Abstract
Traveling to and from university-based clinics is a major health care barrier for children with sickle cell disease in Alabama. To reduce this barrier, the University of Alabama at Birmingham (UAB) developed satellite clinics. This study seeks to determine if these satellite clinics provide a similar level of comprehensive care when compared with the university-based clinic using four surrogate markers: 1) attendance rates, 2) percentage of patients on hydroxyurea, 3) percentage of screening MRIs obtained, and 4) percentage of transcranial dopplers (TCD) completed. A retrospective review of sickle cell visits from June 1, 2012 to May 31, 2013 demonstrated that satellite clinics can provide levels of medical care for children with sickle cell disease similar to those provided by university-based clinics.
Keywords: sickle cell disease, satellite clinics, hydroxyurea, rural, urban
Sickle cell disease (SCD) is a hereditary condition associated with high morbidity and mortality secondary to recurrent episodes of acute illness and progressive organ damage.1,2 It is vital that all SCD patients receive appropriate medical care including regular patient evaluations, patient and family education, psychosocial care, and genetic counseling as previous studies show that comprehensive medical care can decrease the adverse outcomes associated with SCD. 3,4 Two university-based centers in the state of Alabama provide comprehensive medical care to pediatric patients with SCD. These centers are located in urban areas of the state, specifically Birmingham and Mobile, and are approximately 250 miles apart. According to a 2010 report, the estimated number of individuals living with sickle cell disease (SCD) in the state of Alabama is 3,500, of which, 1,200 pediatric patients are cared for at UAB Pediatric Sickle Cell clinics.5 Thirty percent of our sickle cell patient population lives in rural Alabama, making travel time to either of these two centers cumbersome and expensive.
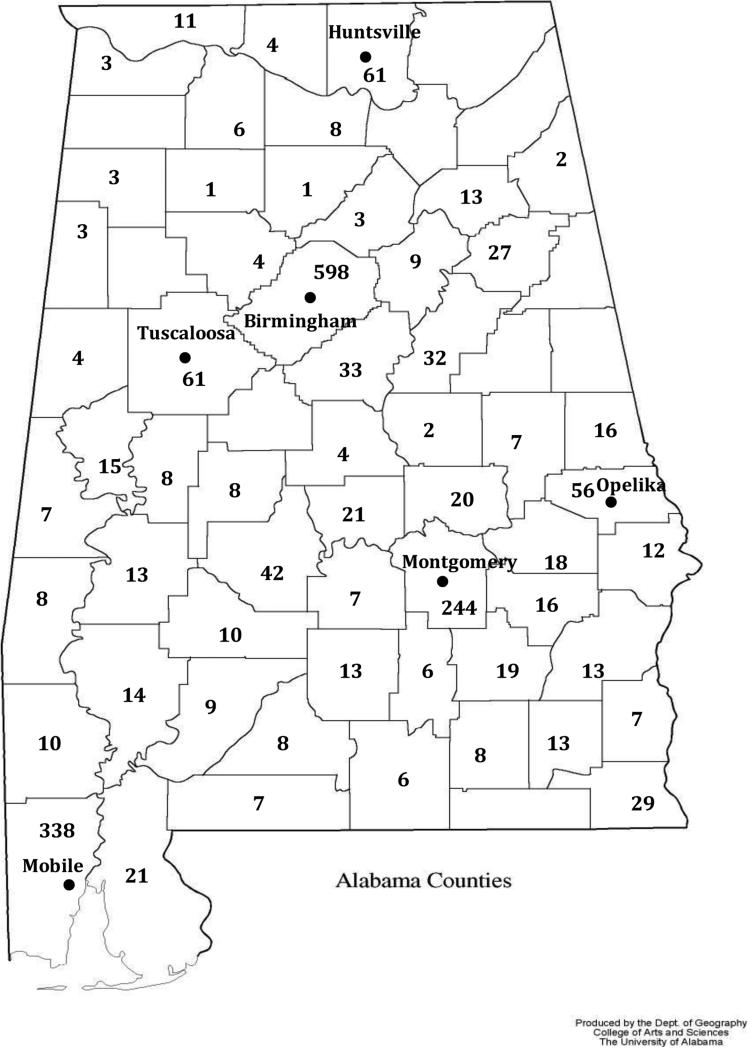
In 1995, the University of Alabama at Birmingham Division of Pediatric Hematology and Oncology established satellite sickle cell clinics in Montgomery (100 miles south of UAB), Opelika (110 miles southeast of UAB), Huntsville (100 miles north of UAB), and Tuscaloosa (60 miles west of UAB). (Figure 1) Prior to opening the satellite clinics, only 50% of patients with sickle positive newborn screens in the state of Alabama were seen by a pediatric hematologist prior to their second birthday. Upon review of our institutional database and grant progress reports, we determined that within five years of opening these clinics, the sickle cell patient population cared for by the UAB Pediatric Sickle Cell clinics doubled, and in 2014, 97% of infants with positive newborn screen referred to the UAB Pediatric Sickle Cell clinics were seen before their first birthday. While our referral process for infants identified with SCD has improved, the type of care provided at these satellite clinics compared with the university-based clinic has not previously been explored.
Figure 1.
Map of Alabama demonstrating county lines along with cities associated with UAB clinics. The numbers in each county represent the number of known individual pediatric SCD patients.
The UAB Pediatric Sickle Cell program has institutional guidelines for comprehensive care that apply to all clinics, on and off site. Thus, we have a consistent framework to compare the delivery of care between the urban clinic located in Birmingham and the satellite clinics. Specific examples include offering hydroxyurea to patients based on clinical indications that take age, degree of anemia, and clinical severity of disease into consideration. 6,7 Our guidelines also screen patients at highest risk of stroke with transcranial dopplers (TCD) by offering TCD to patients with hemoglobin (Hb) SS and Sβ0 thalassemia between two and 16 years of age in keeping with the STOP study criteria.8,9 Additionally, we offer a non-sedated magnetic resonance imaging (MRI) and magnetic resonance angiogram (MRA) to every patient with Hb SS and Sβ0 thalassemia between six and 15 years of age in effort to evaluate for silent cerebral infarct and CNS vasculopathy.10,11 This MRI/MRA is completed at the university campus in Birmingham. These guidelines constitute the framework which guided our choice of surrogate markers used to compare the consistency of care provided at the university-based clinic and the care provided at our satellite clinics.
In the literature, several adult studies discuss the use of satellite clinics while only a few have documented benefit in pediatrics. 12-28 From the pediatric literature, outcomes for pediatric obesity are improved when care and education are delivered closer to where the children live.26 Additionally, satellite clinics are shown beneficial and a successful way to handle a growing demand in pediatric cardiology.25 In a quality questionnaire, parents suggested opening more satellite clinics for pediatric subspecialties. 27 A search of PubMed with key words, sickle cell, pediatric, urban, rural, satellite, outreach, and clinic or a combination thereof revealed only one relevant pediatric sickle cell manuscript. This manuscript came out of Georgia and evaluated the use of TCD exams at their satellite clinics. 28 While the idea of providing care at satellite clinics is not new, the ability of academic centers to document successful use of care guidelines is vital to sustaining such clinics. Our study fills a gap in the literature by not only discussing satellite clinics in the pediatric sickle cell population, but also by attempting to compare the services provided at both types of clinics using multiple surrogate markers.
Therefore, the goal of this retrospective review is to evaluate the efficacy of these satellite clinics compared with the Birmingham clinic in providing care consistent with our institutional guidelines for SCD patients. We chose four outcomes as important measures of comprehensive care: 1) attendance rates, 2) percentage of patients on hydroxyurea, 3) percentage of screening MRIs performed, and 4) percentage of TCDs completed. We hypothesize that both university-based and satellite clinics provide a similar level of comprehensive care.
Methods
An institutional review board-approved retrospective review of all well child sickle cell clinic visits from June 1, 2012 to May 31, 2013 was performed using data from the main medical campus in Birmingham and the offsite sickle cell clinics in Montgomery, Opelika, Tuscaloosa, and Huntsville. The Birmingham sickle cell clinic is conducted on the first four Thursdays of each month, while satellite clinics are conducted on the first four Fridays of each month. Of note, patients with all sickle cell genotypes (SS, SC, Sβ+/Sβ0 thalassemia, SD) attend these clinics. Funding for the satellite clinics has been provided through multiple grants, including local grants and those from the Health Resources and Service Administration, the National Institute of Health Comprehensive Sickle Cell Centers, the Alabama Department of Health, and the State Sickle Cell Oversight Regulatory Commission.
The first measured outcome, attendance rate, is defined as the number of scheduled appointments that the patient attended over the total number of appointments scheduled. Patients with uncomplicated sickle cell disease, not currently on therapy, are scheduled for clinic visits three times/year for the first three years of life and then two times/year until transition of care to an adult provider. To evaluate attendance rates, the two investigators (JH and JL) went through hand-written records from each sickle cell clinic. Second, we calculated the proportion of patients on hydroxyurea out of the total number of individuals registered at each clinic during the study time period by reviewing the institutional list of all patients prescribed hydroxyurea. Once hydroxyurea is initiated, patients are generally seen monthly for the first six months, then every two months for the next six months, and then every three months thereafter. This schedule is subject to change based upon dosing changes and need to ensure balance between toxicity and maximal tolerated dose. The monthly satellite clinics are staffed by the same physicians and nurse practitioners which staff the university-based clinic. Therefore, we assume that uniformly implemented practice guidelines should result in similar percentages of patients on hydroxyurea therapy at each clinic. Initiating hydroxyurea therapy is based on sickle cell phenotype; therefore, patients with all genotypes are potentially offered hydroxyurea at our institution. After final analysis was complete, we queried the database once more in attempt to define the genotypic breakdown of patients on hydroxyurea. This new query represents patients on hydroxyurea at each clinic in September of 2015.
In contrast to offering hydroxyurea, screening MRI/MRAs and TCDs are only offered to patients with Hb SS or Sβ0 thalassemia. Our institutional guidelines mandate every patient undergo at least one non-sedated MRI (age ≥ 6) to screen for silent cerebral infarct. We identified all patients eligible for a screening MRI/MRA by reviewing our institutional database and then reviewed his or her medical record for completion or failure to complete this screening. Patients who had completed the screening MRI/MRA prior to this study period were not considered eligible for an MRI/MRA during this analysis. Finally, all appointments with planned TCD studies were reviewed for completion of this study. The principal investigator was able to gather this data from the hand-written records of TCDs performed at each clinic visit. The annual TCD studies are completed at the location of the clinics and are performed by the same staff at all location sites. In cases where the TCD study was abnormal, charts were reviewed for proper follow-up care.
The first three markers of care—attendance, hydroxyurea use, TCD screenings—are all deemed important factors in providing the standard of care to sickle cell patients and all have been shown to improve SCD outcomes.7,29 The fourth measure, MRI compliance, was chosen as a marker of complexity of care and the difficulty that comes with providing that complex care over a large geographical area. To enhance the reliability of this dataset, two members of the UAB Pediatric Sickle Cell program independently reviewed the data for accuracy. We evaluated the difference in outcome measures between the university clinic and the combined measures from all satellite clinics as the hypothesis of this paper deals with university-based clinics compared with all satellite clinics. A p-value <.05 was considered statistically significant. All results were analyzed by either the chi-square test or Fisher's exact test when the chi-square test was not feasible. All analyses were conducted using JMP Pro software (version 10; SAS Institute, Cary, NC).
Results
The data show that the Pediatric Sickle Cell Clinic at UAB cared for 1,191 patients with SCD in this one-year period. Five hundred and ninety-four (49.9%) of those patients are cared for in the Birmingham clinic, 334 (28.0%) in Montgomery, 103 (8.6%) in Opelika, 90 (7.6%) in Tuscaloosa, and 70 (5.9%) in Huntsville. The attendance rate is 59.8% in Birmingham, while the Montgomery, Opelika, Tuscaloosa, and Huntsville attendance rates are 57.7%, 73.1%, 70.3, and 59.4% respectively (p=.002). (Table 1) The percentage of patients on hydroxyurea therapy in Birmingham is 22.2%, while the percentages in Montgomery, Opelika, Tuscaloosa, and Huntsville are 21.6%, 32.0%, 24.4% and 21.4%, respectively (p =.27). Among Birmingham patients that are on hydroxyurea, 89.7% have HbSS or Sβ0 thalassemia and 10.3% have HbSC or Sβ+ thalassemia while the HbSS or Sβ0 thalassemia genotypes represent 77.8% of the patients at satellite clinics and the HbSC or Sβ+ thalassemia genotypes represent 23.2% of the satellite clinic patients on hydroxyurea (p=0.03). Among the satellite clinics, Tuscaloosa (88.5%) and Huntsville (84.2%) have the highest percentage of patients with HbSS or Sβ0 thalassemia on hydroxurea while Montgomery (74.3%) and Opelika (73.7%) have lower percentages of patients with HbSS or Sβ0 thalassemia on hydroxyurea.
Table 1.
Raw data, the percentages, and the p values for the four surrogate markers of care.
| Appointment Show Rate by Clinic | Number of Appointments Attended/Scheduled | Percent Show Rate | p value |
|---|---|---|---|
| Birmingham | 354/592 | 59.8% | |
| Montgomery | 281/487 | 57.7% | |
| Opelika | 125/171 | 73.1% | |
| Tuscaloosa | 64/91 | 70.3% | |
| Huntsville | 41/69 | 59.4% | |
| Combined Satellite Data | 511/818 | 62.5% | .0019 |
| Hydroxyurea Use by Clinic | Number of Patients on HU | Percent of Patients on HU | p value |
| Birmingham | 132/594 | 22.2% | |
| Montgomery | 72/334 | 21.6% | |
| Opelika | 33/103 | 32.0% | |
| Tuscaloosa | 22/90 | 24.4% | |
| Huntsville | 15/70 | 21.4% | |
| Combined Satellite Data | 142/597 | 23.8% | .2673 |
| MRI Screening by Clinic | Number of MRIs Completed | Percent MRI Completed | p value |
| Birmingham | 28/44 | 63.6% | |
| Montgomery | 22/33 | 66.7% | |
| Opelika | 15/18 | 83.3% | |
| Tuscaloosa | 10/15 | 66.7% | |
| Huntsville | 8/16 | 50.0% | |
| Combined Satellite Data | 55/82 | 67.1% | .3351 |
| TCD Screening by Clinic | Number of TCDs Completed | Percent TCD Completed | p value |
| Birmingham | 54/66 | 75.8% | |
| Montgomery | 42/49 | 81.6% | |
| Opelika | 17/18 | 94.4% | |
| Tuscaloosa | 19/22 | 86.4% | |
| Huntsville | 5/6 | 83.3% | |
| Combined Satellite Data | 83/95 | 87.4% | .707 |
The p values compare the combined satellite data to the Birmingham clinic data.
The percentage of eligible patients with completed MRI/MRA screening in Birmingham is 63.6%, while the percentage in Montgomery, Opelika, Tuscaloosa, and Huntsville is 66.7%, 83.3%, 66.7%, and 50.0% respectively (p=.34). Finally, the percent of completed TCD studies in Birmingham is 75.8%, while the Montgomery, Opelika, Tuscaloosa, and Huntsville percentages are 81.6%, 94.4%, 86.4%, and 83.3% respectively (p=.71) (Table 1). There are two TCD studies (4%) with abnormal results from the Birmingham data. One of these neccessitated a repeat TCD while the other required an immediate follow-up MRI/MRA (TCD>220). Secondary to poor patient adherance, the repeat TCD was not performed within our recommended time frame; however, at the time of this publication, the patient has a normal repeat TCD after initiating hydroxyurea. The MRI/MRA is complete and does not demonstrate any abnormalities. In comparison, five of 83 (6%) screening TCD scans from the satellite clinics are abnormal. Of these abnormal scans, four require confirmatory TCDs and one, an MRI/MRA for TCD>220. Like that of the the above patient, none of the four TCD scans were repeated within our recommended time frame secondary to poor patient adherance. However, at the time of this publication, all four patients have documented normal TCD screens on repeat and one of those patients is now on hydroxyurea for repeat episodes of acute chest syndrome. Despite multiple attempts to reach out to the family, the patient who needed an MRI/MRA did not complete this study within the two years following the abnormal TCD screen and has now relocated to a different state.
Discussion
It is vital that patients with SCD receive a high level of comprehensive medical care.30 This care should be available to all SCD patients; however, travel distance to clinic is a barrier for some patients. For this reason, UAB opened satellite clinics. Prior to choosing places for these clinics, UAB worked with local sickle cell foundations to determine optimal locations based on geographical locations of the sickle cell population as well as ease of transportation as determined by public transportation modalities. The goal for these clinics is to provide care comparable to the central site which includes initiating and monitoring hydroxyurea therapy, offering screening MRI/MRAs, providing TCD studies, promoting penicillin prophylaxis, and providing SCD education to parents through the same program offered at the university based clinic.8,10,31,32
One statistically significant difference identified in this study concerned attendance rates: overall the satellite clinics are better attended than the university-based clinic. We hypothesize this difference could be related to less time and cost of travel associated with the satellite clinics. The barrier associated with cost and time related to travel is not unique to Alabama.33 In fact, other pediatric sickle cell programs report this barrier as a concern, and more specifically note that distance to an academic center may inhibit the delivery of standard of care TCD studies for stroke prevention.34,35 A second difference in this analysis is that while no statistically significant difference exists in the total number or percent of patients on hydroxyurea at satellite clinics when compared to the Birmingham clinic, we do identify a higher percent of patients with HbSS or Sβ0 thalassemia compared to SC and Sβ+ thalassemia using hydroxyurea in Birmingham than in satellite clinics. While we use the same criteria for offering hydroxyurea among all clinics, we value parent and patient opinion and use a shared decision making approach to starting hydroxyurea. One potential hypothesis for this difference in acceptance of hydroxyurea among patients with HbSS and Sβ0 thalassemia is that we consistently discuss hydroxyruea during hospitalization for patients admitted to Children's of Alabama but cannot perform this same education among satellite clinic patients admitted to outside hospitals. Therefore, the acceptance of starting hydroxyurea among patients and families with HBSS and Sβ0 thalassemia may be higher in Birmingham as the patients have already been educated about the medication during a crisis or other admission as compared to satellite clinics who are being educated about hydroxyuea during a well-child SCD visit. However, this result in difference by clinic/genotype may be limited either by the small number of patient on hydroxyurea with HbSC or Sβ+ thalassemia or the large proportion of patients in the Montgomery clinic that have SC/Sβ+ thalassemia on hydroxyrea (38% of all HU for SC/ Sβ+ thalassemia patients attend Montgomery clinic). The remaining data in this study do not demonstrate statistically significant differences, suggesting that satellite clinics can provide similar SCD therapies and surveillance for populations that are located long distances from a specialized, university-based SCD clinic. A recent study also suggests similar levels of care using TCD can be provided at both rural and urban clinics in the pediatric SCD population.28 Our research mirrors the results of that paper, while also demonstrating the ability of outreach sickle cell clinics to achieve a comparable level of care based upon multiple surrogate markers in the delivery of specialized services to this population.
The recently published NHLBI evidence-based expert panel report on the management of SCD reinforces the point that TCDs should be performed annually for patients beginning at the age of two and continuing until at least 16 years of age to identify patients at highest risk for stroke.9 Thus, it is imperative that all comprehensive care locations offer TCD studies. This study demonstrates that it is feasible to perform TCD screening at satellite clinics at similar rates as at a larger, university-based clinic. One major concern from the satellite TCD data is that no patient with an abnormal TCD had the necessary follow-up within two months of the initial screen which is our institutional standard of care. Review of these cases shows that lack of follow-up care during those two months was due to patient poor adherence; however, the reasons for this non-adherence are not known, but may be due to the follow-up care being at the university-based clinic. If so, this represents a remaining barrier despite the success of the initial TCD screening. The NHLBI report also recommends offering hydroxyurea to all infants, children, and adolescent patients regardless of clinical severity.9 If most patients and families accept hydroxyurea, SCD centers must have the capacity to administer and monitor hydroxyurea for effect and toxicity. This study highlights that hydroxyurea can be initiated and managed at satellite clinics. Finally, the NHLBI expert panel does not recommend MRI screening (moderate recommendation- low quality evidence) for asymptomatic patients.9 However, due to the disease burden of silent cerebral stroke and evidence that silent cerebral infarct can progress without therapy, our institution offers screening MRI/MRA.36-38 A trained TCD examiner provides TCD screening at both the Birmingham and satellite clinics; however, the MRI/MRA is only available at the main campus in Birmingham. Therefore, while the discussion to obtain an MRI/MRA occurs either during a Birmingham or satellite clinic visit, all patients and parents must follow up with the MRI procedure in Birmingham. These data suggest that eligible participants from satellite clinics are able and willing to travel to Birmingham for this procedure.
Each satellite clinic is held once per month and due to the significant commitment of resources needed, it is not feasible to offer more frequent satellite clinics. For example, staffing these offsite clinics requires, on average, a full day for three nurse practitioners, one physician, and one nurse coordinator/TCD examiner. This personnel commitment puts a strain on the hematology program at the academic center, particularly on the days of the satellite clinics. Therefore, it is essential that these satellite clinics deliver care that justifies the commitment of resources to the academic program. Developing a plan to assess positive outcomes is important for centers that decide to initiate a satellite clinic.
Satellite clinics are not the only method to help ease the medical barriers associated with travel. Other potential opportunities to improve access to care for patients living with sickle cell disease include using community health workers, telemedicine or mobile health centers.39-41 An alternative approach is to develop a network of pediatric providers comfortable caring for children with sickle cell disease, monitoring hydroxyurea use and toxicity, and obtaining standard of care labs and diagnostic tests. Such a care model requires multiple contacts with a willingness to accept sickle cell patients and participate in care plans. Currently, in addition to providing care through our offsite clinics, we are also attempting to re-integrate primary care providers into the medical home for patients with sickle cell disease. However, there is a paucity of data on the barriers and benefits to providing sickle cell care using these alternative strategies, a matter that warrants research prior to wider implementation.
This retrospective review has some limitations worth noting. First, we report data for only four surrogate markers of care but could not compare differences in hospitalization/adverse events. While our practitioners document parent-reported events between clinic visits, our practice does not often receive ER/hospital records for all of our satellite clinic patients because frequently they are admitted to outside hospitals. Therefore, these adverse events cannot be confirmed reliably. Second, attendance rates are evaluated in a broad sense to get raw data on individual clinic attendance rates rather than examining individual patient attendance rates or the percentage of patients seen for a well child sickle cell care visit during the recommended time frame. Third, to assess therapy goals, we provide data on the percent of patients on hydroxyurea rather than evaluating adherence to hydroxyurea therapy or whether hydroxyurea treatment is offered to all patients who are eligible to receive this therapy based on institutional guidelines. Also, we did not have data on the percent of patients offered hydroxyurea as compared to those who are taking hydroxyurea. Finally, regarding TCD data, the data from Birmingham represents only patients not on current therapy as individual practitioners caring for patients on hydroxyurea or transfusion therapy conduct clinic visits separate from the well child sickle cell clinic. In addition, the percentage in this study represents the TCD studies completed out of those planned for patients who attended clinic, and not the percentage completed out of the total number of eligible patients.
This study provides support that satellite SCD clinics can provide a similar level of care compared with major university-based SCD clinic. Therefore, we suggest that other SCD centers that care for large populations of sickle cell patients from distant areas explore the benefits of establishing similar clinics to help reduce this major health care barrier for their populations. Initiating satellite clinics requires significant resources for sickle cell centers including identifying locations/centers to provide care, divisional resources to support travel, and several practitioners willing to travel the distance required to staff these clinics. Future research should evaluate benefits versus costs of satellite clinics as well as how these clinics relate to more detailed patient outcomes.
Abbreviations
- UAB
University of Alabama at Birmingham
- TCD
Transcranial Doppler
- MRI
Magnetic Resonance Imaging
- MRA
Magnetic Resonance Angiogram
- SCD
Sickle Cell Disease
- Hb
Hemoglobin
- NHLBI
National Heart, Lung, and Blood Institue
References
- 1.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010 Dec 11;376(9757):2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 2.Kavanagh PL, Sprinz PG, Vinci SR, et al. Management of children with sickle cell disease: a comprehensive review of the literature. Pediatrics. 2011 Dec;128(6):e1552–1574. doi: 10.1542/peds.2010-3686. [DOI] [PubMed] [Google Scholar]
- 3.Section on Hematology/Oncology Committee on G, American Academy of P. Health supervision for children with sickle cell disease. Pediatrics. 2002 Mar;109(3):526–535. doi: 10.1542/peds.109.3.526. [DOI] [PubMed] [Google Scholar]
- 4.Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood. 1995 Jul 15;86(2):776–783. [PubMed] [Google Scholar]
- 5.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010 Apr;38(4 Suppl):S512–521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010 Jul 1;115(26):5300–5311. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet. 2011 May 14;377(9778):1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998 Jul 2;339(1):5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 9.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014 Sep 10;312(10):1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 10.Lebensburger JD, Hilliard LM, McGrath TM, et al. Laboratory and clinical correlates for magnetic resonance imaging (MRI) abnormalities in pediatric sickle cell anemia. J Child Neurol. 2011 Oct;26(10):1260–1264. doi: 10.1177/0883073811405054. [DOI] [PubMed] [Google Scholar]
- 11.Vendt BA, McKinstry RC, Ball WS, et al. Silent Cerebral Infarct Transfusion (SIT) trial imaging core: application of novel imaging information technology for rapid and central review of MRI of the brain. J Digit Imaging. 2009 Jun;22(3):326–343. doi: 10.1007/s10278-008-9114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinals RS. The satellite arthritis clinic. Arthritis Rheum. 1978 Jul-Aug;21(6):727–730. doi: 10.1002/art.1780210621. [DOI] [PubMed] [Google Scholar]
- 13.Morris CL, Dexter EB. Taking the clinic to the clients: geriatric health care in a residential setting. Gerontologist. 1989 Dec;29(6):822–825. doi: 10.1093/geront/29.6.822. [DOI] [PubMed] [Google Scholar]
- 14.Martin A, Nathan AW, Camm AJ. Cardiac pacing in an elderly population with a satellite clinic in a district general hospital. Age Ageing. 1985 Nov;14(6):333–338. doi: 10.1093/ageing/14.6.333. [DOI] [PubMed] [Google Scholar]
- 15.Kuo IC. Satellite clinics in academic ophthalmology programs: an exploratory study of successes and challenges. BMC Ophthalmol. 2013;13:79. doi: 10.1186/1471-2415-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keough-Ryan TM, Prasad GV, Hewlett T, et al. Similar outcomes for Canadian renal transplant recipients followed up in transplant centers and satellite clinics. Transplantation. 2010 Sep 27;90(6):591–596. doi: 10.1097/tp.0b013e3181e9febd. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Z, Yu X. Advancing the use and quality of peritoneal dialysis by developing a peritoneal dialysis satellite center program. Perit Dial Int. 2011 Mar-Apr;31(2):121–126. doi: 10.3747/pdi.2010.00041. [DOI] [PubMed] [Google Scholar]
- 18.Hankoff LD, Rabiner CJ, Henry CS. Comparison of the satellite clinic and the hospital-based clinic. Arch Gen Psychiatry. 1971 May;24(5):474–478. doi: 10.1001/archpsyc.1971.01750110086014. [DOI] [PubMed] [Google Scholar]
- 19.Diamant MJ, Young A, Gallo K, et al. Hemodialysis in a satellite unit: clinical performance target attainment and health-related quality of life. Clin J Am Nephrol. 2011 Jul;6(7):1692–1699. doi: 10.2215/CJN.07650810. [DOI] [PubMed] [Google Scholar]
- 20.Diamant MJ, Harwood L, Movva S, et al. A comparison of quality of life and travel-related factors between in-center and satellite-based hemodialysis patients. Clin J Am Nephrol. 2010 Feb;5(2):268–274. doi: 10.2215/CJN.05190709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Challenor R, Pinsent S, Baker D. Improving access to genitourinary medicine by satellite clinics: an evaluation of the use of pump-priming funding. Int J STD AIDS. 2005 Jan;16(1):49–51. doi: 10.1258/0956462052932782. [DOI] [PubMed] [Google Scholar]
- 22.Holloway SM, Lampe AK, Lam WW. Paediatric referral and attendance rates for the clinical genetics service in south-east Scotland--a comparison of a regional clinic with satellite clinics. Scott Med J. 2010 Feb;55(1):10–13. doi: 10.1258/RSMSMJ.55.1.10. [DOI] [PubMed] [Google Scholar]
- 23.Ecklund K, Share JC. Extension of academic pediatric radiology to the community setting: experience in two sites. Pediatr Radiol. 2000 Jan;30(1):3–6. doi: 10.1007/s002470050003. [DOI] [PubMed] [Google Scholar]
- 24.Castile JA, Castile RG, O'Connell EJ. Implementation and operation of a hospital pediatric satellite pharmacy. Hosp Pharm. 1980 Sep;15(9):457–462. 466, 468–459. [PubMed] [Google Scholar]
- 25.Wagstaff MH, Rigby ML, Redington AN. Increasing workload and changing referral patterns in paediatric cardiology outreach clinics: implications for consultant staffing. Heart. 1998 Mar;79(3):223–224. doi: 10.1136/hrt.79.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irby MB, Boles KA, Jordan C, et al. TeleFIT: adapting a multidisciplinary, tertiary-care pediatric obesity clinic to rural populations. Telemed J E Health. 2012 Apr;18(3):247–249. doi: 10.1089/tmj.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesney M, Lindeke L, Johnson L, et al. Comparison of child and parent satisfaction ratings of ambulatory pediatric subspecialty care. J Pediatr Health Care. 2005 Jul-Aug;19(4):221–229. doi: 10.1016/j.pedhc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Hussain S, Nichols F, Bowman L, et al. Implementation of transcranial Doppler ultrasonography screening and primary stroke prevention in urban and rural sickle cell disease populations. Pediatr Blood Cancer. 2014 Nov 8; doi: 10.1002/pbc.25306. [DOI] [PubMed] [Google Scholar]
- 29.Adams RJ, McKie VC, Brambilla D, et al. Stroke prevention trial in sickle cell anemia. Control Clin Trials. 1998 Feb;19(1):110–129. doi: 10.1016/s0197-2456(97)00099-8. [DOI] [PubMed] [Google Scholar]
- 30.Telfair J, Haque A, Etienne M, et al. Rural/Urban Differences in Access to and Utilization of Services Among People in Alabama with Sickle Cell Disease. Public Health Rep. 2003 Jan-Feb;118(1):27–36. doi: 10.1093/phr/118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986 Jun 19;314(25):1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 32.Williams CP, Smith CH, Osborn K, et al. Patient-centered Approach to Designing Sickle Cell Transition Education. J Pediatr Hematol Oncol. 2015 Jan;37(1):43–7. doi: 10.1097/MPH.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 33.Haque A, Telfair J. Socioeconomic distress and health status: the urban-rural dichotomy of services utilization for people with sickle cell disorder in North Carolina. J Rural Health. 2000;16(1):43–55. doi: 10.1111/j.1748-0361.2000.tb00435.x. Winter. [DOI] [PubMed] [Google Scholar]
- 34.Raphael JL, Shetty PB, Liu H, et al. A critical assessment of transcranial doppler screening rates in a large pediatric sickle cell center: opportunities to improve healthcare quality. Pediatr Blood Cancer. 2008 Nov;51(5):647–651. doi: 10.1002/pbc.21677. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong-Wells J, Grimes B, Sidney S, et al. Utilization of TCD screening for primary stroke prevention in children with sickle cell disease. Neurology. 2009 Apr 14;72(15):1316–1321. doi: 10.1212/WNL.0b013e3181a110da. [DOI] [PubMed] [Google Scholar]
- 36.Kwiatkowski JL, Zimmerman RA, Pollock AN, et al. Silent infarcts in young children with sickle cell disease. Br J Haematol. 2009 Aug;146(3):300–305. doi: 10.1111/j.1365-2141.2009.07753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeBaun MR, Sarnaik SA, Rodeghier MJ, et al. Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012 Apr 19;119(16):3684–3690. doi: 10.1182/blood-2011-05-349621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014 Aug 21;371(8):699–710. doi: 10.1056/NEJMoa1401731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duncan DE, Scott RB, Castro O. A mobile unit as an adjunct to a community outreach program of education, screening, and counseling for sickle cell disease, nutritional anemia, and hypertension. J Natl Med Assoc. 1982 Oct;74(10):969–977. [PMC free article] [PubMed] [Google Scholar]
- 40.Woods KF, Johnson JA, Kutlar A, et al. Sickle cell disease telemedicine network for rural outreach. J Telemed Telecare. 2000;6(5):285–290. doi: 10.1258/1357633001935923. [DOI] [PubMed] [Google Scholar]
- 41.Woods KF, Kutlar A, Johnson JA, et al. Sickle cell telemedicine and standard clinical encounters: a comparison of patient satisfaction. Telemed J. 1999;5(4):349–356. doi: 10.1089/107830299311916. Winter. [DOI] [PubMed] [Google Scholar]