Abstract
Study Objectives
This study involves the analysis of a secondary outcome of a trial examining whether cognitive behavior therapy for insomnia (CBT-I), a wake-promoting medication (armodafinil), or both results in greater improvement in prospectively assessed sleep continuity and daytime sleepiness than a placebo-alone group among a heterogeneous group of cancer survivors. Whether or not armodafinil alone, and/or when combined with CBT-I, affected adherence with CBT-I was evaluated.
Design
This study is a randomized, placebo-controlled, clinical trial.
Setting
This study was conducted at two northeastern academic medical centers.
Participants
Eighty-eight cancer survivors with chronic insomnia were recruited between October 2008 and November 2012. Participants were assigned to one of four conditions: 1) CBT-I and placebo (CBTI+P); 2) CBT-I and armodafinil (CBT-I+A); 2) armodafinil alone (ARM); or 4) placebo alone (PLA).
Interventions
CBT-I was delivered in seven weekly individual therapy sessions (three in person, four via telephone). The armodafinil dosage was 50 mg BID.
Measurements and Results
Sleep continuity was measured with daily sleep diaries assessing sleep latency (SL), wake after sleep onset (WASO), and total sleep time (TST). The Epworth Sleepiness Scale (ESS) measured daytime sleepiness. Compared to the PLA group, the CBT+P and CBT+A groups reported a significant reduction in SL with effect sizes of 0.67 and 0.58, respectively. A significant reduction was observed in WASO in the CBT+A group with an effect size of 0.64. An increasing trend of TST was observed in the CBT+P, CBT+A, and PLA groups, but not in the ARM group. No statistically significant reductions in daytime sleepiness (ESS) were observed for any of the groups.
Conclusion
CBT-I alone and in combination with armodafinil caused significant improvement in sleep continuity. The addition of armodafinil did not appear to improve daytime sleepiness or enhance adherence to CBT-I.
Keywords: Cancer, insomnia, cognitive behavior therapy, CBT-I, armodafinil, sleepiness
INTRODUCTION
Early cancer detection and treatment advances have resulted in a substantial increase in the number of individuals who survive cancer. This has brought to the fore the need to ensure the health, wellbeing, and quality of life of more than 13 million cancer survivors.1 Perhaps the most prominent residual or long-lasting symptoms of cancer are fatigue and insomnia. With respect to the latter, several large-scale epidemiological studies demonstrate that nearly 60% of people treated for cancer experience insomnia.2,3 Insomnia (sleep continuity disturbance) and daytime sleepiness are especially important consequences of cancer because of their potential to negatively influence physical and psychological well-being and overall quality of life.4,5 While the high incident rate of insomnia in the context of a cancer diagnosis and treatment is not unexpected, it is often the case that sleep continuity disturbance tends to be unremitting long into remission and recovery from cancer.3 In these cases, interventions are required for improved sleep.
Presently, the primary indications for chronic insomnia are medical treatment and cognitive behavioral therapy for insomnia (CBT-I).6,7 Medical treatments include pharmacotherapy with benzodiazepine receptor agonists (BZRAs, e.g., zolpidem/ambien; melatonin agonists, e.g., ramelteon/rozerem; low-dose antidepressants, e.g., doxepin/silenor; and, most recently, an orexin antagonist suvorexant/belsomra). Of these options, the most exhaustively studied medications are the benzodiazepine receptor agonists. These medications produce good effect sizes with respect to sleep continuity,8,9 and evidence suggests that such effects may be maintained over time with continued use of medications.10 CBT-I has also been shown to have similar effect sizes with respect to sleep continuity11,12 and comparable or better effects than BZRA hypnotics.13,14 Unlike medication, the effects of CBT-I are durable and extend beyond acute treatment for measured periods of up to 24 months.15,16 Further, >55% of patients treated with CBT-I reach remission within 6 months of the discontinuation of acute therapy.17,18 Given these outcomes, it can be argued that CBT-I is the treatment of choice for chronic insomnia (in general), especially for patients who prefer not to use medication. CBT-I appears well suited to cancer survivors with insomnia. To date, eight controlled and four uncontrolled trials of CBT-I have been conducted in cancer patients. A systematic review of these studies concluded that CBT-I is effective in this context with one study showing large effect sizes for sleep latency (SL), wake after sleep onset (WASO), and sleep efficiency (SE).19
Despite the evidence that CBT-I is an effective intervention, there remains a significant proportion of individuals whose insomnia does not respond to, or remit after, treatment. Estimates suggest that 32–89% of patients treated with CBT-I (including those with cancer) do not consistently follow treatment recommendations.20 While the reason for this is unknown, it has been suggested that the transient decrease in total sleep and/or the transient increase in daytime sleepiness that is inherent to CBT-I is simply intolerable for some patients,21 particularly those with high basal levels of fatigue and sleepiness. Given this possibility, it follows that daytime use of a wakefulness promoting medication should, by itself, decrease daytime sleepiness (and possibly fatigue) and when combined with CBT-I, prevent the “iatrogenic” effects of CBT-I with respect to daytime sleepiness and potentially increase adherence given the reduced consequences of delaying time to bed (TTB) and the practice of stimulus control.21 These possibilities were evaluated in one study of patients with primary insomnia. In this study, it was found that modafinil (alone or in combination with CBT-I) did not change the outcomes with respect to sleep continuity, but did significantly alter daytime sleepiness and adherence to CBT-I.21 Further, reducing the negative consequences (side effects) of CBT-I should have a positive effect on adherence. These possibilities have been evaluated in non-cancer patients with primary insomnia. In this context, it was found that modafinil,22-24 alone or in combination with CBT-I, did not affect sleep continuity but did significantly alter daytime sleepiness and adherence to CBT-I.21 Our own group also conducted a two-site study in which CBT-I, armodafinil, and the combination (as compared to placebo) were evaluated in cancer survivors with insomnia.25 It was found that subjective insomnia severity and sleep quality as assessed by the Insomnia Severity Index (ISI) and the Pittsburgh Sleep Quality Index (PSQI) were significantly improved in both CBT-I conditions. In the present analysis, a focused and quantitative evaluation of sleep is undertaken using prospective sampling measures (sleep diaries) to evaluate whether 1) sleep continuity was differently affected by the interventions; 2) armodafinil alone, or in combination with CBT-I, effects sleepiness; and 3) armodafinil in combination with CBT-I affected adherence measures as compared to CBT-I alone (with placebo). An examination of sleep via daily diaries provides a more sensitive measure of sleep continuity disturbance than qualitative measures such as the ISI.
METHODS
Design
The parent study for this analysis was a randomized, placebo-controlled, clinical trial of post-treatment cancer survivors with chronic insomnia. The complete methods of this study have been published elsewhere25 and will only be briefly summarized here. Eighty-eight cancer survivors were assigned to one of four conditions: 1) CBT-I plus placebo (CBT-I+P; n = 21); 2) CBT-I plus armodafinil (CBT-I+A; n = 22); 3) armodafinil only (ARM; n = 22); or 4) medication placebo only (PLA; n = 23). Patients were assessed at the following time points: baseline (2 weeks), during the intervention period (weekly for 7 weeks), and post intervention (2 weeks). The reporting of this trial follows the CONSORT guidelines for reporting randomized trials of behavioral and pharmacological interventions. The institutional review boards of the University of Rochester and the University of Pennsylvania approved the protocol, and patients provided written informed consent. This trial is registered with ClinicalTrials.gov, number NCT01091974.
Inclusion Criteria
Cancer survivors with chronic insomnia in two northeastern cities were screened and recruited between October 2008 and November 2012. No restrictions were placed on cancer type but participants must have been presumed cancer-free at the time of enrollment. Participants must have completed all therapeutic cancer treatments not less than a month prior to study start (continued use of tamoxifen or an aromatase inhibitor was permitted). Participants were required to discontinue any prescribed or over-the-counter medications for sleep for the 11-week study period and have a preferred sleep phase between 7:30 pm and 11:00 am. They also had to endorse problems with insomnia for at least 3 months and state that the insomnia began, or became worse, with the onset of cancer or cancer treatment. Insomnia symptoms were assessed with an online screener and were corroborated with 2 weeks of sleep diaries. Participants were required to have reported SL or WASO problems of >30 min, problem frequency of >3 days per week, and problem chronicity of >1 month). Patients who had ever taken modafinil or armodafinil; had undergone CBT-I therapy; had a history of seizures or severe headaches, or uncontrolled cardiac disease or hypertension; had taken amphetamines within the past 30 days; had a history of substance abuse or meet criteria for current alcohol abuse or dependence; or had sleep apnea, were not eligible.
Measures
Sleep diaries
This instrument provides a night-by-night, self-report measure of sleep continuity and is considered the gold standard for the measurement of insomnia.26,27 The specific variables captured are TTB, time out of bed (TOB), SL, WASO including early morning awakenings, and total sleep time (TST). In addition, three variables were calculated based on the diary data including time in bed (TIB), TST, and SE. All sleep continuity variables were converted into weekly averages.
Epworth Sleepiness Scale (ESS)
The ESS is a validated and well-established measure of daytime sleepiness.28 It is a self-administered questionnaire where respondents are asked to rate, on a four-point scale (0–3), their usual chances of dozing off or falling asleep in eight different situations or activities that most people engage in as part of their daily lives. ESS scores range from 0 to 24. Scores of 0–7 represent normal levels of sleepiness, 8–9 represent average sleepiness, 10–15 excessive sleepiness, and 16–24 pathological sleepiness.
Adherence
Diary values for each sleep continuity variable were aggregated by week. Treatment adherence was evaluated by examining the actual time in bed (ATIB) compared to the prescribed time in bed (PTIB) for each week for the CBT-I+P and CBT-I+A groups. Patients were introduced to sleep restriction at their first CBT-I appointment. ATIB was determined from the TTB and TOB questions on the sleep diary. PTIB was recorded by the therapist for each CBT-I session. For example, if the average baseline TST was 6 h, then the PTIB would be 6 h. An individual was deemed adherent if his/her ATIB was ±30 min of his/her PTIB. Adherence was calculated for each week and coded as yes/no. The number of adherent weeks was divided by the total number of sleep diaries returned to calculate an adherence percentage. We assessed compliance with study medication in two ways: 1) The report of pill use on the sleep diaries and 2) whether the pill slot in the returned foil pack was filled or empty.
Intervention Details
CBT-I combines principles from stimulus control therapy (reducing non-sleep behaviors in the bedroom) and sleep restriction therapy (consolidation of sleep periods) with formal cognitive restructuring (reducing misconceptions about sleep) in order to target hyperarousal, maladaptive behaviors, and negative beliefs, and attitudes associated with insomnia. CBT-I was delivered over the course of seven weekly individual sessions. Sessions 1, 2, and 4 were in person (30–60 min in duration) and sessions 3, 5, 6, and 7 (15–30 min in duration) were by phone. The delivery of CBT-I interventions via telephone is comparable to face-to-face delivery29 and has been demonstrated to reduce patient burden and increase retention of participants.30
Armodafinil is a single isomer formulation of modafinil (R-enantiomer of modafinil). It is indicated for the promotion of wakefulness in several sleep disorders including narcolepsy, sleep apnea syndrome, and shift work disorder. It is available in 50, 150, or 250 mg tablets. Medication was begun with a 50-mg dose of armodafinil in the morning (7–9 am) along with an afternoon (12–2 pm) administration of placebo. After 3 days, active doses of armodafinil were administered both in the morning and afternoon (50 mg each). Twice daily treatment was continued for 40 days and then was followed by 4 days of 50 mg in the morning and a placebo in the afternoon. Patients in the PLA group received a placebo capsule in the morning and afternoon to mimic the dosage times of the ARM group, and all patients received information on sleep hygiene.
Study personnel and patients were blinded regarding medication (armodafinil, placebo) assignment but not CBT-I condition, and participants were not randomized until after the completion of their 2-week baseline period. Participants had the option of completing measures using paper and pen on scannable forms or using an internet data portal. Scannable data were electronically transferred to an Access database, and data quality was checked by an information analyst.
Statistical Analyses
Sample size calculations were performed for the primary analysis and have been reported previously.25 ANCOVA (analysis of covariance) was employed on the post-intervention score (average of the 2 post-intervention weeks), controlling for the baseline score. Using appropriate contrasts, the mean post-pre-change was estimated for CBT-I+A versus placebo, CBT-I+P versus placebo, armodafinil versus placebo, and CBT-I+A versus CBT-I+P. We considered 0.0125 as the significance level to control for multiplicity. Analyses were carried out with the intention to treat, although 23 (24%) of the 96 randomized eligible patients did not provide post-intervention data. The missing value patterns were examined through visual inspection and logistic regression of missingness versus treatment arm and demographic characteristics. Examining the reasons for dropout, we found no evidence that the data were not missing at random and therefore assumed a missing at random (MAR) mechanism.26 We therefore applied multiple imputation (MI) using data from all participants who had baseline data. The MI analyses results were similar to the complete case analyses in which only those patients who provided post-intervention data were included. SAS Version 9.2, SPSS version 19, and R Version were used for the present analyses.
RESULTS
Of the 138 patients who consented to screening, 114 were eligible and 96 were randomized; 88 (77% of eligible patients and 92% of randomized patients) began the intervention, and 73 patients (83% of the 88 patients beginning the intervention) completed the 7-week intervention. No serious related adverse events were reported. Average compliance with the study medication, as determined by the returned study medication cards, was >90% for all study arms and did not differ significantly by group. There were no differences between the treatment groups for any baseline characteristics (Table 1). For specific details regarding subject attrition and details on adverse events, please see our prior publication’s CONSORT diagram.25
Table 1.
Characteristics of patients beginning intervention by study group
| CBT-I + Placebo | CBT-I + Armodafinil | Placebo | Armodafinil | ||
|---|---|---|---|---|---|
| N = 21 | N = 22 | N = 22 | N = 23 | ||
| Age: | Mean (SD) | 59 (10.3) | 56 (10.2) | 52 (11.8) | 57 (7.5) |
| Sex: | Male | 3 (14%) | 1 (5%) | 6 (27%) | 1 (4%) |
| Female | 18 (86%) | 21 (95%) | 16 (73%) | 22 (96%) | |
| Ethnicity: | Non-Hispanic | 20 (95%) | 21 (95%) | 21 (95%) | 21 (91%) |
| Unknown | 1 (5%) | 1 (5%) | 1 (5%) | 2 (9%) | |
| Race: | White | 20 (95%) | 20 (91%) | 18 (82%) | 22 (96%) |
| African American | 1 (5%) | 2 (9%) | 3 (14%) | 1 (4%) | |
| Unknown | 1 (5%) | ||||
| Education: | Beyond high school | 18 (86%) | 18 (86%) | 20 (91%) | 23 (100%) |
| High school or less | 3 (14%) | 3 (14%) | 2 (9%) | ||
| Married | Yes | 12 (57%) | 15 (68%) | 16 (73%) | 12 (52%) |
| Time from last cancer Tx to intervention: | Mean (days) | 1,625 | 1,647 | 654 | 1363 |
| Minimum | 136 | 48 | 112 | 104 | |
| Maximum | 7,071 | 10,034 | 1957 | 6115 | |
| Type of cancer | Breast | 15 (71%) | 16 (73%) | 13 (59%) | 16 (70%) |
| Other | 6 (29%) | 6 (27%) | 9 (41%) | 7 (30%) | |
| Type of cancer treatment | Chemotherapy | 16 (76%) | 17 (77%) | 19 (84%) | 21 (92%) |
| Radiotherapy | 17 (81%) | 17 (77%) | 16 (73%) | 16 (70%) | |
| Insomnia at time of consent1 | Mean (SD) | 15.5 (4.7) | 15.0 (5.5) | 14.0 (5.1) | 14.7 (4.5) |
From Insomnia Severity Index
Sleep Continuity
Between Group Effects
Table 2 presents the between-group analyses of treatment condition for the sleep continuity variables. Participants in the CBT-I+A (p < .001) and CBT-I+P (p = 0.003) groups had significantly shorter SLs than those in the PLA group. No significant differences were observed in SL between the ARM and PLA groups (p = .17) or the CBT-I+A and CBT-I+P groups (p = .60). For reducing the amount of time spent awake during the night (WASO), the results suggest a trend with subjects in the CBT-I+A group doing slightly better than those in the PLA group (p = .02). No other between-group differences were found with respect to WASO. There were no significant between-group differences in TST.
Table 2.
Between group changes in sleep continuity variables compared to placebo.
| Estimate | SE | Lower 95% CI | Upper 95% CI | p-value | Effect Size | |
|---|---|---|---|---|---|---|
| Sleep latency | ||||||
| CBT-I + Placebo vs. Placebo | -18.62 | 5.25 | -29.14 | -8.10 | <0.001** | -0.67 |
| CBT-I + Armodafinil vs. Placebo | -16.16 | 5.27 | -26.73 | -5.59 | 0.003** | -0.58 |
| Armodafinil vs. Placebo | 7.98 | 5.68 | -3.45 | 19.41 | 0.17 | 0.29 |
| CBT-I + Armodafinil vs. CBT-I + Placebo | 2.46 | 4.67 | -6.70 | 11.61 | 0.60 | 0.09 |
|
| ||||||
| Wake after sleep onset | ||||||
| CBT-I + Placebo vs. Placebo | -10.00 | 5.50 | -20.96 | 0.96 | 0.07 | -0.48 |
| CBT-I + Armodafinil vs. Placebo | -13.14 | 5.59 | -24.27 | -2.01 | 0.02 | -0.64 |
| Armodafinil vs. Placebo | 10.23 | 7.56 | -5.05 | 25.51 | 0.18 | 0.50 |
| CBT-I + Armodafinil vs. CBT-I + Placebo | -3.14 | 5.16 | -13.24 | 6.97 | 0.54 | -0.15 |
|
| ||||||
| Total sleep time | ||||||
| CBT-I + Placebo vs. Placebo | -28.69 | 19.43 | -67.65 | 10.27 | 0.15 | -0.39 |
| CBT-I + Armodafinil vs. Placebo | -16.00 | 20.36 | -56.93 | 24.93 | 0.44 | -0.22 |
| Armodafinil vs. Placebo | -51.30 | 21.80 | -95.24 | -7.37 | 0.02 | -0.69 |
| CBT-I + Armodafinil vs. CBT-I + Placebo | 12.69 | 17.68 | -21.97 | 47.35 | 0.47 | 0.17 |
P < 0.01,
P < 0.001 A significance level of 0.0125 was used to adjust for multiple comparisons. (Asterisks denote improvements compared to placebo for between group analyses.); All analyses used multiple imputation; P-values for between group analyses are from ANCOVA controlling for values at time of consent; Effect size is Cohen’s D.
Within-Group Effects
Figure 1 presents the weekly data for SL, WASO, and TST. Table 3 presents these data as between-group differences in sleep continuity variables compared to PLA. As can be seen in Fig. 1 and Table 3, subjects receiving CBT-I experienced a statistically significant reduction in SL, with or without the addition of armodafinil (−37 min in CBT-I+P, p < .001; −26 min in CBT-I+A, p < .001). No significant changes in SL in the ARM or PLA groups were observed. CBT-I with or without the addition of armodafinil resulted in comparable changes in time spent awake after sleep onset (−14 min in CBT-I+P, p < .001; −17 min in CBT-T+A, p < .001). There was no significant reduction in the amount of time spent awake during the night in the ARM or PLA groups.
Figure 1.
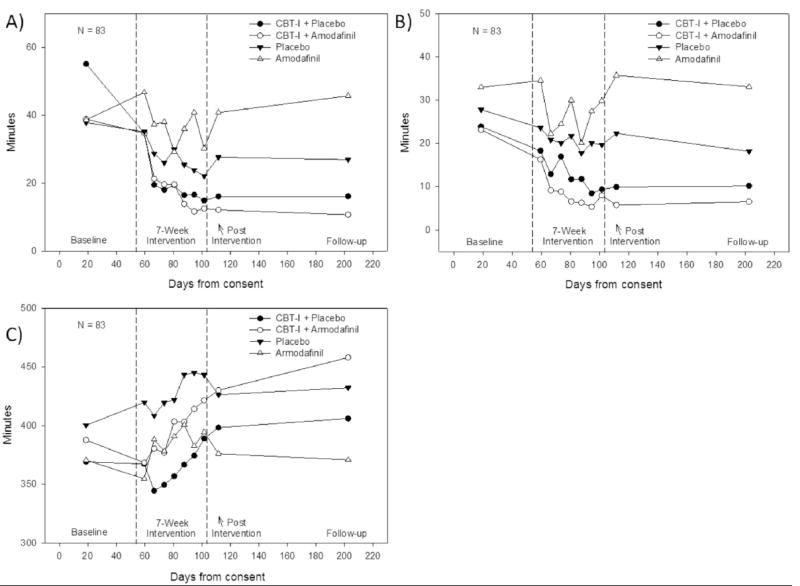
Effect of treatment arm on sleep latency, wake after sleep onset, and total sleep time.
Refer to Table 3 for within-group change values. A) Sleep Latency; B) Wake after Sleep Onset; C) Total Sleep Time.
Data points for baseline, post-program, and follow-up represent the raw average of 2 weeks. N per study arm beginning the intervention, at post intervention and at follow-up includes CBT-I + Placebo, 21, 19, 16; CBT-I + Armodafinil, 22, 17, 16; Placebo, 29, 14, 16; Armodafinil, 21, 14, 15.
Table 3.
Within group changes in sleep continuity variables from pre-to post-intervention
| Pre (SE) | Post (SE) | Post-Pre (SE) | Lower 95% CI for Mean | Upper 95% CI for Mean | p-value | Effect Size | |
|---|---|---|---|---|---|---|---|
| Sleep latency | |||||||
| CBT-I + Placebo | 52.89 (6.14) | 15.47 (2.81) | -37.42 (5.31) | -48.48 | -26.36 | <0.001** | -1.24 |
| CBT-I + Armodafinil | 39.34 (6.20) | 13.52 (2.84) | -25.82 (5.29) | -36.87 | -14.76 | <0.001** | -0.87 |
| Armodafinil | 37.63 (4.26) | 37.11 (5.08) | -0.52 (4.06) | -9.30 | 8.25 | 0.90 | -0.03 |
| Placebo | 41.40 (6.51) | 30.36 (5.16) | -11.04 (6.59) | -24.90 | 2.82 | 0.11 | -0.38 |
|
| |||||||
| Wake after sleep onset | |||||||
| CBT-I + Placebo | 23.25 (3.15) | 9.30 (2.36) | -13.95 (2.88) | -19.97 | -7.93 | 0.001** | -0.91 |
| CBT-I + Armodafinil | 22.59 (3.01) | 5.83 (1.73) | -16.76 (3.12) | -23.30 | -10.23 | <0.001** | -1.16 |
| Armodafinil | 32.95 (5.17) | 34.41 (8.66) | 1.45 (8.18) | -16.24 | 19.15 | 0.86 | 0.06 |
| Placebo | 29.56 (5.77) | 22.47 (4.22) | -7.09 (3.89) | -15.27 | 1.08 | 0.09 | -0.27 |
|
| |||||||
| Total sleep time | |||||||
| CBT-I + Placebo | 378.12 (17.29) | 405.45 (16.94) | 27.32 (9.88) | 6.48 | 48.17 | 0.01 | 0.33 |
| CBT-I + Armodafinil | 395.47 (16.14) | 430.75 (16.07) | 35.29 (13.06) | 7.38 | 63.19 | 0.02 | 0.46 |
| Armodafinil | 371.45 (13.72) | 377.98 (19.56) | 6.53 (15.74) | -27.73 | 40.79 | 0.69 | 0.10 |
| Placebo | 394.17 (16.31) | 445.81 (19.80) | 51.64 (19.00) | 11.10 | 92.18 | 0.02 | 0.71 |
P < 0.01,
P < 0.001;
A significance level of 0.0125 was used to adjust for multiple comparisons. (Asterisks denote improvements from pre-intervention for within group analyses.); All analyses used multiple imputation; Effect size is Cohen’s D.
Daytime Sleepiness
As can be seen in Table 4, participants in the CBT-I+P (p = 0.02) and CBT-I+A (p = 0.03) groups reported reductions in daytime sleepiness, but this did not reach statistical significance. There was no significant reduction in daytime sleepiness in the ARM or PLA groups. No between-group differences were observed.
Table 4.
Comparison of Epworth Sleepiness Scale at post-intervention by study conditions, as well as within group changes from pre-to post-intervention
| Epworth Sleepiness Scale | Estimate | SE | Lower 95% CI | Upper 95% CI | p-value | Effect Size |
|---|---|---|---|---|---|---|
| Between Group | ||||||
| CBT-I + Placebo vs. Placebo | -0.42 | 1.12 | -2.64 | 1.81 | 0.71 | -0.09 |
| CBT-I + Armodafinil vs. Placebo | -0.67 | 1.12 | -2.90 | 1.56 | 0.55 | -0.15 |
| Armodafinil vs. Placebo | -0.31 | 1.36 | -3.04 | 2.42 | 0.82 | -0.07 |
| CBT-I + Armodafinil vs. CBT-I + Placebo | -0.26 | 1.12 | -2.44 | 1.93 | 0.82 | -0.06 |
|
| ||||||
| Post-Pre | SE | Lower 95% CI | Upper 95% CI | p-value | Effect Size | |
|
| ||||||
| Within Group | ||||||
| CBT-I + Placebo | -1.91 | 0.73 | -3.47 | -0.35 | 0.02 | -0.51 |
| CBT-I + Armodafinil | -1.57 | 0.64 | -2.93 | -0.21 | 0.03 | -0.07 |
| Armodafinil | -1.71 | 1.29 | -4.51 | 1.10 | 0.21 | -0.47 |
| Placebo | -1.31 | 0.87 | -3.14 | 0.53 | 0.15 | -0.24 |
P < 0.01,
P < 0.001;
A significance level of 0.0125 was used to adjust for multiple comparisons. (Asterisks denote improvements compared to placebo for between group analyses and from pre-intervention for within group analyses and improvements compared to placebo for between group analyses.); All analyses used multiple imputation; P-values for between group analyses are from ANCOVA controlling for values at time of consent; Effect size is Cohen’s D.
Adherence
No significant differences were noted in the overall adherence observed between the patients assigned to CBT-I+A (M = 67.67, SD = 29.79) or CBT-I+P (M = 54.78; SD = 30.73), t (38) = 1.34, p = 0.19. Figure 2 plots adherence over time for both CBT-I groups. Average adherence with study medication was >90% for all study arms and did not differ significantly by group.
Figure 2.
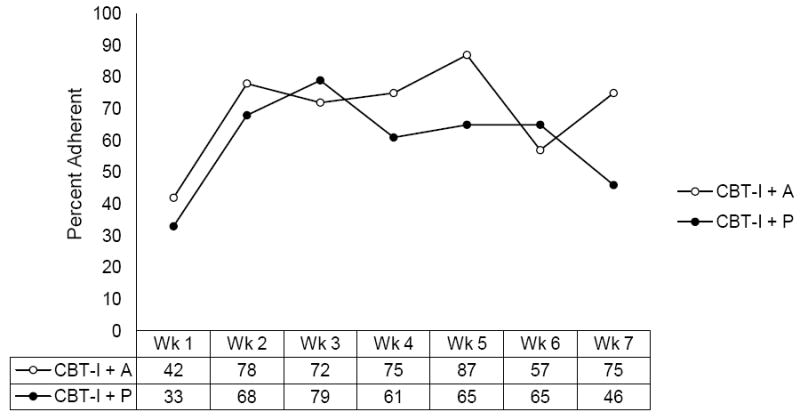
Weekly CBT-I adherence percentages by group.
Note: Adherence was evaluated by examining the actual time in bed compared to the prescribed time in bed for each week. Sleep restriction was initiated in the first session for the CBT-I+P and CBT-I+A groups only. Actual time in bed (ATIB) was determined from the time to bed and time out of bed questions on the sleep diary. Prescribed time in bed (PTIB) was recorded by the therapist for each CBT-I session. An individual was deemed adherent if his/her ATIB was +/-30 min of his/her PTIB. Adherence is relative to sample size.
DISCUSSION
This study examined the effect of CBT-I +/-− armodafinil on sleep continuity variables, daytime sleepiness, and adherence to sleep restriction instructions in a heterogeneous group of cancer survivors. The results from this study show that CBT-I (with or without armodafinil when compared to placebo) reduces SL in cancer survivors. Supplementing CBT-I with the wake-promoting medication armodafinil did not differentially influence sleep continuity variables, reduce sleepiness, or increase adherence. CBT-I, with or without armodafinil, decreased SL by ~30 min, reduced time spent awake during the night by ~15 min, increased TST by ~30 min, and decreased daytime sleepiness by an average 1.75 points. These findings correspond to large pre-to-post effect sizes for SL and WASO and a moderate (but not statistically significant) effect size for TST. The findings are consistent with other trials of CBT-I in cancer.31-33 The lack of effect for TST (during the acute treatment phase) is common to most CBT-I trials and represents the transient effects of sleep restriction (restricting TIB).13
Of note, patients in the placebo condition (in the absence of improvement on SL and WASO) also exhibited a trend toward increased TST with an average increase of 52 min of sleep per night. This effect may be ascribable to expectancy or the effects of increasing sleep opportunity (extending TIB) over time. In the case of the former, this is in line with recent studies that demonstrate strong subjective responses to placebos in the treatment of insomnia34,35 and highlights the importance of patient expectancy on outcomes in medication trials.
There are a number of possible reasons behind the inability of armodafinil to produce an additive effect on sleep continuity variables. First, several well-conducted trials have concluded that CBT-I produces robust clinical improvements in sleep outcomes in cancer survivors,19 and it may be difficult to improve sleep much beyond these effects, especially in the context of cancer. Alternatively, the standardized dosing protocol might have contributed to the lack of effect. In clinical practice, armodafinil dosing is individualized according to patient report of difficulty with either daytime sleepiness or difficulty staying awake until prescribed bedtime. Potentially, this problem could be resolved with a flexible dosing trial. Another (and related) possibility is that daytime sleepiness and high rates of daytime napping are predictive of treatment intolerance, attrition, and nonadherence. This, in combination, with the relatively low dose of armodafinil may have resulted in the lack of therapeutic effects.
The lack of finding with respect to sleepiness is surprising; there is, however, a precedent for modafinil/armodafinil’s lack of effect on fatigue. Several other trials in cancer have shown modafinil (and its enantiomer armodafinil) to be largely ineffective for cancer-related fatigue – a similar yet conceptually different phenomenon from daytime sleepiness.36-40 Both fatigue and sleepiness have physical and mental weariness as a component feature, but only sleepiness addresses the issue of the individual’s ability to stay wake when sleep is unwanted, inappropriate, or dangerous. It is also possible that patients in this trial were not pathologically sleepy (with or without the transient adverse effects of CBT-I). Consistent with this possibility is that the baseline ESS in this study was 8, which is almost identical to ESS scores in adult community samples,41 thereby indicating the possibility of a floor effect. By contrast, reductions in fatigue and/or sleepiness have been reported for patients with obstructive sleep apnea, ALS, major depressive disorder, and human immunodeficiency virus (HIV).42-45 Future studies are required to understand the exact nature of sleepiness and fatigue in cancer patients in order to design effective interventions.
The inability to demonstrate an effect of armodafinil on adherence is less surprising, especially given the nonsignificant effects on daytime sleepiness. While adherence to prescribed bed and wake times is an identified mediator of treatment effect,46 it is possible that our measure of adherence was not sensitive enough to assess the relative benefit (or lack thereof) of armodafinil in patients also receiving CBT-I. This may be true for two reasons: First, our measure of adherence (PTIB) was a global construct that did not allow for the assessment of patient adherence specifically to the prescribed TTB, the time spent out of bed at night, and the prescribed “rise time” (TOB). Thus, it was only possible to assess adherence to reduced TIB and not whether subjects were staying up to the appointed hour, leaving the bedroom when awake, or rising at the appointed hour regardless of the prior night’s sleep. A sharper resolution on these factors may have revealed changes in adherence where the global measure did not. Second, measuring adherence as a dichotomous variable may not adequately capture the complexity inherent in adherence to a behavioral intervention. Lastly, while adherence to CBT-I has room for improvement, it is comparable to other behavioral interventions (e.g., physical activity47 and lymphedema self-care48). As such, future efforts may focus on identifying those at risk for poor adherence and providing individual intervention instead of applying these techniques across all participants.
In summary, CBT-I was associated with clinically significant reductions in insomnia symptoms, specifically in SL and WASO in cancer survivors. The addition of armodafinil did not appear to enhance the overall efficacy of CBT-I on sleep continuity or daytime sleepiness. Armodafinil also did not significantly improve patient adherence with TIB prescriptions when administered in conjunction with CBT-I. These findings are consistent with other trials demonstrating that CBT-I causes robust and durable improvement in terms of cancer-related insomnia. Our results also add to a growing body of literature suggesting that armodafinil and/or modafinil are not effective pharmacological interventions for improving sleep and daytime function in cancer survivors. Future efforts should be focused on improving the availability of CBT-I within cancer centers, health networks, and in the community.
Highlights.
Cognitive behavior therapy for insomnia (CBT-I) is effective but adherence could be improved.
A wake-promoting medication (armodafinil) may help patients adhere to treatment.
CBT-I, with or without armodafinil, improves sleep continuity and sleepiness in cancer survivors.
Armodafinil did not increase adherence to CBT-I recommendations.
Future efforts should focus on identifying those at risk for poor adherence.
Acknowledgments
Supported by NCI grants 5 R01 CA126968, 2R25CA102618-01A1 UG1 CA18961, and R01AG041783. Study medication was provided by Teva Pharmaceuticals, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Global cancer facts & figures. 2. Atlanta, GA: American Cancer Society; 2011. [Google Scholar]
- 2.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester cancer center-community clinical oncology program. J Clin Oncol. 2010;28(2):292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM. Natural course of insomnia comorbid with cancer: An 18-month longitudinal study. J Clin Oncol. 2011;29(26):3580–3586. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 4.Palesh O, Aldridge-Gerry A, Ulusakarya A, Ortiz-Tudela E, Capuron L, Innominato PF. Sleep disruption in breast cancer patients and survivors. J Natl Compr Canc Netw. 2013;11(12):1523–1530. doi: 10.6004/jnccn.2013.0179. [DOI] [PubMed] [Google Scholar]
- 5.Forsythe LP, Helzlsouer KJ, MacDonald R, Gallicchio L. Daytime sleepiness and sleep duration in long-term cancer survivors and non-cancer controls: Results from a registry-based survey study. Support Care Cancer. 2012;20(10):2425–2432. doi: 10.1007/s00520-011-1358-7. [DOI] [PubMed] [Google Scholar]
- 6.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 7.NIH state-of-the-science conference statement on manifestations and management of chronic insomnia in adults. NIH Consens State Sci Statements. 2005;22(2):1–30. [PubMed] [Google Scholar]
- 8.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162(2):225–233. [PMC free article] [PubMed] [Google Scholar]
- 9.Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Reynolds CF, 3rd, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: A meta-analysis of treatment efficacy. JAMA. 1997;278(24):2170–2177. [PubMed] [Google Scholar]
- 10.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: Results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26(7):793–799. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 11.Murtagh DR, Greenwood KM. Identifying effective psychological treatments for insomnia: A meta-analysis. J Consult Clin Psychol. 1995;63(1):79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- 12.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: A meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151(8):1172–1180. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 13.Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: A systematic review. BMC Fam Pract. 2012;13(1):40. doi: 10.1186/1471-2296-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morin CM, Kowatch RA, Barry T, Walton E. Cognitive-behavior therapy for late-life insomnia. J Consult Clin Psychol. 1993;61(1):137–146. doi: 10.1037//0022-006x.61.1.137. [DOI] [PubMed] [Google Scholar]
- 16.Morin CM, Mimeault V, Gagne A. Nonpharmacological treatment of late-life insomnia. J Psychosom Res. 1999;46(2):103–116. doi: 10.1016/s0022-3999(98)00077-4. [DOI] [PubMed] [Google Scholar]
- 17.Morin CM, Vallieres A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: A randomized controlled trial. JAMA. 2009;301(19):2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey AG, Belanger L, Talbot L, et al. Comparative efficacy of behavior therapy, cognitive therapy, and cognitive behavior therapy for chronic insomnia: A randomized controlled trial. J Consult Clin Psychol. 2014;82(4):670–683. doi: 10.1037/a0036606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garland SN, Johnson JA, Savard J, et al. Sleeping well with cancer: A systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113–1124. doi: 10.2147/NDT.S47790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews EE, Schmiege SJ, Cook PF, Berger AM, Aloia MS. Adherence to cognitive behavioral therapy for insomnia (CBTI) among women following primary breast cancer treatment: A pilot study. Behav Sleep Med. 2012;10(3):217–229. doi: 10.1080/15402002.2012.666220. [DOI] [PubMed] [Google Scholar]
- 21.Perlis ML, Smith MT, Orff H, et al. The effects of modafinil and cognitive behavior therapy on sleep continuity in patients with primary insomnia. Sleep. 2004;27(4):715–725. doi: 10.1093/sleep/27.4.715. [DOI] [PubMed] [Google Scholar]
- 22.Drake C, Gumenyuk V, Roth T, Howard R. Effects of armodafinil on simulated driving and alertness in shift work disorder. Sleep. 2014;37(12):1987–1994. doi: 10.5665/sleep.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz JR, Khan A, McCall WV, Weintraub J, Tiller J. Tolerability and efficacy of armodafinil in naive patients with excessive sleepiness associated with obstructive sleep apnea, shift work disorder, or narcolepsy: A 12-month, open-label, flexible-dose study with an extension period. J Clin Sleep Med. 2010;6(5):450–457. [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz JR, Roth T, Drake C. Armodafinil in the treatment of sleep/wake disorders. Neuropsychiatr Dis Treat. 2010;6:417–427. doi: 10.2147/ndt.s3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roscoe JA, Garland SN, Heckler CE, et al. Randomized placebo-controlled trial of cognitive behavioral therapy and armodafinil for insomnia after cancer treatment. J Clin Oncol. 2015;33(2):165–171. doi: 10.1200/JCO.2014.57.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Hammond GC, Croudace TJ, Radhakrishnan M, et al. Comparative effectiveness of cognitive therapies delivered face-to-face or over the telephone: An observational study using propensity methods. PLoS One. 2012;7(9):e42916. doi: 10.1371/journal.pone.0042916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho FY, Chung KF, Yeung WF, Ng TH, Cheng SK. Weekly brief phone support in self-help cognitive behavioral therapy for insomnia disorder: Relevance to adherence and efficacy. Behav Res Ther. 2014;63C:147–156. doi: 10.1016/j.brat.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26(28):4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 32.Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: A randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32(5):449–457. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- 33.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. J Clin Oncol. 2005;23(25):6083–6096. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 34.Winkler A, Rief W. Effect of placebo conditions on polysomnographic parameters in primary insomnia: A meta-analysis. Sleep. 2014 doi: 10.5665/sleep.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perlis ML, McCall WV, Jungquist CR, Pigeon WR, Matteson SE. Placebo effects in primary insomnia. Sleep Med Rev. 2005;9(5):381–389. doi: 10.1016/j.smrv.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Hossain JL, Ahmad P, Reinish LW, Kayumov L, Hossain NK, Shapiro CM. Subjective fatigue and subjective sleepiness: Two independent consequences of sleep disorders? J Sleep Res. 2005;14(3):245–253. doi: 10.1111/j.1365-2869.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- 37.Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer. 2014;22(5):1233–1242. doi: 10.1007/s00520-013-2076-0. [DOI] [PubMed] [Google Scholar]
- 38.Spathis A, Fife K, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: Results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol. 2014;32(18):1882–1888. doi: 10.1200/JCO.2013.54.4346. [DOI] [PubMed] [Google Scholar]
- 39.Boele FW, Douw L, de Groot M, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: A multicenter randomized controlled trial. Neuro Oncol. 2013;15(10):1420–1428. doi: 10.1093/neuonc/not102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berenson JR, Yellin O, Shamasunder HK, et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support Care Cancer. 2015;23(6):1503–1512. doi: 10.1007/s00520-014-2486-7. [DOI] [PubMed] [Google Scholar]
- 41.Buysse DJ, Hall ML, Strollo PJ, et al. Relationships between the Pittsburgh sleep quality index (PSQI), Epworth sleepiness scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4(6):563–571. [PMC free article] [PubMed] [Google Scholar]
- 42.Krystal AD, Harsh JR, Yang R, Rippon GA, Lankford DA. A double-blind, placebo-controlled study of armodafinil for excessive sleepiness in patients with treated obstructive sleep apnea and comorbid depression. J Clin Psychiatry. 2010;71(1):32–40. doi: 10.4088/JCP.09m05536gry. [DOI] [PubMed] [Google Scholar]
- 43.Rabkin JG, Gordon PH, McElhiney M, Rabkin R, Chew S, Mitsumoto H. Modafinil treatment of fatigue in patients with ALS: A placebo-controlled study. Muscle Nerve. 2009;39(3):297–303. doi: 10.1002/mus.21245. [DOI] [PubMed] [Google Scholar]
- 44.Rabkin JG, McElhiney MC, Rabkin R, McGrath PJ. Modafinil treatment for fatigue in HIV/AIDS: A randomized placebo-controlled study. J Clin Psychiatry. 2010;71(6):707–715. doi: 10.4088/JCP.09m05171bro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(1):85–93. doi: 10.4088/jcp.v66n0112. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz DR, Carney CE. Mediators of cognitive-behavioral therapy for insomnia: A review of randomized controlled trials and secondary analysis studies. Clin Psychol Rev. 2012;32(7):664–675. doi: 10.1016/j.cpr.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Kampshoff CS, Jansen F, van Mechelen W, May AM, Brug J, Chinapaw MJ, Buffart LM. Determinants of exercise adherence and maintenance among cancer survivors: a systematic review. Int J Behav Nutr Phys Act. 2014 doi: 10.1186/1479-5868-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown JC, Cheville AL, Tchou JC, Harris SR, Schmitz KH. Prescription and adherence to lymphedema self-care modalities among women with breast cancer-related lymphedema. Support Care Cancer. 2014;22(1):135–43. doi: 10.1007/s00520-013-1962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


