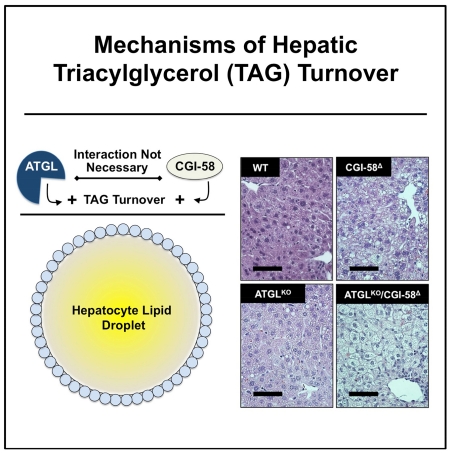
SUMMARY
Adipose Triglyceride Lipase (ATGL) and Comparative Gene Identification 58 (CGI-58) are critical regulators of triacylglycerol (TAG) turnover. CGI-58 is thought to regulate TAG mobilization by stimulating the enzymatic activity of ATGL. However, it is not known whether this coactivation function of CGI-58 occurs in vivo. Moreover, the phenotype of human CGI-58 mutations suggests ATGL-independent functions. Through direct comparison of mice with single or double deficiency of CGI-58 and ATGL, we show here that CGI-58 knockdown causes hepatic steatosis in both the presence and absence of ATGL. CGI-58 also regulates hepatic diacylglycerol (DAG) and inflammation in an ATGL-independent manner. Interestingly, ATGL deficiency, but not CGI-58 deficiency, results in suppression of the hepatic and adipose de novo lipogenic program. Collectively, these findings show that CGI-58 regulates hepatic neutral lipid storage and inflammation in the genetic absence of ATGL, demonstrating that mechanisms driving TAG lipolysis in hepatocytes differ significantly from those in adipocytes.
Keywords: Triacylglycerol, Lipase, Neutral Lipid Storage Disease
eTOC BLURB
Comparative Gene Identification 58 (CGI-58) is thought to regulate triacylglycerol mobilization by stimulating the enzymatic activity of Adipose Triglyceride Lipase (ATGL). Lord et al. now show that CGI-58 regulates hepatic triacylglycerol turnover and inflammation in the genetic absence of ATGL, demonstrating an ATGL-independent role for CGI-58 in mouse liver.
INTRODUCTION
The mobilization of triacylglycerol (TAG) from cellular lipid droplets has become an area of intense research and has been linked to a large number of physiological processes, including energy balance, signaling, gene transcription, and cell-cycle progression (Zechner et al., 2012; Greenberg et al., 2011). Recently, a rare autosomal-recessive disorder of TAG accumulation known as Neutral Lipid Storage Disease (NLSD) was linked to mutations in either CGI-58 or ATGL (Lefevre et al., 2001; Fischer et al., 2007), linking these proteins to TAG storage in humans. ATGL (Adipose Triglyceride Lipase, also known as PNPLA2 or desnutrin) functions as a critical lipase with specificity toward TAG (Zimmermann et al., 2004). CGI-58 has been shown to indirectly promote TAG turnover by serving as a co-activator of ATGL, and this function is lost with CGI-58 mutations that cause NLSD (Lass et al., 2006). Furthermore, the addition of purified CGI-58 can increase TAG hydrolase activity in white adipose tissue lysates from wild-type mice but not from mice lacking ATGL, indicating specificity for ATGL and suggesting that endogenous levels of CGI-58 limit maximal lipolysis (Schweiger et al., 2006).
While loss of ATGL co-activation is a possible explanation for TAG accumulation in NLSD patients with CGI-58 mutations, there is also strong evidence that CGI-58 has ATGL-independent functions (Lord et al., 2012a). For example, CGI-58 mutations always cause icthyosis and frequently result in severe fatty liver disease and neurological defects, whereas ATGL mutations primarily cause myopathies and rarely result in fatty liver disease or icthyosis (Lord et al., 2012a). The metabolic phenotypes of CGI-58 and ATGL deficiency in mice also differ significantly (Haemmerle et al., 2006; Haemmerle et al., 2011; Radner et al., 2010; Schreiber et al., 2015; Ahmadian et al., 2011; Brown et al., 2010; Lord et al., 2012b; Cantley et al., 2013). For instance, global CGI-58 knockout mice die shortly after birth due to a skin barrier defect (Radner et al., 2010), while ATGL global knockout mice survive for several months before ultimately succumbing to a lethal cardiomyopathy (Haemmerle et al., 2006). Furthermore, hepatocyte-specific (Guo et al., 2013; Hoy et al., 2011) or macrophage-specific (Goeritzer et al., 2014; Lammers et al., 2011; Aflaki et al., 2011a; Aflaki et al., 2011b; Miao et al., 2014) deletion of CGI-58 or ATGL results in divergent effects on inflammatory processes in the liver and artery wall in the context of atherosclerosis. Adipose-selective overexpression of CGI-58 in mice does not increase basal or hormone-stimulated lipolysis, calling into question the physiological importance of CGI-58 as a rate-limiting co-activator of ATGL (Caviglia et al., 2011). To determine whether ATGL-mediated lipolysis actually requires CGI-58 under physiological conditions and to directly test whether CGI-58 has any ATGL-independent functions, we generated mouse models with single or double deficiency of CGI-58 and ATGL. This could not be achieved through breeding because CGI-58 global knockout mice die at birth due to a skin barrier defect (Radner et al., 2010). Therefore, we used second generation antisense oligonucleotides (ASOs), which selectively target liver and adipose tissue, to deplete CGI-58 in either ATGL global knockout mice (ATGLKO) or wild-type littermates. As a result, we were able to directly compare wild-type (WT), CGI-58 single-deficient (CGI-58Δ), ATGL single-deficient (ATGLKO), and double-deficient (ATGLKO/CGI-58Δ) mice. These mouse models were used to test the co-activation hypothesis in vivo.
RESULTS
CGI-58 Regulates Hepatic TAG and Diacylglycerol (DAG) Storage via a Mechanism Distinct from ATGL Co-activation
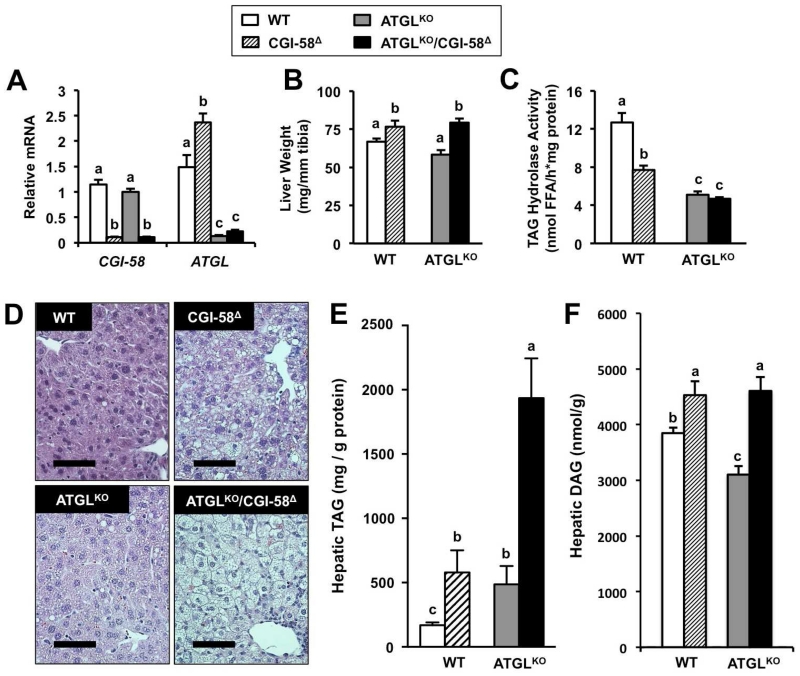
CGI-58 expression in the liver was reduced by over 90% with CGI-58 ASO treatment, and ATGL global knockout mice had expected reductions in ATGL mRNA levels (Figure 1A). ATGL deficiency alone did not change liver weight compared to WT mice, but CGI-58 knockdown resulted in hepatomegaly in both the presence and absence of ATGL (Figure 1B). Compared to WT mice, TAG hydrolase activity in liver was decreased by 40% in CGI-58Δ mice and 60% in ATGLKO mice, with no further decrease in ATGLKO/CGI-58Δ mice (Figure 1C), indicating both CGI-58 and ATGL regulated TAG turnover in the liver. Liver histology revealed striking steatosis in ATGLKO/CGI-58Δ mice compared to CGI-58Δ mice and ATGLKO mice (Figure 1D). Compared to WT mice, hepatic TAG levels were on average 3.5-fold higher in CGI-58Δ mice and 3-fold higher in ATGLKO mice (Figure 1E). Remarkably, ATGLKO/CGI-58Δ mice had 11.5-fold higher TAG, indicating an additive effect of double deficiency (Figure 1E). Interestingly, ATGL and CGI-58 exert reciprocal effects on hepatic DAG levels. ATGL single deficiency resulted in reduced levels of hepatic DAG, while CGI-58 knockdown increased hepatic DAG levels both in the genetic presence or absence of ATGL (Figure 1F). Therefore, CGI-58 knockdown causes hepatomegaly and hepatic steatosis through a mechanism separate from ATGL activity.
Figure 1. CGI-58 Regulates Hepatic Triacylglycerol Storage Via a Mechanism Distinct From ATGL Co-Activation.
At 4-6 weeks of age, male wild-type or ATGLKO mice were treated with either a non-targeting control ASO (WT, ATGLKO) or an ASO targeting CGI-58 (CGI-58Δ, ATGLKO/CGI-58Δ) in conjunction with high fat diet feeding for 6-8 weeks.
(A) Relative mRNA expression of CGI-58 and ATGL in whole liver.
(B) Liver weight normalized to tibia length.
(C) Total TAG hydrolase activity in cytosolic extracts of whole liver.
(D) Representative liver sections stained with hematoxylin and eosin (H&E); bar=75μm.
(E) Hepatic triacylglycerol (TAG) levels.
(F) Hepatic diacylglycerol (DAG) levels.
Data represent the mean ± S.E.M. from three to seven mice per group, and values not sharing a common superscript differ significantly (p<0.05).
Divergent Control of Hepatic Inflammation and Lipogenesis by CGI-58 and ATGL
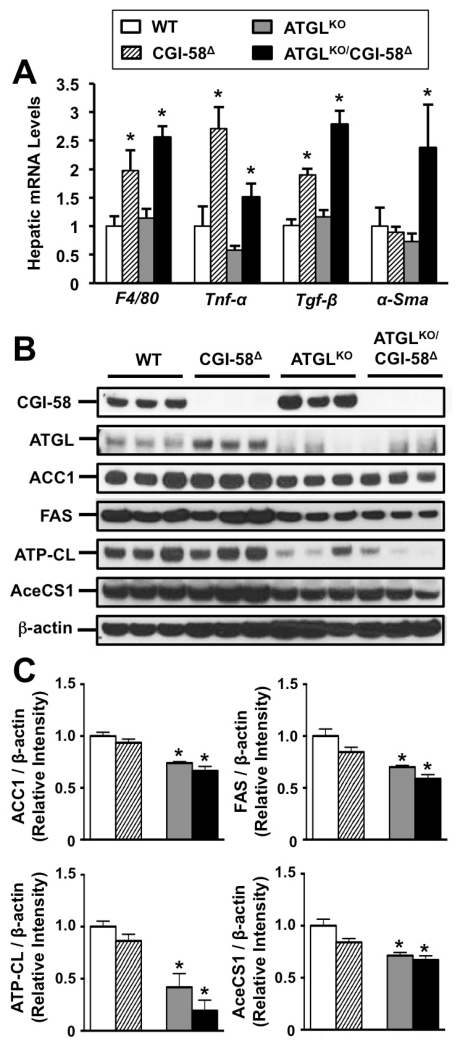
Based on our previous findings that CGI-58 regulates systemic inflammation (Lord et al., 2012b), we investigated whether hepatic inflammation differed between CGI-58 and ATGL deficiency. CGI-58 knockdown, but not ATGL knockout, was associated with higher hepatic expression of TH1-skewed macrophage markers such as F4/80 and tumor necrosis factor (TNF)-α (Figure 2A). Likewise, increased transforming growth factor β (TGF-β) expression (Figure 2B) suggested progression to non-alcoholic steatohepatitis (NASH). Furthermore, ATGLKO/CGI-58Δ mice had significantly higher expression of α-SMA (Figure 2A), a marker of hepatic stellate cell activation. Collectively, these data demonstrate that CGI-58 regulates hepatic inflammation via an ATGL-independent mechanism.
Figure 2. Divergent Control of Hepatic Inflammation and Lipogenesis by CGI-58 and ATGL.
At 4-6 weeks of age, male wild-type or ATGLKO mice were treated with either a non-targeting control ASO (WT, ATGLKO) or an ASO targeting CGI-58 (CGI-58Δ, ATGLKO/CGI-58Δ) in conjunction with high fat diet feeding for 6 weeks.
(A) Inflammatory gene expression in whole liver was quantified by qPCR.
(B) Western blotting for lipogenic proteins in whole liver.
(C) Densitometric quantification of lipogenic protein expression in whole liver.
Abbreviations: α-Sma, α smooth muscle actin; ACC1, acetyl-CoA carboxylase 1; AceCS1, Acetyl-CoA synthetase 1; ATP-CL, ATP-citrate lyase; FAS, fatty acid synthase; F4/80, EGF-like module-containing mucin-like hormone receptor-like 1; Tgf-β, transforming growth factor β; Tnf-α, tumor necrosis factor α. Data represent the mean+S.E.M. of 3-5 mice per group; *= significantly different WT group (p<0.05).
Given that de novo lipogenesis is a major determinant of hepatic DAG and TAG levels (Cohen et al., 2011), we set out to determine whether lipogenic enzyme expression was altered by CGI-58 or ATGL deficiency. Interestingly, the expression of enzymes involved in de novo lipogenesis including acetyl-CoA carboxylase (ACC1), fatty acid synthase (FAS), ATP-citrate lyase (ATP-CL), and acetyl-CoA synthetase (AceCS1) were repressed by ATGL deficiency, yet not affected by CGI-58 ASO treatment (Figure 2B, 2C). These data suggest that blocking TAG turnover via ATGL deficiency results in a compensatory decrease in the expression of proteins involved in de novo lipogenesis through an unknown mechanism, but this feedback regulation is not apparent when CGI-58-driven TAG hydrolysis is limited.
Hepatic Expression of Key TAG Lipases are Reduced in CGI-58 and ATGL Loss of Function Mice
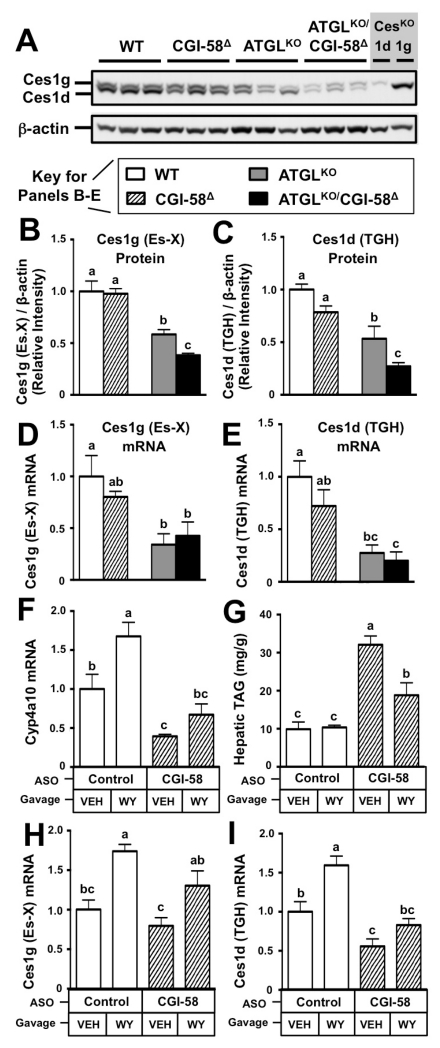
Given that we saw an additive effect of CGI-58 and ATGL deficiency on hepatic TAG accumulation (Figure 1D, 1E), we interrogated whether the expression of several other TAG lipases known to regulate hepatic TAG turnover. To our surprise the protein levels of the hepatic TAG hydrolases of the carboxylesterase family (Ces1g/Es-X and Ces1d/TGH) were markedly reduced, especially in mice lacking ATGL (Figure 3A-3C). Likewise, the mRNA levels of Ces1g/Es-X and Ces1d/TGH were significantly lower in ATGL null mice (Figure 3D, 3E) These data indicate that secondary alterations in hepatic lipase expression may contribute in part to the steatosis seen in ATGLKO and double deficient mice. Given that previous reports have demonstrated that ATGLKO mice have defective activation of the nuclear hormone receptor peroxisome proliferator activated-receptor α (PPARα) (Haemmerle et al., 2011), we interrogated whether CGI-58 likewise regulates PPARα activation and whether this underlies the altered expression of Ces1g/Es-X and Ces1d/TGH. To accomplish this we provided the exogenous PPARα agonist WY-14643 to chow-fed CGI-58 ASO-treated mice (Figure 3F-3I). In agreement with our previous work showing PPARα target gene expression is suppressed in CGI-58 ASO-treated mice (Brown et al., 2010), the canonical PPARα target gene Cyp4a10 was lower in CGI-58 ASO treated mice, yet rescued back to control levels with WY-14643 administration (Figure 3F). Importantly, CGI-58 ASO-driven hepatic TAG accumulation was significantly blunted by WY-14643 treatment (Figure 3G). Interestingly, mRNA expression of both Ces1g/Es-X and Ces1d/TGH were stimulated by WY-14643 in control ASO-treated mice (Figure 3H, 3I), indicating PPARα responsiveness. Furthermore, Ces1d/TGH was significantly reduced in chow-fed CGI-58 ASO treated mice, but rescued back to control levels with WY-14643 treatment (Figure 3I). Collectively, these data suggest that PPARα activation is blunted with both ATGL (Haemmerle et al., 2011) and CGI-58 (Brown et al., 2010) deficiency, which has the potential to impact fatty acid oxidation (Haemmerle et al., 2011; Brown et al., 2007) and the expression of TAG lipases (Figure 3H, 3I).
Figure 3. Hepatic Expression of Key TAG Lipases are Reduced in CGI-58 and ATGL Loss of Function Mice, Which Can Be Reversed by Provision of an Exogenous PPARα Agonist.
For panels A-E male wild-type or ATGLKO mice were treated with either a non-targeting control ASO (WT, ATGLKO) or an ASO targeting CGI-58 (CGI-58Δ, ATGLKO/CGI-58Δ) in conjunction with high fat diet feeding for 6 weeks.
(A) Carboxylesterase (Ces1g/Es-X and Ces1d/TGH) protein expression in whole liver was determined by Western blotting, with Ces1g or Ces1d knockout mouse liver used as genetic controls.
(B,C) Densitometric analysis of hepatic carboxylesterase (Ces1g/Es-X and Ces1d/TGH) protein expression.
(D) qPCR quantification of hepatic Ces1g/Es-X.
(E) qPCR quantification of hepatic Ces1d/TGH.
For panels F-Imale C57BL/6N mice were maintained on a chow diet in conjunction with biweekly injections (25 mg/kg) of either Control ASO or CGI-58 ASO for 3 weeks. During the third week of ASO treatment, the mice were orally gavaged daily with either vehicle (VEH) or 50 mg/kg BW of the exogenous PPARα agonist WY-14643 (WY).
(F) qPCR quantification of the PPARα target gene Cyp4a10 in mouse liver.
(G) Hepatic triacylglycerol (TAG) levels.
(H) qPCR quantification of hepatic Ces1g/Es-X.
(I) qPCR quantification of hepatic Ces1d/TGH
Data represent the mean+S.E.M. of 3-5 mice per group; and values not sharing a common superscript differ significantly (p<0.05).
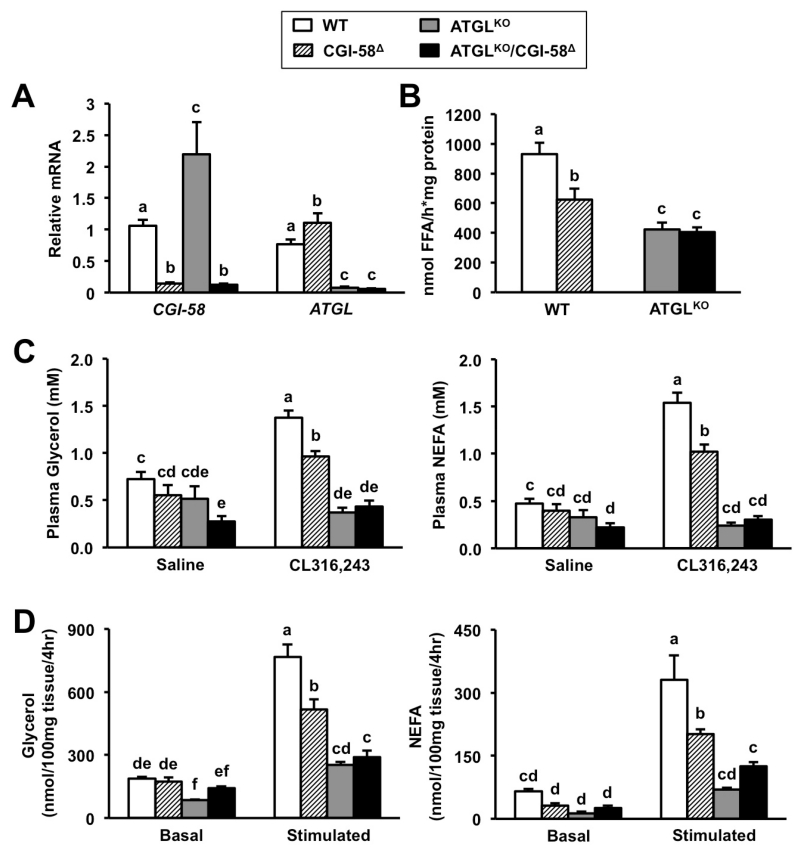
CGI-58 Knockdown in White Adipose Tissue Limits ATGL-mediated Lipolysis
Much like the liver (Figure 1A), we achieved expected reductions of CGI-58 and ATGL also in WAT. Compared to normal activity in WT mice, TAG hydrolase activity was significantly decreased by 33% in CGI-58Δ mice and 55% in ATGLKO mice (Figure 4B). However, double deficiency in ATGLKO/CGI-58Δ mice did not further decrease TAG hydrolase activity compared to ATGLKO mice (Figure 4B). To test whether CGI-58 knockdown would limit stimulated lipolysis in vivo, we measured plasma glycerol and non-esterified fatty acids (NEFA) in mice treated with either saline or a β3-adrenergic receptor agonist (CL-316,243). Stimulated lipolysis was significantly blunted in CGI-58Δ mice compared to WT mice but was not further decreased in ATGLKO/CGI-58Δ mice compared to ATGLKO mice (Figure 4C). Likewise, stimulated lipolysis in CGI-58Δ adipose explants was significantly lower compared to WT explants, but lipolysis in ATGLKO/CGI-58Δ explants did not differ from ATGLKO explants (Figure 4D). These data support the model the maximal stimulation of ATGL-mediated lipolysis in WAT requires CGI-58 expression, and CGI-58 is a specific co-activator of ATGL in WAT under physiological conditions.
Figure 4. CGI-58 Knockdown Limits ATGL-Mediated Lipolysis in White Adipose Tissue.
At 4-6 weeks of age, male wild-type or ATGLKO mice were treated with either a non-targeting control ASO (WT, ATGLKO) or an ASO targeting CGI-58 (CGI-58Δ, ATGLKO/CGI-58Δ) in conjunction with high fat diet feeding for 6 weeks.
(A) Relative mRNA expression of CGI-58 and ATGL in whole epididymal WAT.
(B) Total TAG hydrolase activity in cytosolic extracts of whole epididymal WAT.
(C) Fed mice were treated with either saline vehicle or CL316243 agonist (0.1 μg/g body weight) to stimulate lipolysis, and plasma glycerol and NEFA were measured after 15 minutes.
(D) Surgically-dissected epididymal WAT explants were incubated in basal media (Basal) or media supplemented with 100 μM isoproterenol (Stimulated) for 4 hours.
Data represent the mean+S.E.M. from four to seven mice per group, and values not sharing a common superscript differ significantly (p<0.05).
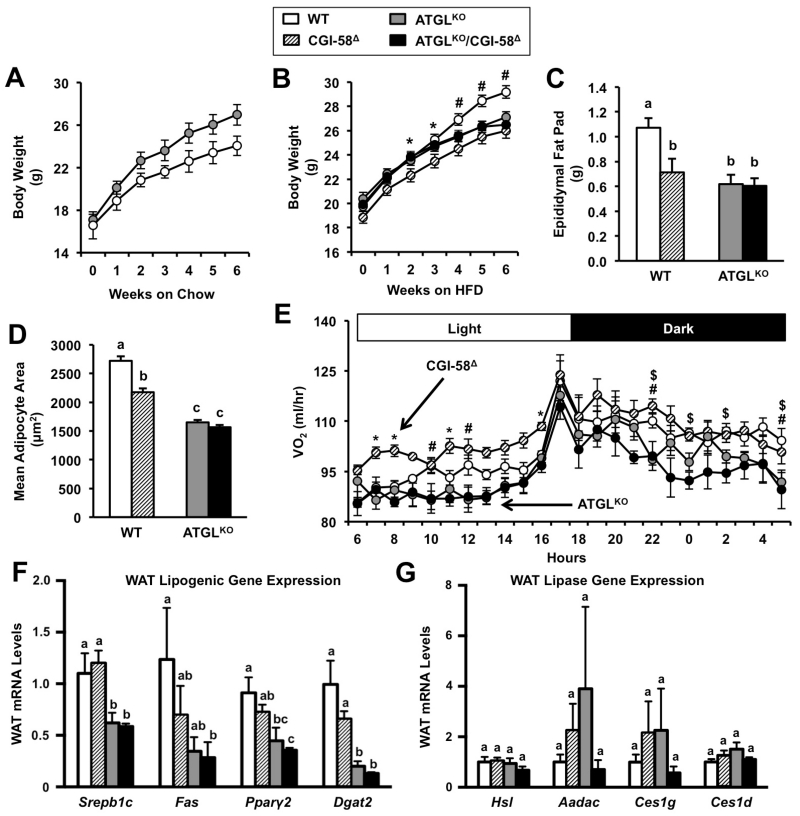
CGI-58 or ATGL Deficiency Attenuates High Fat Diet-induced Obesity Through Distinct Mechanisms
Given the blunted lipolysis in mice lacking CGI-58 and ATGL, we examined adiposity and energy balance in these mice in response to high fat diet (HFD, 45%).In agreement with previous studies (Haemmerle et al., 2006; Kienesberger et al., 2009), we observed mildly elevated body weight in ATGLKO mice maintained on chow diet (Figure 5A). However, global ATGL deficiency unexpectedly reduced body weight in HFD-fed mice (Figure 5B). As previously described (Brown et al., 2010; Cantley et al., 2013), CGI-58 knockdown also reduced body weight in HFD-fed mice (Figure 5B). In addition, CGI-58 and ATGL deficiency reduced epididymal fat pad weight (Figure 5C), and adipocyte size in HFD-fed mice (Figure 5D). We recently showed that this paradoxical lean phenotype in high fat-fed ATGLKO mice is driven in large part to compensatory down-regulation of de novo lipogenesis in WAT (Schreiber et al., 2015). In contrast to significantly reduced expression in high fat-fed ATGLKO mice, CGI-58 knockdown did not alter WAT lipogenic gene expression (Figure 5F). In contrast to the liver (Figure 3A-3E), ATGL or CGI-58 deficiency did not result in alterations of lipase gene expression (Figure 5G). Rather than reducing WAT lipogenesis, CGI-58 knockdown appeared to attenuate diet-induced obesity by increasing energy expenditure (Figure 5E). In contrast, energy expenditure was reduced in high fat-fed ATGLKO mice (Figure 5E), as previously reported (Hoy et al., 2011). Interestingly, CGI-58 knockdown did not increase energy expenditure in ATGLKO mice (Figure 5E). Collectively, these data suggest that CGI-58 and ATGL regulate high fat diet-induced obesity and energy balance via distinct mechanisms.
Figure 5. Single CGI-58 or ATGL Deficiency Prevents High Fat Diet-Induced Obesity Via Distinct Mechanisms.
At 4-6 weeks of age, male wild-type or ATGLKO mice were treated with either a non-targeting control ASO (WT, ATGLKO) or an ASO targeting CGI-58 (CGI-58Δ, ATGLKO/CGI-58Δ) in conjunction with either chow or high fat diet feeding for 6 weeks.
(A) Body weight of WT and ATGLKO mice fed chow diet and treated with control ASO, n=5 mice per group.
(B) Body weight of mice fed high fat diet, n=20-25 mice per group.
(C) Epididymal fat pad weight after 6 weeks of high fat diet, n=8-10 mice per group.
(D) Adipocyte cross-sectional area after 6 weeks of high fat diet was measured in epididymal WAT sections from 5 individual mice per group and a total of 500-550 adipocytes per group.
(E) Following 2-3 weeks of treatment and high fat diet, oxygen consumption (VO2) was measured over a 24 hour period, n=4-5 mice per group; *= p<0.05 WT vs. CGI-58Δ, #= p<0.05 WT vs. ATGLKO, $= p<0.05 WT vs. ATGLKO/CGI-58Δ.
(F) Lipogenic gene expression in epididylmal WAT was measured by qPCR, n=5 per group.
(G) Lipase gene expression in epididymal WAT was measured by qPCR, n=5 per group.
Abbreviations: Aadac, arylacetamide deacetylase; Ces1g, carboxylesterase 1g (also known as Es-X); Ces1d, carboxylesterase 1d (also known as TGH); Dgat2, diacylglycerol acyltransferase 2; Fas, fatty acid synthase; Hsl, hormone-sensitive lipase; Pparγ2, peroxisome proliferator-activated receptor γ; SREBP1c, sterol regulatory element-binding protein 1c. Data represent the mean+S.E.M., and values not sharing a common superscript differ significantly (p<0.05).
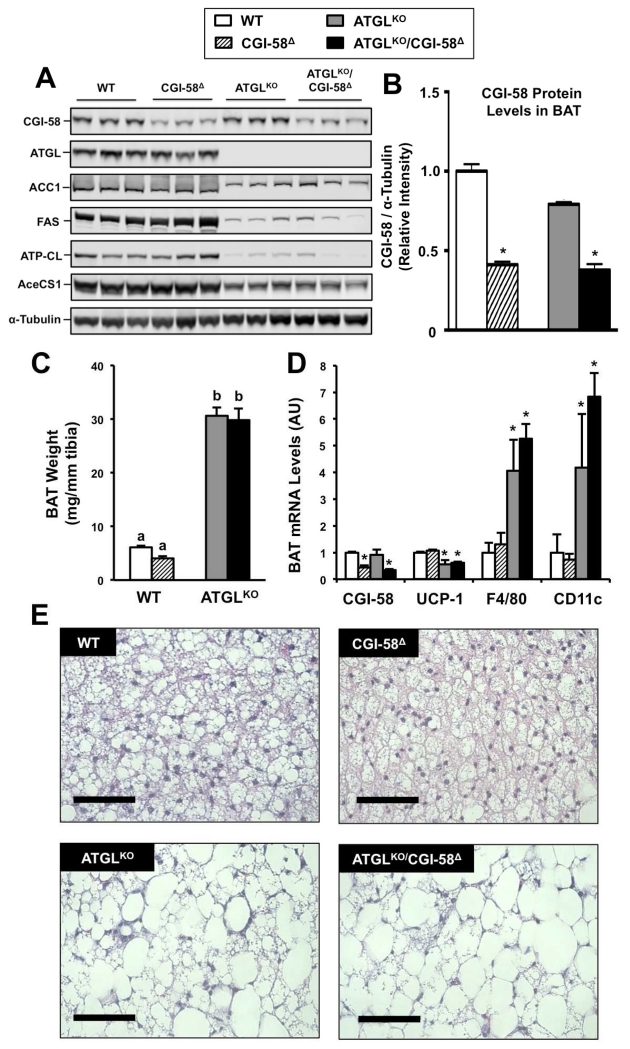
ATGL Deficiency Promotes Brown Adipose Tissue (BAT) Lipid Storage, but CGI-58 ASO-Treatment Does Not
Given the differential phenotypes seen in energy expenditure, we also examined alterations in BAT function in single and double deficient mice. One potential limitation to note is that CGI-58 was only partially suppressed by ASO treatment in BAT, where CGI-58 ASO treatment reduced CGI-58 protein expression by ~60% in both WT and ATGLKO mice (Figure 6A, 6B). In agreement with findings in liver (Figure 2B, 2C) and WAT (Figure 5F), ATGLKO mice had marked reductions in the expression of enzymes involved in de novo lipogenesis, yet CGI-58 ASO treatment did not result in similar repression of lipogenesis (Figure 6A). As has been previously shown (Haemmerle et al., 2006; Haemmerle et al., 2011; Schreiber et al., 2015; Ahmadian et al., 2011), ATGL deficiency significantly increases sub-scapular BAT weight (Figure 6C), which is accompanied by marked brown adipocyte hypertrophy and enhanced lipid droplet size (Figure 6E). In contrast CGI-58 knockdown had no effect on BAT weight or brown adipocyte hypertrophy, either in the presence or absence of ATGL (Figure 6C, 6E). Interestingly, ATGLKO mice also had significantly increased expression of macrophage markers (F4/80 and CD11c), but this was not seen with CGI-58 ASO treatment (Figure 6D). These data suggest quite different roles for ATGL and CGI-58 in BAT inflammation and lipid metabolism.
Figure 6. ATGL Deficiency, but not CGI-58 Knockdown, Promotes Brown Adipocyte Hypertrophy and Suppreses Lipogenic Protein Expression.
At 4-6 weeks of age, male wild-type or ATGLKO mice were treated with either a non-targeting control ASO (WT, ATGLKO) or an ASO targeting CGI-58 (CGI-58Δ, ATGLKO/CGI-58Δ) in conjunction with high fat diet feeding for 6 weeks.
(A) Western blot analysis of brown adipose tissue (BAT).
(B) Densitometric quantification of CGI-58 protein expression.
(C) Interscapular fat pad weight normalized to tibia length.
(D) Relative gene expression in whole interscapular BAT.
(E) Representative H&E-stained BAT sections; bar=75μm.
Data represent mean+S.E.M. of n=5-9 mice per group, and values not sharing a common superscript letter differ significantly (p<0.05). *= p<0.05 vs. WT.
Abbreviations: ACC1, acetyl-CoA carboxylase 1; AceCS1, Acetyl-CoA synthetase 1; ATGL, adipose triglyceride lipase; ATP-CL, ATP-citrate lyase; FAS, fatty acid synthase.
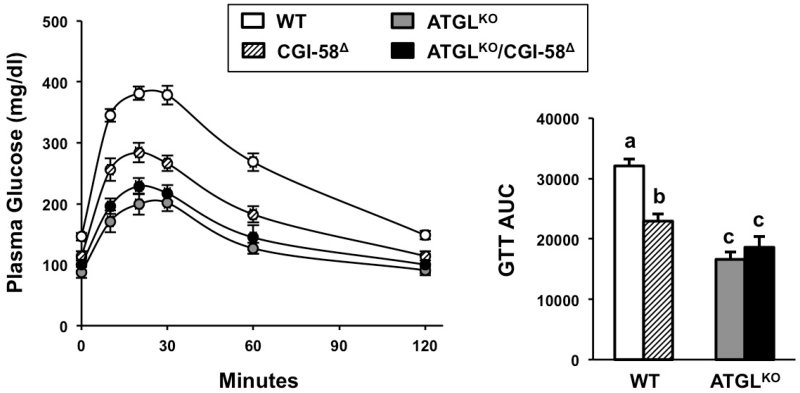
Single and Double CGI-58 or ATGL Loss of Function Results in Marked Improvements in Glucose Tolerance
It has previously been reported that CGI-58 ASO treatment (Brown et al., 2010; Lord et al., 2012b; Cantley et al., 2013) and global deficiency of ATGL (Haemmerle et al., 2006) paradoxically results in improvements in glucose tolerance, despite both models exhibiting marked hepatic steatosis. In agreement, we show that CGI-58 or ATGL loss of function alone results in marked improvements in systemic glucose tolerance, (Figure 7). Despite striking hepatic steatosis in ATGLKO/CGI-58Δ mice, glucose clearance was similar to ATGLKO mice (Figure 7). These data confirm previous reports (Haemmerle et al., 2006; Kienesberger et al., 2009; Brown et al., 2010; Lord et al., 2012b; Cantley et al., 2013) that hepatic TAG accumulation can be dissociated from diminished glucose tolerance. However, CGI-58 and ATGL likely regulate glucose tolerance through distinct mechanisms (Cantley et al., 2013; Kienesberger et al., 2009), as discussed in detail below.
Figure 7. Either CGI-58 or ATGL Loss of Function Improves Glucose Tolerance.
At 4-6 weeks of age, male wild-type or ATGLKO mice were treated with either a non-targeting control ASO (WT, ATGLKO) or an ASO targeting CGI-58 (CGI-58Δ, ATGLKO/CGI-58Δ) in conjunction with either chow or high fat diet feeding for 4-5 weeks.
(A) Glucose tolerance test curves.
(B) Area under the curve (AUC) analysis for glucose tolerance test (GTT).
Data represent the mean+S.E.M. n=5-9 per group, and values not sharing a common superscript differ significantly (p<0.05).
DISCUSSION
Although it has become generally accepted that CGI-58 regulates TAG hydrolysis by co-activating ATGL, results from this study suggest that CGI-58 can regulate TAG metabolism and hepatic inflammation via mechanisms that do not involve ATGL. The major findings of theses studies are that: 1) CGI-58 regulates hepatic TAG storage through an ATGL-independent mechanism, 2) ATGL and CGI-58 reciprocally regulate hepatic DAG levels, 3) ATGL deficiency, but not CGI-58 loss of function, results in suppression of lipogenic enzyme expression in liver, WAT, and BAT, 4) The hepatic expression of key TAG lipases (Ces1g/Es-X and Ces1d/TGH) are reduced in CGI-58 and ATGL loss of function mouse models, 5) Provision of an exogenous PPARα agonist can reverse CGI-58 ASO-driven hepatic steatosis and altered lipase expression 6) CGI-58 knockdown in WAT limits ATGL-mediated lipolysis, supporting a co-activation model, 7) ATGL deficiency promotes (BAT) lipid storage, but CGI-58 ASO treatment does not, 8) Single CGI-58 or ATGL deficiency similarly protects against high fat diet-induced obesity via increased energy expenditure or reduced de novo lipogenesis, respectively, and 9) Single and double CGI-58 or ATGL loss of function results in marked improvements in glucose tolerance, despite severe hepatic lipotoxicity. Collectively, this work supports distinct roles for ATGL and CGI-58 in hepatic and adipose tissue lipid metabolism, insulin action, and inflammation. In NLSD patients, fatty liver disease and hepatomegaly occur more frequently with CGI-58 mutations than with ATGL mutations (Lord et al., 2012a), suggesting that CGI-58 has an ATGL-independent function in the liver. However, single CGI-58 or ATGL deficiency in mice appears to increase hepatic TAG levels to a similar extent (Figure 1E), and no previous studies have directly tested whether CGI-58 is a specific co-activator of ATGL in the liver. We found that maximal ATGL hydrolase activity in the liver required co-activation by endogenous CGI-58 (Figure 1C). Likewise, CGI-58 knockdown did not further decrease hydrolase activity in ATGL deficient livers (Figure 1C), suggesting that CGI-58 does not co-activate other hepatic TAG hydrolases. Since hepatic TAG levels did not correlate with hydrolase activity in CGI-58 knockdown mice, a distinct pathway must account for TAG accumulation.
Although these results clearly demonstrate the existence of a function of CGI-58 distinct from the co-activation of ATGL, the ATGL-independent mechanism by which CGI-58 regulates TAG metabolism and inflammation remains unknown. CGI-58 has previously been reported to possess lysophosphatidic acid acyltransferase (LPAAT) activity (Ghosh et al., 2008; Montero-Moran et al., 2010), suggesting a potential role in lipid synthesis or signaling. However, subsequent investigation has revealed that the LPAAT activity originally associated with recombinant CGI-58 (Ghosh et al., 2008; Montero-Moran et al., 2010) was due to a bacterial contaminant acquired during the affinity purification process (McMahon et al., 2014). This was initially suggested by the fact that mutations of the predicted acyltransferase active site of CGI-58 did not reduce LPAAT activity (McMahon et al., 2014). More importantly, affinity purification of recombinant CGI-58 from a bacterial strain that lack the sole LPAAT found in the Escherichia coli genome (plsC) yielded no detectable LPAAT activity (McMahon et al., 2014). It is important to note that other proteins have been mistakenly identified as LPAAT enzymes due to similar problems with affinity co-purification of bacterial LPAATs (Gallop et al., 2005). It is now clear that the ATGL-independent function of CGI-58 does not stem from intrinsic acyltransferase activity, but instead involves a separate activity of CGI-58 that requires further investigation. In the continued search for an ATGL-independent mechanism for CGI-58 it is important to consider early studies in human skin fibroblasts with mutations in CGI-58 (Igal and Coleman 1996; Igal and Coleman 1998). These studies identified a primary defect in the recycling of TAG-derived acylglycerols into phospholipids, rather than defective TAG hydrolysis per se (Igal and Coleman 1996; Igal and Coleman 1998). Although lipase activity was not defective in NLSD skin fibroblasts (Igal and Coleman 1996), it is important to point out that multiple CGI-58 mutations cause NLSD (Lord et al., 2012a). Some CGI-58 mutations could exclusively affect either ATGL co-activation or ATGL-independent functions. In contrast, CGI-58 knockdown in mice abolishes all functions of CGI-58, and we have demonstrated that CGI-58 knockdown does indeed decrease ATGL activity in liver (Figure 1C).
One unexpected finding that may shed light into how CGI-58 regulates TAG metabolism in the liver is that CGI-58 knockdown results in reorganization of hepatic lipase expression (Figure 3). Our studies show that the hepatic expression of several key TAG lipases (Ces1g/Es-X, Ces1d/TGH) were reduced in CGI-58 and ATGL loss of function mice (Figure 3). Our data also support a model whereby, the reduced expression of Ces1g/Es-X and Ces1d/TGH likely involves reduced PPARa activity in the liver of CGI-58 ASO-treated mice (Figure 3H, 3I). Provocatively, these findings indicate that secondary changes in other TAG lipases may in part contribute to the hepatic steatosis seen in CGI-58 and ATGL loss of function mice. Although both Ces1d and Ces1g have TAG hydrolase activity, mice lacking these TAG hydrolases have very different metabolic phenotypes (Quiroga et al., 2012; Wei et al., 2010; Lian et al., 2012). Ces1d knockout mice present with decreased lipogenesis, increased fatty acid oxidation and resistance to HFD-induced hepatic steatosis (Wei et al., 2010; Lian et al., 2012). In contrast, Ces1g knockout mice fed chow diet present with increased lipogenesis and insulin resistance (Quiroga et al., 2012). Collectively, our studies demonstrate that complex alterations in both lipase (Figure 3) and lipogenic (Figures 2B, 2C,, 5F, 6A) gene expression may in part contribute to altered TAG accumulation in CGI-58 and ATGL loss of function mice.
Given the product of TAG hydrolysis is diacylglycerol (DAG), it is important to consider the shared and divergent effects of ATGL and CGI-58 on hepatic DAG metabolism. First, CGI-58 knockdown in mice leads to increased hepatic DAG levels (Brown et al., 2010; Cantley et al., 2013) and prevents the inflammatory cytokine-driven generation of several signaling lipids that can be derived from DAG (Lord et al., 2012b). Two recent reports also support the possibility that CGI-58 could play a direct role in the regulation of DAG localization or utilization. First, CGI-58 knockdown causes hepatic DAG accumulation in lipid droplets/endoplasmic reticulum, while preventing accumulation of DAG at the plasma membrane (Cantley et al., 2013). Second, CGI-58 co-activation broadens the selectivity of ATGL for the sn-2 position of TAG to include the sn-1 position, resulting in the generation of both sn-1,3 and sn-2,3 DAG (Eichmann et al., 2012). Interestingly, here we show that ATGL deficiency decreases hepatic DAG levels compared to control mice, whereas CGI-58 knockdown increases hepatic DAG both in the presence or absence of ATGL (Figure 1F). Although the mechanism by which CGI-58 knockdown causes DAG accumulation within the lipid droplet/endoplasmic reticulum compartment is unknown, the reciprocal reduction in hepatic DAG by ATGL deficiency likely directly stems from defective TAG to DAG conversion in hepatocytes. In support of this concept, adipocyte-specific deletion of ATGL likewise reduces total adipose DAG levels (Ahmadian et al., 2011). Given that ATGL and CGI-58 reciprocally regulate hepatic DAG levels, and the fact that CGI-58 knockdown increases hepatic DAG in both the presence and absence of ATGL (Figure 1F), it is tempting to speculate that CGI-58 plays an ATGL-independent role in DAG shuttling or metabolism.
Undoubtedly, there is strong evidence that CGI-58 co-activates ATGL in certain tissues to promote TAG hydrolysis (Lass et al., 2006; Granneman et al., 2007; Granneman et al., 2009; Schweiger et al., 2008; Yang et al., 2013; Cornaciu, et al., 2011; Wang et al., 2013; MacPherson et al., 2013; Zierler et al., 2013). However, results from this study indicate that CGI-58 can suppress hepatic inflammatory responses in vivo via a mechanism not involving ATGL. In fact, there is a clear distinction between ATGL and CGI-58 in their ability to impact systemic inflammatory responses and the progression of simple fatty liver to steatohepatitis or fibrosis. In agreement with the data presented here, hepatocyte-specific CGI-58 deficiency promotes marked fatty liver which progresses into steatohepatitis and fibrosis with age (Guo et al., 2013). In contrast, hepatocyte-specific ATGL deficiency results in a mild fatty liver phenotype, which does not progress to steatohepatitis or fibrosis, even after one year (Wu et al., 2011). When challenged acutely with lipopolysaccharide (LPS), mice lacking ATGL or CGI-58 in the liver respond quite differently (Lord et al., 2012b; Jha et al., 2014). While both ATGL and CGI-58 loss of function models have increased levels of circulating TH1 cytokines when challenged with LPS, the tissue source of these cytokines seems to be quite different (Lord et al., 2012b; Jha et al., 2014). In this case, ATGL deficient mice have elevated LPS-induced cytokine gene expression in the liver (Jha et al., 2014), whereas CGI-58 ASO-treated mice have blunted LPS-induced cytokine gene expression in the liver (Lord et al., 2012b). In the case of CGI-58 ASO-treated mice, it seems that the major source of elevated circulating TH1 cytokines is from white adipose tissue (WAT) (Lord et al., 2012b). A major contributing factor to differences in acute inflammatory responses between ATGL and CGI-58 is the divergent role these proteins play in macrophage function (Miao et al., 2014; Chandek et al., 2010; Aflaki et al., 2011a; Aflaki et al., 2011b). Macrophage-selective deficiency of ATGL skews macrophages towards the M2-like phenotype without altering inflammasome activation (Chandek et al., 2010; Aflaki et al., 2011a; Aflaki et al., 2011b). In contrast, macrophage-specific deletion of CGI-58 causes macrophages to acquire an M1-like phenotype, and results in reactive oxygen species-driven activation of the NLRP3 inflammasome (Miao et al., 2014). Therefore, much like data shown here in mouse liver, the roles of CGI-58 and ATGL in macrophages are likely independent from one another.
Even though ATGL or CGI-58 loss of function causes ectopic TAG accumulation in multiple tissues, it has historically been paradoxical why this is not associated with systemic insulin resistance (Haemmerle et al., 2006; Brown et al., 2010; Lord et al., 2012b; Cantley et al., 2013). In agreement with previous studies (Haemmerle et al., 2006; Brown et al., 2010; Lord et al., 2012b; Cantley et al., 2013), we show here that CGI-58 ASO treatment alone improves glucose tolerance to a similar degree seen in ATGL global knockout mice (Figure 7). Although both of these mouse models of defective TAG hydrolysis exhibit a similar improvement in glucose tolerance, the mechanisms underlying these improvements likely stem from different sources. In the case of CGI-58, it has previously been shown that despite large accumulation of hepatic DAG in CGI-58 ASO-treated mice, they are protected from DAG-induced insulin resistance due to sequestration of hepatic DAGs in the lipid droplet/endoplasmic reticulum (Cantley et al., 2013). This sequestration of DAGs within hepatocytes results in prevention of high fat diet-induced accumulation of DAGs at the plasma membrane where they normally act to negatively regulate insulin receptor-driven signaling events (Perry et al., 2014). Collectively, the improvements in glucose tolerance seen in CGI-58 ASO treated mice stem almost exclusively from improvements in hepatic insulin action via a mechanism involving DAG sequestration (Cantley et al., 2013). In contrast, the improvements in glucose tolerance in global ATGL knockout mice have been linked to improvements in skeletal muscle insulin action and improved insulin-stimulated glucose uptake (Kienesberger et al., 2009; Sitnick et al., 2013). Therefore, the striking improvements in glucose tolerance seen with either ATGL or CGI-58 deficiency derive from skeletal muscle-specific or liver-specific improvements in insulin signaling, respectively (Brown et al., 2010; Lord et al., 2012b; Cantley et al., 2013; Kienesberger et al., 2009; Sitnick et al., 2013).
In conclusion, these studies clearly demonstrate the existence of biochemical functions for CGI-58 that does not rely on ATGL co-activation in mouse liver. While CGI-58 can indeed directly interact to co-activate ATGL-mediated TAG hydrolysis (Lass et al., 2006; Granneman et al., 2007; Granneman et al., 2009; Schweiger et al., 2008; Yang et al., 2013; Cornaciu, et al., 2011; Wang et al., 2013; MacPherson et al., 2013; Zierler et al., 2013), this mechanism is likely only relevant in certain cellular contexts. The ATGL co-activation mechanism undoubtedly occurs in adipocytes (Lass et al., 2006; Granneman et al., 2007; Granneman et al., 2009; Schweiger et al., 2008; Yang et al., 2013; Cornaciu, et al., 2011; Wang et al., 2013; MacPherson et al., 2013; Zierler et al., 2013), but the elusive ATGL-independent function of CGI-58 predominates in many other cell types to regulate both TAG metabolism and inflammation. Moving forward, it is critical to make the distinction that molecular mechanisms regulating catecholamine-stimulated lipolysis in adipocytes may be quite different than mechanisms driving lipolysis in non-adipocytes. Future studies to understand the ATGL-independent function(s) of CGI-58 in the liver could lead to future therapies for NLSD, non-alcoholic fatty liver disease, and more advanced forms of end stage liver disease.
EXPERIMENTAL PROCEDURES
Animal Studies
Global ATGLKO mice were previously generated on a mixed background (Haemmerle et al., 2006) and subsequently backcrossed onto a C57BL/6N background more than ten generations (Kienesberger et al., 2009). Breeding was performed as previously described (Kienesberger et al., 2009). At 4-6 weeks of age, male WT and ATGLKO mice were subjected to high fat diet feeding (45% energy as fat from lard) and biweekly intraperitoneal injections (25 mg/kg) of either a non-targeting second generation control ASO or a second generation ASO specifically targeting CGI-58 as previously described (Brown et al., 2010). For studies examining PPARα signaling, 6 week old male C57BL6 mice were injected with control or CGI-58 ASOs and maintained on a standard chow diet for a period of 3 weeks to achieve adequate knockdown. During the last week of ASO injection (week 3), mice were orally gavaged with either vehicle (1.0% carboxymethylcellulose [CMC] and 0.1% Tween 80) or WY-14643 (50 mg/kg/day) once daily for 7 days. All mice were strictly necropsied between 9:00-10:00 a.m. Biochemical assays for glucose, non-esterified fatty acids, and glycerol have been previously described (Brown et al., 2010; Lord et al., 2012b; Cantley et al., 2013). All experimental procedures were approved by the Institutional Animal Care and Use Committee at Wake Forest University School of Medicine or the Cleveland Clinic.
TAG Hydrolase Assays
Total TAG hydrolase activity was measured as previously described (Schweiger et al., 2004) in frozen liver from mice fasted for 4 hours.
Liver Lipid Analyses
Liver TAG and diacylglycerol (DAG) level were extracted and measured as previously described (Brown et al., 2010; Lord et al., 2012b; Cantley et al., 2013).
Histological Analysis
Hematoxylin and eosin staining of paraffin-embedded liver sections was performed as previously described (Brown et al., 2010).
RNA and Protein Methods
Tissue RNA extraction and real time PCR were conducted as previously described (Brown et al., 2010; Lord et al., 2012b) using the Applied Biosystems 7500 Real-Time PCR System. Primer information is available upon request. For Western blotting, whole-tissue homogenates were made from multiple tissues in a modified radioimmunoprecipitation assay buffer, as previously described (Brown et al., 2010; Lord et al., 2012b). Proteins were separated by 4–12% SDS-PAGE and transferred to polyvinylidene difluoride membranes, and proteins were detected after incubation with specific antibodies, for which detailed information is available upon request.
Glucose Tolerance Testing
Glucose tolerance was measured as previously described (Brown et al., 2010; Lord et al., 2012b; Cantley et al., 2013) in male mice following 4-7 weeks of HFD and ASO treatment.
Indirect Calorimetry and Metabolic Cage Measurements
Weight matched mice were acclimated to metabolic cages for 48 hours, and on day 2 were injected with ASO. Thereafter, physical activity, oxygen consumption (VO2), carbon dioxide production (VCO2), and respiratory exchange ratio (RER) were continually monitored for an additional 48 hr using the Oxymax CLAMS system (Columbus Instruments) at a temperature of 22°C. Data represent the last 6 a.m. to 6 a.m. period after adequate acclimation.
Statistical Analysis
Data are expressed as the mean ± standard error of the mean (SEM). All data were analyzed using two-way analysis of variance followed by Student’s t tests for post hoc analysis. Differences were considered significant at p <0.05. All analyses were performed using JMP version 5.0.12 (SAS Institute; Cary, NC) software.
HIGHLIGHTS.
CGI-58 is thought to regulate triacylglycerol hydrolysis by co-activating ATGL
CGI-58 regulates triacylglycerol turnover both in the presence and absence of ATGL
CGI-58 and ATGL regulate obesity and insulin sensitivity via distinct mechanisms
Mechanisms of triacylglycerol turnover differ between hepatocytes and adipocytes
ACKNOWLEDGEMENTS
The authors thank Dr. Erin Kershaw (University of Pittsburg) for providing the ATGLKO breeder mice. Furthermore, we thank Dr. Jennifer Cantley and Gerald I. Shulman (Howard Hughes Medical Institute; Yale University School of Medicine) for measuring hepatic diacylglycerol, which was supported by NIH grant U24-DK-59635. The remainder of this work was supported by grants from the National Institutes of Health (NIH-K99/R00-HL096166 to J.M.B.; NIH-R01-HL122283 to J.M.B.; NIH-P50-AA024333 to J.M.B.) and the American Heart Association (Predoctoral Fellowship 12PRE11910081 to C.C.L.; Beginning Grant in Aid 7840072 to J.M.B.; Grant in Aid 13GRNT17050074 to J.M.B).
ABBREVIATIONS USED
- ABHD5
alpha beta hydrolase domain 5
- ACC1
acetyl-CoA carboxylase 1
- AceCS1
acetyl-CoA synthase 1
- ASO
antisense oligonucleotide
- α-SMA
alpha smooth muscle actin
- ATP-CL
ATP citrate lyase
- ATGL
adipose triglyceride lipase
- CGI-58
comparative gene identification 58
- DAG
diacylglycerol
- FAS
fatty acid synthase
- LPAAT
lysophosphatidic acid acyltransferase
- LPS
lipopolysaccharide
- NASH
non-alcoholic steatohepatitis
- NLSD
neutral lipid storage disease
- PNPLA2
patatin-like phospholipase domain containing 2
- TAG
triacylglycerol
- TGF-β
transforming growth factor beta
- TNF-α
tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
All authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
C.C.L. and J.M.B. planned the project, designed experiments, analyzed data, and wrote the manuscript; J.M.B., R.Z. and G.H. designed experiments and provided useful discussion directing the project; C.C.L., G.T., D.F., S.M., A.L.B., R.C.S., A.D.G., J.B., C.N., J.S., J.S.H., M.S., J.L.C., J.D.L., R.W, R.L., and T.F.S. conducted mouse experiments, performed biochemical workup of mouse tissues, analyzed data, and aided in manuscript preparation; R.G.L., R.M.C., and M.J.G. provided antisense oligonucleotides; All authors were involved in the editing of the final manuscript.
REFERENCES
- Aflaki E, Radovic B, Chandak PG, Kolb D, Eisenberg T, Ring J, Fertschai I, Uellen A, Wolinski H, Kohlwein SD, Zechner R, Levak-Frank S, Sattler W, Graier WF, Malli R, Madeo F, Kratky D. Triacylglycerol accumulation activates the mitochondrial apoptosis pathway in macrophages. J. Biol. Chem. 2011a;286:7418–7428. doi: 10.1074/jbc.M110.175703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aflaki E, Balenga NA, Luschnig-Schratl P, Wolinski H, Povoden S, Chandak PG, Bogner-Strauss JG, Eder S, Konya V, Kohlwein SD, Heinemann A, Kratky D. Impaired Rho GTPase activation abrogates cell polarization and migration in macrophages with defective lipolysis. Cell Mol. Life Sci. 2011b;68:3933–3947. doi: 10.1007/s00018-011-0688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, Sul HS. Desnutrin/ATGL is regulated by AMPK and is required for brown adipose phenotype. Cell Metab. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Chung S, Das A, Shelness GS, Rudel LL, Yu L. CGI-58 facilitates the mobilization of cytoplasmic triglyceride for lipoprotein secretion in hepatoma cells. J. Lipid Res. 2007;48:2295–2305. doi: 10.1194/jlr.M700279-JLR200. [DOI] [PubMed] [Google Scholar]
- Brown JM, Betters JL, Lord C, Ma Y, Han X, Yang K, Alger HM, Melchior J, Sawyer J, Shah R, Wilson MD, Liu X, Graham MJ, Lee R, Crooke R, Shulman GI, Xue B, Shi H, Yu L. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J. Lipid Res. 2010;51:3306–3315. doi: 10.1194/jlr.M010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley JL, Yoshimura T, Camporez JP, Zhang D, Jornayvaz FR, Kumashiro N, Guebre-Egziabher F, Jurczak MJ, Kahn M, Guigni BA, Serr J, Hankin J, Murphy RC, Cline GW, Bhanot S, Manchem VP, Brown JM, Samuel VT, Shulman GI. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc. Natl. Acad. Sci. U.S.A. 2013;110:1869–1874. doi: 10.1073/pnas.1219456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviglia JM, Betters JL, Dapito DH, Lord CC, Sullivan S, Chua S, Yin T, Sekowski A, Mu H, Shapiro L, Brown JM, Brasaemle D. Adipose-selective overexpression of ABHD5/CGI-58 does not increase lipolysis or protect against diet-induced obesity. J. Lipid Res. 2011;52:2032–2042. doi: 10.1194/jlr.M019117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandek PG, Radovic B, Aflaki E, Kolb D, Buchebner M, Frohlich E, Magnes C, Sinner F, Haemmerle G, Zechner R, Tabas I, Levak-Frank S, Kratky D. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 2010;285:20192–20201. doi: 10.1074/jbc.M110.107854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornaciu I, Boeszoermenyi A, Lindermuth H, Nagy HM, Cerk IK, Ebner C, Salzburger B, Gruber A, Schweiger M, Zechner R, Lass A, Zimmermann R, Oberer M. The minimal domain of adipose triglyceride lipase (ATGL) ranges until leucine 254 and can be activated and inhibited by CGI-58 and G0S2, respectively. PLoS One. 2011;6:e26349. doi: 10.1371/journal.pone.0026349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann TO, Kumari M, Haas JT, Farese RV, Jr., Zimmermann R, Lass A, Zechner R. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J. Biol. Chem. 2012;287:41446–41457. doi: 10.1074/jbc.M112.400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Lefevre C, Morava E, Mussini JM, Laforet P, Negre-Salvayre A, Lathrop M, Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nature Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- Gallop JL, Butler PJ, McMahon HT. Endophilin and CtBP/BARS are not acyltransferases in endocytosis or Golgi fission. Nature. 2005;438:675–678. doi: 10.1038/nature04136. [DOI] [PubMed] [Google Scholar]
- Goeritzer M, Schlager S, Radovic B, Madreiter CT, Rainer S, Thomas G, Lord CC, Sacks J, Brown AL, Vujic N, Obrowsky S, Sachdev V, Kolb D, Chandek PG, Graier WF, Sattler W, Brown JM, Kratky D. Deletion of CGI-58 or adipose triglyceride lipase differently affects macrophage function and atherosclerosis. J. Lipid Res. 2014;55:2562–2575. doi: 10.1194/jlr.M052613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J. Biol. Chem. 2007;282:5726–5735. doi: 10.1074/jbc.M610580200. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Moore HP, Krishnamoorthry R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J. Biol. Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Invest. 2011;121:2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J. Hepatology. 1995;22:28–36. [PubMed] [Google Scholar]
- Guo F, Ma Y, Kadegowda AK, Betters JL, Xie P, Liu G, Liu X, Miao H, Ou J, Su X, Zheng Z, Xue B, Shi H, Yu L. Deficiency of liver Comparative Gene Identification-58 causes steatohepatitis and fibrosis in mice. J. Lipid Res. 2013;54:2109–2120. doi: 10.1194/jlr.M035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, Schreiber R, Eichmann T, Kolb D, Kotzbeck P, Schweiger M, Kumari M, Eder S, Schoiswohl G, Wongsiriroj N, Pollak NM, Radner FP, Preiss-Landl K, Kolbe T, Rulicke T, Pieske B, Trauner M, Lass A, Zimmermann R, Hoefler G, Cinti S, Kershaw EE, Schrauwen P, Madeo F, Mayer B, Zechner R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy AJ, Bruce CR, Turpin SM, Morris AJ, Febbraio MA, Watt MJ. Adipose triglyceride lipase-null mice are resistant to high-fat diet-induced insulin resistance despite reduced energy expenditure and ectopic lipid accumulation. Endocrinology. 2011;152:48–58. doi: 10.1210/en.2010-0661. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Ramakrishnan G, Chandramohan C, Rajasekharan R. CGI-58, the causative gene for Chanarin-Dorfman syndrome, mediates acylation of lysophosphatidic acid. J. Biol. Chem. 2008;283:24525–24533. doi: 10.1074/jbc.M801783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igal RA, Coleman RA. Acylglycerol recycling from triacylglycerol to phospholipid, not lipase activity, is defective in neutral lipid storage disease fibroblasts. J. Biol. Chem. 1996;271:16644–16651. doi: 10.1074/jbc.271.28.16644. [DOI] [PubMed] [Google Scholar]
- Igal RA, Coleman RA. Neutral lipid storage disease: a genetic disorder with abnormalities in the regulation of phospholipid metabolism. J. Lipid Res. 1998;39:31–43. [PubMed] [Google Scholar]
- Jha P, Claudel T, Baghdasaryan A, Mueller M, Halibasic E, Das SK, Lass A, Zimmermann R, Zechner R, Hoefler G, Trauner M. Role of adipose triglyceride lipase (PNPLA2) in protection from hepatic inflammation in mouse models of steatohepatitis and endotoxemia. Hepatology. 2014;59:858–869. doi: 10.1002/hep.26732. [DOI] [PubMed] [Google Scholar]
- Kienesberger PC, Lee D, Pulinilkunnil T, Brenner DS, Cai L, Magnes C, Koefeler HC, Streith IE, Rechberger GN, Haemmerle G, Flier JS, Zechner R, Kim YB, Kershaw EE. Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J. Biol. Chem. 2009;284:30218–30229. doi: 10.1074/jbc.M109.047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers B, Chandak PG, Aflaki E, Van Puijvelde GH, Radovic B, Hildebrand RB, Meurs I, Out R, Kuiper J, Van Berkel TJ, Kolb D, Haemmerle G, Zechner R, Levak-Frank S, Van Eck M, Kratky D. Macrophage adipose triglyceride lipase deficiency attenuates atherosclerosis lesion development in low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 2011;31:67–73. doi: 10.1161/ATVBAHA.110.215814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Lefevre C, Jobard F, Caux F, Bouadjar B, Karaduman A, Heilig R, Lakhdar H, Wollenberg A, Verret JL, Weissenbach J, Ozguc M, Lathrup M, Prud’homme JF, Fischer J. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am. J. Hum. Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J, Wei E, Wang SP, Quiroga AD, Li L, Di Pardo A, van der Veen J, Sipione S, Mitchell GA, Lehner R. Liver specific inactivation of carboxylesterase 3/triacylglycerol hydrolase decreases blood lipids without causing severe steatosis in mice. Hepatology. 2012;56:2154–2162. doi: 10.1002/hep.25881. [DOI] [PubMed] [Google Scholar]
- Lord CC, Brown JM. Distinct roles for alpha-beta hydrolase domain 5 (ABHD5/CGI-58) and adipose triglyceride lipase (ATGL/PNPLA2) in lipid metabolism and signaling. Adipocyte. 2012a;1:123–131. doi: 10.4161/adip.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CC, Betters JL, Ivanova PT, Milne SB, Myers DS, Madenspacher J, Thomas G, Chung S, Liu M, Davis MA, Lee RG, Crooke RM, Graham MJ, Parks JS, Brasaemle DL, Fessler MB, Brown HA, Brown JM. CGI-58/ABHD5-derived signaling lipids regulate systemic inflammation and insulin action. Diabetes. 2012b;61:355–363. doi: 10.2337/db11-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson RE, Ramos SV, Vandenboom R, Roy BD, Peters SJ. Skeletal muscle PLIN proteins, ATGL, and CGI-58, interactions at rest and following stimulated contraction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304:44–50. doi: 10.1152/ajpregu.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon D, Dinh A, Kurz D, Shah D, Han GS, Carman GM, Brasaemle DL. Comparative gene identification 58/ab hydrolase domain 5 lacks lysophosphatidic acid acyltransferase activity. J. Lipid Res. 2014;55:1750–1761. doi: 10.1194/jlr.M051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Ou J, Ma Y, Guo F, Yang Z, Wiggins M, Liu C, Song W, Han X, Wang M, Cao Q, Chung BH, Yang D, Liang H, Xue B, Shi H, Gan L, Yu L. Macrophage CGI-58 deficiency activates ROS-inflammasome pathway to promote insulin resistance in mice. Cell Rep. 2014;7:223–235. doi: 10.1016/j.celrep.2014.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Moran G, Caviglia JM, McMahon D, Rothenberg A, Subramanian V, Xu Z, Lara-Gonzalez S, Storch J, Carman GM, Brasaemle DL. CGI-58/ABHD5 is a coenzyme A-dependent lysophosphatidic acid acyltransferase. J. Lipid Res. 2010;51:709–719. doi: 10.1194/jlr.M001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga AD, Li L, Trotzmuller M, Nelson R, Proctor SD, Kofeler H, Lehner R. Deficiency of carboxylesterase 1/esterase-x results in obesity, hepatic steatosis, and hyperlipidemia. Hepatology. 2012;56:2188–2198. doi: 10.1002/hep.25961. [DOI] [PubMed] [Google Scholar]
- Radner FP, Streith IE, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, Rechberger G, Koefeler HC, Eder S, Schauer S, Theussl HC, Preiss-Landl K, Lass A, Zimmermann R, Hoefler G, Zechner R, Haemmerle G. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58) J. Biol. Chem. 2010;285:7300–7311. doi: 10.1074/jbc.M109.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Hofer P, Taschler U, Voshol PJ, Rechberger GN, Kotzbeck P, Jaeger D, Preiss-Landl K, Lord CC, Brown JM, Haemmerle G, Zimmermann R, Vidal-Puig A, Zechner R. Hypophagia and metabolic adaptations in mice with defective ATGL-mediated lipolysis cause resistance to HFD-induced obesity. Proc. Natl. Acad. Sci. USA. 2015;112:13850–13855. doi: 10.1073/pnas.1516004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M, Eichmann TO, Taschler U, Zimmermann R, Zechner R, Lass A. Measurement of lipolysis. Methods Enzymol. 2004;538:171–193. doi: 10.1016/B978-0-12-800280-3.00010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- Schweiger M, Schoiswohl G, Lass A, Radner FP, Haemmerle G, Malli R, Graier W, Cornaciu I, Oberer M, Salvayre R, Fischer J, Zechner R, Zimmermann R. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J. Biol. Chem. 2008;283:17211–17220. doi: 10.1074/jbc.M710566200. [DOI] [PubMed] [Google Scholar]
- Sitnick MT, Basantani MK, Cai L, Schoiswohl G, Yazbeck CF, Distefano G, Ritov V, DeLany JP, Schreiber R, Stolz DB, Gardner NP, Kienesberger PC, Pulinilkunnil T, Zechner R, Goodpaster BH, Coen P, Kershaw EE. Skeletal muscle triacylglycerol hydrolysis does not influence metabolic complications of obesity. Diabetes. 2013;62:3350–3361. doi: 10.2337/db13-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bell M, Sreenivasan U, Hu H, Liu J, Dalen K, Londos C, Yamaguchi T, Rizzo MA, Coleman R, Gong D, Brasaemle D, Sztalryd C. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J. Biol. Chem. 2013;286:15707–15715. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei E, Ben Ali Y, Lyon J, Wang H, Nelson R, Dolinsky VW, Dyck JR, Mitchell G, Korbutt GS, Lehner R. Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab. 2010;11:183–193. doi: 10.1016/j.cmet.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang S, Alvarez F, Casavant S, Gauthier N, Abed L, Soni KG, Yang G, Mitchell G. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54:122–132. doi: 10.1002/hep.24338. [DOI] [PubMed] [Google Scholar]
- Yang X, Heckmann BL, Zhang X, Smas CM, Liu J. Distinct mechanisms regulate ATGL-mediated adipocyte lipolysis by lipid droplet coat proteins. Mol. Endocrinol. 2013;27:116–126. doi: 10.1210/me.2012-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. FAT SIGNALS - lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierler KA, Jaeger D, Pollak NM, Eder S, Rechberger GN, Radner FP, Woelkart G, Kolb D, Schmidt A, Kumari M, Preiss-Landl K, Pieski B, Mayer B, Zimmermann R, Lass A, Zechner R, Haemmerle G. Functional cardiac lipolysis in mice critically depends on comparative gene identification-58. J. Biol. Chem. 2013;288:9892–9904. doi: 10.1074/jbc.M112.420620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]