Abstract
Exosomes are important contributors to cell−cell communication and their role as diagnostic markers for cancer and the pathogenesis for cancer is under intensive investigation. Here, we focus on their role in metastasis-related processes. We discuss their impact regarding promotion of invasion and migration of tumor cells, conditioning of lymph nodes, generation of premetastatic niches and organotropism of metastasis. Furthermore, we highlight interactions of exosomes with bone marrow and stromal components such as fibroblasts, endothelial cells, myeloid- and other immune-related cells in the context of metastases. For all processes as described above, we outline molecular and cellular components for therapeutic intervention with metastatic processes.
Keywords: Exosome interaction with stromal cells, organ tropism of metastasis, pre- and metastatic niche, review
Metastases are responsible for the death of a large majority of cancer patients despite considerable progress in surgical techniques, radiotherapy, chemotherapy and targeted therapies including immuno-therapy (1). A dramatic reduction of metastatic burden has been observed, however, tumor elimination is almost always incomplete. This phenomenon is based on drug resistance, which is due to adaptation of intracellular pathways or on activation of survival-supporting autocrine and paracrine pathways and several secreted factors expressed by drug-sensitive tumor cells after therapy (2). Metastasis can occur through release of cancer cells from the primary tumor into body cavities that holds true for ovarian and CNS tumors, or via hematogeneous and lymphatic vessels of the circulatory system (3). Metastases of some tumors are directed besides to lymph nodes to mainly one type of organ only, such as prostate cancer to the bones, pancreatic cancer and uveal melanoma to the liver, whereas tumors such as melanoma, breast- and lung cancer can colonize several types of organs (3). Tumor cells have been found in the blood of patients with early-stage cancer and in some cases even before the primary tumor has been diagnosed (4,5). Recently, making use of single-cell expression profiling, it has been shown that early metastatic cells possess a stem-like gene signature and give rise to heterogeneous metastases (6). The metastatic process is characterized by a defined sequence of events (7,8). Initial steps are detachment from the extracellular matrix (ECM), invasion into the surrounding tissue and proteolysis of the basement membrane. After intravasation, survival of circulating tumor cells (CTCs) is achieved by forming clusters, binding to platelets and immune evasion. Subsequently, they arrest in distal microvascular beds and extravasation can be achieved either by migration through intercellular junctions of endothelial cells (EC) or penetration of a single EC (9). CTCs can also colonize their tumors of origin, a process referred to as “tumor self-seeding“, selecting for cancer cell populations more aggressive than those present in the primary tumor (10). After extravasation, colonization and outgrowth in the parenchyma of distant organs are the following steps. A common theme of the metastatic process is settlement of disseminated tumor cells (DTCs) into latency (dormancy), which can last from several months to decades (11). It has been observed that DTCs are recruited into pre-metastatic niches that support their survival by interactions with endothelial, myeloid cells, fibroblasts and other types of cells. After adaptation to the host microenvironment and suppression of an anti-tumoral immune response, DTCs are activated by not yet completely resolved mechanisms. Finally their outgrowth is based on an angiogenic switch mediated by pro-angiogenic factors and establishment of a vascular network to support the metabolic demands of colonizing tumor cells (12). In the following we focus on the role of exosomes in the context of the metastatic process.
General Features of Exosomes
Exosomes are spherical to cup-shaped nanovesicles 40-100 nm in diameter, that are secreted by many cells and can be found in most body fluids such as urine and blood as well as in supernatants of cultured cells (13). They are end-products of the recycling endosomal pathway which involves endocytosis, formation of early and late endosomes, multivesicular bodies (MVB) and secretion with the help of Rab family GTPases such as Rab 27a and Rab 27b (14). Exosomes have to be differentiated from other secreted cellular entities such as microvesicles (50-1,000 nm in diameter) and ectosomes, which are microvesicles derived from neutrophils or monocytes and apoptotic bodies (500-2,000 nm in diameter). In contrast to exosomes they bud off directly from the cell membrane (15). Exosomes consist of a lipid bilayer, transmembrane and non-membrane bound proteins and nucleic acids. Lipids (e.g. cholesterol and ceramide), membrane fusion-related proteins (Rab GTPases, flotillins and connexins), proteins involved in vesicle formation (Alix, Tsg 101), integral membrane proteins such as tetraspanins (CD9, CD63, CD 81) and major histocompatibility complex (MHC) class I and II as well as proteins related to the cytoskeleton and the cell metabolism have been identified (16). Also proteins involved in the pathogenesis of cancer such as oncoproteins MET and mutant KRAS have been found in exosomes (17,18). As nucleic acid-related cargo, mRNA, miRNA, long non-coding RNAs as well as DNA have been detected (19). Exosomes can transfer their constituents and cargo to neighbouring or distant cells with preservation of their function (20). Several mechanisms for the uptake of exosomes by recipient cells, such as exosome fusion with the membrane of the recipient cell, endocytosis by phagocytosis and receptor-ligand interaction (Tim1/4 on B cells, ICAM-1 on antigen-presenting cells) have been discussed (20-22).
In order to elucidate the mode of action of exosomes and their constituents, tracking of exosomes in vitro and in vivo is essential. This can be achieved by two different technologies (23). The first one is based on incorporation of small fluorophores into the membrane of exosomes resulting in strong fluorescent signals in the exosome membrane. However, this technology is restricted to the use of purified exosomes. The second one is based on expression of fusion proteins between exosome marker transmembrane proteins such as CD63 and fluorescent proteins (GFP and/or red fluorescent protein RFP) which are expressed in transfected cells and incorporated into exosomes allowing their tracing. The latter technology has allowed for visualization of transfer of tumor-derived exosomes to cells of the tumor-microenvironment, distant sites and blood in tumor-bearing mice (23,24).
Exosomes may have a significant impact on cancer diagnosis and treatment monitoring. The detection of exosomes containing Glypican-1, a glycosylphosphadidyl-inositol anchored proteoglycan, in the blood of patients with precancerous pancreatic lesions and not in those with benign pancreatic lesions is an example emphasizing this issue (25).
Exosomes – Functions in Cancer
It has been noted that cancer cells secrete much higher amounts of exosomes in comparison to non-transformed cells (26). Exosomes are involved in initiation, growth, progression and drug-resistance of tumors involving interactions with the microenvironment by transferring oncogenic proteins and nucleic acids (15,19,27,28). Their role in metastasis will be discussed in the following chapters. Here, we briefly comment on selected additional important functions of exosomes in cancer. The generation of an immuno-suppressive environment is an important issue for the pathogenesis of tumors. Exosomes have been shown to be implicated in induction of apoptosis of cytotoxic T-cells, expansion and function of regulatory T-cells (Tregs), induction of M2 polarization of macrophages, inhibition of cytotoxicity of natural killer (NK) cells, inhibition of differentiation of dendritic cells (DC), expansion and activation of myeloid-derived suppressor cells (MDSC) and mobilization of neutrophils (27,28). Exosomes released under hypoxic conditions can stimulate angiogenesis through interactions with endothelial cells (EC) (29). Exosomal transforming growth factor β (TGFβ) can induce differentiation of fibroblasts into tumor-supporting myofibroblasts (30) and exosomes from ovarian cancer are able to convert adipose-derived mesenchymal stem cells (MSC) into myofibroblast-like cells, supporting tumor growth and angiogenesis (31). Angiogenesis and myofibroblast-related aspects in the context of metastasis will be discussed in more detail in the following chapters. Recently, a role of breast cancer-secreted exosomes in tumorigenesis was demonstrated (32). These exosomes were shown to contain pre-miRNA associated with the RNA-induced silencing loading complex (RISC). They could mature pre-miRNA into miRNA in a cell-independent manner. Due to silencing of mRNAs in non-malignant target cells, their genome was re-programmed. Exosomes derived from patients with breast cancer were found to instigate non-tumorigenic epithelial cells to form tumors in mice in a Dicer-dependent manner (32). Further implications of these challenging findings remain to be investigated. In addition, exosomes derived from MDA-MB 231 breast cancer cells and U87 glioblastoma cells were able to induce transformation of recipient fibroblasts, dependent on continuous supply of exosomes (33). The phenomenon is caused by tissue transglutaminase cross-linked fibronectin (FN) in cancer vesicles, which activates mitogenic signaling. Another important aspect is the role of exosomes as mediators of drug resistance. Transfer of multi-drug resistant protein Pgp to drug-sensitive cells conferring drug-resistent properties and directing cytotoxic drugs such as cisplatin away from the nucleus have been reported (34,35). Stromal derived exosomes were shown to regulate therapy resistance pathways in breast cancer cells (36). Expansion of chemotherapy-resistant tumor cells was mediated by activation of the pattern recognition receptor RIG-1, which triggers STAT-1 signaling (antiviral) and induces the interaction of stromal JAG-1 with Notch3 on breast cancer cells (36). In the following chapters we focus on the role of exosomes in tumor metastases.
Exosomes Derived from Cancer Cells as Mediators of Migration and Invasion
Migration and invasion is a prerequisite for metastasis (37,38). We discuss here cancer cell migration and invasion promoting effects due to cancer-cell derived exosomes. Migration and invasion of cancer cells mediated by stromal-cell related exosomes are covered in the following chapters of this review.
The possible role of breast cancer cell released exosomes in metastasis was supported by the demonstration that exosomes from intermediate-metastatic (MCF-7 transfected with Rab27) and highly metastatic breast cancer cells (MDA-MB-231) could transfer invasion-promoting properties to tumorigenic, but not metastatic MCF-7 breast cancer cells (39). miR-10b was identified as an important component promoting invasion of breast cancer cells (40). Transfer of MDA-MB 231 overexpressed miR-10b in exosomes to immortalized mammary epithelial cells (HMLE) induced invasion in this cell line. Also exosome-mediated activation of EGFR-signaling by its ligands was identified as a pathway supporting cancer cell invasion (41). Amphiregulin-expressing exosomes derived from Madin-Darby Canine Kidney (MDCK) cells increased invasiveness of recipient breast cancer cell lines four-fold over TGFα or heparin-binding EGF-like growth factor exosomes and five-fold over equivalent amounts of recombinant amphiregulin. Another study has identified exosomes as mediators of plasmin-dependent cell motility of breast cancer cells (42). In this context a complex consisting of hsp90α, tissue-type plasminogen activator and annexin II was identified in breast cancer-derived exosomes which mediated plasmin activation. Metastasis is a process promoted by the interplay of invasive tumor cells and motile host cells (43-45). The impact of cancer cell-derived exosomes on host cells was investigated in a rat pancreatic carcinoma cell (ASML)-based system (46). Exosomes derived from this cell line were shown to modulate the ECM of rat host cells such as lung fibroblasts, lymph node stromal cells and aorta endothelial cells by degradation of ECM components such as collagens, laminin and fibronectin due to their protease-based cargo. It was shown that exosome-modulated ECM promotes stromal cell proliferation, migration and survival by inducing resistance against apoptosis. Modulation of the ECM creates new space for migrating tumor cells and attracts tumor, stroma and inflammatory cells and is possibly involved in liberation of chemokines and growth factors (47).
Concept of Pre- and Metastatic Niche
In order to understand the role of exosomes in metastasis, to be discussed in the following chapters of this review, we outline here the concept of the pre- and metastatic niche. The notion metastatic niche has been coined to describe the establisment and bookmarking of a conducive microenvironment in distant organs that enables survival and outgrowth as a “landing dock” for DTCs (48-51). Experimental evidence suggests that metastatic niches are initiated by an interplay of factors secreted by tumor cells, the recruitment of hematopoietic progenitor cells (HPC) as well as myeloid cells and mesenchymal stem cells (MSC) from the bone marrow, enabling engraftment of DTCs, and subsequently their outgrowth promoted by endothelial precursor cells (EPC) and finally angiogenic factors. Initial work has focussed on premetastatic niche formation in the lungs with B16 melanoma cells and Lewis Lung Carcinoma (LLC) cells (52,53). The instigators and promoters of niche formation are dependent on the experimental models involving the type of tumors cells and the distant organs to be colonized.
A brief summary of temporal and spatial events in niche generation is described in the following. Tumor-derived secreted factors (TDSF) secreted in part due to activation by HIF-1α, a transcription factor regulating expression of hypoxia-related genes, are able to initiate and regulate formation of pre-metastatic niches in distant organs (54). TDSFs include pro-angiogenic factors such as VEGF-A and PLGF, inflammation-promoting cytokines and chemokines such as TNFα, TGFβ, SDF-1 and CCL2/MCP1 (48-51). The existence of a premetastatic niche was first demonstrated by the seminal work of Kaplan et al. (52). Bone marrow (BM)-derived HPCs expressing VEGFR1 were shown to precede arrival of DTCs at distant sites in the lungs. S100A8 and S100A9 acting as inflammatory chemoattractants and inducers of inflammatory pathways were identified as key molecules for implementation of metastatic niches (55,56). They act as inducers of serum amyloid A3 (SAA3) which mediates accumulation of CD11+ myeloid cells via interaction with TLR4 and stimulation of NF-ĸB signaling (57). Recruitment of CD11+ myeloid cells to metastatic sites is further promoted by LOX activity which cross-links collagen type IV, thereby acting as a substrate for adherence of CD11+ myeloid cells (58,59). Deposition of FN, tenascin C, periostin and versican by recruited myeloid cells is another important step in formation of metastatic niches (52,60-62). The creation of an immune sanctuary at metastatic sites is a complimentary strategy for survival of DTC in the niche. Myeloid-derived suppressor cells (MDSCs) derived from HPC have been shown to accumulate in metastatic niches and to suppress cytotoxic CD8+ T-cells through production of reactive oxygen species (ROS) as well as NK cell cytotoxicity and maturity (63). Cancer-associated fibroflasts (CAFs), derived from MSCs contribute to niche formation by excessive deposition of constituents of the ECM, by secretion of MMPs that mediate degradation and remodeling of the ECM as well as by promoting proliferation, invasion and motility of niche-related tumor cells (64). In the course of the maturation of the pre-metastatic niche, DTCs are recruited to the pre-metastatic niche (PMN) for example by interaction between niche-derived SDF-1 and CXCR4 on tumor cells (48-50). Activation of the angiogenic switch by secretion of pro-angiogenic factors such as VEGF-A and PLGF and the recruitment of endothelial progenitor cells (EPCs) expressing VEGFR2, as a prerequiste of their outgrowth (65). In contrast to these findings, da Palma et al. (66,67) reported that Tie-2 expressing monocytes (TEMs) homed to metastatic tumors and interacted with vascular endothelial cells promoting their growth. The reasons for the discrepancy of these findings have not yet been resolved.
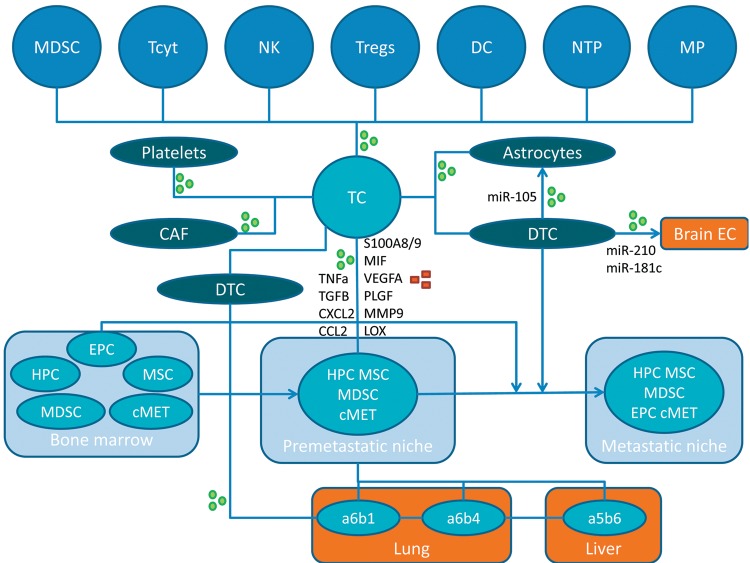
Importantly, bone marrow-derived dendritic cell (BMDC) clusters consisting of VEGFR1+ EPC have been observed in both primary tumors and premetastatic tissue in patients with breast-, lung- and esophageal carcinoma (52). In the following we discuss examples for identification and functional roles of metastatic niches. The concept of the generation of pre- and metastatic niches involving the interplay of different types of cells including tumor cells, exosomes and secreted factors, is outlined in Figure 1.
Figure 1. Interplay between tumor cells, other cell types, secreted factors and exosomes in the creation of pre- and metastatic niches. Exosomes and secreted factors are indicated by green circles or red squares, respectively. α5β6, α6β1 and α6β4,integrins; CAF, cancer-associated fibroblast; CCL2, CC chemokine ligand 2; cMET, tyrosine kinase receptor c-MET; CXCL2, chemokine (C-X-C motif) ligand 2; DC, dendritic cell; DTC, disseminated tumor cell; EC, endothelial cell; EPC, endothelial progenitor cell; HPC, hematopoietic progenitor cell; LOX, lysyl oxidase; MDSC, myeloid-derived suppressor cell; MIF, macrophage migration inhibitory factor; MMP9, matrix metalloprotease 9; MP, macrophage; MSC, mesenchymal stem cell; NK, natural killer cell; NTP, neutrophile; PLGF, pacental growth factor; S100A8/9, S100 calcium binding protein A8 or A9; TC, tumor cell; Tcyt, cytotoxic T-cell; TGFβ, transforming growth factor β; TNFα, tumor necrosis factor α; Tregs, regulatory T-cells; VEGFA, vascular endothelial growth factor, isoform A.
Pre-metastatic Niche of Melanoma in Lymph Nodes
The process of preconditioning of sentinel lymph nodes by melanoma exosomes as a prerequisite for recruitment and growth of melanoma cells was investigated (68). B16 melanoma cell exosomes were injected into the footpads of mice and lymph nodes were dissected and frozen for further analysis, which revealed a well-defined sequence of events. First, melanoma exosomes derived from the primary tumor are recruited to sentinal lymph nodes with a microenvironment consisting of endothelial cells (EC), lymphocytes and myeloid cells. Subsequently, ECM factors are produced, angiogenesis is initiated, lymphocyte anergy is induced and MDSC are generated from myeloid cells and melanoma cells are recruited to sites covered with exosomes in the lymph nodes, deposited and their further blood supply is established. Transcriptional analysis has revealed that exosome-dependent lymph node metastasis is driven by multiple metastatic pathways. Three groups of genes with functions in cell recruitment, ECM formation and angiogenesis have been identified (68). Genes encoding factors responsible for recruitment of melanoma cells such as stabilin 1 (69), ephrin receptor β4 (70) and vitronectin (71) were up-regulated. Additional up-regulated genes identified are involved in remodeling of stroma such as collagen 18 (72), laminin 5 (73), uPA (74) and p38 (75), enabling trapping of melanoma cells in the lymph nodes. Finally, up-regulation of angiogenic factors such as VEGF isoforms (76), which are induced by melanoma cells by hypoxia-inducible factor 1α (HIF1α), was noted (77). Interestingly, further analysis of data revealed simultaneous up-regulation of angiogenesis promoting and immuno-suppressive factors such as GM-CSF and TNFα (78).
CD44v6-promoted Metastatic Niche Formation in a Rat Pancreatic Tumor Model
Rat metastasis models based on intrafoot pad injection of metastatic pancreatic adenocarcinoma BSp73ASML(wt) and weakly metastasizing cell line ASML(kd) knockdown of CD44v4-7 (79), conditioned media (CM) and their fractions such as exosomes and soluble matrix was the basis for the study of premetastatic niches in the lymph nodes and the lungs (80). ASML(wt) CM, but not ASML(kd) CM promoted recruitment of ASML(kd) cells to the lymph nodes and the lungs. Complementation experiments with exosomes from ASML(kd) cells and soluble matrix fractions from CD44v6-containing ASML(wt) cells have identified exosomes as the final actors of pre-metastatic niche formation. In this system, corresponding changes in the pre-metastatic organs were observed only when both exosomes and CD44v6 containing soluble matrix were co-injected. In other words, exosomes independent of their origin from poorly or highly metastatic cells mediate tumor cell embedding and growth in pre-metastatic niches in a CD44v6-dependent manner. The fact that CD44v6, c-MET and uPAR are highly expressed in exosomes and soluble fractions of ASML(wt) cells and are absent in corresponding fractions of ASML(kd) cells, resulted in a hypothesis how these components might be involved in pre-metastatic niche formation. It was previously observed that CD44v6 can initiate c-MET activation through HGF binding (81,82). Therefore, in the absence of CD44v6, c-MET activation might be compromized, resulting in reduced transcription of c-MET and genes regulated by c-MET (81,82). It was shown that uPAR expression is regulated by c-MET at the mRNA and protein level (83,84). Binding of uPA to uPAR resulting in plasminogen activation and uPAR-mediated signaling through its association with integrins, EGFR, PDGFR and vitronectin and following activation of signal transducing kinases such as focal adhesion kinase (FAK), src and Akt (80) might contribute to (pre-)metastatic niche formation. The suggested interplay was supported by co-precipitation of CD44, uPAR and c-MET after chemical cross-linking and c-MET activation through CD44 cross-linking with hyaluronic acid in ASML cells (80). The role of CD44v6 competent exosomes was also investigated in human pancreatic and colorectal CD44v6 knock-out cell lines with reduced motility, invasion and cancer-initiating cell marker expression (85). These exosomes reconstituted cancer-initiating cell marker expression as well as migratory, invasive and metastatic competence due to protease expression and re-established the distorted cooperation between CD44v6 and tetraspanin 8 (Tsp8) with integrins (85).
Metastatic Niche of Melanoma in the Lung
Establishment of pre-metastatic niches was analyzed by the i.v. injection of B16-F10 fluorescently-labeled exosomes and rapid detection of these exosomes in the organ blood vessels and subsequently in the target organs. Enhanced permeability of lung ECs at exosome-induced pre-metastatic niches was observed with the extravasation of fluorescently labeled dextran (86). Gene expression profiling of lung tissue before and after injection of B16-F10 exosomes revealed up-regulation of genes involved in ECM remodeling and inflammation, effectors of pre-metastatic niche formation such as S100A8 and S100A9 (57) and TNFα as a mediator of vascular permeability (87,88). In order to assess the metastatic propensity of exosomes, mice were intravenously inocculated with exosomes produced from poorly (B16-F1) and highly metastatic (B16-F10) melanoma cells and subsequently luciferase-expressing B16-F10 cells were implanted by tail vein injection. A 240-fold increase in luciferase activity was observed in the lungs of mice with B16-F10 primary tumors when injected with B16-F10 exosomes in comparison to B16-F1 exosomes. Since the contribution of BMDCs in pre-metastatic niche formation is well documented (49,88), the hypothesis that tumor-derived exosomes might “educate“ BMDCs, was investigated. For this purpose, C57B1/6 mice were reconstituted with bone marrow from GFP-expressing mice treated with B16 exosomes (BM educated) after lethal irradiation. In these mice an increase in size and number (3 fold-higher metastatic burden) in the lungs and ipsilateral lymph nodes was noted after challenge with B16-F10mCherry cells. Interestingly, BM education with B16-F10 exosomes could increase the metastatic burden of Lewis lung carcinoma cells by a factor of ten (86). A 2-fold increase in pro-angiogenic cKIT+TIE2+ cells in the BM was observed 28 days after treatment in the melanoma exosome-based system. These cells can be recruited to the primary tumor as well as to metastatic niches. Proteomic profiling revealed increased expression of MET (89-91) in B16-F10 exosomes. Reduction of MET and phospho-MET levels by shRNA in B16-F10 exosomes led to a six-fold decrease of cKIT+MET+ BM progenitors in BM and peripheral blood, indicating horizontal transfer of exosomal MET to BM progenitors. The role of exosomes as mediators of the phenomena as described above was further corroborated by the fact that reduction of exosome production by inhibition of Rab27a (92,93) decreased recruitment of BMDCs necessary for metastatic progression. Also TLRs have been shown to be involved in premetastatic niche formation in the lung. The role of TLR3 in the formation of a PMN in the lung was shown with TLR3 knock-out mice (94). TLR3 activation in lung epithelial cells by tumor-derived exosomal RNAs triggers neutrophil recruitment by induction of PMN markers such as S100A8, S100A9, MMP9, Bv8 and FN and secretion of cytokines such as CXCL1, CXCL2, CXCL5 and CXCL12 (94).
Metastatic Niche of Pancreatic Carcinoma in the Liver
Pancreatic ductal adenocarcinoma (PDAC) is highly metastatic and is associated with a dismal prognosis due to delayed detection (95,96). Preferential target organs for metastasis are the liver, peritoneum and the lungs (97). Therefore, models which recapitulate early steps of pathogenesis of PDAC might be useful for diagnostic and therapeutic purposes. It was shown that PDAC-derived exosomes induce pre-metastatic niche formation in the liver of naive mice and subsequent injection of pancreatic tumor cells leads to enhanced metastatic burden in comparison to injection of the tumor cells in absence of exosomes (98). Mechanism-based studies revealed uptake of exosomes by Kupffer cells of the liver, resulting in secretion of factors associated with liver fibrosis including TGFβ. The latter induces deposition of FN by stellate cells and influx of bone marrow-derived macrophages. Treatment of mice with a TGFβ type I receptor inhibitor (A83-01) during exosome education mediated a decrease of stellate cells, FN deposition and macrophage migration to the liver. Analysis of PDAC-derived exosomes revealed high expression of macrophage-inhibitory factor (MIF). Knock-down of MIF in exosomes was accompanied with a pronounced reduction of TGFβ, FN deposition, macrophage recruitment and metastatic liver burden without affecting binding of exosomes to Kupffer cells. In a genetic model of PDAC, increase in exosomal MIF and TGFβ expression by liver Kupffer cells was observed in early pretumoral stages and expression levels correlated with disease progression (98). Preliminary data with patient-derived sera and quantitation of exosome MIF levels could be useful as a biomarker for clinicians to monitor disease progression (98). MIF, through binding to its receptor CD74, has been found to be implicated in cell-mediated immunity, immuno-regulation and inflammation (99). In the highly aggressive 4T1 breast cancer model, MIF was shown to be involved in suppression of recruitment of monocytic MDSC and metastasis without impacting tumor growth (100,101). The phenomena as described were dependent on the tautomerase activity of MIF, since neutralization of its tautomerase activity by the cancer preventive agent sulforaphane abolished the observed MIF-related effects. Taken together, exploration of MIF as a diagnostic and therapeutic target in PDAC, based on the models as described, deserves further investigations.
Exosome Mediated Interplay Between Tumor Cells with Fibroblasts, Astrocytes and Adipocytes Resulting in Metastasis
In the following we outline that exosomes derived from tumor cells or from fibroblasts can function as mediators of metastasis. CAFs have been identified as potent stimulators of cancer metastasis (102,103). They contribute to remodeling of the tumor microenvironment and promote its stiffness mediating a mesenchymal phenotype in epithelial cells by loosing their cell-cell junctions and polarity (104,105). The generation of CAFs by tumor cell-derived exosomes has been described in numerous examples. Exosomes derived from a variety of cancer cells can induce differentiation of stromal fibroblasts to myofibroblasts by exosome-associated TGFβ and subsequent SMAD-dependent signaling (30,106). Further examples are differentiation of adipose-derived MSCs to myofibroblasts by breast- and ovarian cancer derived exosomes (31,107) and the generation of tumor-supporting fibroblasts from umbilical cord derived MSCs by gastric cancer exosomes (108). In addition, the transfer of MMP-inducer EMMPRIN by exosomes derived from lung cancer cells to fibroblasts has been shown to stimulate expression of MMPs, leading to tumor-cell ECM-remodeling, invasion and metastasis (109). Making use of imaging techniques as described in a previous chapter of this review, it was observed in orthotopic breast cancer models that exosomes from breast cancer cells can move to other cancer and stromal cells at metastatic sites (23,24). Vice versa, exosomes from tumor-associated fibroblasts can profoundly enhance the metastatic properties of recipient tumor cells (110,111). Fibroblast secreted exosomes (L-cell and human CAF-derived), promote breast cancer cell protrusive motility and metastasis via the Wnt-planar cell polarity (PCP) signaling pathway (110,111). Fibroblast-secreted CD81-positive exosomes are tethered to tumor cells through Wnt 11, leading to autocrine activation of the PCP signaling pathway. Hallmarks of this pathway are the asymmetric subcellular localization across polarized tissue of complexes comprising seven pass Frizzled (Fzd) receptors, four-pass Van-Gogh-like receptors (Vangl) and cytoplasmic proteins such as prickle (Pk) and dishevelled (Dvd) (111). Several PCP components have been shown to be overexpressed in human tumors (112). In addition to fibroblasts, astrocytes have been identified as mediators of metastasis. Astrocyte-derived exosomes loaded with miR-19a induce loss of PTEN in tumor cells resulting in secretion of the chemokine CCL2, which recruits myeloid cells enhancing outgrowth of brain metastatic tumor cells (113). Loss of PTEN is often observed in brain metastases and cell lines derived from brain metastases (113). Also exosomes derived from adipocytes have been shown to confer migration-promoting properties to melanoma cells through transfer of proteins involved in fatty acid oxidation (114). These observations might explain why obese melanoma patients have a poorer prognosis in comparison to non-obese melanoma patients (114).
Metastasis-related Interactions Between Cancer Cell-derived Exosomes and Endothelial Cells
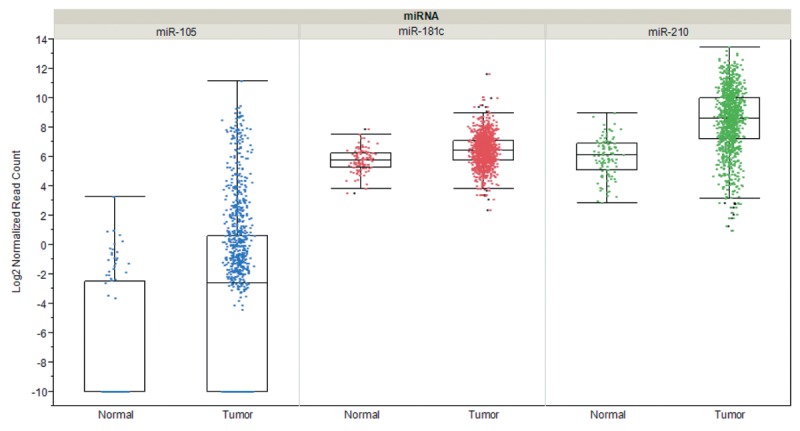
Metastasis-related interactions of tumor-cell derived exosomes with ECs involve the increase of vascular permeability, transfer of mRNAs, miRs and proteins to ECs and degradation of EC tight junction proteins by miR-related cargos. Enhancement of vascular permeability mediated by tumor cell-derived exosomes has been observed with melanoma and breast cancer-derived exosomes in vitro and in vivo (86,115). Several miRs as exosome cargos have been implicated as mediators of angiogenesis and metastasis. Melanoma-derived exosomes containing miR-9 have been shown to activate JAK-STAT signaling in ECs (116). Exosomal cancer cell-derived miR-210 has been identified as another angiogenesis and metastastis-promoting miRNA (117), which is upregulated in exosomes derived under hypoxic conditions (118), and its transfer to ECs was shown to be dependent on exosome-formation promoting neutral sphingomyelinase 2 (nSMase 2) (119). Also exosome-based protein transfer as a stimulator of angiogenesis and metastasis was observed. Exosomes derived from several types of cancer cells deliver EGFR to ECs and induce angiogenesis through VEGF/VEGFR2 signaling (120). Glioblastoma cells grown under hypoxic conditions were shown to produce exosomes that deliver pro-angiogenic factors such as IL8 and PDGF to ECs (121). An additional example is the transfer of tissue factor by tumor-cell derived exosomes to ECs, resulting in activation of GPCR protease-activated receptor 2 and subsequent upregulation of HB-EGF, promoting an angiogenic EC phenotype (122). Promotion of brain metastasis by breast cancer cell-derived exosomal miRs after transfer to ECs was documented in two reports (115,123). Degradation of tight junction proteins of ECs of the BBB such as claudin 5, occludin and ZO-1 by miR-105 and miR-181c promoted brain metastasis of breast cancer cells (115,123). Interestingly, miR-105, miR-181c and miR-210 are significantly overexpressed in breast cancer tissue in comparison to matching normal tissues (Figure 2).
Figure 2. Expression of selected microRNAs in breast cancer in comparison to matched normal tissues. Steady-state RNA levels corresponding to miR-105, miR-181c and miR-210 based on 1,084 invasive breast cancer samples and 104 matched normal samples derived from cohorts of The Cancer Genome Atlas (TCGA) are shown. Expression was quantified by RNA sequencing and is shown as log2 of normalized read counts. The red lines indicate low versus higher expression. Expression data are shown as box plots. The line in the middle of the box represents the data median, the rectangles show the upper and lower 25% quartile, therefore 50% of all data points are included in the rectangle. All other data points, except for outliers are located within the upper and lower whiskers.
Metastasis Promotion by Exosomes Based on Immuno-suppression
Immuno-suppression by exosomes resulting in promotion of cancer pathogenesis is a well-documented phenomenon (15). A variety of immune effector cells can be affected, resulting in induction of apoptosis of cytotoxic T-cells, promotion of expansion and function of regulatory T-cells (Tregs), mobilisation of neutrophils, induction of polarisation of macrophages to the M2 subtype, inhibition of NK cell cytotoxicity, expansion and activation of MDSCs and inhibition of differentiation of dendritic cells (DCs) (15). Here we focus on examples in which promotion of metastasis has been documented based on immuno-suppressive actions of exosomes. Exosomes derived from activated T-cells were shown to promote B16 melanoma and Lewis lung cell metastasis to the lung (86). Activation of the Fas/FasL pathway in tumor cells, increase in FLICE inhibitory proteins, activation of ERK- and NFĸB signaling and the resulting increase in MMP9 activity were shown to be responsible for promotion of metastasis (124). However, most investigations on immune-mechanisms based promotion of metastasis were performed with TC-derived exosomes. Melanoma cell line B16-derived exosomes induced a switch in differentiation to COX2, IL6, VEGF and arginase expressing MDSCs, in a manner that was dependent on MyD-88, an adaptor molecule that is involved in the Toll-like receptor family signaling (125,126). Inhibition of T-cell activation and acceleration of metastasis to the lung was noted in mice treated with TC-derived exosomes and the effects were blunted in MyD88 knock-out mice. In addition, breast cancer cell-derived exosomes, induced proinflammatory cytokines such as IL6, TNFα, G-CSF and CCL2 in distant macrophages, dependent on TLR2 or MyD88 as shown by genetic ablation experiments (127). The alterations, as described, resulted in enhancement of metastases in human breast cancer xenograft models. Another example is enhancement of metastases by TC-derived exosomes after co-culturing of 4T1 breast cancer cells with immuno-suppressive leukocytes (128). It was shown that TC-derived exosomes take-up FN and after fusion with other TCs are able to activate production of pro-inflammatory cytokines and MMP9 as well as FAK signaling, resulting in promotion of metastasis.
Exosome-mediated Organotropism of Metastasis
Previous investigations on organotropism of metastasis have focussed on cell-intrinsic components. They have identified guidance molecules such as CXCR4, CCR7 and their chemokine ligands CXCL12 and CCL21 as mediators of lung tropism of breast cancer metastasis, osteoclast stimulating factors for bone-related metastasis and integrins, tenascin and periostin as important drivers of organotropism of metastasis for different types of tumors (2,60,129,130-133). As previously outlined, pre-metastatic niche formation can be induced by tumor-derived factors including cytokines and lysyl-oxidase and additionally by exosomes. A new twist in this scenario is the demonstration of involvement of exosomes as mediators of organotropism of metastasis (134). Exosomes derived from variants of breast cancer cell line MDA-MB 231 with tropism of metastasis to the lungs and brain were homing to the corresponding target organs after tail vein, intracardial or retro-orbital injection. Conditioning of metastatic sites for subsequent colonization was also demonstrated by education of stromal cells with exosomes derived from pancreatic tumor cells, BxPC3 and HPAFII, which metastasize to the liver. It was shown that exosomes can redirect metastatic distribution. Metastasis of an MDA-MB 231 subline with tropism of metastasis to the bone could be redirected to metastasize to the lungs after creating a metastatic niche with exosomes from a MDA-MB 231 subline known to metastasize to the lungs. Proteomic analysis of exosomes identified integrins α6β1 and α6β4 as determinants of metastasis to the lungs and correspondingly, αvβ6, as the driver for liver metastasis. Uptake of exosomes was mediated by distinct cells, such as S100A4 expressing fibroblasts in the lung, Kupffer cells in the liver and CD31-positive brain endothelial cells. Gene expression analysis revealed activation of S100 genes and Src signaling in the pre-metastatic niches in the lung and the liver. An important translational aspect is the finding that colonization of organs with tumor cells could be inhibited with corresponding integrin-specific mAbs and decoy peptides. Expression patterns of integrins could play a role as organotropic biomarkers since a correlation between expression of integrin β4 and αv in exosomes from patients with lung or liver metastasis was found in comparison to patients with no metastasis. Integrins and exosome-inducible S100 proteins might be relevant targets for metastasis-preventing combination therapies. However, many advanced cancers disseminate to different organs, limiting the potential of therapeutics with an organ-specific mode of action.
Translational Aspects
Exosomes as mediators of metastasis is a field still in its infancy, despite groundbreaking findings over the last couple of years. The emerging diagnostic aspects (135) were not in the focus of this review. The complexicity of exosome-based interaction between tumor cells and other cell types is underlined by recent findings that exosomes from activated platelets can enhance metastases of lung cancer cells based on transfer of platelet CD41 (136).
In this review we focused on pre- and -metastatic niche formation in breast cancer, melanoma and pancreas carcinoma. Steady-state levels of mRNAs encoding niche-related factors expressed in these tumor entities were derived form The Cancer Genome Atlas (TCGA) and are displayed in Figure 3. It shows that none of these tumor types exhibits a pattern of preferential expression of defined factors, pronounced heterogeneous expression within a tumor entity and in each tumor type a subpopulation with exceptionally high expression of a specific factor in comparison to the median expression of the factor can be identified. G-CSF and TNFα are weakly expressed in breast cancer, melanoma and pancreatic cancer, however with outliers exhibiting strongly increased expression. Differential expression can be noted for CXCL2 and S100A8 with low average expression in breast cancer and melanoma, but significantly increased levels of corresponding mRNAs in pancreatic carcinoma. Overall the data as shown in Figure 3 would support a model of incremental contribution of specific factors to formation of pre- and metastatic niches. The major achievements driving the study of the role of exosomes in metastasis from our point of view are summarized in Table I.
Figure 3. Expression of niche-related factors in breast cancer, melanoma and pancreatic tumors. Steady-state levels form RNA encoding the corresponding factors are shown. Three cohorts derived from The Cancer Genome Atlas (TCGA) are displayed: breast invasive carcinoma (n=1,100), melanoma (n=472) and pancreatic adenocarcinoma (n=179). Expression was quantitated by RNA sequencing and is shown as log2 normalized read counts. The red lines indicate low versus higher expression. Expression data are shown as box plots. The line in the middle of the box represents the data median, the rectangles show the upper and lower 25% quartile, therefore 50% of all data points are included in the rectangle. All other data points, except for outliers are located within the upper and lower whiskers. CCL2, CC chemokine ligand 2; CXCL2, chemokine (C-X-C motif) ligand 2; G-CSF, granulocyte stimulating factor; LOX, lysine oxidase; MIF, macrophage migration inhibitory factor; MMP9, macrophage migration inhibitory factor; TGFβ, transforming growth factor β1; TNFα, tumor necrosis factor α.
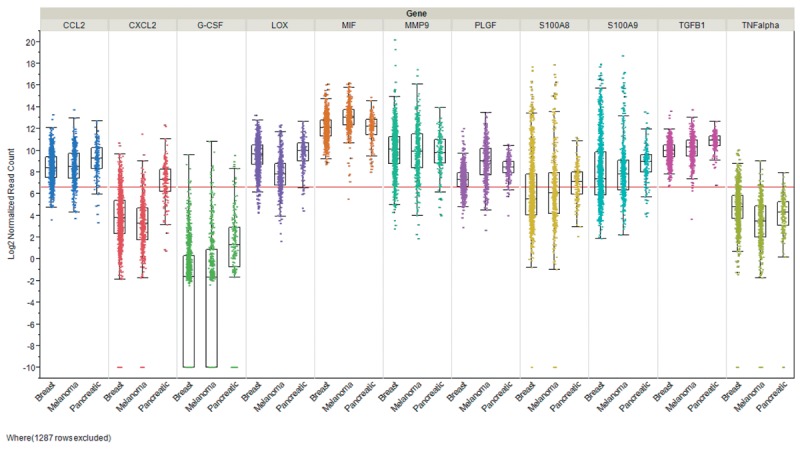
Table I. Major achievements in the field of exosome-related metastasis.
With respect to the potential impact of the findings discussed in this review for therapy, many questions still have to be addressed. The potential impact relies on intervention at different stages of niche formation, at an early stage before tumor cells have reached the pre-metastatic niche or at the stage of stromal progression of the metastatic sites (49). Is it possible to follow niche formation by monitoring growth factors and cytokines in the blood? Biomarkers as a surrogate measure for niche formation also would have an important impact on clinical studies targeting metastases (51). A couple of molecular and cellular targets mediating distinct steps of niche formation, such as vascular leakiness, stromal education in organotropic sites and BM-derived cell education and recruitment should be validated in more detail. Among the targets to be explored in more detail are molecular targets such as stromal FN, LOX, S100 proteins, MIF, CD81 and cellular targets such as VEGFR1+ myeloid cells, MDSC, activated fibroblasts and EPC. Also, technical aspects such as detection of pre-metastatic sites by immuno-histochemistry (IHC), have to be resolved. Myeloid cell clusters, activated fibroblasts and stromal FN are possible candidates for IHC-based detection of pre-metastatic niches. What determines the specific localization of niches within an organ? Are they newly formed, or do pre-formed, organ-type specific stem cell-related niches exist? Do premetastatic niches persist, after tumor-derived secreted factors have been removed? Which are the determinants of dormancy? Regarding organotropism of metastases, integrins mediating tropism of metastasis to single organs such as liver or lung might be of limited therapeutic value as discussed previously. Therefore, the question whether integrins with the propensity of mediating metastasis to several types of organs should be addressed in further studies. Some promising achievements regarding translation of exosome-related metastasis targets have been documented. TSU68, a small molecule inhibiting VEGFR1, PDGFRβ and FGFR1 has been shown to suppress CXCL1/CXCR2-dependent niche formation in the liver by inhibition of the inflammatory response through reduction of CXCL1 and IL12 (137). Furthermore, antibodies directed against αv on breast cancer cells prevented metastasis to other organs, but not to the lung (138,139,140). However, a serious limitation for translational research is the complexicity of pre-metastatic and metastatic niches with respect to its architecture and the interplay between secreted factors and cellular components, resulting in difficulties in setting up in vitro systems for its recapitulation for discovery of inhibitory molecules.
References
- 1.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastases. Endocr Reviews. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 2.Obenauf AC, Massague J. Surviving at a distance. Trends Cancer. 2015;1:76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massague J, Obenauf AC. Metastatic colonization by circulating tumor cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irima D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Pyan P, Balis UJ, Tomkins RG, Haber DA, Toner M. Isolation of rare circulating tumor cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hüsemann Y, Geigl JB, Schubert F, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Rietmüller G, Klein CA. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Lawson DA, Bhakta NR, Kessenbrock k, Prummel KD, Yu Y, Takai K, Zhou A, Eyob H, Balakrishnan S, Wang CY, Yaswen P, Goga A, Werb Z. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langley RR, Fidler IJ. The seed and soil hypothesis revisited – the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell. 2013;24:410–421. doi: 10.1016/j.ccr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawaguchi T, Nakamura K. Analysis of the lodgement and extravasation of tumor cells in experimental models of hematogenous metastasis. Clin Metastasis Rev. 1986;5:77–94. doi: 10.1007/BF00046424. [DOI] [PubMed] [Google Scholar]
- 10.Comen E, Norton L, Massague J. Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011;8:369–377. doi: 10.1038/nrclinonc.2011.64. [DOI] [PubMed] [Google Scholar]
- 11.Josse SA, Gorges TM, Pantel K. Biology, detection and clinical implications of circulating tumor cells. EMBO Mol Med. 2014;14:1–11. doi: 10.15252/emmm.201303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimova I, Popivanov G, Djonov V. Angiogenesis in cancer – general pathways and their therapeutic implications. J BUON. 2014;19:15–21. [PubMed] [Google Scholar]
- 13.Thery C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 14.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab 27a and Rab 27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83. doi: 10.1186/s13045-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azmi AS, Bao Band Sarkar FH. Exosomes in cancer development, metastasis and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji H, Greening DW, Barnes TW, Lim JW, Tauro BJ, Rai A, Xu R, Adda C, Mathivanan S, Zhao W, Xue Y, Xu T, Zhu HJ, Simpson RJ. Proteomic profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics. 2013;13:1672–1686. doi: 10.1002/pmic.201200562. [DOI] [PubMed] [Google Scholar]
- 18.Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ. Proteomic analysis of exosomes of mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12:343–355. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosaka N. Decoding the secret of cancer by means of extracellular vesicles. J Clin Med. 2016;5:E22. doi: 10.3390/jcm5020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125–134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 21.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamua T, Nagata S. Identification of Tim4 as phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 22.Segura E, Nicco C, Lombard B, Vernon P, Raposo G, Batteux F, Amigorena S, Thery C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 23.Suetsugu A, Honma K, Saji S, Moriwaki H, Ochiya T, Hofman RM. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev. 2013;65:383–390. doi: 10.1016/j.addr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Mittelbrunn M, Gutierrez-Vazquez C, Villaroy-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Benrad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Comm. 2011;2 doi: 10.1038/ncomms1285. doi:10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–185. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tickner JA, Urquhart AJ, Stephenson SA, Richard DJ, O’Byrne KJ. Functions and therapeutic roles of exosomes in cancer. Front Oncol. 2014;4:127. doi: 10.3389/fonc.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roma-Rodrigues C, Fernandes AR, Baptista PV. Exosome in tumor microenvironment: overview of the crosstalk between normal and cancer cells. Biomed Res Int. 2014;2014:179486. doi: 10.1155/2014/179486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Sem Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kholoa S, Rangino A, Granieri P, Lopatina T, Deregibus MC, Rispoli P, Brizzi MF, Camussi G. Extracellular vesicles as new players in angiogenesis. Vascul Pharmacol. 2016;86:64–70. doi: 10.1016/j.vph.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 31.Cho JA, Park H, Lim EH, Kim KH, Choi JS, Lee JH, Shin JW, Lee KW. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol Oncol. 2011;123:379–386. doi: 10.1016/j.ygyno.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci USA. 2011;108:4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bebawy M, Combes V, Lee E, Jaiswal R, Gong G, Bonhoure A, Grau GE. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia. 2009;23:1643–1649. doi: 10.1038/leu.2009.76. [DOI] [PubMed] [Google Scholar]
- 35.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S., Naerdemann W, Howell SB. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4:1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 36.Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wieman BZ, Ishwaran H, Ter Brugge PJ, Jonkers J, Slingerland J, Minn AJ. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159:499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greening DW, Gopal SK, Mathias RA, Liu L, Zhu HJ, Simpson RJ. Emerging roles of exosomes during epithelial-mesenchymal transition and cancer progression. Sem Cell Dev Biol. 2015;40:60–71. doi: 10.1016/j.semcdb.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Vella LJ. The emerging role of exosomes in epithelial-mesenchymal transition in cancer. Front Oncol. 2014;4:361. doi: 10.3389/fonc.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris DA, Patel SH, Gucek M, Hendrix A, Westbroek W, Taraska JW. Exosomes released from breast carcinomas stimulate cell movement. PLoS One. 2015;10:e0117495. doi: 10.1371/journal.pone.0117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh R, Pochampally R, Watanabe K, Lu Z, Mo YY. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer. 2014;13:256. doi: 10.1186/1476-4598-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD, McConnell RE, Tyska MJ, Coffey RJ. Amphiregulin exosomes increase cancer cell invasion. Curr Biol. 2011;21:779–786. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294. doi: 10.1186/1471-2407-10-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu H, Yang H, Zhang X, Xu W. The emerging roles of exosomes in tumor-stroma interaction. J Cancer Res Clin Oncol. 2016;142:1897–1907. doi: 10.1007/s00432-016-2145-0. [DOI] [PubMed] [Google Scholar]
- 44.Soung YH, Nguyen T, Cao H, Lee J, Chung J. Emerging roles of exosomes in cancer invasion and metastasis. BMB Rep. 2016;49:18–25. doi: 10.5483/BMBRep.2016.49.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goubran HA, Kotb RR, Stakiw J, Emara ME, Burnouf T. Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth and Metastasis. 2014;7:9–18. doi: 10.4137/CGM.S11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarbe N, Lösch S, Burtscher H, Jarsch M, Weidle UH. Identification of rat pancreatic carcinoma genes associated with lymphogenous metastasis. Anticancer Res. 2002;22:2015–2027. [PubMed] [Google Scholar]
- 47.Mu W, Rana S, Zöller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15:875–887. doi: 10.1593/neo.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peinado H, Lavotskin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Seminars in Cancer Biol. 2011;21:139–146. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Psila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sceneay J, Symth MJ, Möller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32:449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 51.Sleeman JP. The lymph node pre-metastatic niche. J Mol Med. 2015;93:1173–1184. doi: 10.1007/s00109-015-1351-6. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port YL, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 54.Wong CC, Gilkes DM, Zhang H, Chen J, Wie H, Chaturvedi P, Fraley SI, Wong CM, Khoo US, Ng IO, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA. 2011;108:16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer. 2015;15:96–109. doi: 10.1038/nrc3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghavami S, Chitayat S, Hashemi M, Esraghi M, Chazin WJ, Halayko AJ, Kerckhoff C. S100A8/A9: a Janus-faced molecule in cancer therapy and tumorgenesis. Eur J Pharmacol. 2008;625:73–83. doi: 10.1016/j.ejphar.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 57.Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, Maru Y. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic niche. Nat Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 58.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giacca AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox TR, Gartland A, Erler JT. Lysyl Oxidase, a targetable secreted molecule involved in cancer metastasis. Cancer Res. 2016;76:188–192. doi: 10.1158/0008-5472.CAN-15-2306. [DOI] [PubMed] [Google Scholar]
- 60.Oskarsson T, Acharyya S, Zang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RG, Monova-Todorova K, Brogi E, Massague J. Breast cancer cells produce tenascin C as a metastatic component to colonize the lungs. Nat Med. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2011;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 62.Gao D, Joshi N, Choi H, Ryu S, Hahn M, Catena R, Sadik H, Argani P, Wagner P, Vahdat LT, Port JL, Stiles B, Sukumar S, Altorki NK, Rafii S, Mittal V. Myeloid progenitor cells in the premetastatic lung promote metastasis by inducing mesenchymal to epithelial transition. Cancer Res. 2012;72:1384–1394. doi: 10.1158/0008-5472.CAN-11-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sceneay J, Parker BS, Smyth MJ, Möller A. Hypoxia-driven immunosuppression contributes to the pre-metastatic niche. Oncoimmunology. 2013;2:e22355. doi: 10.4161/onci.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Del Pozo Martin Y, Park D, Ramachandran A, Ombrato L, Calvo F, Chakravary P, Spencer-Dene B, Derzsi S, Hill CS, Sahai E, Malanchi I. Mesenchymal cancer cell-stroma crosstalk promotes niche activation, epithelial reversion and metastatic colonization. Cell Rep. 2015;13:2456–2469. doi: 10.1016/j.celrep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ‘premetastatic niche’ within bone and beyond. Cancer Metastasis Rev. 2006;25:521–529. doi: 10.1007/s10555-006-9036-9. [DOI] [PubMed] [Google Scholar]
- 66.De Palma M, Venneri MA, Galli R, Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 67.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 69.Goerdt S, Bhardway R, Sorg C. Inducible expression of MS-1 high-molecular-weight protein by endothelial cells of continuous origin and by dendritic cells/macrophages in vivo and in vitro. Am J Pathol. 1993;142:1409–1422. [PMC free article] [PubMed] [Google Scholar]
- 70.Yang NY, Lopez-Bergami P, Goydos JS, Yip D, Walker AM, Pasquale EB, Ethell IM. The EphB4 receptor promotes the growth of melanoma cells expressing the ephrin B2 ligand. Pigment Cell Melanoma Res. 2010;23:684–687. doi: 10.1111/j.1755-148X.2010.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nip J, Shibata H, Loskutoff DJ, Cheresh DA, Brodt P. Human melanoma cells derived from lymphatic metastases use integrin alpha v beta 3 to adhere to lymph node vitronectin. J Clin Invest. 1992;90:1406–1413. doi: 10.1172/JCI116007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sund M, Kalluri R. Tumor stroma derived biomarkers in cancer. Cancer Metastasis Rev. 2009;28:177–183. doi: 10.1007/s10555-008-9175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patarroyo M, Trggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Sem Cancer Biol. 2002;12:197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 74.Carriero MV, Stoppelli MP. The urokinase-type plasminogen activator and the generation of inhibitors of urokinase activity and signaling. Curr Pharm Des. 2011;17:1944–1961. doi: 10.2174/138161211796718143. [DOI] [PubMed] [Google Scholar]
- 75.Wagner EF, Nebreda AR. Signal integration by JNK and p38MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 76.Eswarappa SM, Fox PL. Antiangiogenic VEGF-Ax: A new participant in tumor angiogenesis. Cancer Res. 2015;75:2765–2769. doi: 10.1158/0008-5472.CAN-14-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumor growth and progression. Nat Rev Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hood JL, Pan H, Lanza GM, Wickline SA. Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89:1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matzku S, Komitowski D, Mildenberger M, Zöller M. Characterization of Bsp73, a spontaneous rat tumor and its in vivo selected variants showing different metastasizing capacities. Invasion Metastasis. 2009;3:109–123. [PubMed] [Google Scholar]
- 80.Jung T, Castellana D, Klingbeil P, Cuesta Hernandez I, Vitacollona M, Orlicky DJ, Roffler SR, Brodt P, Zöller M. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11:1093–1105. doi: 10.1593/neo.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Orian-Rousseau V, Ponta H. Adhesion proteins meet receptors: a common theme. Adv Cancer Res. 2008;101:63–92. doi: 10.1016/S0065-230X(08)00404-1. [DOI] [PubMed] [Google Scholar]
- 82.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signaling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 83.Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504–516. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- 84.Lee KH, Choi EY, Hyun MS, Jang BI, Kim TN, Lee HJ, Eun JY, Kim HG, Yoon SS, Lee DS, Kim JH, Kim JR. Role of hepatocyte growth factor/c-MET signaling in regulating urokinase plasminogen activator on invasiveness in human hepatocellular carcinoma: a potential therapeutic target. Clin Exp Metastasis. 2008;25:89–96. doi: 10.1007/s10585-007-9106-6. [DOI] [PubMed] [Google Scholar]
- 85.Wang Z, von Au A, Schnölzer M, Hackert T, Zöller M. CDv66 competent tumor exosomes promote motility, invasion and cancer-initiating cell marker expression in pancreatic and colorectal cancer cells. Oncotarget. 2016;7:55409–55436. doi: 10.18632/oncotarget.10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos C, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan Y, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 18;2012:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lucas R, Verin AD, Black SM, Catravas JD. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol. 2009;77:1763–1772. doi: 10.1016/j.bcp.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garajova I, Giovanetti E, Biasco G, Peters GJ. c-Met as a target for personalized therapy. Transl Oncogenomics. 2015;7(Suppl 1):13–31. doi: 10.4137/TOG.S30534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 91.Stella GM, Benvenuti S, Comoglio PM. Targeting the MET oncogene in cancer and metastases. Expert Opin Investig Drugs. 2010;19:1381–1394. doi: 10.1517/13543784.2010.522988. [DOI] [PubMed] [Google Scholar]
- 92.Wang JS, Wang FB, Zhang QG, Shen ZZ, Shao ZM. Enhanced expression of Rab 27A gene by breast cancer cells promoting invasiveness and the metastasis potential by secretion of insulin-like growth factor-II. Mol Cancer Res. 2008;6:372–382. doi: 10.1158/1541-7786.MCR-07-0162. [DOI] [PubMed] [Google Scholar]
- 93.Hendrix A, Maynyrd D, Pauwels P, Braems G, Denys H, Van den Broecke R, Lambert J, Van Belle S, Cocquyt V, Gespach C, Bracke M, Seabra MC, Gahl WA, De Wever O, Westbroek W. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion and metastasis. J Natl Cancer Inst. 2010;102:866–880. doi: 10.1093/jnci/djq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, Huang B, Xu X, Zheng J, Cao X. Tumor exosomal RNAs promote lung metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30:243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 95.Teague A, Lim KH, Wang-Gillam A. Advanced pancreatic adenocarcinoma: a review of current treatment strategies and developing therapies. Ther Adv Med Oncol. 2015;7:68–84. doi: 10.1177/1758834014564775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 97.Singh D, Upadhyay G, Srivastawa RK, Shankar S. Recent advances in pancreatic cancer: biology, treatment and prevention. Biochim Biophys Acta. 2015;1856:13–27. doi: 10.1016/j.bbcan.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 98.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsorth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Richard V, Kindt N, Saussez S. Macrophage migration inhibitory factor involvement in breast cancer. Int J Oncol. 2015;47:1627–1633. doi: 10.3892/ijo.2015.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simpson KD, Templeton DJ, Cross JV. Macrophage migration inhibitory factor promotes growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J Immunol. 2012;189:5533–5540. doi: 10.4049/jimmunol.1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simpson KD, Cross JV. MIF: metastasis/MDSC-inducing factor. Oncoimmunology. 2013;2:e23337. doi: 10.4161/onci.23337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jung Y, Kim JK, Shinozawa Y, Wang Y, Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, Wang J, Jin T, Zhang H, Dai J, Krebsbach PH, Keller ET, Pienta KJ, Taichman RS. Recruitment of mesenchymal stem cells into prostate tumors promotes metastasis. Nat Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, Byrom D, Riera A, Rossell D, Mangues R, Massague J, Sancho E, Batlle E. Dependancy of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Otranto M, Sarrazy V, Bonte F, Hinz B, Gabbiani G, Desmouliere A. The role of the myofibroblast in tumor stroma remodeling. Cell Adh Migr. 2012;6:203–219. doi: 10.4161/cam.20377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thuma F, Zöller M. Outsmart tumor exosomes to steal the cancer initiating cell its niche. Sem Cancer Biol. 2014;28:39–50. doi: 10.1016/j.semcancer.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 106.Webber JP, Spary LK, Sanders AJ, Chowdury R, Jiang WG, Steadman R, Wymant J, Jones AT, Kynaston H, Mason MD, Tabi Z, Clayton A. Differentiation of tumor-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34:290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 107.Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012;40:130–138. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- 108.Gu J, Qian H, Shen L, Zhang X, Zhu W, Huang L, Yan Y, Mao F, Zhao C, Shi Y, Xu W. Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-β/Smad pathway. PLoS ONE. 2012;7:e52465. doi: 10.1371/journal.pone.0052465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sidhu SS, Mengistab AT, LaVail J, Basbaum C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene. 2004;23:956–963. doi: 10.1038/sj.onc.1207070. [DOI] [PubMed] [Google Scholar]
- 110.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 111.Luga V, Wrana JL. Tumor-stroma interaction: Revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res. 2013;73:6843–6847. doi: 10.1158/0008-5472.CAN-13-1791. [DOI] [PubMed] [Google Scholar]
- 112.Sebbagh M, Borg JP. Insight into planar cell polarity. Exp Cell Res. 2014;328:284–295. doi: 10.1016/j.yexcr.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 113.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, Wang H, Ellis K, Cheerathodi M, McCarty JH, Palmieri D, Saunus J, Lakhani S, Huang S, Sahinn AA, Aldape KD, Steeg PS, Yu D. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lazar I, Clement E, Dauvillier S, Milhas D, Ducoux-Petit M, LeGonidec S, Moro C, Soldan V, Dalle S, Balor S, Golzio M, Burlet-Schiltz O, Valet P, Muller C, Nieto L. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: a novel mechanism linking obesity and cancer. Cancer Res. 2016;76:4051–4057. doi: 10.1158/0008-5472.CAN-16-0651. [DOI] [PubMed] [Google Scholar]
- 115.Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lötvall J, Nakagama H, Ochiya T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Comm. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gajos-Michniewicz A, Duechler M, Czyz M. MiRNA in melanoma-derived exosomes. Cancer Letters. 2014;347:29–37. doi: 10.1016/j.canlet.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 117.Camacho L, Guerrero P, Marchetti D. MicroRNA and protein profiling of brain metastasis competent cell-derived exosomes. PLoS One. 2013;8:e73790. doi: 10.1371/journal.pone.0073790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Rigner M, Mörgelin M, Bourseau-Guilman E, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci USA. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang X, Zhang Y, Yang Y, Lim S, Cao Z, Rak J, Cao Y. Vascular endothelial growth factor-dependent spatiotemporal dual roles of placental growth factor in modulation of angiogenesis and tumor growth. Proc Natl Acad Sci USA. 2013;110:13932–13937. doi: 10.1073/pnas.1309629110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Svensson KJ, Kucharzwska P, Christianson HC, Sköld S, Löfstedt T, Johansson MC, Mörgelin M, Bengzon J, Ruf W, Belting M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci USA: 2011;108:13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O’Connor ST, Chin AR, Yen Y, Wang Y, Marcusson EG, Chu P, Wu J, Li AX, Li Z, Gao H, Ren X, Boldin MP, Lin PC, Wang SE. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, Yang Y, Wang L, Cao X, Wang J. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol. 2012;188:5954–5961. doi: 10.4049/jimmunol.1103466. [DOI] [PubMed] [Google Scholar]
- 125.Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, Grizzle WE, Mobley J, Zhang HG. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu Y, Xiang X, Zhuan X, Zhang S, Liu C, Michaelek S, Grizzle W, Zhang HG. Contribution of MyD88 to the tumor-exosome mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176:2490–2499. doi: 10.2353/ajpath.2010.090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, Huang W, Ngo V, Kortylewski M, Wang SE. Macrophage immuno-modulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-ĸB. Sci Rep. 2014;4:5750. doi: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deng Z, Cheng Z, Xiang X, Yan J, Zhuang X, Liu C, Jiang H, Ju S, Zhang L, Grizzle W, Mobley J, Roman J, Miller D, Zhang HG. Tumor cell cross talk with tumor-associated leukocytes leads to induction of tumor exosomal fibronectin and promotes tumor progression. Am J Pathol. 2012;180:390–398. doi: 10.1016/j.ajpath.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 129.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 130.Weidle UH, Birzele F, Kollmorgen G, Rüger R. Molecular mechanisms of bone metastasis. Cancer Genomics Proteomics. 2016;13:1–12. [PubMed] [Google Scholar]
- 131.Fukuda K, Sugihara E, Ohta S, Izahara K, Funakoshi T, Amagai M, Saya H. Periostin is a key niche component for wound metastasis of melanoma. PLoS One. 2015;10:e0129704. doi: 10.1371/journal.pone.0129704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hoye AM, Erler JT. Structural ECM components in the pre-metastatic and metastatic niche. Am J Physiol Cell Physiol. 2016;310:C955–967. doi: 10.1152/ajpcell.00326.2015. [DOI] [PubMed] [Google Scholar]
- 133.Glavey SV, Huynh D, Reagan MR, Manier S, Moschetta M, Kawano Y, Roccara AM, Ghobrial IM, Joshi L, O’Dwyer ME. The cancer glycome: carbohydrates as mediators of metastasis. Blood Rev. 2015;29:269–279. doi: 10.1016/j.blre.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 134.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Araso Y, Zhang T, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumor exosome integrins determine organotropic metastases. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Proporzi F, Logozzi M, Fais S. Exosomes: the future of biomarkers in medicine. Biomark Med. 2013;7:769–778. doi: 10.2217/bmm.13.63. [DOI] [PubMed] [Google Scholar]
- 136.Janowska-Wiezirek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratacjczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 137.Yamamoto M, Kikuchi H, Ohta M, Kawabata T, Hirmatsu Y, Kondo K, Baba M, Kamiya K, Tanaka T, Kitagawa M, Konno H. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res. 2008;68:9754–9762. doi: 10.1158/0008-5472.CAN-08-1748. [DOI] [PubMed] [Google Scholar]
- 138.Bäuerle T, Komlyenovic D, Merz M, Berger MR, Goodman SL, Semmler V. Cilengitide inhibits progression of experimental breast cancer bone metastases as imaged noninvasively using VCT, MRI and DCE-MRI in a longitudinal in vivo study. Int J Cancer. 2011;128:2453–2462. doi: 10.1002/ijc.25563. [DOI] [PubMed] [Google Scholar]
- 139.Wu YJ, Muldoon LL, Gahramanov S, Kraemer DF, Marshall DJ, Neuwelt EA. Targeting αV-integrins decreased metastasis and increased survival in a nude rat breast brain metastasis model. J Neurooncol. 2012;110:27–36. doi: 10.1007/s11060-012-0942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao Y, Bachelier R, Treilleux I, Puguguet P, Peyruchaud O, Baron R, Clement-Lacroix P, Clezardin P. Tumor alphavbeta3 integrin is a therapeutic target for breast cancer bone metastasis. Cancer Res. 2007;67:5821–5830. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]