Abstract
Conclusion
Replacement of vestibular hair cells induced by atoh1 driven by the tissue-specific GFAP promoter was significantly more efficient than use of the cBA or hCMV promoter.
Objective
To test whether expression level, persistence, or selectivity from adenovirus vectors delivered in the inner ear can be altered by changing the adenovector backbone or by using different cellular and viral promoters.
Materials and methods
Adenovector and promoter modifications were tested for differences in transgene expression in adult macular organs. The effect of using an E1/E3 deleted vector was compared to E1/E3/E4 deleted vectors. The effect of using viral and cellular promoters to modify transgene expression was tested in explanted adult mouse macular organs. Based on these results three different promoters were tested for efficacy of atonal gene.
Results
Use of adenovectors containing human CMV, the hybrid cBA and ubiquitin promoters driving transgene expression resulted in different types of transgene expression. While several viral and cellular promoters provided broad cell type expression, expression driven by the GFAP promoter was limited to vestibular supporting cells, demonstrating the specificity of this promoter.
Keywords: Adenovector, promoter, green fluorescent protein, glial fibrillary acidic protein, tissue-specific promoter, hair cell regeneration
Introduction
Few drugs are currently available for the treatment of inner ear disease. Delivery of atoh1 to the inner ear triggering the transdifferentiation of supporting cells to hair cells has been suggested as a possible treatment for loss of sensory hair cells [1]. In the design of such a treatment for hearing loss and balance disorders it may be beneficial to specify the level of expression, duration of expression, and localization of transgene expression. To begin the design of adenovectors for delivering genes to the vestibular system we analyzed the impact of vector backbone and promoter combinations. The basic construction of Ad5 serotype vectors involves deleting different portions of the adenovirus genome necessary for virus replication and placing these genes into the genome of cell lines used to propagate the vectors. It has been previously reported that modifications of the adenovector polymerase gene [2] or deletion of the adenovirus E4 region, in addition to deletion of the E1 and E3 regions, result in vectors that have different levels and duration of transgene expression in specific tissues [3]. In addition, the promoter used to drive the delivered transgene can influence the expression of the transgene in a tissue-specific fashion.
Our results indicate that deletion of the E4 region from the adenovirus vector does not compromise location or level of transgene expression and may provide greater duration of gene expression in the inner ear. In addition, the selection of promoters impacted the location, duration, and level of gene expression. Delivery of atoh1 using the tissue-selective GFAP promoter induced the most efficient recovery of hair cells. These results suggest that vector designs including a tissue-selective promoter driving atoh1 with an E1/E3/E4 deleted vector is likely to have both safety and efficacy advantages.
Materials and methods
Macular organ culture
All animal studies were approved by the Kansas University School of Medicine Institutional Animal Care and Use Committee. Adult C57Bl6 mice were anesthetized with intraperitoneal avertin and decapitated. The otic capsule was exposed and the macular organs were identified by finding the otolithic membranes. Using no. 5 watchmaker forceps, the utricles were removed and the otolith layer was dissected away. Explants were exposed to vector in a 50 μl volume of Dulbecco’s modified Eagle’s medium (DMEM) for 24 h. The organs were then washed in DMEM and cultured on a Millicel membrane (Millipore, Bedford, MA, USA) suspended in 1500 μl of DMEM supplemented with N1 (Sigma, St Louis MO, USA) plus 100 u/ml penicillin and 5.5 μl/ml of 30% glucose and maintained in vitro (37°C, 5% CO2). Medium was exchanged every 7 days for the duration of the experiment. Five explants were placed on each membrane.
Vector production
E1/E3 deleted (Ad) or E1/E3/E4 (Ad.11D) deleted vectors were used with an expression cassette of green fluorescent protein (f), or luciferase (L). A third set of vectors expressed atoh1 (NM 007500). Promoters used included human CMV promoter (hCMV), CMV enhancer/chicken β-actin (cBA), ubiquitin (Ub) or glial fibrillary acidic protein promoter (GFAP). The production system for these adenovectors provides robust replication of the adenovector and purified stocks at 5×1011 to 2×1012 total particles (particle unit, pu) per ml with a total particle to active particle (fluorescent focus unit, ffu) ratio ranging from 3 to 10 pu/ffu. Total particles (pu) are determined by a spectrophotometric assay. Adenovector lots are purified, aliquoted, and stored at −80°C. Individual aliquots were used for each experiment to prevent loss of activity associated with freeze–thaw cycles.
Effect of E4 deletion on expression
Macular explants were treated with 1×105 to 5×106 pu of either Adf (E1/E3 deleted, hCMV promoter, GFP transgene) or Adf.11D (E1/E3/E4 deleted, hCMV promoter, GFP transgene). At weekly intervals, the explants (n = 10 each group) were imaged using an inverted fluorescent microscope. Counts of total GFP-positive cells in each explant were obtained. Relative GFP fluorescence per explant was determined by imaging the explant under low power and collecting total GFP fluorescence for each explant for 5 s at a fixed gain ratio. Data were analyzed using NIH image.
Evaluation of promoters in vitro
Macular organ explants were harvested as described above and treated with 1×108 pu of E1/E3/E4 deleted adenovector with luciferase driven either by hCMV, cBA, Ub or GFAP promoters. Expression of the luciferase transgene was quantified at 2, 7, and 14 days post vector delivery. Explants were washed in phosphate-buffered saline (PBS). The entire explant was directly extracted in reporter lysis buffer, the amount of total protein was determined by protein assay, and the amount of luciferase activity was determined by luminescence and expressed as relative light units per microgram of total protein (Applied Biosystems, Bedford, MA, USA). Controls consisted of untreated, cultured macular organs.
A subset of explants was treated with neomycin 10−3 M for 48 h followed by E1/E3/E4 deleted adenovectors using either hCMV or cBA promoters to drive GFP (1×108 pu). At 48 h post transfection the explants were fixed in 4% paraformaldehyde for 20 min, washed in PBS, and embedded in OCT mounting medium. After rapid freezing 10 μm sections were cut and mounted. Sections were stained with Hoechst stain 33342 and imaged for GFP fluorescence and nuclear position. A series of randomly selected sections were immunostained with anti-myosin VIIa and counterstained with Hoechst stain 33342 to evaluate the efficacy of neomycin treatment.
For evaluation of atoh1-expressing vectors, macular organ cultures were treated with neomycin 10−3 M for 48 h. Explants were washed in medium and exposed to 1×108 pu of hCMV-, cBA- or GFAP-driven atoh1. Explants were then cultured on Millicel membranes for 14 days. Subsequently explants were fixed and immunostained for myosin VIIa. Controls consisted of untreated explants, explants treated with neomycin for 48 h, explants treated with neomycin for 48 h and cultured for 14 days, and explants treated with neomycin followed by exposure to a GFP-expressing vector. Total myosin VII-positive hair cell counts were determined for each explant. To control for damaged hair cells or hair cell debris only whole myosin VII-positive cells with a visible nucleus were counted.
Evaluation of constitutive versus tissue-specific promoters in vivo
C57BL6 mice (3 months old) were anesthetized using an intraperitoneal (IP) injection of a weight-adjusted dose of avertin 0.5 mg/g. A dorsal post auricular approach allowed exposure of the facial nerve, the posterior semicircular canal, and the bulla. A diamond drill was used to open the bulla. The stapedial artery and round window (RW) niche were identified and animals were then mounted in a stereotactic head frame using a snout holder. Vector injections into the scala tympani were carried out using a Hamilton micro syringe with 0.1 μl graduations. Either 1×108 pu of Adf.11D or Ad.GFAP.f.11D were injected through the RW into the scala tympani. The RW was sealed with a small patch of connective tissue and the animals were allowed to recover for 48 h. The animals were then anesthetized as described and sacrificed via intracardiac perfusion with 4% PBS-buffered paraformaldehyde. The temporal bones were removed, post-fixed, decalcified, and embedded in paraffin. After cutting 7 μm sections and de-waxing, immunohistochemistry for GFP was carried out using rabbit anti-GFP monoclonal antibody (AbCam Inc., Boston, MA, USA). An immunohistochemical approach was used to detect GFP, since the GFAP promoter is a very low activity promoter. Sections were stained with fluorescent labeled secondary antibody (Vectastain Inc.).
Evaluation of relative expression levels of atoh1 by semi-quantitative RT-PCR
Macular organs were harvested and cultured as described above. The explants were treated with neomycin 10−3 M for 48 h and subsequently transfected with 1×109 pu adenovector expressing atoh1 driven by the hCMV, CBA or GFAP promoters. An empty capsid vector served as a control. After 48 h total mRNA was extracted from the cultures. Atoh1 expression levels were determined using a Taqman system RT-PCR assay (ampliTaqGold DNA polymerase 40 cycles, 95°C for 15 s, 60°C for 1 min). Atoh1 primers used were AACGGCGCAGGATGCA (sense), TTGAAGGACGGGATAACGTTG (antisense), and 6FAM CTGAACCACGCCTTCGACCAGCTG MGBNQF (probe). Results were normalized to a relative standard curve using mbActin (Applied Biosystems) and negative (neomycin plus control vector) controls.
Statistics
Statistical significance was determined by t test and ANOVA using SPSS version 12.
Results
Efficient gene delivery with adenovector to inner ear tissue
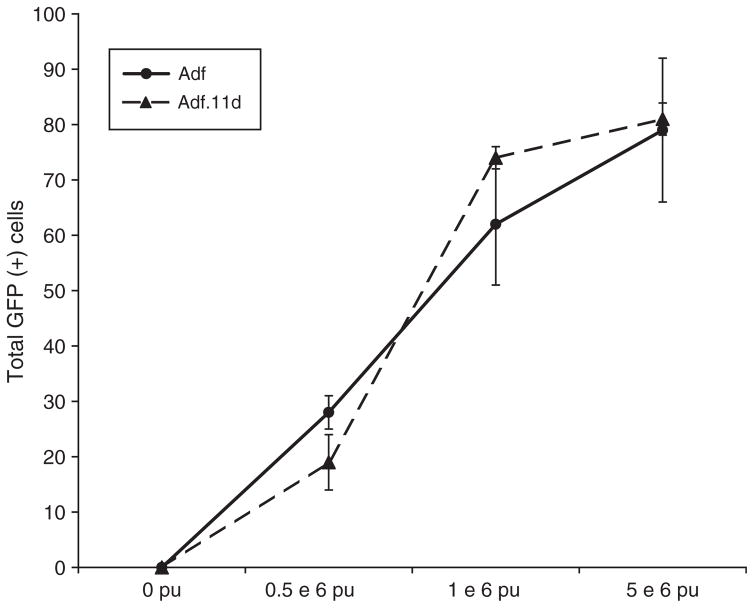
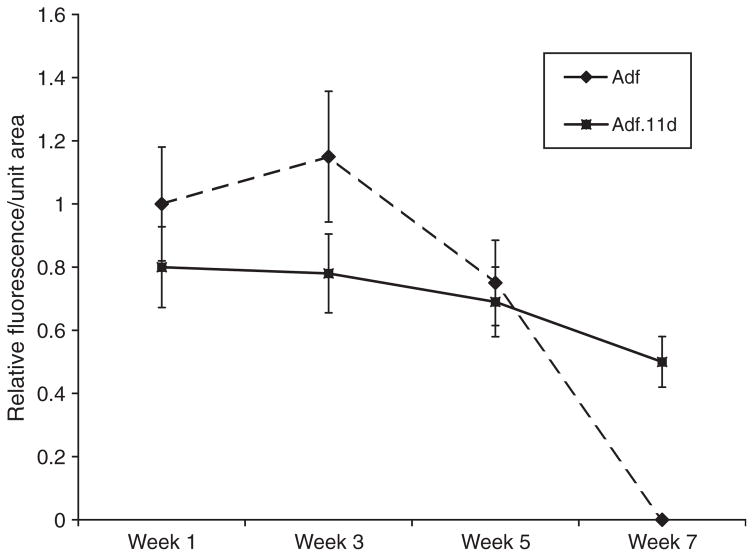
A dose-finding experiment was undertaken in adult macular organ explant cultures to determine the vector dose for efficient transgene delivery. A clear dose response was seen with both adenovector bases delivered. Lower total titers of vector resulted in progressively lower numbers of GFP transduced cells (Figures 1 and 2). Explants treated with 0.5–5×106 pu of Adf.11D demonstrated diffuse transfection of the vestibular explants (Figure 1). Expression of GFP was followed over time and shown to persist for up to 5 weeks in explants treated with Adf and up to 7 weeks in explants treated with Adf.11D (Figure 3). This result suggests that the E1/E3/E4 deleted adenovector with the hCMV promoter may provide extended persistence of expression as compared with the same expression cassette contained within an E1/E3 deleted adenovector. No difference in total cells transfected was seen when comparing the two constructs (Figure 2).
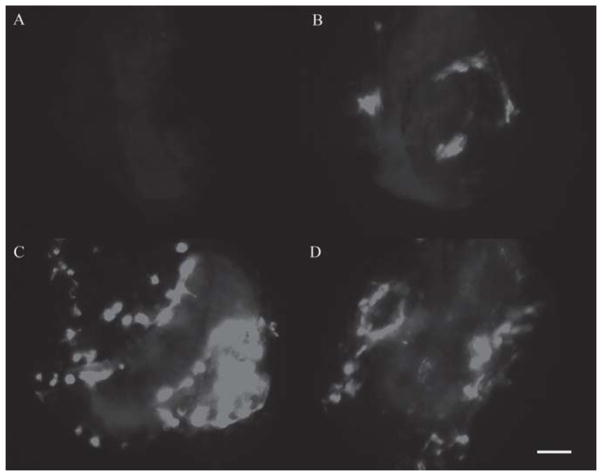
Figure 1.
Fluorescence view of macular organ whole mounts transfected with Adf.11D at 1 week post transfection. Explants are visualized on a Millicel membrane using an inverted fluorescence microscope. A control (no vector administration) explant is seen in (A). Increasing doses of vector result in increasing number of GFP-positive cells. Delivery of 1×105 pu of Adf.11D is seen in (B), 1×106 pu in (C) and delivery of 5×106 pu of Adf.11D is shown in (D). These cultures can be repeatedly imaged over a period of several weeks, allowing the evaluation of different vector constructs for transgene expression in the vestibular neuroepithelium. n = 10 for each condition. Bar = 200 μm.
Figure 2.
Cell counts of adult macular explants treated with Adf or Adf.11D. No difference was seen in transduction efficiency when comparing E1/E3 to E1/E3/E4 deleted vectors. n = 10 for each condition.
Figure 3.
Time course of GFP expression in macular organ cultures. Relative fluorescence intensity was determined by measuring total fluorescence/unit area for six utricles. The highest dose of vector (5×106 pu) for each culture condition was used. The Adf (+) cultures at 1 week were used to normalize fluorescence levels. Advanced generation vectors that carried an E4 region deletion expressed GFP at a slightly lower level but yielded a longer expression period. Expression at the 3 and 7 week time points were significantly different (p < 0.01). n = 10 for each condition.
Changes in promoter yield different levels and duration of expression
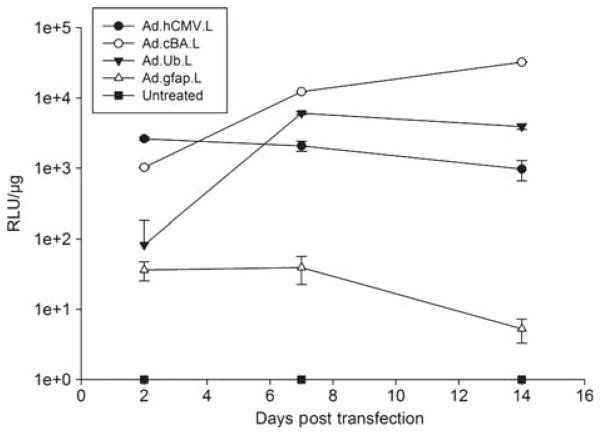
Using an E1/E3/E4 deleted vector system we expressed luciferase in adult macular organ cultures using three different constitutively active promoter systems to determine the role the promoter may have in transgene expression in this system. Expression of luciferase was driven by the hybrid CMV enhancer/chicken β-actin, human CMV or ubiquitin promoters. Additionally we tested the activity of the supporting cell-specific GFAP promoter. As can be seen in Figure 4, among the constitutively active promoters, the ubiquitin promoter initially yielded the lowest level of expression and reached steady state at day 7. Luciferase expression was initially driven at higher levels by both the human CMV and the cBA promoter. Expression of luciferase driven by the CMV promoter slowly declined whereas the cBA promoter drove progressively higher expression of luciferase. The tissue-specific GFAP promoter yielded only a very low level of luciferase production compared with the constitutively active promoters.
Figure 4.
Activity of different promoters in inner ear tissue was assessed by using luciferase-expressing vector in macular organ cultures and measuring the average luciferase activity per μg of extracted protein over time. Results are expressed in a logarithmic scale due to significant differences in expression when comparing promoter types. Use of the human CMV promoter resulted in a steady level of luciferase production (Ad.hCMV.L.). The ubiquitin promoter demonstrated a slow decline of luciferase production over the 2 week study period (AdUb.L.). Expression of luciferase driven by the hybrid CMV enhancer/chicken β-actin promoter resulted in a steady increase in luciferase production (AdcBA.L.). Use of a supporting cell-specific promoter (GFAP) resulted in very low levels of luciferase production (Ad.GFAP.L). At 7 and 14 days post delivery, luciferase levels were significantly different for all groups (p < 0.01). n = 10 for each condition.
Distribution of hCMV and GFAP promoter-driven vectors in vivo
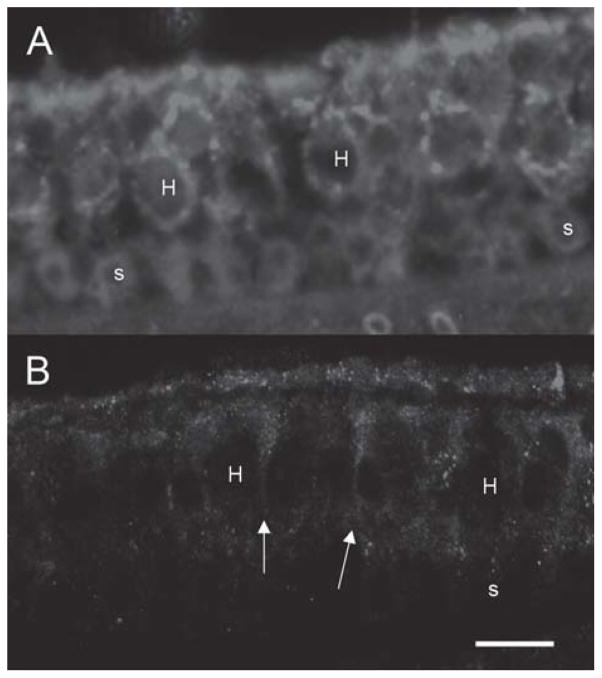
To determine if cell type-specific expression could be elicited after in vivo delivery to the inner ear we compared expression from the tissue-selective GFAP promoter to the ubiquitous hCMV promoter. Expression from the hCMV promoter was seen in multiple cell types of the inner ear, as previously described, and immunohistochemical labeling of GFP was noted in vestibular hair cells and supporting cells (Figure 5A). In contrast to the hCMV promoter profile, expression from the GFAP promoter after delivery of AdGFAP.-f.11D resulted in GFP immunolabeling in the vestibular supporting cells (Figure 5B).
Figure 5.
Effect of a tissue-specific promoter (GFAP promoter) on GFP delivery in vivo. GFP expression using an hCMV-driven vector is seen in (A). Both hair cells and supporting cells demonstrate immunoreactivity to GFP. GFP driven by the GFAP promoter appears to weakly stain supporting cells, extending to the apical surface of the neuroepithelium (arrows). Hair cells are not stained. n=for 3 each condition. Hair cells are designated by ‘H’ and supporting cells by ‘s’. Bar = 20 μm.
Effect of atoh1 delivery on macular organ cultures
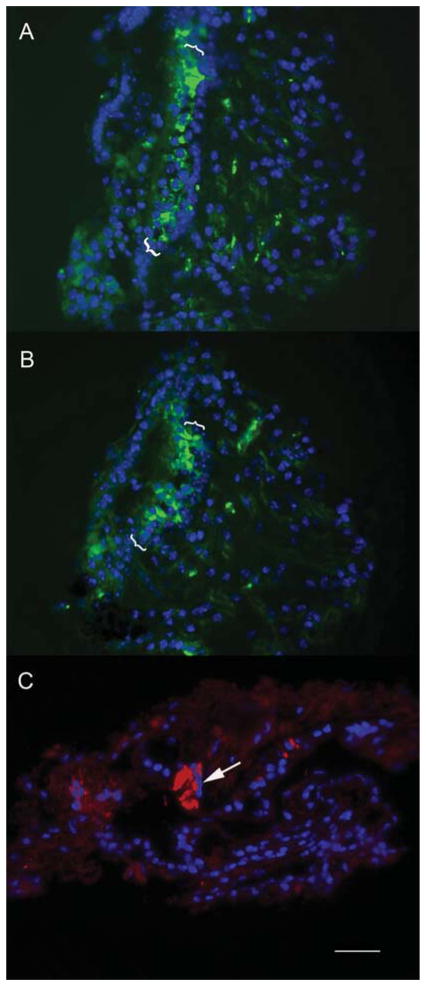
The efficacy of transfection in neomycin-treated explants was tested by evaluating GFP expression patterns in explants treated with neomycin for 48 h followed by delivery of Adf.11D or Ad.cBA.11D. After 48 h explants were fixed, sectioned, and counterstained with a nuclear stain. As seen in Figure 6A and B, both constitutively active promoters led to expression of GFP in the residual cells in the damaged neuroepithelium. Immunostaining for myosin VII in these explants demonstrated only occasional surviving hair cells (Figure 6C).
Figure 6.
Transfection distribution vectors using constitutively active promoters. The efficacy of transfection in neomycin-treated explants was tested by evaluating GFP expression patterns in explants treated with neomycin for 48 h followed by delivery of Adf.11D or Ad.cBA.11D. After 48 h explants were fixed, sectioned, and counterstained with a nuclear stain. As seen in (A) and (B) both constitutively active promoters lead to expression of GFP in the residual cells in the damaged neuroepithelium. Immunostaining for myosin VII in these explants demonstrates only occasional surviving hair cells (arrow, C), suggesting that the GFP-expressing cells seen in (A) and (B) mainly represent supporting cells or other components of the damaged neuroepithelium.
To test whether the differences in transgene expression could impact recovery of sensory cells in damaged sensory epithelium, three different promoters driving atoh1 were evaluated (hCMV, cBA, and GFAP). These were chosen so that we tested a low and high level constitutively active promoter as well as a supporting cell-specific promoter. Atoh1 levels were determined 48 h after gene delivery to neomycin-treated explants using semi-quantitative RT-PCR. In utricles treated with the hCMV-driven atoh1, 8.2-fold elevation of atoh1 over neomycin-only-treated controls was seen. The cBA promoter induced a 3.8-fold increase in atoh1 expression, whereas the GFAP promoter induced only a 0.3-fold increase in atoh1 over controls.
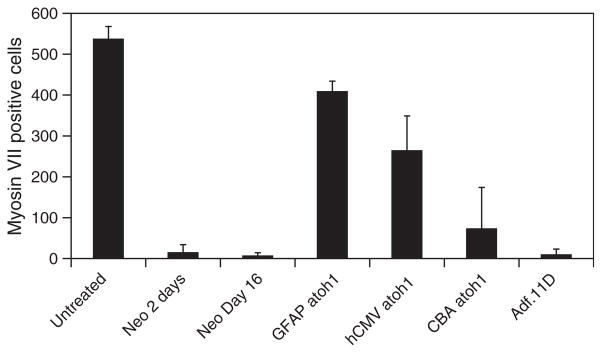
Using the same constructs, we next evaluated the effect of different levels of atoh1 delivery on neomycin-treated macular organ cultures. Outcome measures consisted of counts of whole myosin VII-positive cells from whole mount cultures. This was done to avoid counting damaged cells or cellular debris. At 48 h after neomycin exposure most myosin VII-positive cells had been eliminated (Figure 6C and 8). The damaged macular explant cultures showed no significant spontaneous recovery of hair cells after 14 days in vitro (Figure 7B and Figure 8). Explants treated with neomycin followed by delivery of the control adenovector expressing GFP did not show any recovery of hair cells (Figure 7), suggesting that transfection alone and vector backbone effects do not induce recovery of hair cells. Explants treated with neomycin followed by delivery of vectors expressing atoh1 all demonstrated recovery of hair cells (Figure 8). Use of the cBA promoter resulted in large abnormal appearing myosin VII-positive cells (Figure 7C, arrow). Overall counts of myosin VII-positive cells demonstrated a significant number of regenerated myosin VII-positive cells in cultures treated with either hCMV- or cBA-driven atoh1 (p < 0.01) compared with untreated neomycin controls or cultures treated with neomycin and a control vector (Figure 8). Delivery of atoh1 using the GFAP promoter produced the most robust recovery of normal appearing hair cells (Figure 7) and yielded a threefold increase in hair cell counts compared with the hCMV promoter and a fourfold increase compared with cBA-driven atoh1 treated cultures (Figure 8).
Figure 8.
Hair cell counts for macular organ cultures treated with neomycin followed by atoh1 delivered by adenovectors with hCMV, cBA, and GFAP promoters. Controls consisted of untreated macular organ cultures, macular organ cultures treated with neomycin for either 2 days or 2 days followed by 14 days in vitro, or macular organ cultures treated with neomycin followed by exposure to an adenovector expressing GFP (Adf.11D). Hair cell counts were determined by counting total number of myosin VII-positive cells in the explant (n=5). Delivery of Ad.GFAP.atoh1 resulted in significantly better regeneration than cultures treated with hCMV or cBA driven by atoh1 (p< 0.01). In all cases atoh1 delivery resulted in significantly improved recovery of hair cells when compared with neomycin only or neomycin plus control vector-treated cultures (p< 0.01).
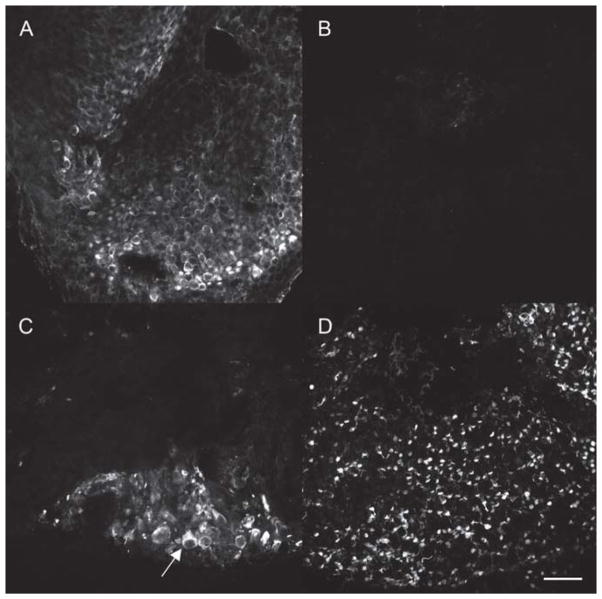
Figure 7.
Effect of promoters on hair cell regeneration in vitro. Whole mount macular organ cultures maintained in vitro for 14 days and immunostained for the presence of myosin VII demonstrated a dense population of hair cells (A). Treatment with neomycin resulted in destruction of hair cells with no evidence of spontaneous recovery of myosin VII-positive cells at 14 days post aminoglycoside (B). Cultures treated with atoh1 driven by the cBA promoter after neomycin treatment resulted in regeneration of few hair cells that appeared abnormally large (arrow) (C). Delivery of atoh1 using the GFAP promoter resulted in the production of a robust number of hair cells (D). n=10 for each condition. Bar=200 μm.
Discussion
Control of vector dose, location of transgene expression, and level of transgene expression may be very important to develop effective, safe, and specific gene delivery drugs. The binding characteristics of a vector determine which cell it will enter, whereas the vector backbone and promoter used determine the level of expression of the transgene. One of the challenges in vector design to achieve these goals is rapid assessment of vector alterations and comparison of different vector properties. This is especially challenging in a complex organ like the inner ear. In most gene therapy research, vector design is initially tested on cell or tissue culture. For application in the inner ear we are limited to postnatal mouse organ of Corti cultures, cultures of dissociated auditory neurons, and cultures of macular epithelium [4,5]. Macular epithelial cultures are derived from adult animals and have mature supporting and hair cells. Earlier studies have demonstrated the utility of these cultures [6]. In this study we demonstrated the ability to utilize these cultures for long-term gene transfer studies. Using the Millicell membrane culture system, explants can be stably maintained for long time periods (Figure 3). The cultures are amenable to examination with a fluorescence inverted microscope, allowing the study of expression time courses in individual explants (Figure 1). The use of both a GFP and a luciferase-based reporter system in these studies allows for both the evaluation of cell types transduced and easy quantification of overall expression level and duration. GFP allows for easy examination of the living explants over time (Figure 3) and for determination of localization of expression (Figures 5, 6A and Figure 6B). Determination of luciferase levels in tissue gives better quantification of transgene expression and allows a direct and accurate relationship to be established between vector dose and level of transgene expression per total tissue (Figure 4). In our studies we found that a key component for effective transfection is to carry the initial vector delivery out in a small (50 μl) volume, rather than adding the vector to the whole volume of the culture (1500 μl).
We demonstrated that E1, E3, and E4 deleted adenovectors yielded robust expression of a delivered transgene in tissue and these may persist for longer time periods than E4+ vectors, as described in other systems [7]. The rationale for using an E4 deleted vector is that less cellular disturbance is expected due to the lack of expression of E4 genes from the adenovirus [8,9]. It is likely that E4 deletion will result in a reduced impact on cells of the inner ear and thus should improve tolerability. Short-term transfection studies (Figure 2) suggest that there are no dose-related differences when comparing the E1/E3 and E1/E3/E4 deleted vectors. However, the E1/E3/E4 deleted vectors expressed GFP for a longer time period (Figure 3), leading us to adopt this as the standard vector backbone for our experiments.
We showed that the expression characteristics from adenovectors delivered to the inner ear can be altered by changing the promoter. Using the explant culture system, we demonstrated that different promoters clearly give different levels of transgene expression, giving us the opportunity to modify expression levels of genes driven by constitutively active promoters such as ubiquitin and the hybrid CMV enhancer/chicken β-actin (Figure 4). We additionally wanted to determine whether tissue-specific promoters would give us a further opportunity to alter the specificity and expression characteristics of the vector. GFAP has been previously shown to be expressed in the supporting cells of the inner ear and has been used as the source of a promoter to drive transgene expression in supporting cells using an adeno-associated vector [10]. Using an E1, E3, E4 deleted GFP expressing adenovector driven by the GFAP promoter, we determined that expression of GFP was limited to the same cells expressing GFAP from the endogenous gene (Figure 5A). As suggested by the luciferase studies (Figure 4), GFAP drives gene expression at a very low level. In comparison, vector containing an hCMV driven transgene results in diffuse expression in both hair cells and supporting cells (Figure 5B). These results demonstrate that cell-specific expression can be achieved using tissue-specific promoters in the inner ear.
One of the potential advantages of cell-specific promoters is the expression of the transgene in a specific site. This may provide a means by which to target the gene delivery and hence provide a means to prevent side effects of the delivery. Delivery of the atonal homolog atoh1 has been demonstrated to result in the replacement of hair cells after ototoxic destruction of hair cells in both auditory and vestibular neuroepithelium [11,12]. In this study we demonstrated that hCMV, cBA, and GFAP promoters are capable of driving regeneration in macular organ cultures treated with neomycin (Figures 7 and 8). Interestingly, the cBA promoter did not perform as well as the other promoters (Figure 7C and 8), despite its high level of expression for a longer period of time as judged by expression of luciferase (Figure 4) and the level of atoh1 mRNA produced. In this study the GFAP promoter driving atoh1 provided the highest level of hair cell recovery (Figure 8), despite being the weakest promoter of the group that we tested (Figure 4) and producing the lowest level of atoh1 mRNA. Many hair cell regeneration studies suggest that hair cell replacement is due to supporting cell transdifferentiation [1,13]. In this case the use of a supporting cell-specific promoter expressed at a low level provided a significantly improved hair cell regeneration rate and also produced cells that appear morphologically more normal than a high strength promoter such as the cBA promoter (Figure 7D vs Figure 7C). Therefore, modification of vector backbone and promoter choice impacts the expression characteristics of the transgene. For optimal gene delivery in the ear, the expression time, expression dynamics, and location of expression will need to be taken into account.
Acknowledgments
Supported by NIDCD R01DC008424 and NICHD HD02528.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–6. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 2.Amalfitano A, Begy CR, Chamberlain JS. Improved adenovirus packaging cell lines to support the growth of replication-defective gene-delivery vectors. Proc Natl Acad Sci U S A. 1996;93:3352–6. doi: 10.1073/pnas.93.8.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Greenburg G, Bunch D, Farson D, Finer MH. Persistent transgene expression in mouse liver following in vivo gene transfer with a delta E1/delta E4 adenovirus vector. Gene Ther. 1997;4:393–400. doi: 10.1038/sj.gt.3300404. [DOI] [PubMed] [Google Scholar]
- 4.Gillespie LN, Clark GM, Bartlett PF, Marzella PL. LIF is more potent than BDNF in promoting neurite outgrowth of mammalian auditory neurons in vitro. Neuroreport. 2001;12:275–9. doi: 10.1097/00001756-200102120-00019. [DOI] [PubMed] [Google Scholar]
- 5.Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–79. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham LL. The adult mouse utricle as an in vitro preparation for studies of ototoxic-drug-induced sensory hair cell death. Brain Res. 2006;1091:277–81. doi: 10.1016/j.brainres.2006.01.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorziglia MI, Lapcevich C, Roy S, Kang Q, Kadan M, Wu V, et al. Generation of an adenovirus vector lacking E1, e2a, E3, and all of E4 except open reading frame 3. J Virol. 1999;73:6048–55. doi: 10.1128/jvi.73.7.6048-6055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji L, Bouvet M, Price RE, Roth JA, Fang B. Reduced toxicity, attenuated immunogenicity and efficient mediation of human p53 gene expression in vivo by an adenovirus vector with deleted E1–E3 and inactivated E4 by GAL4-TATA promoter replacement. Gene Ther. 1999;6:393–402. doi: 10.1038/sj.gt.3300825. [DOI] [PubMed] [Google Scholar]
- 9.Lusky M, Christ M, Rittner K, Dieterle A, Dreyer D, Mourot B, et al. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J Virol. 1998;72:2022–32. doi: 10.1128/jvi.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone IM, Lurie DI, Kelley MW, Poulsen DJ. Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol Ther. 2005;11:843–8. doi: 10.1016/j.ymthe.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–6. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 12.Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–79. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 13.Oesterle EC, Cunningham DE, Westrum LE, Rubel EW. Ultrastructural analysis of [3H]thymidine-labeled cells in the rat utricular macula. J Comp Neurol. 2003;463:177–95. doi: 10.1002/cne.10756. [DOI] [PubMed] [Google Scholar]