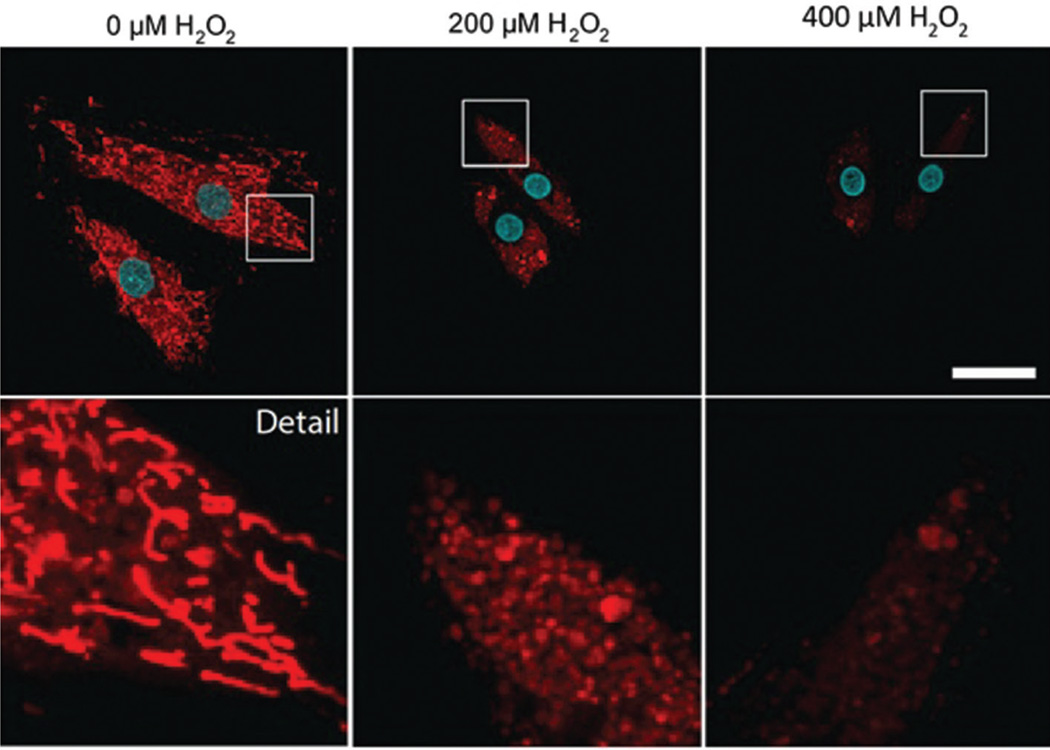
At the same time that oxidative radicals are damaging mtDNA, they are also attacking the mitochondrial network as a whole. While cardiomyoblasts maintain a balance of fusion and fission, mitochondria become completely fragmented upon exposure to concentrations of H2O2 equivalent to those described above (Figure 1), indicating that oxidative damage has a profound effect on the mitochondrial network, disrupting steady-state fusion/fission balance. Moreover, it has been shown that defects in either fission or fusion can also disrupt mtDNA: decreased mitochondrial fission through downregulation of DRP1 causes decreased mtDNA content (93) likely through replication stress (94), while deletion of mitofusins 1 and 2 causes massive loss of mtDNA content (95). Thus, in addition to direct damage to mtDNA, oxidant-mediated disruption of fission and fusion factors also are likely to contribute to the loss of mtDNA that occurs as part of ROS-mediated damage.
Figure 1.
Impact of oxidative stress on mitochondrial morphology. Confocal microscopy of H9C2 cardiomyoblasts stained with MitoTracker (red) and DAPI (cyan) following treatment with H2O2 at concentrations of 0, 200, and 400 µm for 1 hour. Size bar = 10 µm.
While mitochondrial OXPHOS is a significant source of ROS production, other cellular stresses are emerging as potential mediators of mtDNA and mitochondrial damage, indicating that the mitochondrial network is both a target and participant in cytokine-mediated inflammation. Tumor necrosis factor-alpha (TNF-α) is a pro-inflammatory cytokine, requiring Toll-like receptors to initiate inflammation, producing a burst of ROS within the cell. Upon incubation with increasing concentrations of TNF-α, significant increases in ROS and concomitant decreases in mtDNA copy number were clearly evident in cardiac myocytes (96). Subsequent work showed that TNF-α-mediated mtDNA damage requires TNF receptors and ROS production; moreover, this mtDNA damage involves binding of p53 to TFAM (97). Screening for novel mtDNA repair factors has identified the novel mitochondrial DNA polymerase theta (POLθ) that is recruited to the mitochondria when under oxidative stress (98). Moreover, damaged mtDNA appears to be released from the organelle, where it acts as a pro-inflammatory signal in a variety of contexts (99). The mitochondrial network, and mtDNA specifically, are thus a highly sensitive indicator of cellular stress, directly integrated into critical stress-signaling pathways.
5. CONCLUDING REMARKS
Our understanding of the mitochondrial genome and its maintenance within a highly dynamic organellar network has evolved to show that mtDNA is actively packaged and dynamically regulated, interacting with an increasing number of cellular pathways to coordinately participate in mitochondrial responses to a wide range of cellular stimuli. As a critical component of the pleiomorphic, responsive mitochondrial network, mtDNA is emerging as a critical indicator of cell stress, with new insights revealing this small genome to have an outsized impact on metabolism and cellular homeostasis.
Acknowledgments
This work was supported by National Institute of General Medical Sciences 1SC3GM116669-01 (RG) and Diabetes Action Research and Education Foundation Grant 409 (RG). Additional training support provided by USDA 2015-38422-24061 (for IG), National Institute of General Medical Sciences 5R25GM100866-03 (for EJ), and UTRGV Science Foundation Grant 52007568 from the Howard Hughes Medical Institute (for MR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- mtDNA
mitochondrial DNA
- OXPHOS
oxidative phosphorylation
- Δψm
mitochondrial transmembrane potential
- TFAM
Transcription factor A mitochondrial
- POLG
polymerase γ
- mtTFB
mitochondria transcription factor
- mtSSB
mitochondrial single-stranded DNA binding protein
- OPA1
optic atrophy-1
- DRP1
dynamin-related protein-1
- PGC-1α
PPAR-gamma related cofactor-1 alpha
- ROS
reactive oxygen species
- POLRMT
mtRNA polymerase
- NRF-1, NRF-2
nuclear respiratory factors-1 and -2
- CREB
cyclic AMP response binding element-binding protein
- MEF2D
myocyte enhancer factor-2D
- NFκB
nuclear factor kappa B
- MFN1 and MFN2
mitofusins 1 and 2
- Δ-mtDNAs
deletions of mtDNA
- BER
base excision repair
- MMR
mismatch repair
- POLθ
polymerase theta
- TNF-α
tumor necrosis factor-alpha
- APE1
apurinic/apyrimidinic endonuclease 1
REFERENCES
- 1.Sagan L. On the origin of mitosing cells. J Theor Biol. 1967;14(3):255–274. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 2.Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467(7318):929–934. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- 3.Kasamatsu H, Vinograd J. Replication of Circular DNA in Eukaryotic Cells. Annual Review of Biochemistry. 1974;43(1):695–719. doi: 10.1146/annurev.bi.43.070174.003403. [DOI] [PubMed] [Google Scholar]
- 4.Anderson S, Bankier AT, Barrell BG, Debruijn MHL, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJH, Staden R, Young IG. Sequence and Organization of the Human Mitochondrial Genome. Nature. 1981;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 5.Birky CW. Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(25):11331–11338. doi: 10.1073/pnas.92.25.11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giles RE, Blanc H, Cann HM, Wallace DC. Maternal inheritance of human mitochondrial DNA. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(11):6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato M, Sato K. Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochimica et biophysica acta. 2013;1833(8):1979–1984. doi: 10.1016/j.bbamcr.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz M, Vissing J. Paternal inheritance of mitochondrial DNA. The New England journal of medicine. 2002;347(8):576–580. doi: 10.1056/NEJMoa020350. [DOI] [PubMed] [Google Scholar]
- 9.Korhonen JA, Gaspari M, Falkenberg M. TWINKLE Has 5’ -> 3’ DNA helicase activity and is specifically stimulated by mitochondrial single-stranded DNA-binding protein. The Journal of biological chemistry. 2003;278(49):48627–48632. doi: 10.1074/jbc.M306981200. [DOI] [PubMed] [Google Scholar]
- 10.Milenkovic D, Matic S, Kühl I, Ruzzenente B, Freyer C, Jemt E, Park CB, Falkenberg M, Larsson N-G. TWINKLE is an essential mitochondrial helicase required for synthesis of nascent D-loop strands and complete mtDNA replication. Human Molecular Genetics. 2013;22(10):1983–1993. doi: 10.1093/hmg/ddt051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannwarth S, Berg-Alonso L, Augé G, Fragaki K, Kolesar JE, Lespinasse F, Lacas-Gervais S, Burel-Vandenbos F, Villa E, Belmonte F, Michiels J-F, Ricci J-E, Gherardi R, Harrington L, Kaufman BA, Paquis-Flucklinger V. Inactivation of Pif1 helicase causes a mitochondrial myopathy in mice. Mitochondrion. 2016 doi: 10.1016/j.mito.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copeland WC, Longley MJ. DNA polymerase gamma in mitochondrial DNA replication and repair. TheScientificWorldJournal. 2003;3:34–44. doi: 10.1100/tsw.2003.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legros F, Malka F, Frachon P, Lombes A, Rojo M. Organization and dynamics of human mitochondrial DNA. J Cell Sci. 2004;117(Pt 13):2653–2662. doi: 10.1242/jcs.01134. [DOI] [PubMed] [Google Scholar]
- 14.Parisi MA, Clayton DA. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science. 1991;252(5008):965–969. doi: 10.1126/science.2035027. [DOI] [PubMed] [Google Scholar]
- 15.Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, Rustin P, Gustafsson CM, Larsson NG. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Human molecular genetics. 2004;13(9):935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- 16.Freyer C, Park CB, Ekstrand MI, Shi Y, Khvorostova J, Wibom R, Falkenberg M, Gustafsson CM, Larsson N-G. Maintenance of respiratory chain function in mouse hearts with severely impaired mtDNA transcription. Nucleic Acids Research. 2010;38(19):6577–6588. doi: 10.1093/nar/gkq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda M, Ide T, Fujino T, Arai S, Saku K, Kakino T, Tyynismaa H, Yamasaki T, Yamada K-i, Kang D, Suomalainen A, Sunagawa K. Overexpression of TFAM or Twinkle Increases mtDNA Copy Number and Facilitates Cardioprotection Associated with Limited Mitochondrial Oxidative Stress. PLoS ONE. 2015;10(3):e0119687–e0119687. doi: 10.1371/journal.pone.0119687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushima Y, Goto Y, Kaguni LS. Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM) Proc Natl Acad Sci U S A. 2010;107(43):18410–188415. doi: 10.1073/pnas.1008924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu B, Lee J, Nie X, Li M, Morozov YI, Venkatesh S, Bogenhagen DF, Temiakov D, Suzuki CK. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA(+) Lon protease. Molecular cell. 2013;49(1):121–132. doi: 10.1016/j.molcel.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madeira VMC. Overview of Mitochondrial Bioenergetics. In: Palmeira CM, Moreno JA, editors. Mitochondrial Bioenergetics: Methods and Protocols. Totowa, NJ: Humana Press; 2012. [Google Scholar]
- 21.Saraste M. Oxidative Phosphorylation at the fin de siècle. Science. 1999;283(5407):1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- 22.DiMauro S, Schon EA. Mitochondrial DNA mutations in human disease. American journal of medical genetics. 2001;106(1):18–26. doi: 10.1002/ajmg.1392. [DOI] [PubMed] [Google Scholar]
- 23.Taylor SW, Fahy E, Zhang B, Glenn GM, Warnock DE, Wiley S, Murphy AN, Gaucher SP, Capaldi RA, Gibson BW, Ghosh SS. Characterization of the human heart mitochondrial proteome. Nat Biotechnol. 2003;21(3):281–286. doi: 10.1038/nbt793. [DOI] [PubMed] [Google Scholar]
- 24.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong S-E, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134(1):112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sickmann A, Reinders J, Wagner Y, Joppich C, Zahedi R, Meyer HE, Schonfisch B, Perschil I, Chacinska A, Guiard B, Rehling P, Pfanner N, Meisinger C. The proteome of Saccharomyces cerevisiae mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(23):13207–13212. doi: 10.1073/pnas.2135385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forner F, Foster LJ, Campanaro S, Valle G, Mann M. Quantitative proteomic comparison of rat mitochondria from muscle, heart, and liver. Molecular & cellular proteomics: MCP. 2006;5(4):608–619. doi: 10.1074/mcp.M500298-MCP200. [DOI] [PubMed] [Google Scholar]
- 27.Ren J, Li Q, Wu S, Li S-Y, Babcock SA. Cardiac overexpression of antioxidant catalase attenuates aging-induced cardiomyocyte relaxation dysfunction. Mechanisms of ageing and development. 2007;128(3):276–285. doi: 10.1016/j.mad.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 29.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 30.Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proceedings of the National Academy of Sciences. 1994;91(4):1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rantanen A, Jansson M, Oldfors A, Larsson NG. Downregulation of Tfam and mtDNA copy number during mammalian spermatogenesis. Mammalian genome: official journal of the International Mammalian Genome Society. 2001;12(10):787–792. doi: 10.1007/s00335-001-2052-8. [DOI] [PubMed] [Google Scholar]
- 32.Kelly RDW, Mahmud A, McKenzie M, Trounce IA, St John JC. Mitochondrial DNA copy number is regulated in a tissue specific manner by DNA methylation of the nuclear-encoded DNA polymerase gamma A. Nucleic acids research. 2012;40(20):10124–10138. doi: 10.1093/nar/gks770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jäger S, Handschin C, St.-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proceedings of the National Academy of Sciences. 2007;104(29):12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1(alpha) and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 35.Ryu H, Lee J, Impey S, Ratan RR, Ferrante RJ. Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):13915–13920. doi: 10.1073/pnas.0502878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Kim C-H, Simon DK, Aminova LR, Andreyev AY, Kushnareva YE, Murphy AN, Lonze BE, Kim K-S, Ginty DD, Ferrante RJ, Ryu H, Ratan RR. Mitochondrial cyclic AMP response element-binding protein (CREB) mediates mitochondrial gene expression and neuronal survival. The Journal of biological chemistry. 2005;280(49):40398–40401. doi: 10.1074/jbc.C500140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.She H, Yang Q, Shepherd K, Smith Y, Miller G, Testa C, Mao Z. Direct regulation of complex I by mitochondrial MEF2D is disrupted in a mouse model of Parkinson disease and in human patients. The Journal of Clinical Investigation. 2011;121(3):930–940. doi: 10.1172/JCI43871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cogswell PC, Kashatus DF, Keifer JA, Guttridge DC, Reuther JY, Bristow C, Roy S, Nicholson DW, Baldwin AS., Jr NF-kappa B and I kappa B alpha are found in the mitochondria. Evidence for regulation of mitochondrial gene expression by NF-kappa B. The Journal of biological chemistry. 2003;278(5):2963–2968. doi: 10.1074/jbc.M209995200. [DOI] [PubMed] [Google Scholar]
- 39.Johnson RF, Witzel I-I, Perkins ND. p53-dependent regulation of mitochondrial energy production by the RelA subunit of NF-kappaB. Cancer research. 2011;71(16):5588–5597. doi: 10.1158/0008-5472.CAN-10-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olichon A, ElAchouri G, Baricault L, Delettre C, Belenguer P, Lenaers G. OPA1 alternate splicing uncouples an evolutionary conserved function in mitochondrial fusion from a vertebrate restricted function in apoptosis. Cell Death Differ. 2006;14(4):682–692. doi: 10.1038/sj.cdd.4402048. [DOI] [PubMed] [Google Scholar]
- 42.Griparic L, Kanazawa T, van der derBliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178(5):757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guillery O, Malka F, Landes T, Guillou E, Blackstone C, Lombès A, Belenguer P, Arnoult D, Rojo M. Metalloprotease-mediated OPA1 processing is modulated by the mitochondrial membrane potential. Biology of the Cell. 2008;100(5):315–325. doi: 10.1042/BC20070110. [DOI] [PubMed] [Google Scholar]
- 44.Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187(7):1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187(7):959–966. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19(6):2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen Q, Yamano K, Head BP, Kawajiri S, Cheung JTM, Wang C, Cho J-H, Hattori N, Youle RJ, van derder Bliek AM. Mutations in Fis1 disrupt orderly disposal of defective mitochondria. Molecular Biology of the Cell. 2014;25(1):145–159. doi: 10.1091/mbc.E13-09-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. The Journal of Cell Biology. 2005;170(7):1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smirnova E, Griparic L, Shurland D-L, van der Bliek AM. Dynamin-related Protein Drp1 Is Required for Mitochondrial Division in Mammalian Cells. Molecular Biology of the Cell. 2001;12(8):2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amchenkova AA, Bakeeva LE, Chentsov YS, Skulachev VP, Zorov DB. Coupling membranes as energy-transmitting cables. I. Filamentous mitochondria in fibroblasts and mitochondrial clusters in cardiomyocytes. The Journal of Cell Biology. 1988;107(2):481–495. doi: 10.1083/jcb.107.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilkerson RW, Margineantu DH, Capaldi RA, Selker JM. Mitochondrial DNA depletion causes morphological changes in the mitochondrial reticulum of cultured human cells. FEBS Lett. 2000;474(1):1–4. doi: 10.1016/s0014-5793(00)01527-1. [DOI] [PubMed] [Google Scholar]
- 52.Legros F, Lombès A, Frachon P, Rojo M. Mitochondrial Fusion in Human Cells Is Efficient, Requires the Inner Membrane Potential, and Is Mediated by Mitofusins. Molecular Biology of the Cell. 2002;13(12):4343–4354. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cereghetti GM, Stangherlin A, de Brito OM, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proceedings of the National Academy of Sciences. 2008;105(41):15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO reports. 2007;8(10):939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kashatus DF, Lim K-H, Brady DC, Pershing NLK, Cox AD, Counter CM. RALA and RALBP1 regulate mitochondrial fission at mitosis. Nat Cell Biol. 2011;13(9):1108–1115. doi: 10.1038/ncb2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO Journal. 2008;27(2):433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katajisto P, Döhla J, Chaffer CL, Pentinmikko N, Marjanovic N, Iqbal S, Zoncu R, Chen W, Weinberg RA, Sabatini DM. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science. 2015;348(6232):340–343. doi: 10.1126/science.1260384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proceedings of the National Academy of Sciences. 2011;108(25):10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narendra D, Tanaka A, Suen D-F, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. The Journal of Cell Biology. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oettinghaus B, D’Alonzo D, Barbieri E, Restelli LM, Savoia C, Licci M, Tolnay M, Frank S, Scorrano L. DRP1-dependent apoptotic mitochondrial fission occurs independently of BAX, BAK and APAF1 to amplify cell death by BID and oxidative stress. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2016;1857(8):1267–1276. doi: 10.1016/j.bbabio.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC, Gupta MP. SIRT3 Deacetylates and Activates OPA1 To Regulate Mitochondrial Dynamics during Stress. Molecular and Cellular Biology. 2014;34(5):807–819. doi: 10.1128/MCB.01483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alam TI, Kanki T, Muta T, Ukaji K, Abe Y, Nakayama H, Takio K, Hamasaki N, Kang D. Human mitochondrial DNA is packaged with TFAM. Nucleic acids research. 2003;31(6):1640–1645. doi: 10.1093/nar/gkg251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Margineantu DH, Gregory Cox W, Sundell L, Sherwood SW, Beechem JM, Capaldi RA. Cell cycle dependent morphology changes and associated mitochondrial DNA redistribution in mitochondria of human cell lines. Mitochondrion. 2002;1(5):425–435. doi: 10.1016/s1567-7249(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 64.Iborra FJ, Kimura H, Cook PR. The functional organization of mitochondrial genomes in human cells. BMC Biol. 2004;2:9. doi: 10.1186/1741-7007-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He J, Cooper HM, Reyes A, Di Re M, Sembongi H, Litwin TR, Gao J, Neuman KC, Fearnley IM, Spinazzola A, Walker JE, Holt IJ. Mitochondrial nucleoid interacting proteins support mitochondrial protein synthesis. Nucleic Acids Res. 2012;40(13):6109–6121. doi: 10.1093/nar/gks266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gilkerson RW, Schon EA, Hernandez E, Davidson MM. Mitochondrial nucleoids maintain genetic autonomy but allow for functional complementation. J Cell Biol. 2008;181(7):1117–1128. doi: 10.1083/jcb.200712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kukat C, Wurm CA, Spahr H, Falkenberg M, Larsson NG, Jakobs S. Super-resolution microscopy reveals that mammalian mitochondrial nucleoids have a uniform size and frequently contain a single copy of mtDNA. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(33):13534–13539. doi: 10.1073/pnas.1109263108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proceedings of the National Academy of Sciences. 2009;106(29):11960–11965. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adams DW, Wu LJ, Errington J. Nucleoid occlusion protein Noc recruits DNA to the bacterial cell membrane. The EMBO Journal. 2015;34(4):491–501. doi: 10.15252/embj.201490177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu LJ, Ishikawa S, Kawai Y, Oshima T, Ogasawara N, Errington J. Noc protein binds to specific DNA sequences to coordinate cell division with chromosome segregation. The EMBO Journal. 2009;28(13):1940–1952. doi: 10.1038/emboj.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- 72.Moraes CT, Ciacci F, Silvestri G, Shanske S, Sciacco M, Hirano M, Schon EA, Bonilla E, DiMauro S. Atypical clinical presentations associated with the MELAS mutation at position 3243 of human mitochondrial DNA. Neuromuscular Disorders. 1993;3(1):43–50. doi: 10.1016/0960-8966(93)90040-q. [DOI] [PubMed] [Google Scholar]
- 73.Yoneda M, Miyatake T, Fau - Attardi G, Attardi G. Heteroplasmic mitochondrial tRNA(Lys) mutation and its complementation in MERRF patient-derived mitochondrial transformants. Muscle Nerve Suppl. 1995 doi: 10.1002/mus.880181420. [DOI] [PubMed] [Google Scholar]
- 74.Ono T, Isobe K, Nakada K, Hayashi J-I. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet. 2001;28(3):272–275. doi: 10.1038/90116. [DOI] [PubMed] [Google Scholar]
- 75.Nakada K, Inoue K, Ono T, Isobe K, Ogura A, Goto Y-I, Nonaka I, Hayashi J-I. Inter-mitochondrial complementation: Mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nat Med. 2001;7(8):934–940. doi: 10.1038/90976. [DOI] [PubMed] [Google Scholar]
- 76.Yang L, Long Q, Liu J, Tang H, Li Y, Bao F, Qin D, Pei D, Liu X. Mitochondrial fusion provides an ‘initial metabolic complementation’ controlled by mtDNA. Cellular and Molecular Life Sciences. 2015;72(13):2585–2598. doi: 10.1007/s00018-015-1863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gilkerson RW, De Vries RL, Lebot P, Wikstrom JD, Torgyekes E, Shirihai OS, Przedborski S, Schon EA. Mitochondrial autophagy in cells with mtDNA mutations results from synergistic loss of transmembrane potential and mTORC1 inhibition. Hum Mol Genet. 2012:978–990. doi: 10.1093/hmg/ddr529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harman D. Free radical theory of aging: dietary implications. Am J Clin Nutr. 1972;25(8):839–843. doi: 10.1093/ajcn/25.8.839. [DOI] [PubMed] [Google Scholar]
- 79.Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94(2):514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown WM, George M, Wilson AC. Rapid evolution of animal mitochondrial DNA. Proceedings of the National Academy of Sciences. 1979;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alexeyev MF. Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS Journal. 2009;276(20):5768–5787. doi: 10.1111/j.1742-4658.2009.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gouliaeva NA, Kuznetsova EA, Gaziev AI. Proteins associated with mitochondrial DNA protect it against X-rays and hydrogen peroxide. Biophysics. 2006;51(4):620–623. [PubMed] [Google Scholar]
- 84.Gredilla R, Bohr VA, Stevnsner T. Mitochondrial DNA repair and association with aging--an update. Experimental gerontology. 2010;45(7–8):478–488. doi: 10.1016/j.exger.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Svilar D, Goellner EM, Almeida KH, Sobol RW. Base Excision Repair and Lesion-Dependent Subpathways for Repair of Oxidative DNA Damage. Antioxidants & Redox Signaling. 2011;14(12):2491–2507. doi: 10.1089/ars.2010.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kazak L, Reyes A, Holt IJ. Minimizing the damage: repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol. 2012;13(11):726–726. doi: 10.1038/nrm3439. [DOI] [PubMed] [Google Scholar]
- 87.Prakash A, Doublie S. Base Excision Repair in the Mitochondria. Journal of cellular biochemistry. 2015;116(8):1490–1499. doi: 10.1002/jcb.25103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Souza-Pinto NC, Mason PA, Hashiguchi K, Weissman L, Tian J, Guay D, Lebel M, Stevnsner TV, Rasmussen LJ, Bohr VA. Novel DNA mismatch-repair activity involving YB-1 in human mitochondria. DNA repair. 2009;8(6):704–719. doi: 10.1016/j.dnarep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mason PA, Matheson EC, Hall AG, Lightowlers RN. Mismatch repair activity in mammalian mitochondria. Nucleic acids research. 2003;31(3):1052–1058. doi: 10.1093/nar/gkg167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mishra M, Kowluru RA. Retinal Mitochondrial DNA Mismatch Repair in the Development of Diabetic Retinopathy, and Its Continued Progression After Termination of HyperglycemiaDiabetic Retinopathy and mtDNA Mismatch. Investigative Ophthalmology & Visual Science. 2014;55(10):6960–6967. doi: 10.1167/iovs.14-15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009;37(8):2539–2548. doi: 10.1093/nar/gkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Furda AM, Marrangoni AM, Lokshin A, Van Houten B. Oxidants and not alkylating agents induce rapid mtDNA loss and mitochondrial dysfunction. DNA Repair. 2012;11(8):684–692. doi: 10.1016/j.dnarep.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, Barja F, Martinou J-C. Preventing Mitochondrial Fission Impairs Mitochondrial Function and Leads to Loss of Mitochondrial DNA. PLoS ONE. 2008;3(9):e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qian W, Choi S, Gibson GA, Watkins SC, Bakkenist CJ, Van Houten B. Mitochondrial hyperfusion induced by loss of the fission protein Drp1 causes ATM-dependent G2/M arrest and aneuploidy through DNA replication stress. Journal of Cell Science. 2013;125(23):5745–5757. doi: 10.1242/jcs.109769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial Fusion Is Required for mtDNA Stability in Skeletal Muscle and Tolerance of mtDNA Mutations. Cell. 2010;141(2):280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, Takeshita A. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107(10):1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. [DOI] [PubMed] [Google Scholar]
- 97.Vadrot N, Ghanem S, Braut F, Gavrilescu L, Pilard N, Mansouri A, Moreau R, Reyl-Desmars F. Mitochondrial DNA maintenance is regulated in human hepatoma cells by glycogen synthase kinase 3beta and p53 in response to tumor necrosis factor alpha. PLoS One. 2012;7(7):e40879. doi: 10.1371/journal.pone.0040879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wisnovsky S, Jean SR, Kelley SO. Mitochondrial DNA repair and replication proteins revealed by targeted chemical probes. Nat Chem Biol. 2016;12(7):567–573. doi: 10.1038/nchembio.2102. [DOI] [PubMed] [Google Scholar]
- 99.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485(7397):251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]