To the Editor
Epidemic transmission of Zika virus (ZIKV) has rapidly occurred in the Americas, with most cases limited to mild or asymptomatic disease.1,2 To date, nine deaths from ZIKV infection that were unrelated to the Guillain–Barré syndrome have been confirmed in adults.1 Here, we report a rapidly progressive, fatal ZIKV infection acquired outside the United States and secondary local transmission in the absence of known risk factors for ZIKV infection.
Patient 1, a 73-year-old man who had emigrated to the United States from Mexico in 2003, was admitted to a hospital in Salt Lake City with hypotension and abdominal pain. Radiation therapy for stage IIB prostate cancer had been completed 1 month earlier, and he was receiving antiandrogen therapy but was otherwise not systemically immunocompromised. Eight days before admission, he had returned from a 3-week trip to the southwest coast of Mexico, where ZIKV transmission had been reported. He was well during his trip but reported being bitten by mosquitoes. After returning home, he reported having abdominal pain, pharyngitis, and fever, which was followed by conjunctivitis, nonbloody diarrhea, and myalgias.
On the day of admission, hypotension and dyspnea had developed. The patient was alert and oriented with no fever but with tachypnea and tachycardia. He remained hypotensive after the administration of intravenous fluids, and vasopressors and broad-spectrum antibiotics were initiated. The physical examination was remarkable for marked erythematous conjunctivitis with profuse tearing and soft-palate petechiae, tachypnea, and moderate, diffuse abdominal pain with mild guarding. A tourniquet test (which is often performed in patients in whom dengue is suspected) was negative.
Laboratory testing revealed metabolic acidosis, an elevated venous lactate level, renal insufficiency, mild hypoglycemia, elevated aminotransferase levels, leukocytosis with 44% band forms, anemia, and marked thrombocytopenia. (Details are provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org.) Testing for malaria and blood cultures were negative. A presumptive diagnosis of dengue shock syndrome was made. The patient’s clinical deterioration progressed, with progressive respiratory and renal failure, metabolic acidosis, and hepatitis. On day 4 of hospitalization, the patient died shortly after care was withdrawn.
Testing was negative for dengue virus (DENV) on polymerase-chain-reaction (PCR) assay. Serologic analysis for DENV was consistent with remote infection, with a highly elevated IgG level and an equivocal IgM level. Serum testing for ZIKV on real-time PCR assay was positive, with a threshold cycle of 17 and a very high estimated viral load of 2.0×108 ZIKV genome copies per milliliter. High-throughput sequencing of RNA revealed the presence of a ZIKV strain that shared 99.8% of the genome sequence with a strain isolated from a mosquito in Chiapas, Mexico, in 2016 (Fig. 1). No other putative pathogen was detected by routine diagnostic testing and RNA sequencing.3
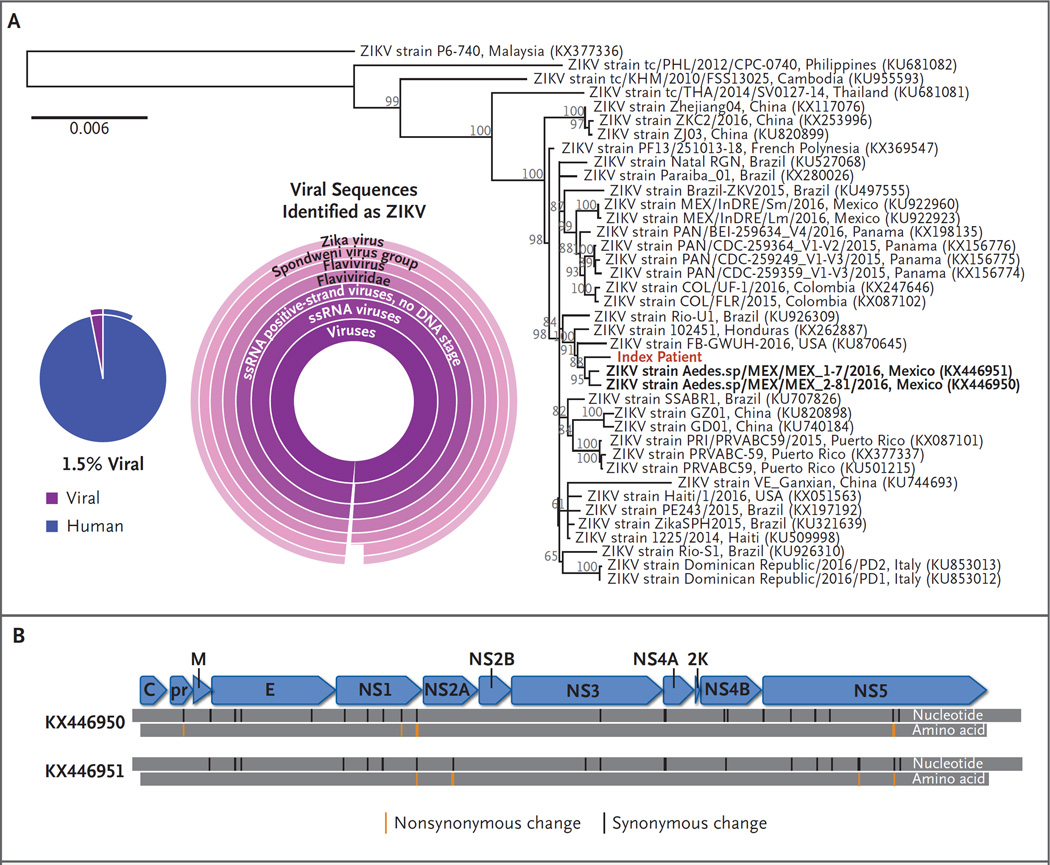
Figure 1. Identification and Characterization of ZIKV on RNA Sequencing of Serum from Patient 1.
Panel A shows that 1.5% of complementary DNA sequencing reads were of viral origin (pie chart). Partial external arcs indicate the proportion of viral reads that could be fully classified and human reads derived from messenger RNA transcripts. Viral sequences were identified as Zika virus (ZIKV) by means of Taxonomer software.3 Classification results for viral sequences are shown as a sunburst graph, in which the relative abundance of read-level classification is shown by the size of a given slice, with the highest taxonomic rank in the center and species-level classification in the periphery. The abbreviation ssRNA denotes single-stranded RNA. Panel B shows synonymous and nonsynonymous changes in the ZIKV isolate genome, as compared with its closest taxonomic relatives. Complete sequence information is available at GenBank (accession number, KX827268).
Five days after Patient 1 died, Patient 2, a previously healthy 38-year-old man with no known coexisting illnesses who had visited Patient 1 in the hospital, reported having conjunctivitis, fevers, myalgia, and facial maculopapular rash. The rash became generalized but resolved within 7 days. On day 7 after the onset of symptoms, urinalysis was positive for ZIKV but serum was negative on PCR assay. Serum IgM antibody to ZIKV was positive. Patient 2 reported having assisted a nurse in repositioning Patient 1 in bed without using gloves. Patient 2 also reported having wiped Patient 1’s eyes during the hospitalization but reported having had no other overt contact with blood or other body fluids, including splashes or mucous membrane exposure. No health care workers who had contact with Patient 1 reported having symptomatic illness.
It is likely that Patient 2 acquired the infection from Patient 1, since Patient 2 had not traveled to an area in which ZIKV is endemic in more than 9 months and had not had sex with a partner who had traveled to such areas. Given the very high level of viremia in Patient 1, infectious levels of virus may have been present in sweat or tears, both of which Patient 2 contacted without gloves. Transmission of the infection through a mosquito bite appears to be unlikely, since aedes species that are known to transmit ZIKV have not been detected in the Salt Lake City area.4 In addition, the second case occurred 7 to 10 days after contact with the index patient in the hospital, which implicates direct contact during hospitalization.
These two cases illustrate several important points. The spectrum of those at risk for fulminant ZIKV infection may be broader than previously recognized, and those who are not severely immunocompromised or chronically ill may nevertheless be at risk for fatal infection. The effect of previous infection with related flaviviruses cannot be assessed and may increase the risk of severe ZIKV infection. The transmission of flaviviruses through intact skin or mucous membranes, although uncommon, has been shown in experimental animal models and in at least one human case.5 Whether contact with highly infectious body fluids from patients with severe ZIKV infection poses an increased risk of transmission is an important question that requires further research.
Supplementary Material
Acknowledgments
Supported by a grant from the National Institutes of Health (RO1-CA-8133, to Dr. Swaminathan).
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Pan American Health Organization, World Health Organization. Cumulative Zika suspected and confirmed cases reported by countries and territories in the Americas, 2015–2016. ( http://ais.paho.org/phip/viz/ed_zika_cases.asp)
- 2.Duffy MR, Chen T-H, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 3.Flygare S, Simmon K, Miller C, et al. Taxonomer: an interactive metagenomics analysis portal for universal pathogen detection and host mRNA expression profiling. Genome Biol. 2016;17:111. doi: 10.1186/s13059-016-0969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraemer MU, Sinka ME, Duda KA, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen LH, Wilson ME. Transmission of dengue virus without a mosquito vector: nosocomial mucocutaneous transmission and other routes of transmission. Clin Infect Dis. 2004;39(6):e56–e60. doi: 10.1086/423807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.