Abstract
Objective
The prevalence of septic acute kidney injury (AKI) and impact on functional status of pediatric intensive care unit (PICU) survivors are unknown. We utilized data from an international prospective severe sepsis study to elucidate functional outcomes of children suffering septic AKI.
Design
Secondary analysis of patients in the Sepsis PRevalence, OUtcomes, and Therapies (SPROUT) point prevalence study. AKI was defined on the study day using Kidney Disease Improving Global Outcomes definitions. Patients with no AKI or stage 1 AKI (“No/mild AKI”) were compared to those with stage 2 or 3 AKI (“Severe AKI”). The primary outcome was a composite of death or new moderate disability at discharge defined as a Pediatric Overall Performance Category score of 3 or higher, and increased by 1 from baseline.
Setting
128 PICUs in 26 countries.
Patients
Children with severe sepsis in the SPROUT study.
Interventions
None
Measurements and Main Results
One hundred two (21%) of 493 patients had Severe AKI. More than twice as many patients with Severe AKI died or developed new moderate disability compared to those with No/mild AKI (64% vs. 30%, p<0.001). Severe AKI was independently associated with death or new moderate disability (adjusted OR 2.5, 95% CI 1.5, 4.2; p=0.001) after adjustment for age, region, baseline disability, malignancy, invasive mechanical ventilation, albumin administration, and the pediatric logistic organ dysfunction score.
Conclusions
In a multi-national cohort of critically ill children with severe sepsis and high mortality rates, septic AKI is independently associated with further increased death or new disability.
Keywords: Sepsis, Acute Kidney Injury, Pediatric Intensive Care Units, Critical Care Outcomes, Renal Replacement Therapy, Epidemiology
INTRODUCTION
Sepsis is a major precipitant of critical illness in children. Current data estimates that 8% of children in pediatric intensive care units (PICUs) around the world have severe sepsis at a given time (1). The incidence of sepsis in children is increasing, and with improvements in sepsis-related mortality, functional outcomes in survivors are increasingly important (2, 3). One common comorbidity of severe sepsis is acute kidney injury (AKI), comprising up to half of critical illness-associated AKI in adults (4–6). AKI occurs in approximately 16% of children in PICUs (7), but the true prevalence of septic AKI is unknown. Few studies prospectively describe the outcomes of children suffering AKI exclusively in the context of sepsis. Most instead evaluate the outcome after AKI in the general PICU population (7–11). Functional status and long term health of survivors of critical illness are increasingly viewed as highly meaningful patient-centered outcomes (12, 13). Septic AKI is associated with increased morbidity and mortality compared to non-septic AKI (4, 14, 15) in adults. Data on functional outcomes of children with septic AKI do not exist.
The Sepsis PRevalence, OUtcomes, and Therapies (SPROUT) point prevalence study enrolled patients with severe sepsis in 128 PICUs across 26 countries worldwide (1). Hospital mortality was 25%, and 17% of survivors had new moderate or worse disability at hospital discharge. We used data from the SPROUT study to determine the global prevalence of pediatric septic AKI. We evaluated the association of septic AKI with the composite outcome of new disability or mortality to determine the additive impact of AKI in the setting of severe sepsis.
METHODS
SPROUT was a prospective, cross-sectional point prevalence study performed at 128 PICUs in 26 countries (1). PICU patients were screened for severe sepsis according to the 2005 International Pediatric Sepsis Consensus Conference criteria (16) using data from the 24 hours preceding the study day, yielding a study cohort with active severe sepsis. For this analysis, patients with a prior history of renal disease were excluded. Ethics approval was obtained at all sites. Written consent was required at three sites. All other sites granted waiver of informed consent.
Data collection
Patient demographics were collected from the time of PICU admission. Vital signs, laboratory data, and therapeutic interventions provided were collected within a 48-hour window around the study day. Systolic blood pressure was categorized as less than or greater than 5th percentile for age and gender assuming 50th percentile for height (17). Severity of illness was measured by the Pediatric Index of Mortality-3 (PIM3) score (18) at PICU admission. Presence of multi-organ dysfunction syndrome (MODS) was assessed on the day of severe sepsis recognition (first hospital day on which patients met consensus criteria for severe sepsis). Degree of organ dysfunction was measured by the Pediatric Logistic Organ Dysfunction (PELOD) score (19) without the renal component of the score (PELOD-R) in the 48-hour window around the study day. Patients were followed until the hospital discharge to determine outcomes, and censored at 90 days after the study day if still hospitalized.
AKI Definition
AKI was defined using the Kidney Disease Improving Global Outcomes (KDIGO) criteria (20) (Supplemental Digital Content – Methods). Patients were categorized as having no AKI or stage 1 AKI (“No/mild AKI” group) versus stage 2 or 3 AKI (“Severe AKI” group) using the maximum serum creatinine measured in the 48-hour data collection window. Baseline renal function was assumed to be normal as children with pre-existing renal comorbidities were excluded. A baseline serum creatinine value was assigned to all patients using average age- and gender-based norms as previously described (9, 21, 22).
Outcomes
The primary outcome was the composite of 1) death, or 2) new moderate or worse disability, measured by a Pediatric Overall Performance Category (POPC) score of 3 or higher, and increased by at least 1 category from baseline (12). The POPC ordinal scale (1-good overall performance, 2-mild disability, 3-moderate disability, 4-severe disability, 5-coma or vegetative state, 6-brain death) was measured at baseline using PICU admission documentation and at hospital discharge or 90 days after the study day to assess change in functional status (23). A priori defined secondary outcomes included PICU and hospital mortality censored at 90 days; PICU and hospital length of stay (LOS); and ventilator-free and vasoactive infusion-free days out of 28 days from the day of severe sepsis recognition.
Statistical Analysis
Data were analyzed using STATA (version 12.1; College Station, TX). Data are presented as medians with interquartile ranges (IQR) for continuous variables and frequencies with proportions for categorical variables. Comparisons between groups were performed using the Wilcoxon rank sum test for continuous variables and Fisher’s exact or chi-squared tests for proportions. Multivariable logistic regression was performed to test the independent association of AKI with binary outcomes, and negative binomial regression was used for count outcomes (Supplemental Digital Content – Methods). Based on a non-significant trend noted in the univariate association of AKI with new moderate disability in survivors, a post hoc analysis of a trichotomous outcome separating death and disability was performed using multinomial regression (Supplemental Digital Content – Methods).
RESULTS
AKI prevalence
In the SPROUT study, 6,925 patients were screened over five days and 569 were identified with severe sepsis (1). For this analysis, 493 patients met inclusion criteria (Supplemental Digital Content - Fig. 1). Three hundred ninety one had No/mild AKI, and 102 (21%) had Severe AKI (Table 1, Supplemental Digital Content – Fig. 1). Sixty two patients were treated with renal replacement therapy (RRT).
Table 1.
Cohort Characteristics
| Variable | No AKI/Stage 1 AKI (n=391) |
Stage 2–3 AKI (n=102) |
p-value |
|---|---|---|---|
| Age in years, median (IQR) | 3 (0.7, 10) | 5 (0.8, 12) | 0.148 |
| Male gender, n (%) | 209 (53) | 58 (57) | 0.538 |
| Weight in kg, median (IQR)a | 14 (7, 33.3) | 20 (8.7, 40) | 0.088 |
| Height in cm, median (IQR)b | 94 (64, 137) | 110 (82.1, 145) | 0.055 |
| Race, n (%) | 0.048 | ||
| White | 156 (40) | 49 (48) | |
| Black | 62 (16) | 7 (7) | |
| Asian | 49 (13) | 19 (19) | |
| Other | 98 (25) | 23 (23) | |
| Unknown | 26 (7) | 4 (4) | |
| Source of PICU admission, n (%) | 0.096 | ||
| Emergency department | 127 (32) | 20 (20) | |
| Hospital ward | 97 (25) | 35 (34) | |
| Operating room | 33 (8) | 9 (9) | |
| Other hospital | 115 (29) | 33 (32) | |
| Other | 19 (5) | 5 (5) | |
| Admission Type, n (%) | 0.640 | ||
| Medical | 313 (80) | 84 (82) | |
| Surgical | 61 (16) | 16 (16) | |
| Trauma | 17 (4) | 2 (2) | |
| Admitted after cardiopulmonary bypass surgery |
19 (5) | 7 (7) | 0.455 |
| Previously healthy, n (%) | 89 (23) | 24 (24) | 0.870 |
| Comorbid conditions, n (%) | |||
| Respiratory | 120 (31) | 13 (13) | <0.001 |
| Cardiovascular | 93 (24) | 23 (23) | 0.793 |
| Gastrointestinal | 76 (19) | 13 (13) | 0.118 |
| Genetic | 74 (19) | 19 (19) | 0.945 |
| Neuromuscular | 72 (18) | 5 (5) | <0.001 |
| Hematologic/Immunologic | 60 (15) | 28 (27) | 0.004 |
| Prematurity | 52 (13) | 7 (7) | 0.074 |
| Neoplastic | 45 (12) | 23 (23) | 0.004 |
| Solid organ/hematopoietic cell transplant | 21 (5) | 20 (20) | <0.001 |
| Baseline POPC score | 0.126 | ||
| Good overall performance | 208 (53) | 60 (59) | |
| Mild overall disability | 51 (13) | 17 (17) | |
| Moderate overall disability | 59 (15) | 17 (17) | |
| Severe overall disability | 72 (18) | 8 (8) | |
| Coma or vegetative state | 1 (<1) | 0 (0) | |
| PIM3, median (IQR) | 3.6 (1.6, 6.9) | 7.2 (3.1, 16.6) | <0.001 |
| PELOD (without renal component), median (IQR) |
11 (2, 12) | 12 (4, 21) | <0.001 |
| MODS at sepsis recognition | 190 (49) | 73 (72) | <0.001 |
| Minimum systolic blood pressure, median (IQR)c |
76 (67, 87) | 70 (59, 88) | 0.014 |
| Minimum systolic blood pressure <5th percentile for age/gender, n (%) |
224 (57) | 72 (71) | 0.015 |
| Capillary refill time >5 seconds | 50 (13) | 35 (34) | <0.001 |
| Minimum ScvO2d, median (IQR)e | 68 (57, 75) | 61 (50, 73) | 0.065 |
| Maximum lactate (mmol/L), median (IQR)f | 1.6 (1.1, 3) | 2.8 (1.7, 4.8) | <0.001 |
| Minimum PF ratio (torr), median (IQR)g | 165 (97, 250) | 142 (79, 236) | 0.192 |
| Bacteremia present | 90 (23) | 32 (31) | 0.082 |
| No infectious organism identified | 138 (35) | 37 (36) | 0.854 |
Data present in 490 of 493 patients,
Data present in 356 of 493 patients,
Data present in 492 of 493 patients,
ScvO2: Central venous oxyhemoglobin saturation,
Data present in 181 of 493 patients,
Data present in 351 of 493 patients,
Data present in 315 of 493 patients
Septic AKI was associated with death/disability
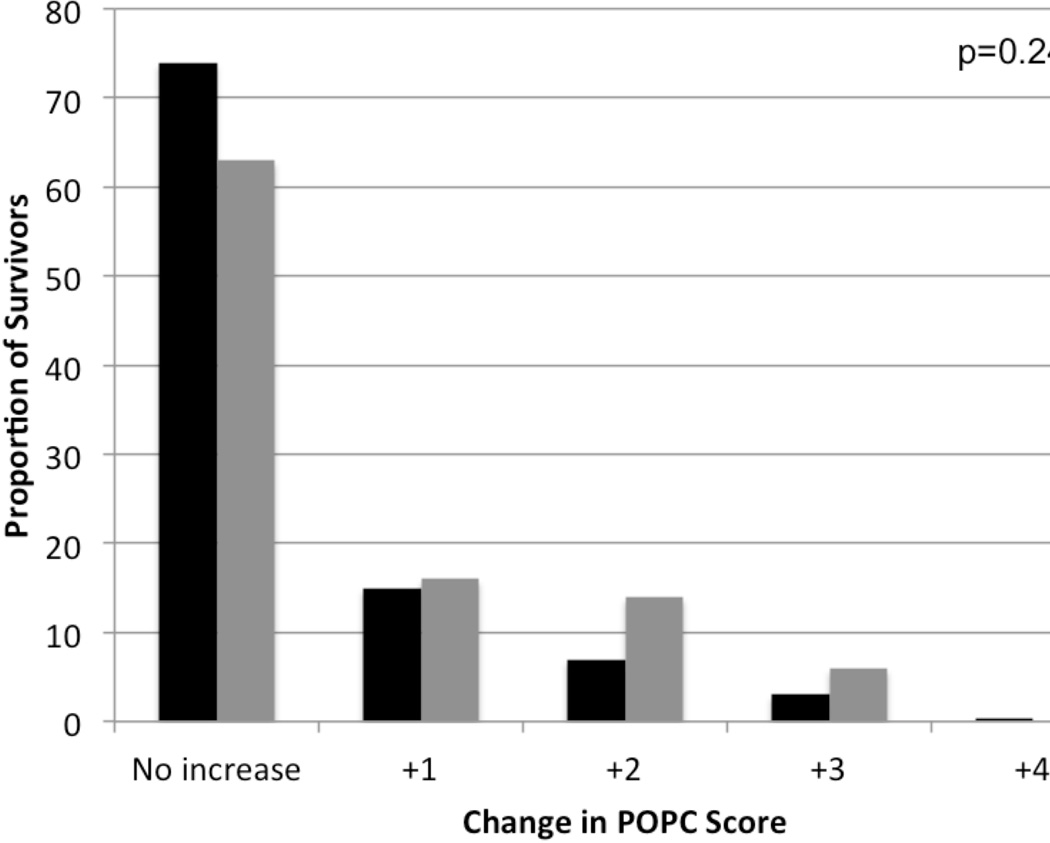
The primary outcome, death or new moderate or worse disability, occurred over twice as often in those with Severe AKI compared to patients with No/mild AKI (64% vs. 30%, p<0.001, Table 2). POPC score worsened in 37% of survivors with Severe AKI and in 26% of survivors with No/mild AKI (Fig. 1). New moderate or worse disability occurred in 24% of survivors with Severe AKI versus 15% of survivors with No/mild AKI (p=0.105). Unadjusted PICU and hospital mortality were nearly three times higher in the Severe AKI group, and these patients had fewer ventilator-free and vasoactive infusion-free days in the 28 days following severe sepsis recognition (Table 2).
Table 2.
Patient Outcomes
| Outcome Measure | No AKI/Stage 1 AKI (n=391) |
Stage 2–3 AKI (n=102) |
p-value |
|---|---|---|---|
| Death or moderate disability, n (%) | 119 (30) | 65 (64) | <0.001 |
| Hospital mortality, n (%) | 70 (18) | 53 (52) | <0.001 |
| PICU mortality, n (%) | 66 (17) | 51 (50) | <0.001 |
| Hospital LOS (days), median (IQR) | 25 (12, 51) | 27 (15, 51) | 0.767 |
| PICU LOS (days), median (IQR) | 14 (6, 32) | 18 (10, 32) | 0.105 |
| Vasoactive-free days, median (IQR) | 25 (19, 28) | 20.5 (11, 24) | <0.001 |
| Ventilator-free days, median (IQR) | 20 (2, 26) | 14.5 (0, 21) | 0.0013 |
Figure 1.
Change in POPC scores from baseline to discharge. Bars represent the proportion of survivors who had increases (worsening) in pediatric overall performance category (POPC) scores from baseline to hospital discharge. The x-axis shows the number of categories of increase in score. Black bars represent patients with No/mild acute kidney injury (AKI) and gray bars represent patients with Severe AKI.
Univariable logistic regression was used to identify variables associated with AKI (Supplemental Digital Content - Table 1) and with death or new moderate or worse disability (Supplemental Digital Content - Table 2). Using multivariable regression modeling, Severe AKI remained independently associated with death or new moderate or worse disability after adjustment for age, geographic region, history of malignancy, baseline POPC score, use of invasive mechanical ventilation, albumin administration, and PELOD-R score (adjusted OR 2.49, 95% CI 1.48, 4.19; p=0.001). In sensitivity analyses, the presence of Severe AKI by urine output criteria or any AKI (stage 1–3) were also independently associated with death or new moderate disability (Supplemental Digital Content – Table 3). In addition, Severe AKI was independently associated with PICU mortality and hospital mortality after adjustment for these variables (Table 3). Similarly, Severe AKI was independently associated with fewer vasoactive infusion-free days (adjusted β −0.27, 95% CI −0.48, −0.05; p=0.015) after adjustment for age, geographic region, use of invasive mechanical ventilation, blood product administration, and PELOD-R score. The association of AKI with fewer ventilator-free days was no longer statistically significant (adjusted β −0.23, 95% CI −0.56, 0.09; p=0.158) after adjustment for confounders (age, geographic region, number of vasoactive infusions, blood product administration, and PELOD-R score).
Table 3.
Logistic Regression for Association of Acute Kidney Injury with Different Outcomes
| Outcome Measure | Adjusteda OR for Association of AKI and Outcome |
95% CI | p-value |
|---|---|---|---|
| Death or new moderate or worse disability |
2.49 | 1.48, 4.19 | 0.001 |
| Hospital mortality | 3.17 | 1.88, 5.36 | <0.001 |
| PICU mortality | 3.13 | 1.84, 5.31 | <0.001 |
Adjusted for age, region, malignancy, baseline Pediatric Overall Performance Category Score, PELOD score without renal component, invasive mechanical ventilation, and albumin administration
A post hoc analysis was performed to evaluate the independent association of Severe AKI with new moderate or worse disability and mortality separately, using the trichotomous outcome alive with no or mild new disability, alive with new moderate or worse disability, or deceased. In this analysis, Severe AKI remained significantly associated with mortality but was not significantly associated with new moderate or worse disability in survivors (Supplemental Digital Content – Table 4).
Patients with Severe AKI had higher illness severity and complexity
PIM3 score at PICU admission was higher in patients with Severe AKI (Table 1). More patients with Severe AKI had MODS at the time of sepsis recognition compared to those with No/mild AKI, and patients with Severe AKI had a higher degree of organ dysfunction as measured by the PELOD-R score on the study day (Table 1). Respiratory, hematologic/immunologic, and neoplastic comorbidities and solid organ or hematopoietic cell transplant were more common in patients with Severe AKI (Table 1).
In keeping with more clinical findings of shock in the Severe AKI group (Table 1), these patients were treated more often with infusions of vasoactive agents, albumin, and blood products on the study day (Table 4). While albumin and blood product administration was quite common in the study (26% and 43%, respectively, out of all patients in the cohort), the use of synthetic colloid was rare at 4% overall, and did not differ in patients with Severe AKI and those with No/mild AKI (Table 4).
Table 4.
Therapies Used
| Therapy | No AKI/Stage 1 AKI (n=391) |
Stage 2–3 AKI (n=102) |
p-value |
|---|---|---|---|
| Invasive mechanical ventilation | 283 (72) | 90 (88) | 0.001 |
| Vasoactive infusions | |||
| Any vasoactive infusion | 204 (52) | 78 (76) | <0.001 |
| Norepinephrine | 79 (20) | 44 (43) | <0.001 |
| Epinephrine | 69 (18) | 48 (47) | <0.001 |
| Milrinone | 64 (16) | 23 (23) | 0.145 |
| Dopamine (>5mcg/kg/min) | 62 (16) | 30 (30) | 0.002 |
| Dopamine (≤5mcg/kg/min) | 20 (5) | 4 (4) | 0.798 |
| Dobutamine | 25 (6) | 10 (10) | 0.232 |
| Vasopressin | 11 (3) | 17 (17) | <0.001 |
| Number of vasoactive infusions, median (IQR) |
1 (0, 1) | 2 (1, 3) | <0.001 |
| Synthetic colloid | 15 (4) | 6 (6) | 0.407 |
| Albumin | 83 (21) | 44 (43) | <0.001 |
| Blood products | 136 (35) | 74 (73) | <0.001 |
| Corticosteroids | 155 (40) | 56 (55) | 0.006 |
| Diuretics | 237 (61) | 56 (55) | 0.295 |
| Insulin infusion | 27 (7) | 23 (23) | <0.001 |
| Extracorporeal membrane oxygenation | 15 (4) | 14 (14) | <0.001 |
| Plasma exchange | 1 (0.3) | 3 (3) | 0.029 |
| Catheters | |||
| Foley | 259 (66) | 89 (87) | <0.001 |
| Arterial line | 237 (61) | 80 (78) | 0.001 |
| CVLa (excluding dialysis catheter) | 318 (81) | 97 (95) | 0.001 |
CVL: Central venous line
Geographic differences
Severe AKI was least frequent in North and South America (16–19%) and most frequent in Europe, Asia, and Australia/New Zealand (29–32%, Supplemental Digital Content - Fig. 2). RRT was not used in Africa (only 13 patients were from this region), and RRT was used in 9–23% of patients in other regions, lowest in North America and highest in Europe and Australia/New Zealand (Supplemental Digital Content - Fig. 2). In the final multivariable model for the primary outcome, South America had an association with good outcome (adjusted OR 0.31, 95% CI 0.13, 0.73, p=0.008) compared to North America as the reference group. Despite higher frequency of Severe AKI and more than double the use of RRT in Europe and Australia/New Zealand compared to North America, neither geographic region had a significant association with outcome in the final multivariable model compared to North America (adjusted OR 1.01, 95% CI 0.56, 1.81, p=0.982, and adjusted OR 0.44, 95% CI 0.15, 1.29, p=0.135, respectively, for Europe and Australia/New Zealand).
DISCUSSION
Severe AKI was present in approximately 20% pediatric patients with severe sepsis and was independently associated with poor outcomes. Children with sepsis-associated severe AKI had more than double the odds of death or new moderate disability at hospital discharge than children with severe sepsis and no or mild AKI. This is the first study to evaluate the association of AKI with a general measure of disability in survivors of pediatric severe sepsis, which is attributable for much of AKI encountered in PICUs. Further research in this area is needed as a post hoc analysis could not confirm a significant association between AKI and new disability.
These results suggest that severe AKI is associated with poorer functional outcomes in survivors of pediatric severe sepsis. There is increasing recognition of the relevance of patient-centered functional outcomes in pediatric severe sepsis research (24, 25), and there is a growing body of literature describing methodology to study these outcomes (12, 13). Long-term follow-up of survivors of pediatric critical illness have shown an association with AKI and chronic kidney disease (26, 27), but have not looked into other measures of overall patient function. Evidence from non-septic animal models of AKI support the extra-renal effects of injury on other vital organs, including the brain (28). Rodent models of renal ischemia show impairment in motor activity, disruption of the blood-brain barrier, and effects on the hippocampus, which is important for learning (29, 30). These studies suggest that investigation of functional outcomes in patients with AKI should be prioritized.
This study demonstrated that children with severe sepsis and severe AKI had an additive increase in risk of death or disability, on top of that imparted by severe sepsis alone. Patients with severe AKI in this study had higher illness severity and were treated more frequently with vasoactive infusions, mechanical ventilation, and colloid administration. The association of severe AKI and poorer outcome, however, persisted after adjusting for increased patient complexity and illness severity in our primary analysis. In post hoc analysis, the association of AKI with increased new disability in survivors was not statistically significant when analyzed using trichotomous outcomes, suggesting mortality is a large driver of our results. While this study was not powered for such a trichotomous analysis, this finding indicates that further research is needed to confirm our conclusions. Previous studies on pediatric AKI survivors were confined to renal outcomes, demonstrating that patients were likely to develop signs of chronic kidney disease as early as one year after discharge (27). Up to 25% of patients leave the PICU with abnormal serum creatinine values (9) although only a minority return for long term Nephrology follow up (26). Our data suggest AKI survivors are also at risk of new disability, further underscoring the importance of long term follow up.
In this international cohort of children with severe sepsis, severe AKI was most common in Australia/New Zealand, Asia, and Europe, and least common in North and South America. The proportion of children with severe AKI treated with RRT varied across geographic region from 0–75%. These geographic differences in AKI prevalence and practice variability in RRT use possibly reflect varying PICU patient populations and PICU admission criteria, and could be a natural setting for comparative effectiveness work in septic AKI.
There are multiple areas for further study of pediatric septic AKI. No targeted therapies for septic AKI exist and optimal treatment of pediatric septic AKI is unknown. The etiology of septic AKI is multifactorial, rather than simply a secondary insult due to ischemia from shock (31–33). Poor perfusion, pro- and anti-inflammatory mediators, and microthrombosis likely contribute synergistically to necrotic and apoptotic renal cell death, leading to AKI (33–35). Implementation of emergency department-based resuscitation bundles with protocolized fluid resuscitation and early antibiotics along with appropriate escalation of vasopressor support has been associated with decreased AKI development in children with septic shock (36). The KDIGO AKI management bundle for patients with AKI, which includes ensuring volume status and perfusion pressure, consideration of functional hemodynamic monitoring, and consideration of RRT for Stage 2 or greater AKI (37) needs to be tested. Controversy remains over the use of colloids versus crystalloids for fluid resuscitation in severe sepsis, and adult evidence shows increased risk of AKI with synthetic colloid use (38–41). Patients with Severe AKI in this study received more colloid, however, this dataset was not designed to compare use of crystalloid versus colloid, and few patients received synthetic colloids, limiting comparisons within our cohort.
A limitation of this study is that baseline serum creatinine data were not collected, thus limiting the cohort to patients with no history of kidney disease. Baseline creatinine was assigned using age and gender norms, an accepted methodology for dealing with missing baseline creatinine data in children (9, 22). Assigning a baseline estimated glomerular filtration rate of 120mL/min/1.73m2 (11, 42), and using the Schwartz formula to assign baseline creatinine values with multiple imputation for missing height data yielded similar results (data not shown). Additionally, we categorized patients as Severe (stage 2 or 3) versus No/mild AKI (no or stage 1) to minimize misclassification bias due to unknown baseline renal function.
AKI was defined by KDIGO criteria as this is the most recently described AKI classification, is applicable to children and adults, and does not require height data. Other AKI staging criteria exist with similar but not identical definitions, yielding different incidences of AKI based on definition (43), possibly limiting comparisons across studies. The primary outcome was evaluated at hospital discharge, censored at 90 days, thus not all patients were followed for 90 days, possibly leading to misclassification bias as new disability at discharge may have resolved by 90 days. This should bias the results toward the null, and therefore not alter the study conclusions.
Other limitations include the point prevalence design, in which the majority of data collection occurred during a brief, cross-sectional time window. This could result in underestimation of the frequency of AKI. The design allows only for measurement of associations between AKI and other clinical variables at the time of the data collection window, precluding attempts to establish exposures leading to AKI. However, patients were followed prospectively for outcomes, and the association of AKI with outcome was adjusted for multiple factors such as pre-existing comorbidities, PELOD-R, and therapeutic interventions.
CONCLUSIONS
Severe AKI is an independent risk factor for death or new moderate disability, a patient-centered functional outcome. Sixty four percent of those with Severe AKI died or developed new moderate or worse disability. Septic AKI survivors need long-term follow-up of renal function and other organ systems impacted by septic AKI. Future study with longer follow-up in pediatric septic AKI survivors is needed to confirm the association of AKI and disability we observed.
Supplementary Material
Acknowledgments
We would like to acknowledge the contributions of all the participating SPROUT investigators.
Financial Support: Financial support was provided by the Endowed Chair, Department of Anesthesia and Critical Care, University of Pennsylvania Perelman School of Medicine and the Center for Pediatric Clinical Effectiveness at The Children’s Hospital of Philadelphia. Dr. Weiss is also supported by NIH K12HD047349-10. Financial support for data collection in all UK centers was provided by the UK National Institute of Health (NIHR) Clinical Research Network and in Southampton by the Southampton NIHR Wellcome Trust Clinical Research Facility. None of the funders participated in the design and conduct of study; collection, management, analysis, and interpretation of the data; or preparation, review or approval of the manuscript.
Dr. Fitzgerald’s institution received funding from the Children's Hospital of Philadelphia Center for Pediatric Clinical Effectiveness. Dr. Akcan-Arikan received funding (Consultant for Baxter). Dr. Sapru received support for article research from the National Institutes of Health (NIH). His institution received funding from the NIH. Dr. Thomas received support from Therabron and CareFusion. His institution received funding from the FDA. Dr. Weiss received funding from Thermo-Fisher Scientific (honoraria for lecture unrelated to current work). His institution received funding from the Center for Pediatric Clinical Effectiveness at The Children's Hospital of Philadelphia. Dr. Furth received support for article research from the NIH.
SPROUT STUDY INVESTIGATORS
North America: Canada: P. Fontela (Montreal Children's Hospital-McGill), M. Tucci, M. Dumistrascu (Sainte Justine Hospital), P. Skippen, G. Krahn (BC Children's Hospital); Puerto Rico: E. Bezares (Hospital Cardiovascular de Puerto Rico y el Caribe), G. Puig, A. Puig-Ramos (San Jorge Children’s Hospital), R. Garcia, M. Villar (University Pediatric Hospital); United States: M. Bigham, T. Polanski, S. Latifi, D. Giebner, H. Anthony (Akron Children's Hospital), J. Hume, A. Galster, L. Linnerud (Amplatz Children’s Hospital), R. Sanders, G. Hefley (Arkansas Children’s Hospital), K. Madden (Boston Children’s Hospital), A. Thompson, S. Shein (Children’s Hospital of Pittsburgh), S. Gertz (Children’s Hospital-Hackensack), Y. Han, T. Williams, A. Hughes-Schalk (Children’s Mercy Hospital), H. Chandler (Children's Healthcare of Atlanta), A. Orioles, E. Zielinski, A. Doucette (Children’s Hospital in Minnesota), A. Orioles, E. Zielinski, A. Doucette (Children’s Hospital St. Paul), C. Zebuhr, T. Wilson (Children's Hospital Colorado), C. Dimitriades, J. Ascani, S. Layburn, S. Valley (Children's Hospital New Orleans), B. Markowitz, J. Terry, R. Morzov (Children’s Hospital of Los Angeles), A. Mcinnes (Children’s Hospital of Monmouth), J. McArthur, K. Woods, K. Murkowski (Children's Hospital of Wisconsin), M. Spaeder, M. Sharron (Children's National Medical Center), D. Wheeler, E. Beckman, E. Frank, K. Howard (Cincinnati Children‘s Medical Center), C. Carroll (Connecticut Children's), S. Nett, D. Jarvis (Dartmouth Hitchcock), V. Patel (Dayton Children's Hospital), R. Higgerson, L. Christie (Dell Children's Medical Center), K. Typpo, J. Deschenes (Diamond Children's Hospital), A. Kirby (Doernbecher Children's Hospital), T. Uhl, K. Rehder, I. Cheifetz, S. Wrenn (Duke Children's Hospital), K. Kypuros (El Paso Children's Hospital), K. Ackerman (Golisano Children’s Hospital), F. Maffei, G. Bloomquist (Janet Weis/Geisinger), N. Rizkalla (Johns Hopkins), D. Kimura, S. Shah, C. Tigges (Le Bonheur Children’s Hospital), F. Su, C. Barlow (Lucile Packard Children’s Hospital), K. Michelson, K. Wolfe, D. Goodman, L. Campbell, L. Sorce (Lurie Children's Hospital of Chicago), K. Bysani, T. Monjure (Medical City Children's-Dallas), M. Evans (Medical University of South Carolina), B. Totapally, M. Chegondi, C. Rodriguez (Miami Children’s Hospital), J. Frazier, L. Steele (Nationwide Children's Hospital), S. Viteri, A. Costarino (Nemours/ Alfred I. duPont Children’s Hospital), N. Thomas, D. Spear (Penn State Hershey Medical Center), E. Hirshberg, J. Lilley (Primary Children's Medical Center), C. Rowan, C. Rider (Riley Hospital for Children), J. Kane (Rush Children's Hospital), J. Zimmerman, C. Greeley (Seattle Children's Hospital), J. Lin, R. Jacobs (St. Louis Children's Hospital), M. Parker, K. Culver (Stony Brook University), L. Loftis, N. Jaimon, M. Goldsworthy (Texas Children's Hospital), J. Fitzgerald, S. Weiss, V. Nadkarni, J. Bush, M. Diliberto (The Children’s Hospital of Philadelphia), C. Alen, M. Gessouroun (Oklahoma University Medical Center), A. Sapru, T. Lang, M. Alkhouli (University of California San Francisco), S. Kamath, D. Friel, J. Daufeldt (University of Iowa), D. Hsing, C. Carlo, S. Pon (Weill Cornell Medical Center), J. Scimeme, A. Shaheen (Wolfson Children’s Hospital), A. Hassinger, H. Qiao (Women and Children’s Hospital of Buffalo), J. Giuliano, J. Tala (Yale Children’s Hospital). South America: Argentina: D. Vinciguerra, A. Fernandez (Hospital Durand); Colombia: R. Carrero (Clínica Infantil Colsubsidio), P. Hoyos (Hospital de San Jose), J. Jaramillo, A. Posada (Hospital General de Medellín), L. Izquierdo (Hospital Militar Central), B.E. Piñeres Olave, J. Donado (Pablo Tobón Uribe); Chile: R. Dalmazzo, S. Rendich (Clínica Las Condes), L. Palma, M. Lapadula (Clínica Santa María), C. Acuna (Hospital Luis Calvo Mackenna), P. Cruces (Hospital Padre Hurtado) Europe: Belgium: S. Clement De Clety, M. Dujardin, C. Berghe, S. Renard (St. Luc University Hospital); Czech Republic: J. Zurek (Masaryk University); Germany: H. Steinherr (Klinikum Augsburg); Greece: K. Mougkou (Aghia Sophia Children’s Hospital), E. Critselis, K. Mougkou (P. & A. Kyriakou Children’s Hospital); Italy: M. Di Nardo, S. Picardo, F. Tortora (Bambino Gesu Area Rossa); E. Rossetti (Bambino Gesu Children’s Hospital); T. Fragasso, P. Cogo, R. Netto (Bambino Gesu Pediatrico); Lithuania: A. Dagys, V. Gurskis, R. Kevalas (Lithuanian University of Health Sciences); Netherlands: C. Neeleman, J. Lemson, C. Luijten (Radboud University Medical Centre); Poland: K. Wojciech, I. Pagowska-Klimek (Polish Mother Memorial Hospital), M. Szczepanska, J. Karpe (Medical University of Silesia) ; Portugal: P. Nunes, H. Almeida (Hospital Prof Dr. Fernando Fonseca), J. Rios, M. Vieira (Centrol Hospitalar Lisboa Norte); Spain: JP García-Iñiguez, P. Madurga-Revilla (Children´s Hospital Miguel Servet), J. Urbano, J. Lopez-Herce, A. Bustinza (Hospital General Universitario Gregorio Marañón), A. Cuesta, S. Hofheinz (Hospital 12 de Octubre), A. Rodriguez-Nunez (Hospital Clínico Universitario), S. Sanagustin, E. Gonzalez (Hospital de la Sant Creu Sant Pau), M. Riaza, R. Piaya (Hospital Universitario Madrid), P. Soler (Hospital Carlos Haya Materno Infantil), E. Esteban (Hospital Sant Joan de Déu), J. Laraudogoitia, C. Monge (Hospital Universitario Donostia), V. Herrera, J. Granados (Hospital Universitario Salamanca), C. Gonzalez (Hospital Virgen de la Arrixaca); Turkey: T. Koroglu, E. Ozcelik (Dokuz Eylul University); United Kingdom: P. Baines (Alder Hey Children’s Hospital), A. Plunkett (Birmingham Children’s Hospital), P. Davis, S. George (Bristol Royal Hospital for Children), S. Tibby, J. Harris (Evelina Children’s Hospital), R. Agbeko, R. Lampitt (Great North Children’s Hospital–Newcastle), J. Brierley, M. Peters, A. Jones, T. Dominguez, T. Thiruchelvam, (Great Ormond Street), A. Deep, L. Ridley, W. Bowen (King’s College Hospital), R. Levin, I. Macleod (Royal Hospital for Sick Children), M. Gray, N. Hemat (St George’s Hospital), J. Alexander, S. Ali (University Hospital of North Staffordshire NHS Trust), J. Pappachan, J. McCorkell (University Hospital Southampton NHS Foundation Trust), P. Fortune, M. MacDonald, P. Hudnott (Royal Manchester Children’s Hospital). Asia: China: Q. Suyun (Beijing Children’s Hospital); India: S. Singhi, K. Nallasamy (Advanced Pediatrics), R. Lodha (All India Institute); Japan: N. Shime, Y. Tabata (Kyoto Prefectural), O. Saito, T. Ikeyama (Tokyo Metropolitan), T. Kawasaki (Shizuoka Children’s Hospital); Malaysia: L. Lum, A. Abidin, S. Kee (University Malaya Medical Center), S. Tang, R. Jalil (Kebangsaan Malaysia Medical Center); Singapore: Y. Guan, L. Yao (KK Women’s and Children’s Hospital), K. Lin, J. Ong (National University Hospital). Africa: South Africa: A. Salloo, L. Doedens, L. Mathivha (Chris Hani Baragwanath), G. Reubenson, S. Moaisi (Rahima Moosa), A. Pentz, R. Green (Steve Biko Academic Hospital). Australia: A. Schibler, A. Fernandez (Mater Children’s Hospital), S. Erickson (Princess Margaret Hospital), J. McEneiry, D. Long, T. Dorofaeff, M. Coulthard (Royal Children’s Hospital Brisbane), J. Millar, C. Delzoppo (Royal Children’s Melbourne), G. Williams, M. Morritt (Sydney Children’s Hospital), N. Watts, M. Morritt (Children’s Hospital Westmead). New Zealand: J. Beca, C. Sherring, T. Bushell (Starship Children’s Hospital)
Footnotes
Copyright form disclosures: The remaining authors have disclosed that they do not have any potential conflicts of interest.
SUPPLEMENTAL DIGITAL CONTENT LEGEND
Supplemental methods
Supplemental digital content figure 1: Flow diagram of patients included in the cohort.
Supplemental digital content table 1: Univariable Logistic Regression for Association of Covariates with Severe Acute Kidney Injury.
Supplemental digital content table 2: Univariable Logistic Regression for Association of Covariates with Death or New Moderate or Worse Disability.
Supplemental digital content table 3: Logistic Regression for Association of Acute Kidney Injury with Death or New Moderate or Worse Disability using Different Definitions of Acute Kidney Injury.
Supplemental digital content table 4: Multinomial Regression for Association of Acute Kidney Injury with Trichotomized Outcome of Alive, Alive with Disability, or Death.
Supplemental digital content figure 2: Geographic differences in AKI. Black bars represent the proportion of patients from each region with Severe acute kidney injury (AKI). Gray bars represent the proportion of patients from each region treated with renal replacement therapy (RRT)
REFERENCES
- 1.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191(10):1147–1157. doi: 10.1164/rccm.201412-2323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balamuth F, Weiss SL, Neuman MI, et al. Pediatric Severe Sepsis in U.S. Children's Hospitals. Pediatr Crit Care Med. 2014;15(9):798–805. doi: 10.1097/PCC.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruth A, McCracken CE, Fortenberry JD, et al. Pediatric Severe Sepsis: Current Trends and Outcomes From the Pediatric Health Information Systems Database. Pediatr Crit Care Med. 2014;15(9):828–838. doi: 10.1097/PCC.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 4.Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12(2):R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz DN, Bolgan I, Perazella MA, et al. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol. 2007;2(3):418–425. doi: 10.2215/CJN.03361006. [DOI] [PubMed] [Google Scholar]
- 6.Kolhe NV, Stevens PE, Crowe AV, et al. Case mix, outcome and activity for patients with severe acute kidney injury during the first 24 hours after admission to an adult, general critical care unit: application of predictive models from a secondary analysis of the ICNARC Case Mix Programme database. Crit Care. 2008;12(Suppl 1):S2. doi: 10.1186/cc7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider J, Khemani R, Grushkin C, et al. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38(3):933–939. doi: 10.1097/CCM.0b013e3181cd12e1. [DOI] [PubMed] [Google Scholar]
- 8.Akcan-Arikan A, Zappitelli M, Loftis LL, et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 9.Alkandari O, Eddington KA, Hyder A, et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 2011;15(3):R146. doi: 10.1186/cc10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plotz FB, Bouma AB, van Wijk JA, et al. Pediatric acute kidney injury in the ICU: an independent evaluation of pRIFLE criteria. Intensive Care Med. 2008;34(9):1713–1717. doi: 10.1007/s00134-008-1176-7. [DOI] [PubMed] [Google Scholar]
- 11.Soler YA, Nieves-Plaza M, Prieto M, et al. Pediatric Risk, Injury, Failure, Loss, End-Stage renal disease score identifies acute kidney injury and predicts mortality in critically ill children: a prospective study. Pediatr Crit Care Med. 2013;14(4):e189–e195. doi: 10.1097/PCC.0b013e3182745675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farris RW, Weiss NS, Zimmerman JJ. Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial. Pediatr Crit Care Med. 2013;14(9):835–842. doi: 10.1097/PCC.0b013e3182a551c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124(1):e18–e28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poukkanen M, Vaara ST, Pettila V, et al. Acute kidney injury in patients with severe sepsis in Finnish Intensive Care Units. Acta Anaesthesiol Scand. 2013;57(7):863–872. doi: 10.1111/aas.12133. [DOI] [PubMed] [Google Scholar]
- 15.White LE, Hassoun HT, Bihorac A, et al. Acute kidney injury is surprisingly common and a powerful predictor of mortality in surgical sepsis. J Trauma Acute Care Surg. 2013;75(3):432–438. doi: 10.1097/TA.0b013e31829de6cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 17.Rosner B, Cook N, Portman R, et al. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167(6):653–666. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 18.Straney L, Clements A, Parslow RC, et al. Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care*. Pediatr Crit Care Med. 2013;14(7):673–681. doi: 10.1097/PCC.0b013e31829760cf. [DOI] [PubMed] [Google Scholar]
- 19.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362(9379):192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 20.Section 2: AKI Definition. Kidney Int Suppl (2011) 2012;2(1):19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colantonio DA, Kyriakopoulou L, Chan MK, et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem. 2012;58(5):854–868. doi: 10.1373/clinchem.2011.177741. [DOI] [PubMed] [Google Scholar]
- 22.Basu RK, Wong HR, Krawczeski CD, et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol. 2014;64(25):2753–2762. doi: 10.1016/j.jacc.2014.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 24.Curley MA, Zimmerman JJ. Alternative outcome measures for pediatric clinical sepsis trials. Pediatr Crit Care Med. 2005;6(3 Suppl):S150–S156. doi: 10.1097/01.PCC.0000161582.63265.B6. [DOI] [PubMed] [Google Scholar]
- 25.Marshall JC, Vincent JL, Guyatt G, et al. Outcome measures for clinical research in sepsis: a report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit Care Med. 2005;33(8):1708–1716. doi: 10.1097/01.ccm.0000174478.70338.03. [DOI] [PubMed] [Google Scholar]
- 26.Askenazi DJ, Feig DI, Graham NM, et al. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69(1):184–189. doi: 10.1038/sj.ki.5000032. [DOI] [PubMed] [Google Scholar]
- 27.Mammen C, Al Abbas A, Skippen P, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59(4):523–530. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 28.Yap SC, Lee HT. Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology. 2012;116(5):1139–1148. doi: 10.1097/ALN.0b013e31824f951b. [DOI] [PubMed] [Google Scholar]
- 29.Adachi N, Lei B, Deshpande G, et al. Uraemia suppresses central dopaminergic metabolism and impairs motor activity in rats. Intensive Care Med. 2001;27(10):1655–1660. doi: 10.1007/s001340101067. [DOI] [PubMed] [Google Scholar]
- 30.Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19(7):1360–1370. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basu RK, Devarajan P, Wong H, et al. An update and review of acute kidney injury in pediatrics. Pediatr Crit Care Med. 2011;12(3):339–347. doi: 10.1097/PCC.0b013e3181fe2e0b. [DOI] [PubMed] [Google Scholar]
- 32.Bellomo R, Wan L, Langenberg C, et al. Septic acute kidney injury: new concepts. Nephron Exp Nephrol. 2008;109(4):e95–e100. doi: 10.1159/000142933. [DOI] [PubMed] [Google Scholar]
- 33.Fortenberry JD, Paden ML, Goldstein SL. Acute kidney injury in children: an update on diagnosis and treatment. Pediatr Clin North Am. 2013;60(3):669–688. doi: 10.1016/j.pcl.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen TC, Han YY, Kiss JE, et al. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med. 2008;36(10):2878–2887. doi: 10.1097/ccm.0b013e318186aa49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronco C, Tetta C, Mariano F, et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: the peak concentration hypothesis. Artif Organs. 2003;27(9):792–801. doi: 10.1046/j.1525-1594.2003.07289.x. [DOI] [PubMed] [Google Scholar]
- 36.Akcan Arikan A, Williams EA, Graf JM, et al. Resuscitation Bundle in Pediatric Shock Decreases Acute Kidney Injury and Improves Outcomes. J Pediatr. 2015;167(6):1301–1305. e1301. doi: 10.1016/j.jpeds.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 37.Section 3: Prevention and Treatment of AKI. Kidney Int Suppl(2011) 2012;2(1):37–68. doi: 10.1038/kisup.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 39.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 40.Bayer O, Reinhart K, Kohl M, et al. Effects of fluid resuscitation with synthetic colloids or crystalloids alone on shock reversal, fluid balance, and patient outcomes in patients with severe sepsis: a prospective sequential analysis. Crit Care Med. 2012;40(9):2543–2551. doi: 10.1097/CCM.0b013e318258fee7. [DOI] [PubMed] [Google Scholar]
- 41.Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2012;367(2):124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 42.Zappitelli M, Parikh CR, Akcan-Arikan A, et al. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3(4):948–954. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554–561. doi: 10.2215/CJN.01900214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.