Abstract
Hypertension is an inflammatory condition controlled by the renin angiotensin system and is linked to kidney disease, diabetes mellitus, and recently to dysfunction of the gut. The aim of this study was to determine what effect antihypertensive drug treatments may have on intestinal function of the spontaneously hypertensive rat (SHR). In the first experiment, SHRs were treated with enalapril, hydralazine, or with no treatment as a control. In the second experiment, SHRs were treated with losartan or with no treatment as a control. All drug treatments led to significant lowering of blood pressure after 16 weeks. At termination, intact tissue sections of the ileum and colon were induced to contract ex vivo by KCl; electrical stimulation; and agonists carbachol, angiotensin II, and prostaglandin E2 (PGE2). There were no differences in ileal or colonic contractility due to hydralazine or enalapril compared with no-treatment SHR control. However, for the ileum, the losartan group responded significantly more to KCl and carbachol while responding less to angiotensin II, with no difference for PGE2 compared with the no-treatment SHR control. In contrast, the colon responded similarly to KCl, electrical stimulation, and PGE2 but responded significantly less to angiotensin II. These results demonstrate that the ileum responds differently (with KCl and carbachol as agonists) to the colon after losartan treatment, whereas there is a reduced contractile response in both the ileum and colon following losartan treatment. Although there are few well documented major contraindications for angiotensin receptor blockers, the modulation of gut contractility by losartan may have wider implications for bowel health.
Introduction
The major bioactive component of the renin-angiotensin system (RAS) is the potent vasoconstrictor angiotensin II (Ang II), which is formed from angiotensin I (Ang I) by angiotensin-converting enzyme (ACE), which is located in lung, kidney, and gut tissue (Duggan et al., 1989). Although the main pathophysiological impact of RAS has been centered on cardiovascular and renal biology, dense populations of angiotensin receptor subtypes have been reported in the mucosa and muscularis layers of human and rat ileum and colon in both human and animal studies alike (Hirasawa et al., 2002; Spak et al., 2008). Further, a human homolog of ACE, angiotensin-converting enzyme 2 (ACE2), constitutes a new enzymatic pathway that generates Ang 1–7 from Ang II and binds to the putative MAS receptor, leading to vasodilation and cardiovascular protection, thus opposing the effects of Ang II (Donoghue et al., 2000; Tipnis et al., 2000; Kuba et al., 2013). In addition, it has been recently revealed that, via its function in amino acid transport, ACE2 controls intestinal inflammation and diarrhea and plays a role in regulating the microbiome (Hashimoto et al., 2012; Perlot and Penninger, 2013). These findings strongly link RAS to regulation and health of the gastrointestinal system (Cole-Jeffrey et al., 2015).
However, there have been a few reports that angiotensin II can elicit direct effects on gut smooth muscle as well as indirect effects via myenteric plexus cholinergic neurons (Spak et al., 2008; Mastropaolo et al., 2015). For the gut, it has been demonstrated that angiotensin II acts mainly at the angiotensin type 1 (AT1) receptor (Sechi et al., 1993) in rodents and humans and has been shown to be antagonized by losartan and its analogs, termed angiotensin receptor blockers (ARBs) (Dickstein et al., 1998; Garg et al., 2012; Mastropaolo et al., 2013, 2015; Patten et al., 2015a). Many drugs can cause pathology and dysmotility of the small and large intestine (Geboes et al., 2006; Zeino et al., 2010). For one of the ARBs, olmesartan, which provides a comparatively high level of antihypertensive efficacy (Wang et al., 2012), some evidence has accumulated associating it with increased risk of hospitalization for intestinal malabsorption and celiac disease (Lagana et al., 2015; Basson et al., 2016), especially in some predisposed individuals (Sciolom et al., 2015).
For the rat, prostaglandin E2 (PGE2) has been shown to induce relaxation of circular muscles and contraction of longitudinal muscle in the small intestine (Bennett et al., 1968; Patten et al., 2004, 2005; Iizuka et al., 2014). We have previously shown that, for the spontaneously hypertensive rat (SHR), the ex vivo contractile response to PGE2 and prostaglandin F2α was lower in the ileum and colon compared with the Wistar Kyoto rat nonhypertensive control. This depression was not apparent for muscarinic or peptide-stimulated contraction of gut tissue (Patten et al., 2004, 2005). It is known that hypotension induced by the ACE inhibitor captopril involves a prostaglandin-dependent component (Pontieri et al., 1990). However, it is unknown to what extent the RAS interacts with prostanoid generation and how antihypertensive agents may influence contractility and motility of the gut.
The aim of this study was to examine the effects of long-term treatment with three classes of antihypertensive agents as: an ACE inhibitor, enalapril; an ARB, losartan; and the arterial relaxant hydralazine as a positive control on SHR gut contractility. Separate no-treatment SHRs were used as controls. To do this, we examined the effects of these antihypertensive agents on the ex vivo induced contractility of intact sections of ileum and colon by KCl; electrical stimulation; and muscarinic, prostanoid, and angiotensin receptor–dependent agonists (Patten et al., 2004, 2005, 2015a,b). We hypothesize that antihypertensive drugs may affect intestinal contractility and motility.
Materials and Methods
Animal Experiments.
Forty 12-week-old SHRs were delivered to the CSIRO small-animal facility from the Animal Resource Centre breeding facility in Western Australia (24 animals for experiment 1 and 16 animals for experiment 2). The animals were allowed 2 weeks to acclimatize in a 12-hour light/dark cycle at 22°C, and were ear-tagged and weighed. Animals were caged in groups of no more than five animals in the standard colony wire cages to decrease coprophagy and prevent the ingestion of bedding materials. Environmental enrichment was provided to allow animals to rest away from the wire-based floor. Animals were fed an AIN93M diet (ICN Biomedicals, Irvine, CA) (13.6% protein, 4.0%, fat, 4.7% crude fiber, and 15.1 MJ/kg digestible energy) ad libitum and were monitored daily and weighed weekly. For experiment 1, at 14 weeks of age, three groups of eight animals were randomly assigned on a weight basis to be administered the antihypertensive agents hydralazine (0.012%, w/v) or enalapril maleate (4 mg/kg per day) in the drinking water given ad libitum for 16 weeks or were assigned as no-treatment SHRs acting as the control group. For experiment 2, at 14 weeks, two other groups of eight animals were randomly assigned on a weight basis to be administered the antihypertensive losartan (10 mg/kg per day) in the drinking water given ad libitum for 16 weeks or were assigned as no-treatment SHRs as control. Sixteen weeks of treatment was undertaken to allow the animals to reach full maturity and stable blood pressure in the no-treatment SHR control groups. All drugs and fine chemicals were from Sigma Australia Pty. Ltd. (Sydney, Australia). All experimental protocols were undertaken in accordance with the Australian code for the care and use of animals for scientific purposes (8th edition, 2013, https://www.nhmrc.gov.au/guidelines-publications/ea28) and approved by the CSIRO Animal Ethics Committee and included power calculations.
Blood Pressure Measurements.
For experiments 1 and 2, systolic blood pressure was measured in conscious rats from 14 weeks, every 2 weeks for the 16-week treatment phase. Blood pressure (BP) was measured using an indirect tail-cuff method at 30°C in measuring tubes using an electro-sphygmomanometer combined with a pneumatic pulse transducer/amplifier (model 6m22931, six-channel NIBP system, Mediquip Pty Ltd, Loganholme, Qld, Australia) using BpMonWin Monitor version 1.33 software (IITC Life Science, Woodland Hills, CA). Rats were acclimatized in the measuring tubes twice before BP measurements were commenced.
Measurement of Ileal and Colonic Tissue Contractility Ex Vivo.
Methodology for inducing contractility and magnitude measurements for both the ileum and colon have been described in detail previously (Patten et al., 2002, 2015; Bajka et al., 2010). Animals were euthanized via exsanguination while still under anesthesia (60 mg/kg Nembutal, sodium pentobarbitone, Sigma Chem Co, Sydney, Australia). Death of the animal was confirmed by the removal of the heart. In brief, sections of both the terminal ileum (4–5 cm) between 2 and 10 cm from the ileo-caecal junction and proximal colon (3–4 cm) were excised 2 cm from the caecal-colonic junction. Both the colon and ileum were mounted in tandem in organ chambers with a modified Krebs-Henseleit bicarbonate buffer, and the colon was excited by an electric field to induce contraction (Bajka et al., 2010). The gastrointestinally active compounds were then added sequentially to both ileal and colonic baths as follows: KCl (5–35 mM), carbachol (0.03–22 µM), angiotensin II (0.001–10 µM), and PGE2 (0.001–10 µM). Dose-response curves were generated by cumulative additions of a small bolus of KCl or agonist to the incubated tissue in the organ bath until contraction reached a plateau (normally after 3–5 minutes). The solution in the organ bath was washed out when maximal contraction was reached, ready for subsequent testing of contractility. No refractoriness was recorded after KCl, carbachol, angiotensin II, or PGE2 treatments. To assess this, 1 µM carbachol was added at the completion of the experiment, and only tissues with 100% recovery of contractile activity were used for experimental determinations.
Statistical Analysis.
Data were summarized as the mean ± S.E.M. Comparisons between two groups were conducted using the unpaired Student’s t test, whereas comparison of groups of three was performed using one-way analysis of variance followed by the Bonferroni multiple comparison test using GraphPad Instat 3.0 (GraphPad Software, La Jolla, CA). Statistical significance was set at P < 0.05.
Results
Blood Pressure.
For experiment 1, the initial and final BP for no-treatment SHR control, enalapril, and hydralazine SHR were 212.4 ± 3.7 and 221.5 ± 1.0, 212.3 ± 4.6 and 171.1 ± 1.8, and 203.2 ± 2.2 and 138.2 ± 2.5, respectively. For experiment 2, the initial and final BP for no-treatment SHR control and losartan SHR were 210 ± 3.6 and 220.9 ± 2.2, and 215 ± 3.3 and 186.4 ± 3.4, respectively. All drug treatments led to significantly lower blood pressure in the SHR (P < 0.05) compared with the no-treatment SHR control group. At the doses used, the hydralazine treatment group had a lower final blood pressure than the enalapril and losartan treatment groups (P < 0.05).
Contractility of Ileum and Colon Ex Vivo, Experiment 1.
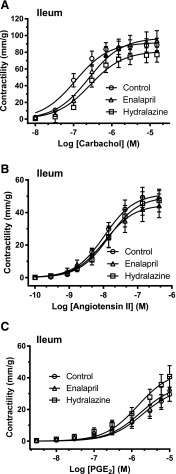
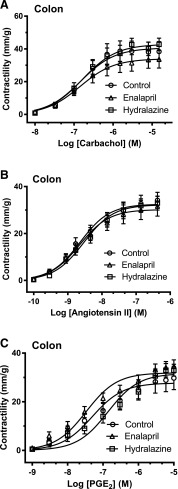
In the ileum, there were no differences in contraction due to KCl for the enalapril and hydralazine groups compared with no-treatment SHR controls (Table 1). For the colon, there were no differences in contraction due to KCl or electrical stimulation for the enalapril- and hydralazine-treated groups compared with no-treatment SHR controls (Table 2). For both the ileum (Fig. 1) and colon (Fig. 2), there were no differences in maximal contractility (mm/g) or sensitivity (EC50) in response to concentration dose curves of carbachol, angiotensin II, and PGE2. There was a depressed response to PGE2 in the ileum and colon compared with what we have found previously for control Wistar Kyoto rats (results not shown) (Patten et al., 2004, 2005).
TABLE 1.
Experiment 1, effects of antihypertensive agents enalapril and hydralazine in drinking water for 16 weeks on the contractility of SHR isolated intact sections of ileum ex vivo by KCl and agonists carbachol, angiotensin II, and PGE2 compared with no-treatment SHR control
Results are the mean ± S.E.M. from six to eight rats. Statistics were performed using one-way analysis of variance. There were no significant differences between the groups (results not shown).
| Control | Enalapril | Hydralazine | |
|---|---|---|---|
| KCl (40 mM) | |||
| Max (mm/g) | 71.2 ± 15.8 | 74.3 ± 12.4 | 63.0 ± 9.5 |
| Carbachol | |||
| EC50 (nM) | 137 ± 26 | 300 ± 70 | 255 ± 24 |
| Max (mm/g) | 92.4 ± 8.7 | 98.9 ± 13.2 | 83.1 ± 9.6 |
| AUC | 141 ± 18 | 169 ± 23 | 137 ± 20 |
| Angiotensin II | |||
| EC50 (nM) | 12.0 ± 1.5 | 17.2 ± 3.7 | 23.2 ± 5.8 |
| Max (mm/g) | 52.9 ± 6.6 | 46.3 ± 7.6 | 51.0 ± 7.4 |
| AUC | 82.1 ± 9.9 | 72.2 ± 14.9 | 74.3 ± 13.6 |
| PGE2 | |||
| EC50 (nM) | 1624 ± 249 | 3813 ± 1002 | 2169 ± 562 |
| Max (mm/g) | 39.0 ± 8.2 | 40.0 ± 6.6 | 47.5 ± 6.7 |
| AUC | 29.0 ± 5.4 | 35.2 ± 12.1 | 43.7 ± 9.0 |
AUC, area under the curve as arbitrary units; Max, maximal derived contraction of the tissue.
TABLE 2.
Experiment 1, effects of antihypertensive agents enalapril and hydralazine in the drinking water for 16 weeks on the contractility of SHR isolated intact sections of proximal colon ex vivo by KCl; electrical stimulation; and agonists carbachol, angiotensin II, and PGE2 compared with no-treatment SHR control
Results are mean ± S.E.M. from six to eight rats. Statistics were performed using one-way analysis of variance. There were no significant differences between the groups (results not shown).
| Control |
Enalapril |
Hydralazine |
|
|---|---|---|---|
| KCl (40 mM) | |||
| Max (mm/g) | 21.2 ± 3.7 | 19.7 ± 3.6 | 20.1 ± 4.5 |
| Electrical stimulation | |||
| Max (mm/g) | 17.1 ± 1.7 | 14.5 ± 3.4 | 13.5 ± 2.4 |
| Carbachol | |||
| EC50 (nM) | 159 ± 27 | 186 ± 57 | 207 ± 77 |
| Max (mm/g) | 40.7 ± 6.6 | 32.9 ± 5.2 | 41.7 ± 4.4 |
| AUC | 79.7 ± 12.8 | 63.5 ± 7.5 | 79.6 ± 11.4 |
| Angiotensin II | |||
| EC50 (nM) | 2.7 ± 0.5 | 2.7 ± 0.7 | 3.3 ± 0.7 |
| Max (mm/g) | 32.7 ± 3.4 | 30.6 ± 4.1 | 32.4 ± 3.0 |
| AUC | 72.7 ± 8.7 | 68.2 ± 8.8 | 68.9 ± 7.0 |
| PGE2 | |||
| EC50 (nM) | 67.3 ± 20.9 | 52.7 ± 17.8 | 208 ± 67 |
| Max (mm/g) | 32.2 ± 1.1 | 32.3 ± 2.4 | 32.2 ± 2.8 |
| AUC | 74.7 ± 6.5 | 79.1 ± 7.5 | 79.6 ± 11.4 |
AUC, area under the curve as arbitrary units; Max, maximal derived contraction of the tissue.
Fig. 1.
Experiment 1. The dose accumulative effects of carbachol (A), angiotensin II (B), and PGE2 (C) on intact ileal sections ex vivo after 16-week treatment with enalapril (triangles) or hydralazine (squares) from 25-week-old SHRs compared with no-treatment SHR controls (circles). The data are expressed as the mean ± S.E.M. from six to eight rats. Using analysis of variance, there were no significant differences in maximal contractions or sensitivities in response to the gastrointestinal active agents across the groups. The calculated maximal contraction (mm/g), sensitivity (EC50), and area under the curve for each gastrointestinally active agent in the ileum are shown in Table 1.
Fig. 2.
Experiment 1. The dose accumulative effects of carbachol (A), angiotensin II (B), and PGE2 (C) on intact colonic sections ex vivo after 16-week treatment with enalapril (triangles) or hydralazine (squares) from 25-week-old SHRs compared with no-treatment SHR controls (circles). The data are expressed as the mean ± S.E.M. from six to eight rats. Using analysis of variance, there were no significant differences in maximal contractions or sensitivities across the groups. The calculated maximal contraction (mm/g), sensitivity (EC50), and area under the curve for each electrical stimulation and the gastrointestinally active agents in the colon are shown in Table 2.
Contractility of Ileum and Colon Ex Vivo, Experiment 2.
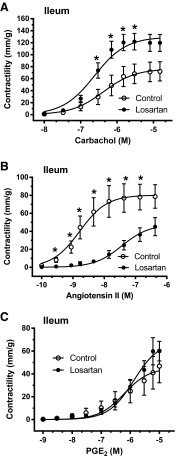
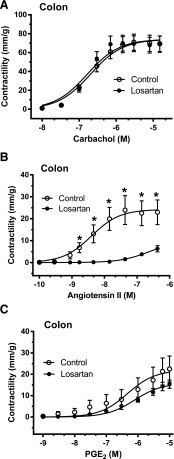
For the ileum, the contractility responses to 10 and 40 mM KCl were higher for the losartan-treated group compared with the no-treatment SHR control group (Table 3). For the colon, however, there were no differences in response to KCl or electrical stimulation between the no-treatment SHR control or losartan groups (Table 3). For the ileum, with respect to losartan treatment, there was a higher contractile response to carbachol (Fig. 3A; Table 3) and lower responses to angiotensin II (Fig. 3B; Table 3), which also demonstrated lower sensitivity (higher EC50) (Fig. 3B; Table 3), with no difference noted for PGE2 (Fig. 3C; Table 3) compared with the no-treatment SHR control group. For the colon, with respect to losartan treatment, there were no differences in contractile responses to carbachol (Fig. 4A; Table 3) or PGE2 (Fig. 4C; Table 3), with lower responses noted for angiotensin II (Fig. 4B; Table 3) with concomitant lower sensitivities (higher EC50) (Table 3) compared with the no-treatment SHR control group. For experiment 2, there were no differences noted in the mean tissue densities of ilea or colons from the losartan-treated group compared with the no-treatment SHR control group (Table 3).
TABLE 3.
Experiment 2, effects of losartan in drinking water for 16 weeks on SHR contractility of intact ileum and proximal colon sections ex vivo by KCl, agonists (carbachol, angiotensin II, and PGE2), and electrically driven colon and tissue densities compared with no-treatment SHR control
Results are mean ± S.E.M. from six to eight rats. Statistics were performed using Student’s t test.
| Control |
Losartan |
P Value |
|
|---|---|---|---|
| Ileum | |||
| KCl (10 mM) Max (mm/g) | 22.6 ± 7.8 | 61.1 ± 10.1 | 0.013 |
| KCl (40 mM) Max (mm/g) | 49.3 ± 11.0 | 108.3 ± 12.2 | 0.005 |
| Carbachol | |||
| EC50 (nM) | 798 ± 343 | 253 ± 23 | ns |
| Max (mm/g) | 79 ± 17 | 130 ± 15 | 0.048 |
| AUC | 117 ± 33 | 226 ± 29 | 0.033 |
| Angiotensin II | |||
| EC50 (nM) | 4.7 ± 2.1 | 43.1 ± 13.2 | 0.017 |
| Max (mm/g) | 82.5 ± 18.1 | 44.6 ± 11.9 | ns |
| AUC | 194.7 ± 55.6 | 49.0 ± 10.5 | 0.028 |
| PGE2 | |||
| EC50 (nM) | 2383 ± 1081 | 1463 ± 266 | ns |
| Max (mm/g) | 48.4 ± 12.3 | 69.1 ± 11.5 | ns |
| AUC | 60.1 ± 24.9 | 68.6 ± 11.5 | ns |
| Colon | |||
| Electrical stimulation | 15.7 ± 5.3 | 14.0 ± 3.0 | ns |
| KCl (10 mM) Max (mm/g) | 8.16 ± 3.04 | 4.11 ± 1.16 | ns |
| KCl (40 mM) Max (mm/g) | 60.7 ± 9.1 | 57.7 ± 8.1 | ns |
| Carbachol | |||
| EC50 (nM) | 209 ± 24 | 170 ± 8 | ns |
| Max (mm/g) | 74.4 ± 9.0 | 74.1 ± 9.0 | ns |
| AUC | 135 ± 16 | 140 ± 18 | ns |
| Angiotensin II | |||
| EC50 (nM) | 4.66 ± 0.66 | 241.0 ± 91.1 | 0.027 |
| Max (mm/g) | 27.1 ± 6.7 | 9.3 ± 2.2 | 0.030 |
| AUC | 54.3 ± 14.4 | 4.5 ± 0.9 | 0.006 |
| PGE2 | |||
| EC50 (nM) | 2078 ± 941 | 934 ± 231 | ns |
| Max (mm/g) | 24.9 ± 5.5 | 16.1 ± 2.0 | ns |
AUC, area under the curve (as arbitrary units); Max, maximal derived contraction of the tissue; ns, not significant.
Fig. 3.
Experiment 2. The dose accumulative effects of carbachol (A), angiotensin II (B), and PGE2 (C) on intact ileal sections ex vivo after 16-week treatment with losartan (filled circles) from 25-week-old SHRs compared with no-treatment SHR controls (open circles). The data are expressed as the mean ± S.E.M. from six to eight rats. Using Student’s t tests, there were significant differences at the doses shown for carbachol and angiotensin II and between the groups (P < 0.05). The calculated maximal contraction (mm/g), sensitivity (EC50), and area under the curve for the gastrointestinally active agents in the ileum are shown in Table 3.
Fig. 4.
Experiment 2. The dose accumulative effects of carbachol (A), angiotensin II (B), and PGE2 (C) on intact proximal colonic sections ex vivo after 16-week treatment with losartan (filled circles) from 25-week-old SHRs compared with no-treatment SHR controls (open circles). The data are expressed as the mean ± S.E.M. from six to eight rats. Using Student’s t tests, there were significant differences at the doses shown for angiotensin II between the groups (P < 0.05). The calculated maximal contraction (mm/g), sensitivity (EC50), and area under the curve for the gastrointestinally active agents and electrical stimulation in the colon are shown in Table 3.
Discussion
The RAS is involved in the development of essential hypertension, which is an inflammatory condition that not only influences the cardiovascular system but dysregulates glucose control and kidney disease and can also influence gut microbiota and the functionality of the gut (Kuba et al., 2013; Yang et al., 2015; Zhu et al., 2016). We examined the effects that three classes of antihypertensive drugs may have on SHR gut function after 16 weeks of treatment. In the first experiment, SHRs were treated with the ACE inhibitor enalapril and the direct-acting smooth muscle arteriole relaxant hydralazine, which resulted in no significant differences in gut contractility in response to all agents tested ex vivo compared with no-treatment SHR controls. In human patients undergoing hypertension treatment, complications to the gut due to ACE inhibitors are uncommon. However, they have been reported to induce intestinal angioedema that may lead to unnecessary invasive procedures, such as exploratory laparotomy (Weingärtner et al., 2009), but to our knowledge, contraindications for gut motility have not been reported. Indeed, in animal models such as the mouse, enalaprilat has been shown to reduce the severity of dextran sulfate sodium–induced colitis and reduce tumour necrosis factor-α levels and epithelial cell apoptosis (Spencer et al., 2007), whereas enalapril was shown to attenuate the upregulation of IκBα phosphorylation and reduced the severity of colitis as assessed by histologic examination (Lee et al., 2014).
In this study, we used hydralazine, a non-nucleoside DNA methyltransferase inhibitor and a potent arterial vasodilator (Knowles et al., 2004), which is approved for the treatment of severe hypertension and heart failure (Graça et al., 2014). Hydralazine is not routinely used as a primary drug for treating human hypertension because it elicits a reflex sympathetic stimulation of the heart (the baroreceptor reflex). The sympathetic stimulation may increase heart rate and cardiac output and, in patients with coronary artery disease, may cause angina pectoris or myocardial infarction. However, in animal models, this class of drug can inhibit gastric emptying in rats and inhibit gastrointestinal propulsion in mice (Chiba et al., 1981), but this dysmotility was not evident for ex vivo intestinal contractility in hydralazine-treated SHRs used in this study.
Losartan is an orally active, nonpeptide AT1 receptor antagonist which provides a more specific and complete blockade of the actions of angiotensin II than renin or ACE inhibitors (Simpson and McClellan, 2000) and is effective in controlling BP and long-term renal damage in hypertensive patients. Although it has been reported that a specific ARB, olmesartan, may interfere with gut immune homeostasis, is known to cause rare cases of sprue-like enteropathy in predisposed individuals, and is associated with an increased risk of hospitalization for intestinal malabsorption and celiac disease (Sciolom et al., 2015; Basson et al., 2016), losartan has been reported to be well tolerated alone and in combination, with only limited reports of gastrointestinal side effects, such as constipation (Weber, 1997; Gokhale et al., 2002), and generally no association with increased risk of cancer for ARBs (Bhaskaran et al., 2012).
In our study with SHRs, losartan treatment led to increased receptor-independent KCl-induced depolarization-driven ileal contractility that was not evident for the colon. There was also an increased ileal contractility after losartan treatment with the muscarinic-mimetic carbachol with no significant change in sensitivity (EC50) and no effects noted for the colon. Electrical-stimulated colon also demonstrated no difference in the response to losartan treatment. Although the prostanoid response is depressed in SHRs (Patten et al., 2004, 2005), there was also no change in ileal or colonic response to the prostanoid PGE2. However, losartan treatment led to a large suppression of both ileal and colonic response to angiotensin II. For the ileum, there was a concomitant decrease in sensitivity to angiotensin II. The colon showed decreased sensitivity to angiotensin II. The changes in contractility due to losartan treatment could not be explained by changes in either ileal or colonic tissue density or muscle mass, which were not significantly different compared with no-treatment SHR controls. It is to be determined if other ARBs currently used in clinical settings have similar effects.
These differences in intestinal tissue responsiveness are difficult to explain, so the knowledge gap requires the investigation of the mechanisms for the losartan-induced changes in intestinal contractility. It is known that, in animal models of disease, transcription and translation of elements of the RAS may be regulated, and that ARBs such as losartan can modulate these effects (Sim and Chen, 2006). In a recent study, cardiac expressions of ACE and Mas were decreased in the hypertrophied left ventricle of SHRs, whereas those of the AT1 receptor and ACE2 were unchanged. Continuous perinatal losartan treatment reduced left ventricular weight but did not influence the altered cardiac RAS expression (Klimas et al., 2015). Since the ileal and colonic preparations undergo extensive washout prior to stimulation, it is very unlikely that residual losartan would explain the decreased angiotensin II–induced contractile responses observed in this study. It is more likely that the AT1 receptor density within the contractile tissue was decreased following the 14-week losartan treatment; however, there is a need to undertake receptor binding studies of ileal and colonic tissue after losartan treatment to determine muscarinic receptor density of: 1) ileal tissue and 2) the profile of AT1 receptors of both the ileum and colon (Leifert et al., 2009). We have previously shown that there is a depressed prostanoid response in young rats (Patten et al., 2006) and SHRs, but have not measured prostanoid receptor profiles (Patten et al., 2004, 2005). In earlier studies, we also demonstrated that dietary fish oil supplementation led to higher receptor-induced contractility in normal (Patten et al., 2002) and hypertensive rats (Patten et al., 2005) that was not explained by changes in receptor number or sensitivity (Patten et al., 2005), although it was determined fish oil could lead to altered sensitivity of muscarinic 1 receptor subtype (Patten et al., 2006). We have recently shown that dietary resistant starch can alter colonic contractility in healthy adult rats, and demonstrated alterations in colonic expression of genes related to systems that influence gastrointestinal contractility using genomic microarray techniques (Patten et al., 2015b) that may be used to discern losartan effects shown in this study on the intestinal genome. It is also to be determined if other ARBs, such as olmesartan, which has been reported to have some evidence associating it with gut disorders (Basson et al., 2016), also influence SHR gut contractility, especially in angiotensin receptor–driven systems (Miura et al., 2011; Singh and Karnik, 2016).
In conclusion, the current study in part supports our hypothesis that antihypertensive drug treatment affects intestinal contractility in SHRs. The ACE inhibitor enalapril and the arterial relaxant hydralazine had no effect on gut tissue contractility ex vivo. However, the ARB losartan increased receptor-independent KCl-induced and muscarinic-induced contractility of the ileum, whereas the angiotensin-receptor system was severely depressed in both ileal and colonic tissue by mechanisms that are to be determined. This is the first description of altered smooth muscle contractility induced by losartan in a hypertensive animal model, and we may speculate that this phenomenon in part explains some adverse side effects of ARB treatment of high blood pressure.
Acknowledgments
The authors thank Julie Dallimore and Michael Adams for their technical assistance with this study.
Abbreviations
- ACE
angiotensin-converting enzyme
- Ang
angiotensin
- ARB
angiotensin receptor blocker
- AT1
angiotensin type 1
- BP
blood pressure
- PGE2
prostaglandin E2
- RAS
renin-angiotensin system
- SHR
spontaneously hypertensive rat
Authorship Contributions
Participated in research design: Patten, Abeywardena.
Conducted experiments: Patten.
Performed data analysis: Patten.
Wrote or contributed to the writing of the manuscript: Patten, Abeywardena.
Footnotes
This work was fully funded by CSIRO Health and Biosecurity, Kintore Avenue, Adelaide, South Australia.
References
- Bajka BH, Clarke JM, Topping DL, Cobiac L, Abeywardena MY, Patten GS. (2010) Butyrylated starch increases large bowel butyrate levels and lowers colonic smooth muscle contractility in rats. Nutr Res 30:427–434. [DOI] [PubMed] [Google Scholar]
- Basson M, Mezzarobba M, Weill A, Ricordeau P, Allemand H, Alla F, Carbonnel F. (2016) Severe intestinal malabsorption associated with olmesartan: a French nationwide observational cohort study. Gut 65:1664–1669. 10.1136/gutjnl-2015-309690. [DOI] [PubMed] [Google Scholar]
- Bennett A, Eley KG, Scholes GB. (1968) Effects of prostaglandins E1 and E2 on human, guinea-pig and rat isolated small intestine. Br J Pharmacol 34:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran K, Douglas I, Evans S, van Staa T, Smeeth L. (2012) Angiotensin receptor blockers and risk of cancer: cohort study among people receiving antihypertensive drugs in UK General Practice Research Database. BMJ 344:e2697 10.1136/bmj.e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Shibamura S, Tanaka M, Yamasaki T, Hashimoto H, Kurebayashi Y, Kasai Y, Ryokawa Y, Tamura K, Hirohashi M, et al. (1981) Antihypertensive and general pharmacological properties of budralazine. Arzneimittelforschung 31:1080–1087. [PubMed] [Google Scholar]
- Cole-Jeffrey CT, Liu M, Katovich MJ, Raizada MK, Shenoy V. (2015) ACE2 and microbiota: emerging targets for cardiopulmonary disease therapy. J Cardiovasc Pharmacol 66:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein K, Timmermans P, Segal R. (1998) Losartan: a selective angiotensin II type 1 (AT1) receptor antagonist for the treatment of heart failure. Expert Opin Investig Drugs 7:1897–1914. [DOI] [PubMed] [Google Scholar]
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, et al. (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 87:E1–E9. [DOI] [PubMed] [Google Scholar]
- Duggan KA, Mendelsohn FA, Levens NR. (1989) Angiotensin receptors and angiotensin I-converting enzyme in rat intestine. Am J Physiol 257:G504–G510. [DOI] [PubMed] [Google Scholar]
- Garg M, Angus PW, Burrell LM, Herath C, Gibson PR, Lubel JS. (2012) Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther 35:414–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geboes K, De Hertogh G, Ectors N. (2006) Drug-induced pathology in the large intestine. Curr Diagn Pathol 12:239–247. [Google Scholar]
- Gokhale N, Shahani S, Pawar D. (2002) Efficacy and safety of losartan-amplodipine combination--an Indian postmarketing surveillance experience. J Indian Med Assoc 100:207–208. [PubMed] [Google Scholar]
- Graça I, Sousa EJ, Costa-Pinheiro P, Vieira FQ, Torres-Ferreira J, Martins MG, Henrique R, Jerónimo C. (2014) Anti-neoplastic properties of hydralazine in prostate cancer. Oncotarget 5:5950–5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, et al. (2012) ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa K, Sato Y, Hosoda Y, Yamamoto T, Hanai H. (2002) Immunohistochemical localization of angiotensin II receptor and local renin-angiotensin system in human colonic mucosa. J Histochem Cytochem 50:275–282. [DOI] [PubMed] [Google Scholar]
- Iizuka Y, Kuwahara A, Karaki S. (2014) Role of PGE2 in the colonic motility: PGE2 generates and enhances spontaneous contractions of longitudinal smooth muscle in the rat colon. J Physiol Sci 64:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimas J, Olvedy M, Ochodnicka-Mackovicova K, Kruzliak P, Cacanyiova S, Kristek F, Krenek P, Ochodnicky P. (2015) Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J Cell Mol Med 19:1965–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles HJ, Tian Y-M, Mole DR, Harris AL. (2004) Novel mechanism of action for hydralazine: induction of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ Res 95:162–169. [DOI] [PubMed] [Google Scholar]
- Kuba K, Imai Y, Penninger JM. (2013) Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J 77:301–308. [DOI] [PubMed] [Google Scholar]
- Lagana SM, Braunstein ED, Arguelles-Grande C, Bhagat G, Green PH, Lebwohl B. (2015) Sprue-like histology in patients with abdominal pain taking olmesartan compared with other angiotensin receptor blockers. J Clin Pathol 68:29–32. [DOI] [PubMed] [Google Scholar]
- Lee C, Chun J, Hwang SW, Kang SJ, Im JP, Kim JS. (2014) Enalapril inhibits nuclear factor-κB signaling in intestinal epithelial cells and peritoneal macrophages and attenuates experimental colitis in mice. Life Sci 95:29–39. [DOI] [PubMed] [Google Scholar]
- Leifert WR, Bucco O, Abeywardena MY, Patten GS. (2009) Radioligand binding assays: application of [(125)I]angiotensin II receptor binding. Methods Mol Biol 552:131–141. [DOI] [PubMed] [Google Scholar]
- Mastropaolo M, Zizzo MG, Auteri M, Caldara G, Liotta R, Mulè F, Serio R. (2015) Activation of angiotensin II type 1 receptors and contractile activity in human sigmoid colon in vitro. Acta Physiol (Oxf) 215:37–45. [DOI] [PubMed] [Google Scholar]
- Mastropaolo M, Zizzo MG, Mulè F, Serio R. (2013) Angiotensin II contractile effects in mouse colon: role for pre- and post-junctional AT(1A) receptors. Acta Physiol (Oxf) 207:337–345. [DOI] [PubMed] [Google Scholar]
- Miura S, Karnik SS, Saku K. (2011) Review: angiotensin II type 1 receptor blockers: class effects versus molecular effects. J Renin Angiotensin Aldosterone Syst 12:1–7 http://www.sagepub.co.uk/journalspermission.nav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten GS, Abeywardena MY, McMurchie EJ, Jahangiri A. (2002) Dietary fish oil increases acetylcholine- and eicosanoid-induced contractility of isolated rat ileum. J Nutr 132:2506–2513. [DOI] [PubMed] [Google Scholar]
- Patten GS, Abeywardena MY, Sundram K, Tan YA, Sambanthamamurthi R. (2015a) Effect of palm oil phenolics on gastrointestinal transit, contractility and motility in the rat. J Funct Foods 17:928–937. [Google Scholar]
- Patten GS, Adams MJ, Dallimore JA, Abeywardena MY. (2004) Depressed prostanoid-induced contractility of the gut in spontaneously hypertensive rats (SHR) is not affected by the level of dietary fat. J Nutr 134:2924–2929. [DOI] [PubMed] [Google Scholar]
- Patten GS, Adams MJ, Dallimore JA, Rogers PF, Topping DL, Abeywardena MY. (2005) Restoration of depressed prostanoid-induced ileal contraction in spontaneously hypertensive rats by dietary fish oil. Lipids 40:69–79. [DOI] [PubMed] [Google Scholar]
- Patten GS, Conlon MA, Bird AR, Adams MJ, Topping DL, Abeywardena MY. (2006) Interactive effects of dietary resistant starch and fish oil on short-chain fatty acid production and agonist-induced contractility in ileum of young rats. Dig Dis Sci 51:254–261. [DOI] [PubMed] [Google Scholar]
- Patten GS, Kerr CA, Dunne RA, Shaw JM, Bird AR, Regina A, Morell MK, Lockett TJ, Molloy PL, Abeywardena MY, et al. (2015b) Resistant starch alters colonic contractility and expression of related genes in rats fed a Western diet. Dig Dis Sci 60:1624–1632. [DOI] [PubMed] [Google Scholar]
- Perlot T, Penninger JM. (2013) ACE2 - from the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect 15:866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri V, Lopes OU, Ferreira SH. (1990) Hypotensive effect of captopril. Role of bradykinin and prostaglandinlike substances. Hypertension 15(2, Suppl)I55–I58. [DOI] [PubMed] [Google Scholar]
- Sciolom S, Malamut G, Meresse B, Guegan N, Brousse N, Verarre V, Derrieux C, Macintyre E, Seksik P, Savoye G, et al. (2015) Gastrointestinal disorder associated with olmesartan mimics autoimmune enteropathy. PLoS One 10:e0125024 DOI: 10.1371/journal.pone.0125024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechi LA, Valentin JP, Griffin CA, Schambelan M. (1993) Autoradiographic characterization of angiotensin II receptor subtypes in rat intestine. Am J Physiol 265:G21–G27. [DOI] [PubMed] [Google Scholar]
- Sim MK, Chen WS. (2006) Effects of losartan on angiotensin receptors in the hypertrophic rat heart. Regul Pept 137:140–146. [DOI] [PubMed] [Google Scholar]
- Simpson KL, McClellan KJ. (2000) Losartan: a review of its use, with special focus on elderly patients. Drugs Aging 16:227–250. [DOI] [PubMed] [Google Scholar]
- Singh KD, Karnik SS. (2016) Angiotensin receptors: structure, function, signaling and clinical applications. J Cell Signal 1:111 10.4172/jcs.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spak E, Casselbrant A, Olbers T, Lönroth H, Fändriks L. (2008) Angiotensin II-induced contractions in human jejunal wall musculature in vitro. Acta Physiol (Oxf) 193:181–190. [DOI] [PubMed] [Google Scholar]
- Spencer AU, Yang H, Haxhija EQ, Wildhaber BE, Greenson JK, Teitelbaum DH. (2007) Reduced severity of a mouse colitis model with angiotensin converting enzyme inhibition. Dig Dis Sci 52:1060–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. (2000) A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275:33238–33243. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhao JW, Liu B, Shi D, Zou Z, Shi XY. (2012) Antihypertensive effects of olmesartan compared with other angiotensin receptor blockers: a meta-analysis. Am J Cardiovasc Drugs 12:335–344. [DOI] [PubMed] [Google Scholar]
- Weber M. (1997) Clinical safety and tolerability of losartan. Clin Ther 19:604–616, discussion 603. [DOI] [PubMed] [Google Scholar]
- Weingärtner O, Weingärtner N, Böhm M, Laufs U. (2009) Bad gut feeling: ACE inhibitor induced intestinal angioedema. BMJ Case Rep 2009:bcr09.2008.0868 10.1136/bcr.09.2008.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, et al. (2015) Gut dysbiosis is linked to hypertension. Hypertension 65:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeino Z, Sisson G, Bjarnason I. (2010) Adverse effects of drugs on small intestine and colon. Best Pract Res Clin Gastroenterol 24:133–141. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Xiong S, Liu D. (2016) The gastrointestinal tract: an initial organ of metabolic hypertension? Cell Physiol Biochem 38:1681–1694. [DOI] [PubMed] [Google Scholar]