STRUCTURED ABSTRACT
Background
Peripheral arterial disease (PAD) is an atherosclerotic vascular disease that affects over 200 million worldwide. The hallmark of PAD is ischemic leg pain and this condition is also associated with an augmented blood pressure response to exercise, impaired vascular function, and high risk of myocardial infarction and cardiovascular mortality. In this study, we tested the hypothesis that coronary exercise hyperemia is impaired in PAD.
Methods
Twelve patients with PAD and no overt coronary disease (65 ± 2 yr, 7 men) and 15 healthy control subjects (64 ± 2 yr, 9 men) performed supine plantar flexion exercise (30 contractions/min, increasing workload). A subset of subjects (N = 7 PAD, N = 8 healthy) also performed isometric handgrip exercise (40% maximum voluntary contraction to fatigue). Coronary blood velocity in the left anterior descending artery was measured by transthoracic Doppler echocardiography; blood pressure and heart rate were monitored continuously.
Results
Coronary blood velocity responses to 4 minutes of plantar flexion exercise (PAD: Δ 2.4 ± 1.2, healthy: Δ 6.0 ± 1.6 cm/s, P = 0.039) and to isometric handgrip exercise (PAD: Δ 8.3 ± 4.2, healthy: Δ 16.9 ± 3.6, P = 0.033) were attenuated in PAD patients.
Conclusions
These data indicate that coronary exercise hyperemia is impaired in PAD, which may predispose these patients to myocardial ischemia.
Keywords: peripheral vascular disease, coronary flow, blood pressure, exercise
1. INTRODUCTION
Peripheral arterial disease (PAD) is an atherosclerotic vascular disease that affects over eight million people in the United States and 202 million worldwide [1, 2]. PAD is characterized by progressive narrowing of the lower extremity arterial vasculature and ischemic leg pain that occurs with walking, termed “intermittent claudication” [3]. Whether or not patients experience claudication, a diagnosis of PAD heightens overall cardiovascular risk. Patients with PAD have a five times greater incidence of cardiovascular mortality in 10 years compared to healthy individuals of similar age [4]. Myocardial infarction accounts for 60% of all deaths in PAD [3] and it is suspected that impaired coronary vasomotor control contributes to these outcomes. A recent study found that occluding the femoral arteries in swine blunts coronary vasodilation [5]. However, very few studies have evaluated coronary blood flow responses in patients with PAD [6].
The coronary blood vessels play a vital role in maintaining oxygen (O2) and substrate delivery to the myocardium. In healthy humans, physiological stress increases myocardial O2 demand, which is compensated by an increase in coronary blood flow since the myocardium cannot significantly increase O2 extraction [7]. Changes in peak coronary blood velocity (CBV) reflect changes in coronary blood flow [8, 9]. Furthermore, CBV responses to pharmacological stimuli are predictive of adverse cardiovascular events in healthy subjects and patients with coronary artery disease [10–14]. PAD patients also have attenuated coronary vasodilation to pharmacological stimuli [6], but whether coronary hyperemia is impaired in these patients during physiological stress is unknown.
Previous studies show that patients with PAD have augmented blood pressure (BP) responses to low-intensity exercise [15–18], which is prognostic of cardiovascular events and all-cause mortality [19]. While the mechanisms contributing to mortality are unknown, an augmented rise in myocardial metabolism coupled with impaired O2 delivery may lead to myocardial ischemia. Therefore, the main purpose of this study was to investigate coronary blood velocity (CBV) responses to plantar flexion exercise in patients with PAD. A subset of subjects also performed isometric handgrip exercise to assess coronary exercise hyperemia without exercising an ischemic leg. We hypothesized that PAD patients have attenuated coronary exercise hyperemia despite an augmented rise in myocardial O2 demand.
2. MATERIAL AND METHODS
2.1 Subjects and Design
These laboratory studies used an independent-subjects design whereby physiological parameters were compared between groups (PAD patients, healthy control subjects). Twelve PAD patients (65 ± 2 yr, 7 men) and 15 healthy control subjects (64 ± 2 yr, 9 men) were enrolled. The sample size was determined after the first four subjects in each group had completed testing. Specifically, we determined that if the true difference in the mean ΔCBV/Δrate pressure product (RPP = HR × SBP; an index of myocardial O2 demand) from baseline to plantar flexion exercise at 2.0 kg was 5.6 a.u. with a standard deviation of 4.0 a.u. then we would need to study 12 subjects in each group to reject the null hypothesis with 90% power and α = 0.05. In order to account for attrition and potential missing data, we enrolled 12 PAD patients and 15 healthy subjects. Subsequently, for the handgrip experiments we determined after the first three subjects that if the true difference in the mean ΔCBV/ΔRPP from baseline to peak handgrip was 5.27 a.u. with a standard deviation of 2.5 a.u. then we would need to study 6 subjects in each group to reject the null hypothesis with 90% power and α = 0.05. We enrolled 7 PAD patients and 8 healthy controls to account for potential missing data.
PAD patients were recruited from the Penn State Hershey Medical Center vascular outpatient clinic lists and from our database of subjects who had previously participated in our studies. We made an effort to match PAD patients to healthy subjects from our database (first by sex, then by age, then BMI). All PAD patients had an ankle-brachial index (ABI) < 0.9 and were classified as Fontaine stage II. All PAD patients in the current study did not have clinically active coronary artery disease, aortic stenosis, or prior myocardial infarction based on medical history, symptoms (no angina with exertion), EKG and resting echocardiogram interpreted by a cardiologist (U. Leuenberger). More specifically, all PAD patients had normal ejection fraction (55–70%) and left ventricle wall thickness (< 1.1 cm) as well as no wall motion abnormalities. Patients with diabetes or were not excluded from the study; two PAD patients were diabetic and one PAD patient was pre-diabetic. Most PAD patients (9/12) had undergone at least one leg vascular intervention prior to enrollment but remained symptomatic with ABI < 0.9. These procedures included femoral-popliteal bypass (N=4), peripheral stents (N=3), angioplasty without stenting (N=3), aortic-bifemoral bypass (N=1), and popliteal endarterectomy (N=1). PAD patients were on several medications including antiplatelet medications (N=8), statins (N=8), aspirin (N=8), ACE inhibitors (N=7), hydrochlorothiazide (N=6), fish oil supplements (N=5), amlodipine (N=4), selective β1 blockers (N=4), proton pump inhibitors (N=3), Angiotensin II receptor blockers (N=2), cilostazol (N=3), and metformin (N=1). PAD patients withheld cardiovascular medications for at least 12 hours prior to the plantar flexion exercise trials but stayed on medications for the handgrip trials. Two PAD patients were active smokers and we did not ask that they refrain from smoking before the study. Healthy subjects were normotensive, non-obese, and non-smokers who were not on any medications and had no chronic illnesses. Healthy subjects were recreationally active but not competitive athletes. All subjects refrained from caffeine, alcohol, and vigorous exercise for 24h prior to the study.
2.2 Study Protocol
All study protocols were approved in advance by the Institutional Review Board of Penn State Hershey and conformed to the Declaration of Helsinki. All subjects provided written and informed consent. The study protocols were performed in the supine position in a thermoneutral laboratory (20–21°C). First, ABIs were assessed at rest in all subjects. Subjects were then instrumented with a three-lead EKG (Cardiocap/5, GE Healthcare), a finger BP cuff (Finometer, FMS), and pneumotrace to monitor respiratory activity; these variables were continuously collected at 200 Hz by PowerLab (ADInstruments) and analyzed offline. Resting BPs were obtained in triplicate by automated oscillometry of the right brachial artery (Phillips Sure Signs VS3) after 15 min of quiet rest and these values were used to verify the Finometer values as previously described [20]. A transthoracic echocardiogram (GE Vivid 7) was performed at rest in the supine posture to determine left ventricular size and function, mitral inflow at the mitral leaflet tips, and Doppler velocities at the septal mitral annulus (table 1). Peak diastolic CBV in the left anterior descending artery (LAD) was obtained from the adjusted apical four-chamber view using a 7S probe (all images acquired by Z. Gao). The specific procedures for measuring CBV in LAD have been previously described by our laboratory and the reproducibility within subjects has been verified [21–24]. Subjects also completed the walking impairment questionnaire (WIQ), a pen and paper test to evaluate walking performance that correlates to peak walking time and absolute claudication distance in PAD [25]. The WIQ contains 14 questions and each response is weighted based on the difficulty of the task; scores range from 0 to 100 with lower scores indicating worse walking ability (greater impairment).
Table 1.
Baseline anthropometric measurements and resting hemodynamics
| PAD (N = 12) |
Healthy (N = 15) |
P-values | |
|---|---|---|---|
| Male / female | 7 / 5 | 9 / 6 | |
| Age, yr | 65 ± 2 | 64 ± 2 | 0.940 |
| Height, m | 1.67 ± 0.03 | 1.72 ± 0.02 | 0.104 |
| Weight, kg | 78.7 ± 3.6 | 75.2 ± 3.3 | 0.481 |
| Body Mass Index, kg/m2 | 28.1 ± 1.2 | 25.2 ± 0.7 | 0.072 |
| ABI exercising leg | 0.58 ± .03* | 1.09 ± .03 | < 0.001 |
| ABI non-exercising leg | 0.72 ± .06* | 1.07 ± .03 | < 0.001 |
| Pack years | 29 ± 8* | 0 ± 0 | < 0.001 |
| Systolic BP, mmHg | 143 ± 7* | 123 ± 3 | 0.014 |
| Diastolic BP, mmHg | 74 ± 2 | 76 ± 2 | 0.351 |
| Mean BP, mmHg | 96 ± 3 | 90 ± 2 | 0.194 |
| Heart Rate, beats/min | 63 ± 3* | 55 ± 2 | 0.020 |
| S’ cm/s | 9.1 ± 0.7 | 8.5 ± 0.5 | 0.497 |
| e’, cm/s | 8.7 ± 0.6 | 9.8 ± 0.6 | 0.209 |
| a’, cm/s | 10.2 ± 0.8 | 10.0 ± 0.7 | 0.877 |
| E, cm/s | 86.1 ± 4.3* | 72.7 ± 3.3 | 0.024 |
| A, cm/s | 90.4 ± 7.7* | 68.8 ± 1.6 | 0.021 |
| E-to-A ratio | 1.00 ± 0.05 | 1.06 ± 0.05 | 0.383 |
| E to e’ | 10.7 ± 0.9* | 7.6 ± 0.4 | 0.012 |
Values are mean ± SEM. ABI, ankle-brachial index; BP, blood pressure; S’, systolic mitral annulus velocity; e’, early diastolic mitral annulus velocity; a’, late diastolic mitral annulus velocity; E, early diastolic mitral inflow; A, late diastolic mitral inflow.
P < 0.05 compared to healthy control subjects
To test our hypothesis, subjects performed plantar flexion exercise by contracting the calf muscles of a single leg 30 times per minute with increasing amounts of resistance attached to the device. The resistance started at 0.5 kg and increased by 0.5 kg every minute until 7 kg, fatigue, significant pain, or inability to maintain the cadence occurred as previously performed in our lab [15, 26, 27]. Ratings of perceived exertion and pain were obtained each minute using the Borg scales [28]. PAD patients performed the exercise with their most symptomatic limb. In a subsequent study on a separate day, a subset of subjects who were willing to participate in another experiment (N=7 PAD, N=8 healthy) performed isometric handgrip exercise at 40% maximal voluntary contraction until fatigue by squeezing a handgrip dynamometer using their right forearm muscles. This stressor was chosen because it raises sympathetic nervous system activity, HR, and BP without evoking ischemic leg pain [29].
2.3 Data collection and statistical analysis
All variables were measured continuously and analyzed offline. An average of the last 20 s of each minute is presented. Rate pressure product (RPP, the product of HR and systolic BP) was used as an index of myocardial O2 demand. CBV was used as an index of myocardial O2 supply. Our primary outcome variable was the ΔCBV to ΔRPP ratio from baseline to exercise, which was calculated as ΔCBV/ΔRPP × 1000. This ratio has been used previously to quantify coronary hyperemia normalized to cardiac metabolism; higher numbers indicate greater myocardial O2 supply relative to demand [30–33]. Statistical analysis was performed using SPSS 22. Mann-Whitney U tests were used to compare anthropometric and non-parametric data between groups. To analyze responses to plantar flexion exercise, two group (PAD, healthy) by 6 time point (baseline, exercise at 0.5 kg, 1.0 kg, 1.5 kg, 2.0 kg, fatigue) repeated measures ANOVA were conducted on the raw physiological variables (figure 1). For significant interactions, post-hoc Tukey-Kramer tests were employed. Changes (Δ) from baseline to plantar flexion exercise at 2.0 kg and from baseline to peak handgrip exercise were compared between groups using Student’s t-tests for independent samples. Cohen’s d (mean 1– mean 2/ combined standard deviation) was calculated on primary outcomes to determine effect size. We also conducted planned correlations between WIQ scores, ABIs, and physiological responses to exercise. All data are shown as mean ± standard error mean (SEM) unless otherwise stated. Significance was set at P < 0.05 for all tests.
Figure 1.
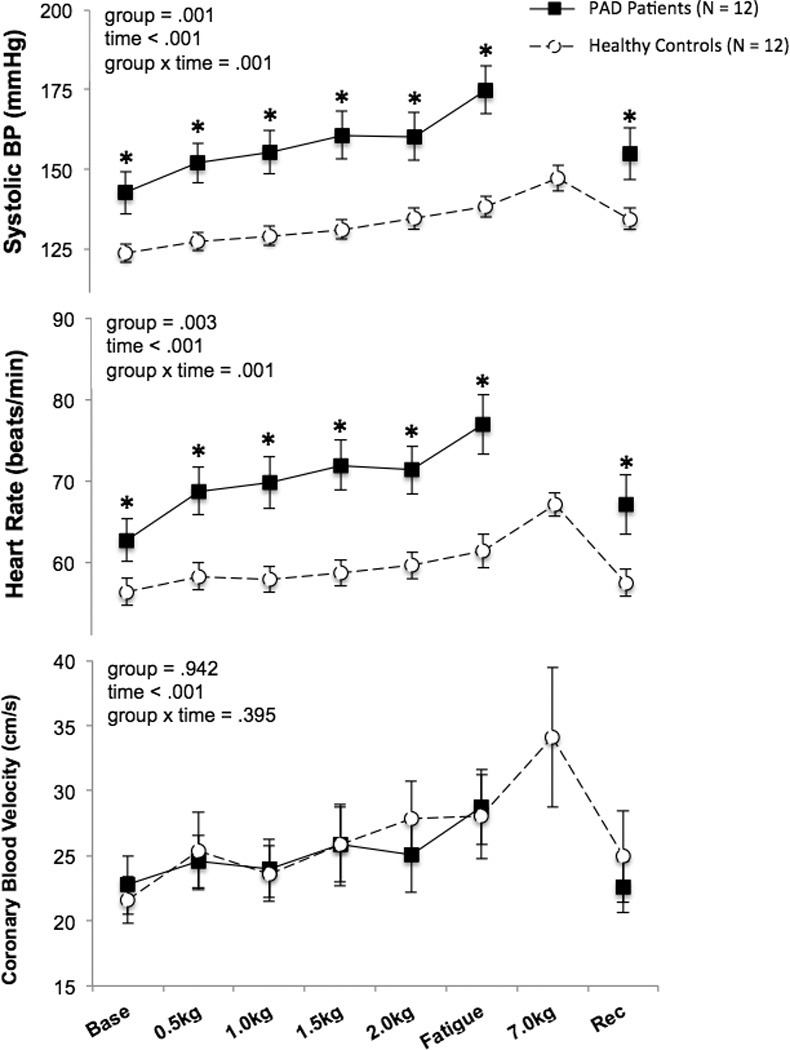
Systolic blood pressure (BP), heart rate, and coronary blood velocity responses to plantar flexion exercise in patients with peripheral arterial disease (PAD) and healthy control subjects (N = 12 in each group). The fatigue time point represents the last workload performed by each PAD patient and the corresponding workload of each matched healthy subject. *P < 0.05 between groups.
3. RESULTS
Table 1 shows anthropometric measurements and resting hemodynamics in PAD patients and healthy control subjects. Age, height, weight, diastolic BP, and mean BP were similar between groups. While BMI was not statistically different between groups, BMI tended to be higher in PAD patients compared to healthy subjects. Systolic BP was higher in PAD patients compared to healthy subjects. The median WIQ score in PAD was 51 (range: 16 to 100). All healthy subjects scored 100 (i.e. no impairment) on the WIQ. There were no significant correlations between WIQ scores, ABIs, and physiological responses to exercise. More specifically, the CBV response to plantar flexion did not correlate to worst ABI (R = −0.093, P = 0.785) or to WIQ scores (R = −.060, P = 0.860).
During plantar flexion exercise, PAD patients experienced more pain and stopped at a lower workload compared to healthy subjects; yet ratings of perceived exertion were similar between groups during the last stage of exercise (table 2). All PAD patients completed the 2.0kg workload (4 minutes) therefore statistical comparisons were made up to this time point. Significant group × time interactions were found for systolic BP and HR, which were higher in PAD compared to healthy subjects at all analyzed time points (Figure 1). Yet, CBV over time was not different between groups (Figure 1). The changes from baseline to exercise at 2.0 kg in systolic BP (PAD: Δ 18 ± 2, healthy: Δ 12 ± 2 mmHg, P = 0.020) and HR (PAD: Δ 9 ± 1, healthy: Δ 3 ± 1 beats/min, P = 0.001) were higher in PAD. However, the change in mean BP from baseline to exercise at 2.0kg was not different between groups (PAD: Δ 12 ± 2, healthy: Δ 8 ± 2 mmHg, P = 0.089). The change in CBV (ΔCBV) from baseline to exercise at 2.0kg was attenuated in PAD vs. healthy subjects (Δ 2.4 ± 1.2 vs. Δ 6.0 ± 1.6 cm/s, P = 0.039, Cohen’s d = 0.7). In addition, ΔCBV/ΔRPP (slope of the line from baseline to exercise at 2.0kg) was lower in PAD vs. healthy subjects (Δ 1.0 ± 0.6 vs. Δ 7.3 ± 2.9 a.u., P = 0.013, Cohen’s d = 0.9, figure 2A).
Table 2.
Perceived Exertion, Pain, and Exercise Duration
| PAD | Healthy | P-values | |
|---|---|---|---|
| Dynamic Plantar Flexion | (N = 13) | (N = 14) | |
| Last workload completed (kg) | 4.0 ± 0.5* | 7.0 ± 0.0 | < 0.001 |
| RPE at end of exercise (a.u., 6 – 20) | 14 ± 1 | 16 ± 1 | 0.212 |
| Pain at end of exercise (a.u., 0 – 10) | 4 ± 1* | 0 ± 0 | 0.011 |
| Isometric Handgrip | (N = 8) | (N = 7) | |
| Time to fatigue (seconds) | 204 ± 15* | 280 ± 24 | 0.010 |
| MVC forearm (kg) | 34 ± 3 | 37 ± 2 | 0.252 |
| RPE at fatigue (a.u., 6 – 20) | 19 ± 1 | 19 ± 0 | 0.694 |
Values are mean ± SEM. RPE, rating of perceived exertion; MVC, maximum voluntary contraction.
P < 0.05 compared to healthy control subjects
Figure 2.
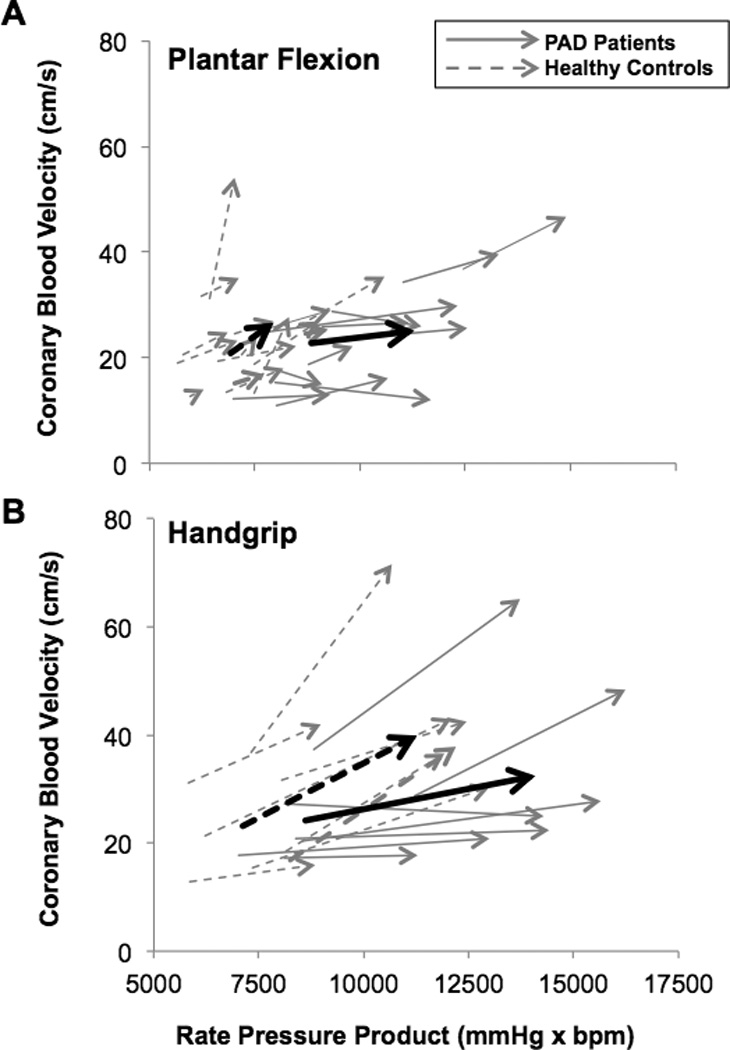
Top graph. Change in coronary blood velocity / rate pressure product from baseline to plantar flexion exercise at 2.0 kg in peripheral arterial disease patients (PAD, N= 12, solid lines) and healthy control subjects (N = 14, dashed lines). Bottom graph. Change in coronary blood velocity / rate pressure product from baseline to peak fatiguing isometric handgrip exercise (40% maximal voluntary contraction) in PAD (N = 7, solid black lines) and healthy subjects (N = 8, gray dashed lines). Gray arrows represent individual responses and black arrows show group averages. A steeper slope indicates better myocardial O2 supply for a given amount of increased metabolic demand.
In response to isometric handgrip exercise, maximum voluntary contraction of the forearm and ratings of perceived exertion at fatigue were similar between groups although healthy subjects exercised about one minute longer (table 2). There were no group differences in mean BP (PAD: Δ 35 ± 3, healthy: Δ 30 ± 4 mmHg, P = 0.223), HR (PAD: Δ 14 ± 2, healthy: Δ 14 ± 3 beats/min, P = 0.701), or RPP (PAD: Δ 5450 ± 487, healthy: Δ 4307 ± 408 beats/min, P = 0.093) responses to handgrip exercise. Despite comparable increases in myocardial O2 demand, ΔCBV from baseline to peak handgrip was attenuated in PAD vs. healthy subjects (Δ 8.3 ± 4.2 vs. Δ 16.9 ± 3.6, P = 0.033, Cohen’s d = 1.2). The ΔCBV/ΔRPP ratio from baseline to peak grip was also attenuated in PAD vs. healthy subjects (Δ 1.7 ± 0.9 vs. Δ 4.5 ± 1.0, P = 0.030, Cohen’s d = 1.1, figure 2B).
4. DISCUSSION
The purpose of this study was to investigate coronary responses to exercise in patients with PAD and in healthy subjects of similar age. Consistent with our hypothesis, we found that the increase in myocardial O2 supply (CBV) to single leg dynamic plantar flexion exercise is attenuated in PAD patients compared to healthy subjects despite a larger increase in metabolic O2 demand (RPP). In addition, we found that the change in CBV to isometric handgrip exercise is also attenuated in PAD, despite similar increases in RPP compared to healthy subjects. These findings indicate that PAD is associated with impaired coronary exercise hyperemia, which may partially explain the high risk of adverse cardiovascular events in this population.
In humans, peak CBV responses to pharmacological agents are predictive of adverse cardiovascular events in healthy subjects and patients with coronary atherosclerosis [10–14]. Specifically, CBV reserve (CBVpeak/CBVbaseline) is lower in patients with coronary artery disease. One study found that PAD patients also have an attenuated increase in CBV to intravenous dipyridamole [6]. However, CBV responses to physiological stimuli in PAD were previously unknown.
With respect to BP and HR, the current plantar flexion experiments are consistent with previous studies from our laboratory, which found that systolic BP and HR responses to plantar flexion exercise up to 2.0kg were augmented in PAD [15, 26, 34]. The primary finding of the current study, which used a longer exercise protocol, is that ΔCBV from baseline to exercise is attenuated in PAD despite higher myocardial metabolism (RPP). In addition, the ΔCBV/ΔRPP ratio was also attenuated in PAD patients compared to healthy subjects as shown by the blunted slope of the line in figure 2. This suggests that reflex increases in systemic hemodynamics during exercise elicit different coronary vascular responses between PAD patients and healthy subjects. To our knowledge, this is the first study to evaluate CBV during plantar flexion or isometric handgrip exercise in PAD patients. We believe our data are clinically important because they imply that PAD patients with local leg atherosclerosis likely have impairments in coronary perfusion during exertion. Furthermore, the PAD patients in the current study were relatively healthy; all had intermittent claudication (Fontaine stage 2), none had rest pain (critical limb ischemia), they were medically stable, and only 3/12 subjects had low walking performance based on WIQ scores < 42.5 [25]. We speculate that coronary exercise hyperemia is further impaired in patients with critical limb ischemia.
It is unlikely that impaired coronary exercise hyperemia in PAD is the result of an altered reflex originating from the leg due to ischemia or pain. Indeed, we found that coronary hyperemia is also impaired in PAD during isometric handgrip exercise. While CBV responses to handgrip were attenuated in PAD, the increase in BP and HR were similar to healthy subjects, which is consistent with other studies [16]. The pressor response generated during plantar flexion exercise is greater in PAD. Thus, the lower coronary velocity responses to plantar flexion exercise in PAD may have been due to greater coronary myogenic influences [35]. However, the handgrip data demonstrates that even when BP responses are not augmented in PAD, coronary velocity responses remain attenuated. These findings suggest that the lower coronary velocity responses observed in PAD are unlikely to be caused by an augmented myogenic flow control in the coronary circulation.
Previous studies found that PAD patients and animal models of peripheral artery insufficiency have decreased coronary blood flow responses to pharmacological stimuli [5, 6]. Our data extend these findings, by showing that PAD patients also have impaired coronary vasomotor responses to physiological stimuli (i.e., exercise). The mechanism of impaired coronary vasodilation in PAD is unknown but may be attributed to global endothelial dysfunction, subclinical coronary atherosclerosis, or altered sympathetic vascular control. Future studies are needed to elucidate the mechanisms of coronary blood flow regulation in PAD.
Limitations
While our sample size was relatively small, our study was powered to assess differences in the primary outcome variable (ie. the ΔCBV/ΔRPP) and our effect size was large for our primary findings (Cohen’s d > 0.7). With transthoracic Doppler echocardiography we are only able to measure CBV not coronary blood flow since we are unable to measure diameter accurately. However, previous studies found that adenosine-induced increases in CBV are correlated to increases in coronary blood flow and the percent change in velocity is 10-fold greater than the increase in diameter [8, 9]. Therefore increases in LAD flow are largely attributed to changes in velocity. It is possible that some of the PAD patients in the current study had significant but subclinical coronary atherosclerosis since atherosclerosis is a systemic disease. However, all PAD patients in this study did not have clinically active coronary atherosclerosis and were screened for coronary artery disease via medical history, EKG, and resting echocardiography. Since coronary vasodilation is impaired in swine with femoral artery occlusion in the absence of any atherosclerosis it is possible that impaired coronary vasodilation in PAD could be an adaptation to leg ischemia rather than generalized atherosclerosis [5]. However, our PAD patients did not have isolated leg ischemia. Some patients in the study did have diabetes, hypertension, hyperlipidemia, and/or history of tobacco use, which may have contributed to changes in coronary flow. Two PAD patients were also active smokers, which may have altered vasomotor tone. In addition, all patients were on medications that could have affected our measurements because it is unethical to have patients withhold medications for enough time to washout the effects. More specifically, four subjects took amlodipine, a dihydropyridine, that can produce vasodilation. While CBV responses were impaired in PAD patients, even ones on amlodipine, it is possible that the impairment of coronary hyperemia was underestimated in these patients. We did not measure disease markers such as HbA1c or lipid levels in our subjects and cannot determine if these influenced their physiological responses. The ratio of early mitral inflow to early diastolic velocity of mitral annulus (E/e’ ratio, Table 1) at rest was higher in PAD patients, which suggests higher left ventricular filling pressure [36]. Yet, left ventricular wall thickness, fractional shortening, and ejection fraction were normal in all PAD patients in the current study and the subjects had no signs of prior myocardial infarction based on resting EKG and echocardiography. In addition, PAD patients did not perform plantar flexion or handgrip exercise as long as healthy subjects but perceived exertion was similar between groups at the end of exercise.
5. CONCLUSIONS
This is the first study to show that coronary blood flow responses to leg and forearm exercise are impaired in patients with peripheral artery disease (PAD) without overt coronary disease. Additional studies with larger sample sizes are needed to validate these findings. Since coronary blood velocity indicates oxygen supply to the myocardium, these findings may partially explain the high risk of myocardial ischemia in PAD. However, whether decreased coronary hyperemia during exercise is associated with greater cardiovascular morbidity or mortality is yet to be determined. Further studies are also needed to elucidate the mechanism of impaired coronary exercise hyperemia in PAD and develop strategies to improve myocardial perfusion during exercise in these patients.
Acknowledgments
The authors would like to thank Dr. Michael Herr for engineering support, Kris Brandt for assistance with echocardiography analysis, and Jen Stoner and Kris Gray for administrative support.
FUNDING SOURCES
This publication was supported, in part, by NIH Grants UL1 TR000127 and P01 HL096570 (to L. I. Sinoway) and KL2 TR000126 from the National Center for Advancing Translational Sciences (NCATS) and also under a grant with the Pennsylvania Department of Health using Tobacco CURE funds (to M.D. Muller). The Pennsylvania Department of Health and the NIH specifically disclaim responsibility for any analyses, interpretations, or conclusions. Also contributing to this project was American Heart Association 15PRE24470033 (to A. J. Ross).
ABBREVIATIONS
- PAD
peripheral arterial disease
- O2
oxygen
- BP
blood pressure
- CBV
coronary blood velocity
- RPP
rate pressure product
- RPE
rating of perceived exertion
- LAD
left anterior descending coronary artery
Appendix
AUTHOR CONTRIBUTIONS
| Task | AJR | ZG | JCL | CAB | AEC | FA | JFR | DNP | UAL | LIS | MDM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conception and Design | X | X | X | X | |||||||
| Perform Experiments | X | X | X | X | X | X | |||||
| Analyze Data | X | X | X | X | X | ||||||
| Interpret Results | X | X | X | X | X | X | X | X | X | X | X |
| Prepare Figures | X | X | |||||||||
| Draft Manuscript | X | X | |||||||||
| Edit/revise Manuscript | X | X | X | X | X | X | X | X | X | X | X |
| Approve Manuscript | X | X | X | X | X | X | X | X | X | X | X |
REFERENCES
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 5.Arce-Esquivel AA, Bunker AK, Simmons GH, Yang HT, Laughlin MH, Terjung RL. Impaired Coronary Endothelial Vasorelaxation in a Preclinical Model of Peripheral Arterial Insufficiency. J Heart Cardiol. 2015;1(2) doi: 10.15436/2378-6914/15/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellegrino T, Storto G, Filardi PP, Sorrentino AR, Silvestro A, Petretta M, et al. Relationship between brachial artery flow-mediated dilation and coronary flow reserve in patients with peripheral artery disease. J Nucl Med. 2005;46(12):1997–2002. [PubMed] [Google Scholar]
- 7.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 8.Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33(6):1469–1475. doi: 10.1016/s0735-1097(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 9.Kiviniemi TO, Toikka JO, Koskenvuo JW, Saraste A, Saraste M, Parkka JP, et al. Vasodilation of epicardial coronary artery can be measured with transthoracic echocardiography. Ultrasound Med Biol. 2007;33(3):362–370. doi: 10.1016/j.ultrasmedbio.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Schindler TH, Hornig B, Buser PT, Olschewski M, Magosaki N, Pfisterer M, et al. Prognostic value of abnormal vasoreactivity of epicardial coronary arteries to sympathetic stimulation in patients with normal coronary angiograms. Arterioscler Thromb Vasc Biol. 2003;23(3):495–501. doi: 10.1161/01.ATV.0000057571.03012.F4. [DOI] [PubMed] [Google Scholar]
- 11.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 12.Rigo F, Gherardi S, Galderisi M, Pratali L, Cortigiani L, Sicari R, et al. The prognostic impact of coronary flow-reserve assessed by Doppler echocardiography in non-ischaemic dilated cardiomyopathy. Eur Heart J. 2006;27(11):1319–1323. doi: 10.1093/eurheartj/ehi795. [DOI] [PubMed] [Google Scholar]
- 13.Rigo F, Cortigiani L, Pasanisi E, Richieri M, Cutaia V, Celestre M, et al. The additional prognostic value of coronary flow reserve on left anterior descending artery in patients with negative stress echo by wall motion criteria. A Transthoracic Vasodilator Stress Echocardiography Study. Am Heart J. 2006;151(1):124–130. doi: 10.1016/j.ahj.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 15.Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, et al. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol. 2012;590(Pt 23):6237–6246. doi: 10.1113/jphysiol.2012.241281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakke EF, Hisdal J, Kroese AJ, Jorgensen JJ, Stranden E. Blood pressure response to isometric exercise in patients with peripheral atherosclerotic disease. Clin Physiol Funct Imaging. 2007;27(2):109–115. doi: 10.1111/j.1475-097X.2007.00720.x. [DOI] [PubMed] [Google Scholar]
- 17.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology. 1999;50(5):361–374. doi: 10.1177/000331979905000502. [DOI] [PubMed] [Google Scholar]
- 18.Lorentsen E. Systemic arterial blood pressure during exercise in patients with atherosclerosis obliterans of the lower limbs. Circulation. 1972;46(2):257–263. doi: 10.1161/01.cir.46.2.257. [DOI] [PubMed] [Google Scholar]
- 19.de Liefde I, Hoeks SE, van Gestel YR, Bax JJ, Klein J, van Domburg RT, et al. Usefulness of hypertensive blood pressure response during a single-stage exercise test to predict long-term outcome in patients with peripheral arterial disease. Am J Cardiol. 2008;102(7):921–926. doi: 10.1016/j.amjcard.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Muller MD, Gao Z, McQuillan PM, Leuenberger UA, Sinoway LI. Coronary responses to cold air inhalation following afferent and efferent blockade. Am J Physiol Heart Circ Physiol. 2014;307(2):H228–H235. doi: 10.1152/ajpheart.00174.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Z, Novick M, Muller MD, Williams RJ, Spilk S, Leuenberger UA, et al. Exercise and diet-induced weight loss attenuates oxidative stress related-coronary vasoconstriction in obese adolescents. Eur J Appl Physiol. 2013;113(2):519–528. doi: 10.1007/s00421-012-2459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Z, Wilson TE, Drew RC, Ettinger J, Monahan KD. Altered coronary vascular control during cold stress in healthy older adults. Am J Physiol Heart Circ Physiol. 2012;302(1):H312–H318. doi: 10.1152/ajpheart.00297.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Z, Spilk S, Momen A, Muller MD, Leuenberger UA, Sinoway LI. Vitamin C prevents hyperoxia-mediated coronary vasoconstriction and impairment of myocardial function in healthy subjects. Eur J Appl Physiol. 2012;112(2):483–492. doi: 10.1007/s00421-011-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Momen A, Mascarenhas V, Gahremanpour A, Gao Z, Moradkhan R, Kunselman A, et al. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol. 2009;296(3):H854–H861. doi: 10.1152/ajpheart.01075.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagar SP, Brown PM, Zelt DT, Pickett WL, Tranmer JE. Further clinical validation of the walking impairment questionnaire for classification of walking performance in patients with peripheral artery disease. Int J Vasc Med. 2012;2012:190641. doi: 10.1155/2012/190641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller MD, Drew RC, Ross AJ, Blaha CA, Cauffman AE, Kaufman MP, et al. Inhibition of cyclooxygenase attenuates the blood pressure response to plantar flexion exercise in peripheral arterial disease. Am J Physiol Heart Circ Physiol. 2015;309(3):H523–H528. doi: 10.1152/ajpheart.00267.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drew RC, Muller MD, Blaha CA, Mast JL, Heffernan MJ, Estep LE, et al. Renal vasoconstriction is augmented during exercise in patients with peripheral arterial disease. Physiol Rep. 2013;1:e00154(6), 1–9. doi: 10.1002/phy2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 29.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57(3):461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- 30.Muller MD, Gao Z, Mast JL, Blaha CA, Drew RC, Leuenberger UA, et al. Aging attenuates the coronary blood flow response to cold air breathing and isometric handgrip in healthy humans. Am J Physiol Heart Circ Physiol. 2012;302(8):H1737–H1746. doi: 10.1152/ajpheart.01195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross AJ, Gao Z, Pollock JP, Leuenberger UA, Sinoway LI, Muller MD. beta-Adrenergic receptor blockade impairs coronary exercise hyperemia in young men but not older men. Am J Physiol Heart Circ Physiol. 2014;307(10):H1497–H1503. doi: 10.1152/ajpheart.00584.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol (1985) 2004;97(1):404–415. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- 33.Muller MD, Gao Z, Patel HM, Heffernan MJ, Leuenberger UA, Sinoway LI. beta-Adrenergic blockade enhances coronary vasoconstrictor response to forehead cooling. Am J Physiol Heart Circ Physiol. 2014;306(6):H910–H917. doi: 10.1152/ajpheart.00787.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drew RC, Muller MD, Blaha CA, Mast JL, Heffernan MJ, Estep LE, et al. Renal vasoconstriction is augmented during exercise in patients with peripheral arterial disease. Physiol Rep. 2013;1(6):e00154. doi: 10.1002/phy2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinotti O, Gattullo D, Linden RJ, Losano G. The myogenic mechanism in the autoregulation of the coronary vascular bed. Cardiologia. 1992;37(4):301–308. [PubMed] [Google Scholar]
- 36.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102(15):1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]


