Abstract
Objectives/Hypothesis
Determine the optimal design characteristics of an adenoviral vector to deliver atoh1 and induce regeneration of vestibular hair cells.
Study Design
Evaluation of a mouse model of intra-labyrinthine gene delivery. Tissue culture of mouse and human macular organs.
Methods
Macular organs from adult C57Bl/6 mice were treated with binding modified and alternate adenovectors expressing green fluorescent protein (gfp) or luciferase (L). Expression of marker genes was determined over time to determine vector transfection efficiency. The inner ear of adult mice was then injected with modified vectors. Expression of gfp and distribution of vector DNA was followed. Hearing and balance function was evaluated in normal animals to ensure safety of the novel vector designs. An optimized vector was identified and tested for its ability to induce hair cell regeneration in a mouse vestibulopathy model. Finally this vector was tested for its ability to induce hair cell regeneration in human tissue.
Results
Ad5 serotype based vectors were identified as having a variety of different binding capacities for inner ear tissue. This makes it difficult to limit the dose of vector due to entry into non-targeted cells. Screening of rare adenovector serotypes demonstrated that Ad28 based vectors were ideally suited for delivery to supporting cells and therefore useful for hair cell regeneration studies. Utilization of an Ad28 based vector to deliver atoh1 to a mouse model of vestibular loss resulted significant functional recovery of balance. This vector was also capable of transfecting human macular organs and inducing regeneration of human vestibular hair cells in vitro.
Conclusions
Improvement in vector design can lead to more specific cell based delivery and reduction of non specific delivery of the trans gene leading to the development of optimized molecular therapeutics to induce hair cell regeneration.
Level of Evidence
N/A Controlled basic science study.
Keywords: Atoh1, hair cell regeneration, viral vector, adenovector, balance
Introduction
Treatment of hearing loss and balance disorders has traditionally relied on rehabilitative therapies such as hearing aids or vestibular physical therapy rather than reversal or cure of a physiologic defect. Regeneration of auditory and vestibular hair cells has been a goal of translational hearing research since the discovery that birds spontaneously regenerate hair cells [1]. A variety of different genes have been evaluated in as potential modulators of hair cell regeneration. The helix loop helix transcription factor atoh1 has been identified as the gene needed for the terminal genesis of auditory and vestibular hair cells [2]. Numerous studies have demonstrated that adenovector mediated delivery of atoh1 can restore auditory and vestibular hair cells and restore lost function [3–6]. To develop these findings into a clinical product we have to produce a vector that is safe (i.e. target specific), effective at the lowest possible dose and produces long lasting and stable return of function.
Adenovectors have been used to efficiently deliver genes to the mouse inner ear [7]. Specificity of gene delivery can be achieved through the use of tissue specific promoters or through retargeting of the adenovector to alternate cellular receptors. Standard adenovectors based on serotype 5 bind to cells via interaction of the fiber knob with cellular coxackie adenovirus receptor (CAR). Some studies have suggested that there are alternative non specific binding and entry mechanisms including interaction of the viral shaft with heparin sulfate [8]. Retargeting of adenovectors can be achieved through a variety of capsid modification strategies or picking alternate adenovectors that have different binding site [9].
It has been previously demonstrated that atoh1 induced regeneration can be influenced by several factors including number of vector particles delivered, binding characteristics of the particle type used, strength of promoter used to drive atoh1 and target factors such duration of hair cell loss and status of the molecules that regulate lateral inhibition [10–12]. In this series of experiments we determined if choice of adenovector capsid or capsid modification could affect transfection. To evaluate Ad5 vector binding effects we compared delivery of green fluorescent protein to the inner ear using native Ad5 capsid vectors to vectors that have ablated CAR and integrin binding [13, 14]. To improve binding specificity one can alter the binding characteristics of Ad5 or identify other adenovector types that could be employed to transfect the inner ear. This would have the advantage of producing a more stable construct and potentially would allow use of a vector to which there are no neutralizing antibodies in the target patient population or may reduce the total number of vector particles needed to effectively deliver to the inner ear.
So far 51 human serotypes that are divided into subgroups A-F have been described. We screened 3 alternate serotype adenovectors for their ability to safely deliver genes to the inner ear. This led to the identification of a vector that targeted supporting cells from which we developed a vector to deliver atoh1 to the inner ear of mice with bilateral vestibulopathy. Outcomes included dose response for high and low doses of vector and counts of type I hair cells, type II hair cells and supporting cells. Balance was measured by rotarod at 4 months post treatment. Finally we tested the optimized vector’s capacity to induce hair cell regeneration in human tissue.
Methods
Vector construction and storage
For Ad5 modification studies, native capsid Ad5 derived vectors carrying with luciferase (AdL) or enhanced GFP (Adf) were used. Capsid double ablated (DA) vectors consisted of Ad5 vector with mutations of the knob and penton domains expressing either luciferase (AdL.DA) or GFP (Adf .DA)as previously described[14]. This double ablated vector could not bind CAR or integrins. A third capsid used carried mutations of the knob, penton and fiber shaft and expressed luciferase (AdL.TA). The adenovector backbone used for all experiments is deleted of adenovirus regions E1A, E1B, E3 and E4. Expression is driven by the hCMV promoter and the expression cassette contains an optimized splice 5′ of the open reading frame (ORF) being expressed and an SV40 polyadenylation site and transcriptional stop 3′ of the ORF.
The production system for these and alternate serotype adenovectors (Ad28, Ad35, Ad41) provides robust replication of the adenovector and purified stocks at 5 × 1011 to 2 × 1012 total particles (particle unit, pu) per mL with a total particle to active particle (fluorescent focus unit, ffu) ratio ranging from 3–10pu/ffu. Total particles (pu) were determined by a spectrophotometric assay. Adenovector lots were purified, aliquoted and stored at −80°C. Individual aliquots were used for each experiment to prevent loss of activity associated with freeze thaw cycles. All of these alternate serotype adenovectors carry GFP driven by the hCMV promoter. For hair cell regeneration studies we utilized an Ad28 serotype capsid with atoh1 driven by the supporting cell specific glial fibrillary acidic protein (gfap) promoter (Ad28.gfap.atoh1).
Evaluation of vector kinetics in vitro
All experiments were approved by the University of Kansas Animal Care and Use Committee. Three month old C57Bl6 mice (Jackson Labs, Maine) were anesthetized with Beuthanasia-D (10ml/kg, Schering-Plough Animal Health) and decapitated. The temporal bones were removed and placed in sterile phosphate buffered saline (PBS) supplemented with 5% glucose. The inner ear was opened and the utricles removed. The otolithic membranes were removed with a sharpened wire pick. For vector delivery the utricles were suspended in 100 μl of DMEM supplemented with N1 (Sigma)+100 u/ml penicillin and glucose. Cultures were maintained under standard conditions (37 °C, 5% CO2). For evaluation of GFP expression, explants were treated with 1 × 107 to 1 × 109 pu of either native capsid vector (Adf) or vector that did not bind CAR or alpha V integrin (Adf.DA) (n=5). Initial vector delivery was carried out in a 100 μl volume. After 24 hours, explants were washed in DMEM and transferred to Millicell membranes for a total of 7 days. Explants were examined and photographed on a daily basis for GFP fluorescence. For determination of luciferase activity, explants were exposed to 1 × 109 pu of either native capsid vector (AdL), vector that did not bind CAR or alpha V integrin (AdL.DA) or the triply ablated vector that carried mutations in the knob, penton and shaft regions (AdL.TA) (n=5 each condition). Explants were incubated with vector for 24 hours or alternately incubated with vector for 1 hour followed by repeated washing with DMEM and 23 hours further time in vitro in DMEM supplemented with N1 (Sigma)+100 u/ml penicillin and glucose. After 24 hrs total culture time protein was extracted and total luciferase/mg protein determined.
For alternative serotype studies, macular organs were prepared as described above. All explants were transfected with 1 × 109 pu of Ad5 (Adf), Ad28, Ad35 and Ad41, respectively. At 1, 2, 7 and 14 days post transfection, explants were imaged to determine degree of GFP expression. A second set of macular organs were prepared and underwent DNA and RNA extraction to quantify vector load and transgene expression.
In vivo delivery of adenovectors to the inner ear
Mice were anesthetized with an intraperitoneally administered mixture of ketamine (100mg/kg), xylazine (5mg/kg) and acepromazine (2mg/kg). A dorsal postauricular incision was made and the bulla exposed. Using an 18# needle a hole was drilled exposing the middle ear space medial to the tympanic ring. The round window niche was identified and the bone overhanging the niche is scraped away revealing the round window membrane. Vector injections consisted of 108 pu of vector in 1 μl of volume (n=5 each condition) delivered into the scala tympani via the round window using a Hamilton microsyringe with 0.1 μl graduations. After delivery the round window was sealed using a muscle plug, the wound was closed and animals allowed to recover. After 48 hours the animals were anesthetized as described above and sacrificed by intracardiac perfusion with ice cold 4% PBS buffered paraformaldehyde.
Induction of vestibular hair cell loss and delivery of atonal expressing vector
3,3′-iminodipropionitrile (IDPN) 6 mg/gm body weight, was administered to mice via oral gavage. Ten days post administration of IDPN mice were anesthetized. The posterior semicircular canal was exposed and fenestrated and either 1 × 106 or 1 × 108 pu (n=4 per group) of Ad28.gfap.atoh1 injected into the canal. The canal was sealed and the animals were allowed to recover. Rotarod times were measured pre IDPN treatment and at 10 days post IDPN then at 1 and 4 months after vector delivery. Controls consisted of IPDN only treated animals injected with 1 μl of artificial perilymph via the posterior canal (n=4). Hair cell counts were determined at 4 months after vector delivery.
Analysis of alternate serotype adenovectors
To test the impact of the alternate adenovector serotypes on the inner ear, hearing and balance function was assessed prior to vector delivery via the posterior canal and seven days after vector injection. To examine the tissue distribution of Ad5, Ad28, Ad35 and Ad41 mice received vector via the round window and allowed to recover for seven days. Mice were anesthetized with beuthanasia-D (10ml/kg, Schering-Plough Animal Health) and perfused with 4% paraformaldehyde. Temporal bones were removed and postfixed in 4% paraformaldehyde overnight. After decalcification in 10% EDTA for 48hrs the tissue was embedded in paraffin. For immunohistochemistry, 10μm sections were cut and deparaffinized. After antigen retrieval (Dako Target Retrieval Solution, Dako, Denmark) and incubation with Image-IT FX signal enhancer (Invitrogen, USA) sections were stained with anti-GFP (rabbit, Invitrogen, USA, 1:50) as a primary antibody and fluorescent labeled secondary antibody (Alexa Fluor 555, goat anti-rabbit IgG, Invitrogen, USA, 1:50). Sections were coverslipped with ProLong Gold antifade medium (Invitrogen, USA) and examined by confocal microscopy (Nikon Confocal Microscope C1, Nikon Corp. Japan).
Balance testing
To evaluate balance function mice were tested on a rotarod treadmill (ENV-575M, Med Associated Inc., Georgia, USA). The rod started at an initial velocity of 4 rpm and accelerated to 40 rpm at 300 sec. The time from the start of acceleration until the mouse fell completely off the rod was recorded. The test was stopped after a maximum of 360 sec. All mice were trained on the rod for two days prior to the baseline recording.
Auditory brainstem response (ABR) and distortion product otoacoustic emissions (DPOAE)
ABRs were measured on the left ear of each animal using the Smart EP Program (Intelligent Hearing Systems Corp, Miami, Florida) at 4–32kHz (pure tone stimulation of 500μs duration, High Frequency Transducer) prior to surgery and 7 days after vector application. For each animal we compared the pre- and postoperative thresholds at each frequency and determined the statistical significance of any change. In addition, we measured the distortion product otoacoustic emissions (DPOAE) at the same time points as the ABRs, using a range of pure tones from 2 to 32 kHz (16 sweeps). The pure tone ratio F2/F1 was set at 1.22 and the pure tone amplitudes were fixed at 65dB for L1 and 55dB for L2 (Intelligent Hearing System, SmartOAE Program, HIS Corp, Miami, Florida).
Tissue preparation for stereology
Mice were anesthetized with beuthanasia-D (10ml/kg, Schering-Plough Animal Health) and perfused with 4% paraformaldehyde in 0.1M PBS. Both temporal bones were extracted and the bulla was opened. All middle ear ossicles and the stapedial artery were removed. The oval and round windows were opened to allow perfusion of fixative. Specimens were kept in fixative overnight at 4°C. Post fixation, mouse temporal bones were decalcified in RDO decalcifier (Apex Engineering Products Corporation, Aurora, Illinois) for 60 minutes. The temporal bones were rinsed in ddH2O, trimmed and osmicated (1% OsO4 in ddH2O) for 30 minutes. After osmication, cochleae were rinsed in PBS and ddH2O and dehydrated in ethanols and propylene oxide. Plastic embedding was carried out in araldite resins (Electron Microscopy Sciences, Hatfield, PA). Cochleae were oriented in a horizontal plane parallel to the spiral axis of the upper turn and sectioned at 40μm with a Leica tungsten carbide steel knife. Sections were stained with toludine blue and rinsed with tap water. Air-dried sections were mounted in permount on microscope slides and coverslipped for light microscopic analysis.
Quantification of hair cells
All stereologic measures were performed using the optical fractionator technique using Stereo Investigator V9 coupled to a Nikon TE 2000 inverted scope for brightfield with a prior stage run by an Oasis controller board. Images were generated with a Q Imaging Retiga 2000R color camera utilizing a Nikon PlanFluor I00X/1.30 oil DIC H/N2 ∞/0.17 WD 0.20 objective. In brief, after setting a reference point, the neuroepithelium of the utricle was identified and outlined. The guard height was set at 10 μm and counting frame size 75μm × 75μm. Supporting cells and type I and II hair cells were identified based on position of the nucleus within the neuroepithelium and the presence or absence of a neuronal calyx.
Tissue preparation and laser capture microdissection (LCM)
Paraffin embedded cochleae were cut into 10μm sections and mounted onto Superfrost/Plus Microscope Slides (Fisher Scientific Int., Pittsburgh, PA). Sections were baked at 60°C (5 min), washed in xylene (2 × 5 min), deparaffinised and stained with hematoxylin and eosin using a Leica ST5020 multistainer (Leica microsystems GmbH, Wetzlar, Germany) and air dried afterwards. LCM was performed using a Veritas Microdissection System (MDS, Inc., Toronto, Canada). Dissected tissue was collected on CapSure Macro LCM Caps (MDS, Inc., Toronto, Canada) and stored at 4°C until the tissue was further processed for quantitative PCR and RT-PCR.
Quantitative PCR and RT-PCR
After vector delivery to macular organ culture, both DNA and RNA were extracted at 1, 4, 7 and 14 days post vector delivery. The amount of vector particles and level of transgene expression was determined by taqman assay as previously described [15]. (SplicePrimer: TGC CAA GAG TGA CˆGT GTC CA; GFP, EGFPaPrimer: TCC TCG CCC TTG CTC A; SpliceProbe: 6FAM CCC AGG TCC AAC TGC AGC CGG MGBNFQ). For LCM specimens, detection of GFP DNA sequences was carried out to determine the presence of vector delivered transgenes.
Human macular organ culture
Utricles were obtained during translabarynthine excision of acoustic neuromas. The utricles were cultured as described above. For ablation of residual hair cells utricles were treated with neomycin 10−3 M for 5 days. After neomycin treatment, explants were either placed in DMEM or exposed to 1 × 108 pu/100 μl medium of Ad28.gfap.atoh1. Explants were fixed and immunostained for myosin VIIa with a DAPI counterstain at 14 days post vector exposure as previously described [16].
Statistics
ANOVA and ANOVA for repeated measures was carried out suing Simga Plot V 12. P<0.05 was considered significant.
Results
Ad5 vectors have multiple binding mechanisms in the inner ear
Adult mice were treated with intracochlear injections of either a native capsid vector (Adf) or Adf.DA, which does not bind CAR or integrin alpha V. GFP expression could be seen in a broad range of tissues within the inner ear after delivery of Adf through the round window (Fig 1A,B,C). Within the cochlea there was clear expression of GFP in inner and outer hair cells, labeling of the spiral ganglion, and the spiral ligament and stria vascularis. Within the vestibular system GFP expression was noted within both hair cells and supporting cells as well as vestibular ganglion cells. Delivery of an equivalent dose of Adf.DA which does not bind CAR or integrin αV did not show significantly different GFP expression patterns (Fig 1D,E,F), suggesting that non CAR and non integrin mediated entry was possible within the inner ear.
Figure 1.
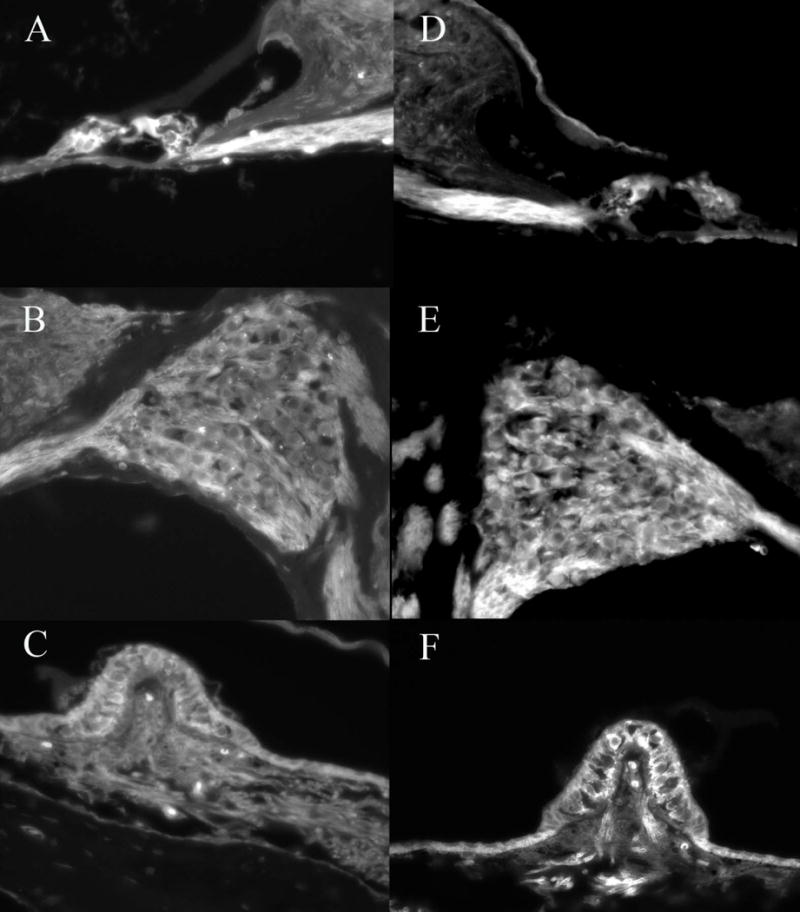
Delivery of Adf to the basal turn of the mouse cochlea resulted in expression of GFP in the inner and outer hair cells (A), auditory neurons (B) and supporting as well as sensory cells in the vestibular system (C). Ablation of CAR and integrin binding capacity results in similar GFP distribution patterns (E,F,G). No clear differences in GFP intensity or distribution were noted with any of the capsid ablated vectors when used in high concentration. This demonstrates that integrin binding or CAR binding is not necessary for entry of the vector into the cells of the inner ear and suggests that secondary vector entry mechanisms exist.
Effect of vector capsid on kinetics of GFP expression in adult macular organs
Adult mouse macular organ explants were treated with either Adf or Adf.DA at three different vector concentrations. At day 3 post vector delivery expression of GFP was noted in the Ad gfp treated explants (Fig 2A). After 7 days in vitro, GFP continued to be expressed (Fig 2C). The Adf.DA vector showed only a very low level of transfection at 3 days post delivery (Fig 2B). By 7 days post delivery expression of GFP in the Adf.DA treated cultures was equivalent to the native capsid (Adf) treated cultures (Fig 2D). This delayed effect of GFP expression was present at all three doses of vector tested suggesting that his delay effect is not related to vector concentration. Ablation of CAR and integrin binding did not eliminate the ability to deliver GFP to macular organs but did demonstrate slower delivery kinetics than a native capsid vector.
Figure 2.
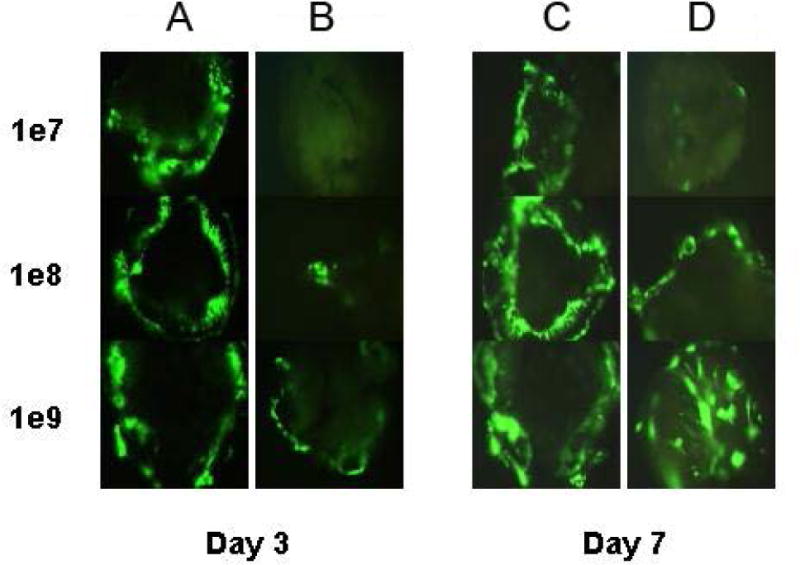
To determine if delivery of high concentrations could account for the efficacy of non CAR non integrin delivery finding noted in Fig 1, adult macular explant cultures were set up and treated with 1 × 10 7 to 1 × 10 9 pu of native capsid vector and the Adf.DA vector which is incapable of binding CAR or integrins. Cultures were examined at 3 and 7 days post delivery using fluorescence microscopy. The Adf.DA vector showed only a very low level of transfection at 3 days post delivery that was independent of dose and overall transduced less cells/explant at the lowest dose of Adf delivered. By 7 days post delivery expression of GFP in the Adf.DA treated cultures was equivalent to the native capsid (Adf) treated cultures. This suggests that the kinetics of delivery is different.
Effect of shaft mutations and vector exposure time on transduction
To determine if the lower affinity delivery seen in vitro by Ad.DA could be eliminated a mutation was introduced into the fiber shaft region resulting in a triple ablated vector (AdL.TA). Luciferase expressing vectors were chosen to allow for quantification of transgene expression levels. Mouse macular organ explants exposed to either AdL, AdL.DA or AdL.TA for 24 hours showed significantly different levels of luciferase expression after 24 hours in vitro (Fig 3A). AdL and AdL.DA showed comparable levels of luciferase expression (7.2 ± 1.8 × 106 RLU/ug protein vs 1.1 ± 0.3 106 RLU/ug protein (p <0.5)). AdL.TA treated explants demonstrated only background levels of luciferase activity (Fig 3) (5.1 ± 1.1 × 103 RLU/ug protein (p<0.01). This suggests that the fiber shaft mutation introduced into Ad.DA vector eliminates all delivery of transgene to the inner ear. Decreasing the vector exposure time from 24 hours to 1 hour (Fig 3B) resulted in reduction of luciferase expression in explants treated with AdL.DA to 0.8 ± 0.2 × 103 RLU/ug protein (p<0.01) levels similar to that of AdL.TA. This demonstrates that delivery to inner ear tissue seen with non CAR and integrin binding vectors requires prolonged contact with the target tissue.
Figure 3.
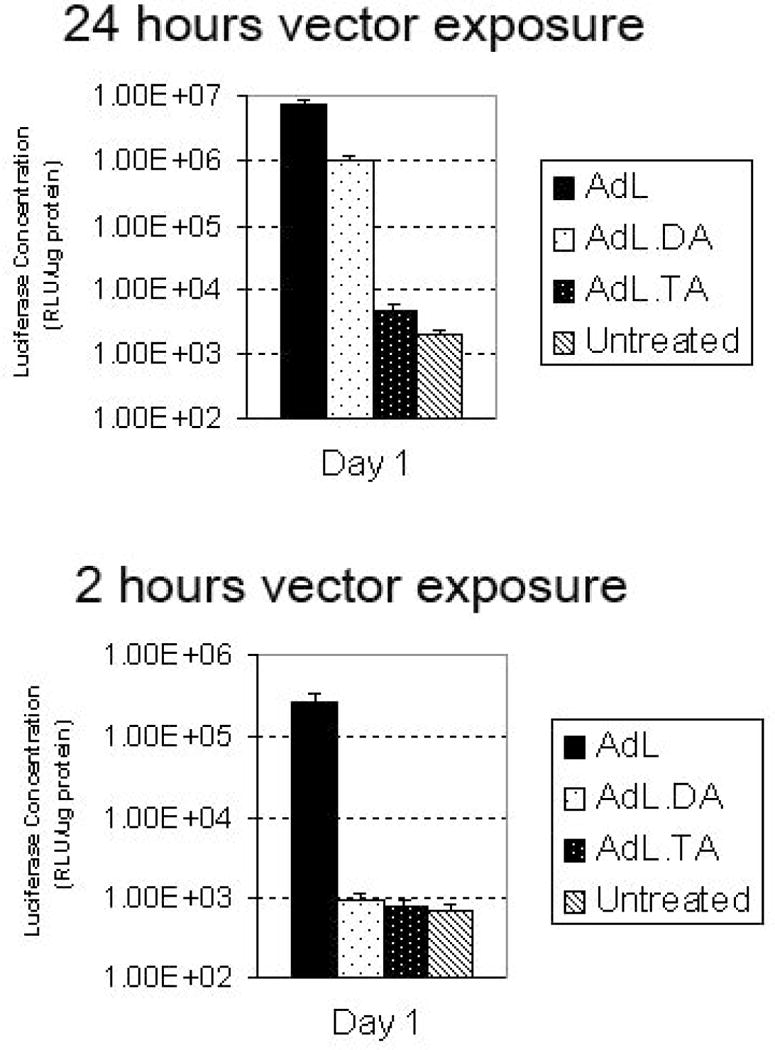
To test if the entry into the inner ear could be prevented a luciferase expressing vector with knob, penton and shaft mutations was designed. Adult mouse macular organ cultures were treated with native capsid vector (AdL), vector incapable of binding CAR and integrin (AdL.DA) and vector incapable of binding CAR, integrin and heparin (AdL.TA). After 24 hours cultures were harvested, protein extracted and luciferase levels determined. Native and CAR/integrin ablated capsid binding showed a high level of luciferase expression with a close to 10 fold difference in luciferase levels between native capsid vector and the CAR/integrin ablated vector. The addition of a mutation of the vector shaft region resulted in extremely low levels of luciferase expression (Fig 3A). These data suggest that an alternative mechanism of entry is directed by the Ad5 shaft interacting with heparin. To test if the kinetics of entry utilized by the Ad5 shaft interaction was indeed altered the time of entry of the vectors was by washing the cultures 2 hours after vector exposure (Fig 3B). After 24 hours cultures were harvested and luciferase levels determined. When the time of vector contact was limited to only 2 hours the overall transduction level of the normal capsid AdL was reduced (Fig 3B) and the modified capsid without CAR and integrin interactions was also limited.
Alternate serotype vectors can transfect macular organ cultures
To evaluate the time course of transgene expression after vector delivery to macular organ explants were imaged for GFP expression at 1, 2, 7 and 14 days post transfection. We used three alternate serotype vectors Ad28, Ad35 and Ad41 and compared them to the Ad5 capsid vector (Adf) that we had used in previous experiments. GFP was expressed evenly in all macular organ explants starting from day 1 and expression maintained on a strong and even level up to day 7 for all adenovector serotypes (Fig 4). On day 14, the level of GFP was higher in Ad28 transfected explants compared to the other serotypes.
Figure 4.
Macular organ cultures transfected with GFP expressing Ad5, Ad28, Ad35 and Ad41 (1 ×109 pu). Images were taken on day 1, 2, 7 and 14 after transfection and show that all vectors transfect the macular organ cultures in a similar way from day 1 till day 7. On day 14 post transfection Ad28 appeared to produce more GFP.
To quantify the transfection efficiency we used quantitative PCR for GFP DNA to detect number of vector genomes in the macular organs. Efficiency of vector function was determined by quantitiative RT PCR of GFP mRNA in transfected macular organ explants. Levels of GFP DNA in macular organ explants after transfection with alternate adenovirus serotypes on day 2 were 121.59 GFP copy numbers/pg for Ad5, 157.22 GFP copy numbers/pg for Ad35, 162.91 GFP copy numbers/pg for Ad41 and 127.16 GFP copy numbers/pg for Ad28. GFP DNA copy numbers for alternate adenovector serotypes are similar suggesting that equal amounts of each vector were present in the tissue after delivery. Delivery of Ad28 resulted in increasing levels of GFP mRNA over the two weeks after transfection. A maximum was reached at 14 days with GFP mRNA levels after Ad28 transfection being 12 times higher than after Ad5 transfection (Fig 5). Ad35 transfection peaked at day 4 after vector delivery which was the only time point GFP mRNA levels after incubation with Ad35 were higher than levels after incubation with Ad5 (Adf). The maximum mRNA levels of Ad41 were found on day 7 but they never reached the mRNA levels of Ad5.
Figure 5.
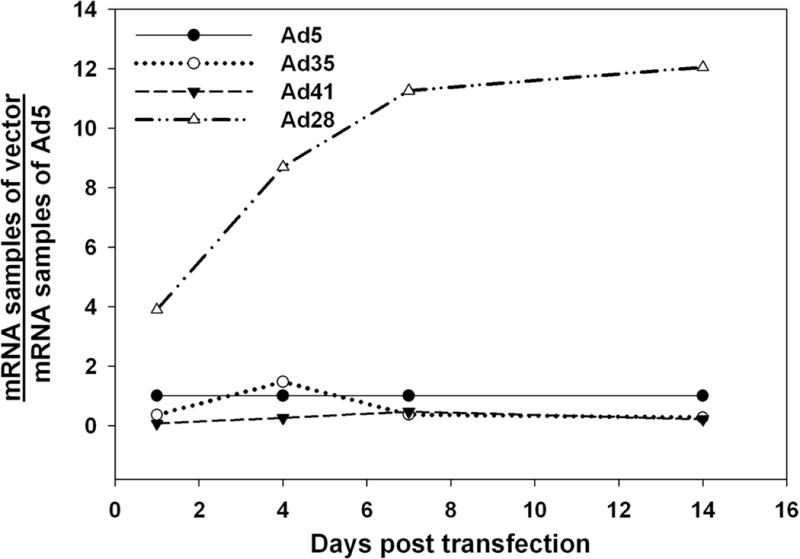
Level of transgene expression in macular organs. Taqman assays for GFP mRNA were carried out at 1, 4, 7 and 14 day intervals post transfection. For Ad35 and Ad41 GFP mRNA levels are lower in relation to Ad5 (Adf) based vectors (Adf). In contrast, after Ad28 mediated delivery of GFP, RNA levels continue to increase over time relative to Ad5.
Spatial expression pattern of alternate adenovector serotypes in healthy inner ears
The tissue distribution of Ad5, Ad28, Ad35 and Ad41 was assessed 7 days after vector delivery. Ad5, Ad35 and Ad41 had a similar distribution (Fig 6). In the organ of Corti intense staining was found in the pillar cells and to a lesser extent in hair cells and other types of supporting cells. Comparison with negative control sections showed that the massive staining of the pillar cells is due to an artifact (data not shown). In these three adenovector serotypes GFP was expressed in the spiral ganglion cells and at a lower level in the stria vascularis. In the macular organs GFP was expressed in all cell types though supporting cells were more intensely stained. In contrast, Ad28 showed a different distribution that was characterized by a preference for vestibular and cochlear supporting cells (Fig 6). No cochlear or vestibular hair cells expressed GFP. Spiral ganglion cells and the stria vascularis were not transfected.
Figure 6.
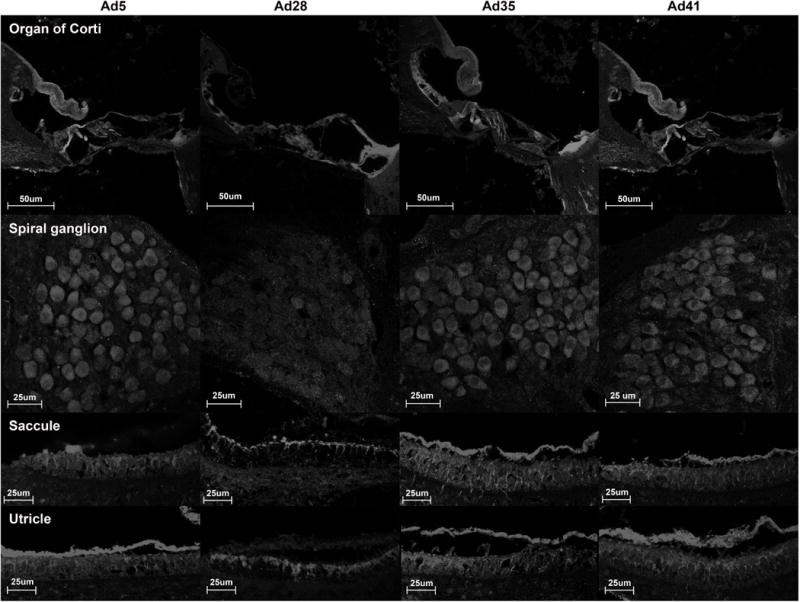
Pattern of tissue distribution of alternate adenovirus serotypes as determined by immunohistochemistry. Tissue sections were stained for GFP and showed a distinct transfection pattern for different adenovector serotypes. The tissue distribution of Ad5, Ad35 and Ad41 is similar, all of them transfecting vestibular and cochlear hair cells and supporting cells as well as spiral ganglion cells. In contrast, Ad28 targets vestibular and cochlear supporting cells. No GFP expression was found in the spiral ganglion cells or in vestibular and cochlear hair cells.
Effect of alternate adenovector serotypes on hearing and balance in normal mice
To test if Ad28, Ad35 or Ad41 affected inner ear function in normal mice we assessed rotarod times, ABR thresholds and DPOAEs 1d before surgery and 7d after surgery (Ad28 n=6, Ad35 n=4, Ad41 n=4). There was no statistically significant change in rotarod times for Ad28 (Fig 7A, 298.9 sec ± 45SD preop vs. 309.1 sec ± 33.5SD postop, P=0.202, n=4), for Ad35 (Fig 7B, 322.6 sec ± 24.2SD preop vs. 327 sec ± 19.8SD postop, P=0.141, n=4) or Ad41 (Fig 7C, Figure 7 shows their average ABR thresholds pre- and postoperatively for Ad28 (Fig 7D), Ad35 (Fig 7E) and Ad41 (Fig 7F). We compared the pre- and postoperative thresholds at each measured frequency but no statistically significant shift in threshold was detectable. In addition DPOAEs were measured on the left ear at the same time points. The hair cell response to the pure tone F2 was not statistically significantly changed after delivery of Ad28 (Fig 7G), Ad35 (Fig 7H) or Ad41 (Fig 7I) at all tested frequencies.
Figure 7.
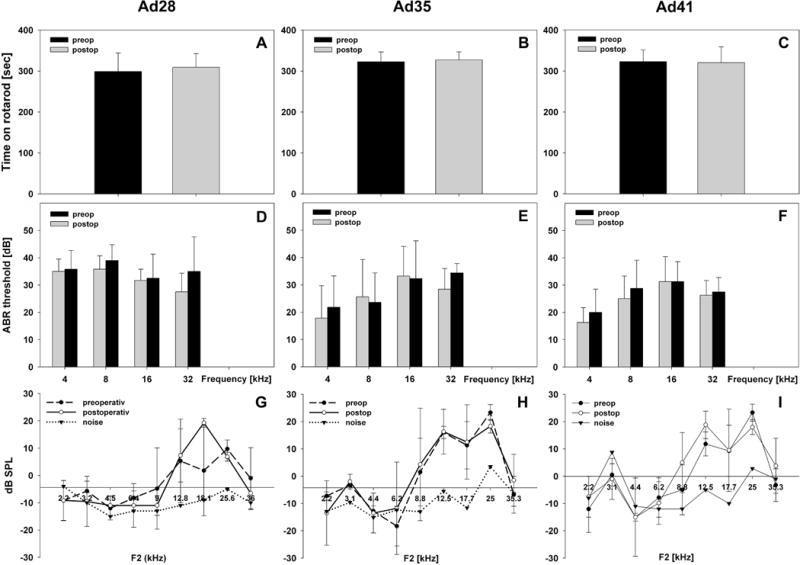
Vestibular and cochlear function before and after delivery of alternate adenovectors. Animals were tested one the day prior to surgery (=preop) and retested 7 days after vector inoculation (=postop). Rotarod times (7A–C) were not statistically significantly affected by vector delivery of Ad28 (7A), Ad35 (7B) or Ad41 (7C). There was also no change in ABR thresholds (7D–F) or in DPOAEs (7G–I) detectable for any of the adenovectors tested (error bar: mean + SD).
Distribution of vector DNA within the inner ear
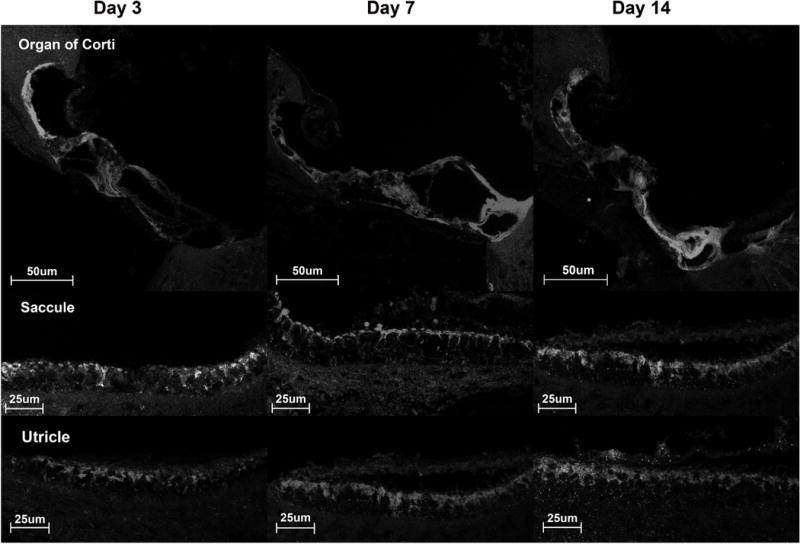
To quantify the tissue distribution of alternate adenovector serotypes samples of different tissues were harvested by laser capture microscopy and analyzed for the presence of GFP DNA by PCR (Table 1). Ad5 transfected the Organ of Corti, the stria vascularis, the spiral ganglion and the macular organs. The tissue distribution of Ad41 was comparable to Ad5. In contrast Ad 28 showed a different distribution pattern with a maximum of GFP DNA in the organ of Corti but no presence of GFP DNA in the stria vascularis or the spiral ganglion. Transfection by Ad35 also differed from Ad5 with low levels of GFP DNA detected in the spiral ganglion and stria vascularis.
Table 1.
Levels of GFP DNA in different tissues after transfection with alternate adenovector serotypes. Laser capture microscopy was used to sample the organ of Corti, saccule, utricle, spiral ganglion and stria vascularis for GFP DNA on day 2 after vector delivery to confirm transfection of these tissues. This table demonstrates differences in transfection patterns for Ad5, Ad28, Ad35 and Ad41 similar to the ones seen in figure 6. Unlike Ad5, Ad35 and Ad41, Ad28 does not transfect spiral ganglion cells or the stria vascularis. All adenovector serotypes
| Ad 5 | Ad 28 | Ad 35 | Ad 41 | |
|---|---|---|---|---|
| Organ of Corti | + | ++ | + | + |
| Stria Vascularis | + | − | +/− | + |
| Spiral Ganglion | + | − | +/− | + |
| Macular Organs | + | + | + | + |
Effect of vector dose on hair cell regeneration in vivo
Since the Ad28 vector appeared to optimize delivery to supporting cells we performed all regeneration experiments with atoh1 in this vector type. We evaluated the effect of Ad28.gfap.atoh1 on hair cell regeneration in the utricle in animals treated with the vestibulotoxin IDPN. Control utricles demonstrated an average of 1977 ± 141 type I hair cells. Treatment with IDPN resulted in a significant reduction of type I hair cells to 817 ± 195 (p<0.01) measured at 4 months post ototoxin administration. Administration of IDPN followed by delivery of 1 × 106 PU of Ad28.gfap.atoh1 via the posterior canal resulted a moderate increase of hair cells to 1126 ± 38 (p<0.05 compared to IDPN only). Delivery of a higher dose of vector (1 × 108 PU) after IDPN treatment led to recovery of the type I hair cell population to 1403 ± 234 cells (p<0.01 compared to IDPN only) (Fig 8A). Type II hair cells were reduced from 812 ± 50 to 242 ± 36 after IDPN treatment. Treatment with low and high dose vector resulted in hair cell counts of 324 ±20 and 382 ± 64 (p<0.05 ; p<0.02 compared to IDPN only) (Fig 8B). Supporting cell populations in controls were 1402 ± 173. IDPN treatment did not result in a reduction in supporting cell counts (1643 ± 238; p>0.05). Treatment with 1 × 106 pu of vector resulted in supporting cell counts of 1582 ± 382 (p>0.05 compared to control). In animals treated with the higher dose of vector after IDPN there was a moderate reduction of supporting cells to 1060 ± 246 (p<0.05 compared to controls) (Fig 8C).
Figure 8.
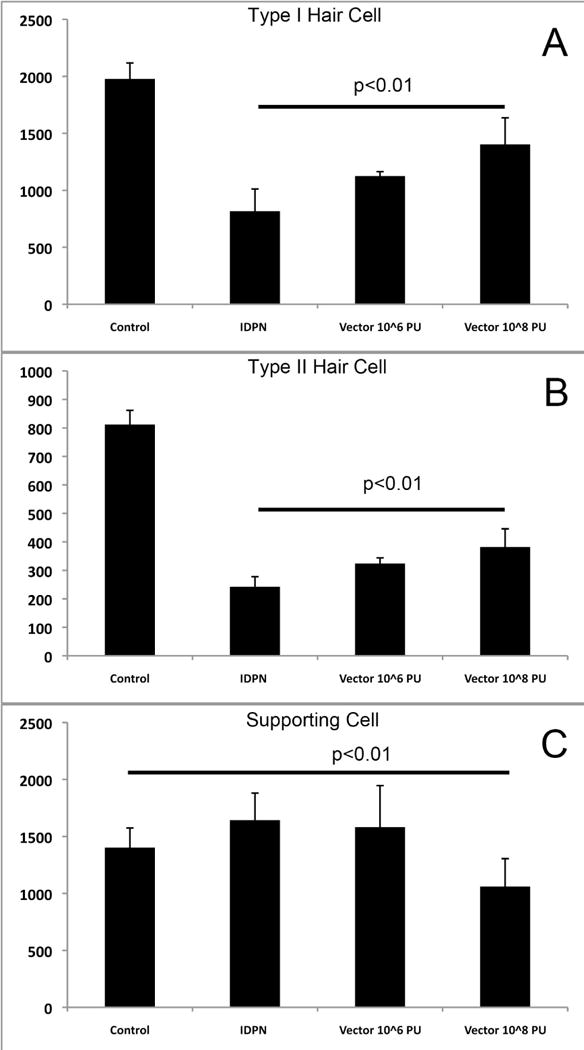
Regeneration of hair cells after IDPN vestibulotoxicity and delivery of Ad28.gfap.atoh1. Treatment of mice with IDPN resulted in a significant reduction in both Type I (8A) and Type II (8B) hair cells while maintaining a stable population of supporting cells (8C). Administration of Ad28.gfap.atoh1 at a low dose resulted in a statistically significant recovery of both type I and type II hair cells for both the low and the high dose of vector administration. Supporting cell numbers were reduced in animals that had been treated with high dose vector (8C).
Effect of vector dose on recovery of rotarod times
Treatment of animals with IDPN resulted in a rapid reduction of rotarod times by 10 days post feeding. By four months post IDPN feeding control animals had recovered to a rotarod time of 35 ± 41s (Fig 9). Animals treated with IDPN followed by low dose vector recovered to 59 ± 40s which was not significantly different from IDPN alone (P>0.05). Treatment with IDPN followed by high dose vector resulted in improvement of rotarod times to 114 ± 39s which was a significant difference from IDPN alone (p<0.05).
Figure 9.
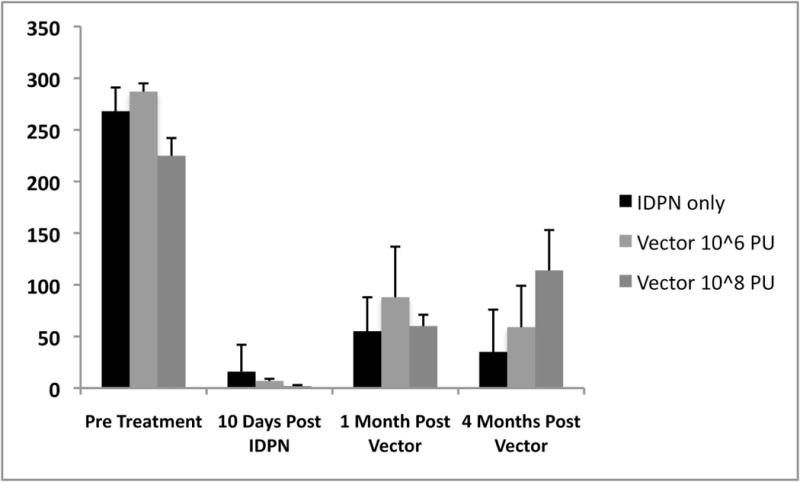
Effect of hair cell regeneration on balance measured by rotarod times. Administration of IDPN resulted in loss of balance and inability to stay on a rotating rod. Administration of high dose Ad28.gfap.atoh1 resulted in a significant recovery of rotarod times at 4 months post vector administration. Lower dose of vector did not significantly improve rotarod times compared to IDPN only treated animals
Atoh1 can regenerate hair cells in human tissue
Human utricles harvested at time of acoustic neuroma surgery were treated with neomycin 10−3 M for 5 days in vitro followed by administration of 1 × 108 pu of Ad28.gfap.atoh1. Explants were then maintained in vitro for 2 weeks, then fixed and immunostained for the presence of myosin VII. Control utricles showed normal appearing myosin VII positive cells (Fig 10A). Treatment with neomycin resulted in ablation of hair cells (Fig 10B). Neomycin treatment followed by administration of Ad28.gfap.atoh1 resulted in restoration of myosin VII positive cells (Fig 10C).
Figure 10.
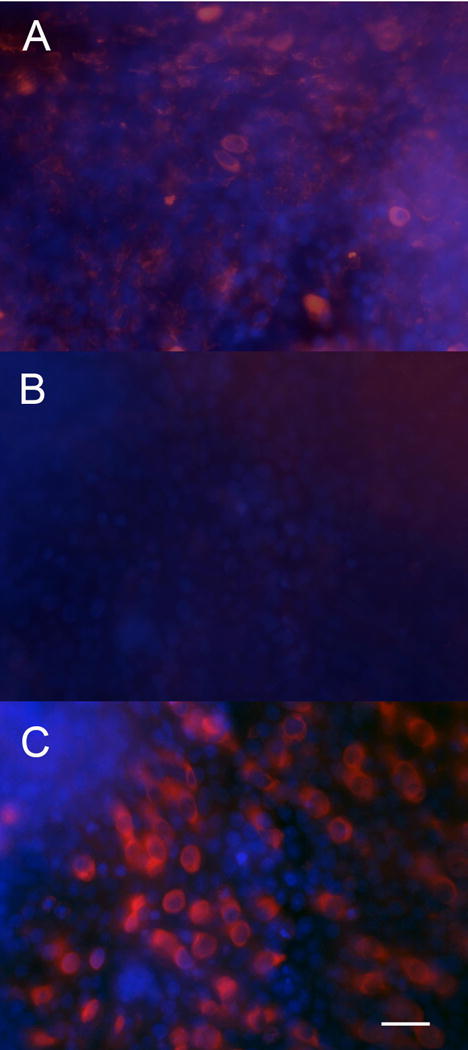
Effect of atoh1 on human utricles. Control utricles kept in vitro for 3 weeks demonstrate a robust population of myosin VII positive hair cells (10A). Treatment of utricles with neomycin resulted in loss of myosin VII positive cells (10B). Explants treated with neomycin followed by Ad28.gfap.atoh1 recovered myosin VII a positive cells (10C) demonstrating that Ad28 mediated delivery of atoh1 can induce hair cell regeneration in human tissue
Discussion
Optimizing gene therapy in the inner ear
A major goal of gene therapy is specificity of drug delivery to subsets of cells within a complex tissue. This is particularly important in the inner ear. The inner ear is a limited volume space with mice having a calculated cochlear perilymph volume of less than 2 μl. There is therefore immediately a limit on total number of vector particles that can be delivered to the inner ear that is dependent on the initial concentration of the vector delivered. Thus adenovectors which can be purified to concentrations of 1012 particles/ml would allow one to deliver 5 × 109 particles if the total cochlear perilymph volume is replaced by concentrated vector [17]. Vectors that have lower final titers such as adeno associated vectors and herpes vectors would thus deliver a lower number of particles. Careful analysis of the in vivo delivery data for adenovectors demonstrates that delivery is not completely uniform across all cells. Other studies have also noted that there is a saturation level reached within the cochlea after gene delivery with Ad 5 capsid vectors and increasing vector delivery does not increase the number of cells transduced [18]. Additional issues to be considered is that large volume deliveries to the inner ear result in functional loss and may result in spread of the vector into the CSF space via the cochlear aqueduct [19–21]. Increasing the overall dose of vector may also increase the immune response induced by the vector. An ideal vector for the inner ear would therefore minimize the number of particles delivered and modify particle binding to targeted cells for specific delivery that does not damage the residual function of the inner ear.
Ad5 capsid vectors have multiple binding and entry pathways
We have demonstrated that delivery of a vector that does not bind CAR or integrins results in very uniform delivery of GFP to a broad range of cells (Fig 1). Careful analysis of GFP expression patterns does not demonstrate any significant differences in the cells transduced (Fig 1 A–C vs D–F). This suggests that there is a non CAR, non integrin mediated adenovector entry system in the inner ear. In order to further characterize this effect we examine the kinetics of Ad 5 and Ad 5 ablated capsids in vitro using adult mouse macular organ cultures. As can be seen in Fig 2, CAR and integrin binding ablated vector requires a longer time to demonstrate GFP expression in cultures compared to native capsid vector, further demonstrating that a different vector cell interaction is responsible. We next constructed a vector with deletions of the fiber shaft region in addition to knob and penton mutations that prevent CAR and integrin binding. Comparison of luciferase activity in macular cultures treated with Ad 5 capsid vector (AdL), knob and penton mutated vector (AdL.DA) and triple ablated vector (AdL.TA) demonstrates only minimal luciferase activity in the explants exposed to the triple ablated vector, suggesting that this third mutation impairs vector binding and or entry (Fig 3A). The lower binding affinity and altered kinetics of the double ablated vector demonstrated in the follow up experiment where explants were only exposed to each capsid for one hour (Fig 3B). This resulted in normal GFP transduction for the native capsid vector but reduction of the ability of the double ablated vector to transducer to similar levels of the triple ablated vector (Fig 3B). Currently no data exists on the time course of vector persistence in the inner ear, but modeling data suggests that high molecular weight substances would diffuse only very slowly through the inner ear [22]. The pattern of GFP expression seen after gfp delivery via the doubly ablated vector thus most likely is the result of prolonged vector contact with inner ear tissue due to poor vector clearance from the inner ear. This suggests that delivery of a gene using an Ad5 construct could result in delivery of the gene to non targeted cells. Alternately use of an Ad5 capsid may require more vector particles to deliver the gene of interest to the target tissue. To optimize the specificity of delivery to cells of the inner ear a vector that specifically binds only supporting cells is needed.
Alternate capsid adenovectors can deliver genes to the inner ear
One approach for improving the specificity of gene delivery is to take advantage of the natural binding characteristics of different vectors. In this study we characterized the expression pattern of the alternate adenovector serotypes in the inner ear of healthy mice. We found that adenovectors had serotype specific transfection kinetics and spatial transfection patterns. Though different the adenovector serotypes Ad5, Ad35, and Ad41 shared more similarities than Ad28 that had a distinctly different sub-localization pattern and time course of gene expression. Balance and hearing tests before and after inoculation of Ad28, Ad35 or Ad41 showed no functional impact on hearing and balance function demonstrating that they were safe to use in a normal inner ear.
Spatial and temporal expression pattern of alternate adenovector serotypes
In vitro and in vivo data displayed differences in the transfection kinetics for the alternate adenovector serotypes tested in this study. In vitro macular organ cultures provide an excellent way to follow the kinetics of transgene expression after vector delivery. Ad35 mediated GFP expression peaked on day 4 after transfection and Ad41 on day 7 but the overall level of GFP expression was low compared to Ad5 (Fig 4 and 5). In contrast Ad28 sustained a significant higher level of GFP expression than Ad5 throughout the whole period and had a slower transfection kinetics with a maximum on day 14, despite the delivery of equivalent vector doses. The tissue distribution of Ad35 and Ad41 were similar to the distribution of Ad5. Transfection occurs throughout the cochlea and macular organs and is not confined to a certain cell type. In contrast, the main targets for Ad28 are vestibular and cochlear supporting cells. Unlike Ad5, Ad35 and Ad41 it does not appear to transfect hair cells.
Ad28, Ad35 and Ad41 vectors have been used in a variety of tissues but until this study had not been tested in the inner ear [23]. Unlike the commonly used vectors Ad5 and Ad2 the prevalence of neutralizing antibodies (NAbs) against Ad28 and Ad35 in the general population is very low [24]. Neutralizing antibodies are directed against serotype-specific structures on the viral hexon and decrease the efficiency of gene transfer in animal models as well as in early human trials [25–27]. As levels of NAbs vary among people the rate of inactivation of a potential vector will also vary. This makes it very difficult to determine the ideal dose of vector needed. It therefore is important to develop a vector that is less likely to be affected by NAbs. Though NAbs against Ad41 are higher than against Ad28 and Ad35, they are still lower compared to Ad5 and Ad2 [28, 29]. Since the tissue distribution of Ad41 is comparable to Ad5 (Fig 6, Table 1) it might be an alternative to Ad5 though it has a lower level of transgene expression. Ad35 is an uncommon adenovirus that often found in immuncompromised hosts. In contrast Ad41 is a quite common natural pathogen of the digestive tract. Both adenoviruses have been established and characterized as vectors. Ad35 has already been successfully used for transfection studies in different tissues but not the inner ear. Ad28 is a rare serotype that belongs to the subgroup C. So far its receptor is unknown. Ad35 binds CD46.
In our study we characterized alternate adenovector serotypes in the inner ear of healthy mice. Ad35 possesses a fast transfection kinetic with a peak at 4 days post vector delivery which could be used for forced short term expression of transgenes. The Ad35 serotype transfected predominately the organ of Corti and macular organs. Another advantage of Ad35 is that its local delivery produces good local transgene expression without any significant distant transfection [30]. Quantitative PCR showed that the transduction efficiency of Ad35 was less compared to Ad5. The primary receptor for Ad35 is CD46, a complement regulatory protein that is ubiquitously expressed in humans [31]. However, in wild-type mice CD46 is predominately expressed in the testis and its protein sequence shares only a homology of 46% to the human CD46 protein which probably accounts for the reduced transfection efficiency. In addition, binding of Ad35 to human CD46 leads to down-regulation of cell-surface CD46 which in turn increases the susceptibility of these cells to complement-mediated cell lysis [32]. A further investigation in CD46-transgenic mice might therefore be necessary to address these two issues and determine if Ad35 is well suited to inner ear gene therapy.
Ad41 and Ad5 enter cells via the CAR-ανβ3/ανβ5-pathway that is also found on sensory hair cells [33]. The receptor for Ad28 is not known yet. As Ad28 has a distinct transfection pattern targeting predominately supporting cells it is likely that this is mediated by a specific receptor. The preference of Ad28 for vestibular and cochlear supporting cells, has major clinical implications. Supporting cells can transdifferentiate into functional hair cells after transfection with the transcription factor Atoh1 [5]. In deaf guinea pigs this approach partially restored hearing. Ad28 would therefore be a suitable vector for regeneration studies. Another characteristic of Ad28 is its high and long-lasting transduction efficiency. Therefore less amounts of vectors are necessary to produce a sufficient level of transfection. As the risk of unwanted side effects is dependent on the amount of injected vector this is an important feature to increase the safety of a vector [34, 35]. To decrease the needed volume of vector to a minimum is especially crucial in the inner ear as large volumes can destroy hearing [19].
Vestibular hair cell regeneration induced by Ad28 mediated delivery of atoh1
The clear advantages of the Ad28 construct led us to utilize this vector for building a vector to drive hair cell regeneration. The supporting cell specific entry characteristics of this vector were further enhanced by driving atoh1 with the supporting cell specific promoter GFAP. In previous studies we had shown this to be more effective in driving regeneration of hair cells in vitro [11, 12]. Treatment of animals with IDPN reliably reduced both type I and type II hair cell counts and gave animals a permanent disturbance of balance as measured by rotarod (Figs 8,9). Delivery of low dose atoh1 vector increased both type I and type II hair cell populations but did not result in a significant improvement of balance (Fig 8,9). Since hair cell counts were conducted at 4 months post treatment we can assume that hair cell survival after regeneration is stable. Use of a higher dose of vector resulted in a significant improvement in type I and type II hair cell counts and a significant recovery in rotarod times (Fig 8,9). In this cohort of animals we also noted a reduction in supporting cell number which is to be expected if the new hair cells repopulate from existing supporting cells. Overall this suggests that a minimum cell number needs to regenerate to restore vestibular function. Possibly increasing their population of functional hair cells by a moderate amount rather than complete restoration of the vestibular neuroepithelium would be adequate to restore their VOR and balance.
Development of a therapeutic for human bilateral vestibular hypofunction may be possible using vector mediated delivery of atoh1. We have demonstrated that human utricles are capable of regenerating hair cells after aminoglycoside damage and delivery of atoh1 (Fig 10). Evaluation of Ad5 capsid vectors has shown that Ad5 is capable of cell entry by non CAR mechanisms including binding to heparin. This could result in non specific delivery of atoh1 to non targeted cells. Screening alternate adenovector serotypes has led us to identify a vector that appears to target supporting cells (Fig 6) and additionally has the property of having low immunogenicity. Adding a supporting cell specific promoter further improves the safety and specificity of the construct. Using a rodent model of vestibulotoxitiy we have demonstrated a dose response effect for Ad28.gfap.atoh1. Although high and low doses of vector result in robust regeneration of hair cells, a higher dose of vector is needed to see functional balance improvement.
Conclusion
Ad 5 vectors have both specific and non specific entry mechanisms for inner ear targets that potentially influence to dose of vector needed to induce hair cell regeneration. Screening of rare serotype vectors demonstrated that Ad28,35 and 41 were able to deliver a transgene to inner ear tissue. Ad28 is the most suited for delivery of atoh1 since it specifically transfects supporting cells. Utilization of this vector coupled to a supporting cell specific promoter was able to restore hair cells in an IDPN model of vestibulopathy even at low vector doses. Functional restoration required higher doses of vector. Finally we additionally demonstrated that our optimized vector, Ad28.gfap.atoh1 was able to regenerate vestibular hair cells in human tissue, making it a good candidate for drug development.
Acknowledgments
Supported by NIDCD grant R01 DC 008424
Financial disclosure: All work was supported by NIDCD grant R01 DC 008424; Chi Hsu and Douglas Brough are employees of GenVec Inc.
Footnotes
Conflict of Interest: None
References
- 1.Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240(4860):1774–6. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- 2.Bermingham NA, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284(5421):1837–41. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 3.Staecker H, et al. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007;28(2):223–31. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- 4.Staecker H, Praetorius M, Brough DE. Development of gene therapy for inner ear disease: Using bilateral vestibular hypofunction as a vehicle for translational research. Hear Res. 2011;276(1–2):44–51. doi: 10.1016/j.heares.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izumikawa M, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11(3):271–6. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 6.Kawamoto K, et al. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23(11):4395–400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss MA, et al. Viral-mediated gene transfer in the cochlea. Int J Dev Neurosci. 1997;15(4–5):577–83. doi: 10.1016/s0736-5748(96)00112-8. [DOI] [PubMed] [Google Scholar]
- 8.Dechecchi MC, et al. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J Virol. 2001;75(18):8772–80. doi: 10.1128/JVI.75.18.8772-8780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Einfeld DA, Roelvink PW. Advances towards targetable adenovirus vectors for gene therapy. Curr Opin Mol Ther. 2002;4(5):444–51. [PubMed] [Google Scholar]
- 10.Izumikawa M, et al. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240(1–2):52–6. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Praetorius M, et al. Adenovector-mediated hair cell regeneration is affected by promoter type. Acta Otolaryngol. 2010;130(2):215–22. doi: 10.3109/00016480903019251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Praetorius M, et al. Adenoviral vectors for improved gene delivery to the inner ear. Hear Res. 2009;248(1–2):31–8. doi: 10.1016/j.heares.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama M, et al. Ablating CAR and integrin binding in adenovirus vectors reduces nontarget organ transduction and permits sustained bloodstream persistence following intraperitoneal administration. Mol Ther. 2004;9(2):218–30. doi: 10.1016/j.ymthe.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Einfeld DA, et al. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J Virol. 2001;75(23):11284–91. doi: 10.1128/JVI.75.23.11284-11291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McVey D, et al. Repeat administration of proteins to the eye with a single intraocular injection of an adenovirus vector. Mol Ther. 2008;16(8):1444–9. doi: 10.1038/mt.2008.124. [DOI] [PubMed] [Google Scholar]
- 16.Schlecker C, et al. Selective atonal gene delivery improves balance function in a mouse model of vestibular disease. Gene Ther. 2011;18(9):884–90. doi: 10.1038/gt.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staecker H, et al. Drug delivery to the inner ear using gene therapy. Otolaryngol Clin North Am. 2004;37(5):1091–108. doi: 10.1016/j.otc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto K, et al. The functional and structural outcome of inner ear gene transfer via the vestibular and cochlear fluids in mice. Mol Ther. 2001;4(6):575–85. doi: 10.1006/mthe.2001.0490. [DOI] [PubMed] [Google Scholar]
- 19.Praetorius M, et al. Hearing preservation after inner ear gene therapy: the effect of vector and surgical approach. ORL J Otorhinolaryngol Relat Spec. 2003;65(4):211–4. doi: 10.1159/000073117. [DOI] [PubMed] [Google Scholar]
- 20.Stover T, Yagi M, Raphael Y. Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther. 2000;7(5):377–83. doi: 10.1038/sj.gt.3301108. [DOI] [PubMed] [Google Scholar]
- 21.Kho ST, et al. Safety of adeno-associated virus as cochlear gene transfer vector: analysis of distant spread beyond injected cochleae. Mol Ther. 2000;2(4):368–73. doi: 10.1006/mthe.2000.0129. [DOI] [PubMed] [Google Scholar]
- 22.Plontke SK, Salt AN. Quantitative interpretation of corticosteroid pharmacokinetics in inner fluids using computer simulations. Hear Res. 2003;182(1–2):34–42. doi: 10.1016/s0378-5955(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 23.Vogels R, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77(15):8263–71. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nwanegbo E, et al. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin Diagn Lab Immunol. 2004;11(2):351–7. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gahery-Segard H, et al. Humoral immune response to the capsid components of recombinant adenoviruses: routes of immunization modulate virus-induced Ig subclass shifts. Eur J Immunol. 1997;27(3):653–9. doi: 10.1002/eji.1830270312. [DOI] [PubMed] [Google Scholar]
- 26.Gahery-Segard H, et al. Phase I trial of recombinant adenovirus gene transfer in lung cancer. Longitudinal study of the immune responses to transgene and viral products. J Clin Invest. 1997;100(9):2218–26. doi: 10.1172/JCI119759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toogood CI, Crompton J, Hay RT. Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. J Gen Virol. 1992;73(Pt 6):1429–35. doi: 10.1099/0022-1317-73-6-1429. [DOI] [PubMed] [Google Scholar]
- 28.Kidd AH, Banatvala JE, de Jong JC. Antibodies to fastidious faecal adenoviruses (species 40 and 41) in sera from children. J Med Virol. 1983;11(4):333–41. doi: 10.1002/jmv.1890110409. [DOI] [PubMed] [Google Scholar]
- 29.Shinozaki T, et al. Antibody response to enteric adenovirus types 40 and 41 in sera from people in various age groups. J Clin Microbiol. 1987;25(9):1679–82. doi: 10.1128/jcm.25.9.1679-1682.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakurai F. Development and evaluation of a novel gene delivery vehicle composed of adenovirus serotype 35. Biol Pharm Bull. 2008;31(10):1819–25. doi: 10.1248/bpb.31.1819. [DOI] [PubMed] [Google Scholar]
- 31.Liszewski MK, et al. Emerging roles and new functions of CD46. Springer Semin Immunopathol. 2005;27(3):345–58. doi: 10.1007/s00281-005-0002-3. [DOI] [PubMed] [Google Scholar]
- 32.Schnorr JJ, et al. Measles virus-induced down-regulation of CD46 is associated with enhanced sensitivity to complement-mediated lysis of infected cells. Eur J Immunol. 1995;25(4):976–84. doi: 10.1002/eji.1830250418. [DOI] [PubMed] [Google Scholar]
- 33.Venail F, et al. Coxsackie adenovirus receptor and alpha nu beta3/alpha nu beta5 integrins in adenovirus gene transfer of rat cochlea. Gene Ther. 2007;14(1):30–7. doi: 10.1038/sj.gt.3302826. [DOI] [PubMed] [Google Scholar]
- 34.Campochiaro PA, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther. 2006;17(2):167–76. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 35.Morral N, et al. Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: comparison of E2a wild type and E2a deleted vectors. Hum Gene Ther. 1997;8(10):1275–86. doi: 10.1089/hum.1997.8.10-1275. [DOI] [PubMed] [Google Scholar]


