Abstract
Building and maintaining a comprehensive yet simple set of standardized protocols for a cross-sectional image can be a daunting task. A single department may have difficulty preventing “protocol creep,” which almost inevitably occurs when an organized “playbook” of protocols does not exist and individual radiologists and technologists alter protocols at will and on a case-by-case basis. When multiple departments or groups function in a large health system, the lack of uniformity of protocols can increase exponentially. In 2012, the University of Colorado Hospital formed a large health system (UCHealth) and became a 5-hospital provider network. CT and MR imaging studies are conducted at multiple locations by different radiology groups. To facilitate consistency in ordering, acquisition, and appearance of a given study, regardless of location, we minimized the number of protocols across all scanners and sites of practice with a clinical indication-driven protocol selection and standardization process. Here we review the steps utilized to perform this process improvement task and insure its stability over time. Actions included creation of a standardized protocol template, which allowed for changes in electronic storage and management of protocols, designing a change request form, and formation of a governance structure. We utilized rapid improvement events (1 day for CT, 2 days for MR) and reduced 248 CT protocols into 97 standardized protocols and 168 MR protocols to 66. Additional steps are underway to further standardize output and reporting of imaging interpretation. This will result in an improved, consistent radiologist, patient, and provider experience across the system.
Keywords: Clinical workflow, Workflow re-engineering, Radiology workflow
Background
Protocol review for CT is mandated by the American College of Radiology [1] and the Joint Commission on the Accreditation of Hospitals [2]. The principle motivation is to achieve diagnostic quality images with the lowest radiation dose possible (ALARA) [3–5]. Most work in protocol management has defined tools to take the protocol output from the scanners and collect it in a standardized format on an electronic tool for the purposes of comparison and auditing [6–9]. However, little has been written about standardization of imaging protocols in order to provide a consistent radiologist, patient, and referring provider experience [4].
In 2012, the University of Colorado Hospital formed a large health system (UCHealth) in the most populous region of the state, known as the Colorado Front Range. The academic, self-contained medical center (University of Colorado Hospital) joined forces with two community health systems (Poudre Valley Health System and Memorial Health System) and became a five-hospital provider network. This network now consists of three distinct regions (Northern Colorado, Metro Denver and Colorado Springs,) with two private and one academic radiology groups. Growth of the health system has continued unabated with plans to triple in size over the next 10 years through build, affiliation, and acquisition. The already extensive collection of CT and MR protocols became a dizzying array of options across the new enterprise. There is a tremendous amount of patient movement within the system, with the same patient having imaging studies at a multitude of locations, often serviced by different radiology groups. This has resulted in an inconsistent experience for patients and providers, and the need for radiologists to have access to images regardless of where they are acquired. The IT vision for the system has provided a unified platform via a single instance of the EMR. The imaging platform has also been standardized so there is one instance of PACS (Phillips Healthcare, Andover, Massachusetts) in which all studies can be viewed by radiologists and referring providers via a single point of access in the EMR or via a single timeline in the PACS.
Three distinct radiology groups can yield a host of factors, resulting in “protocol creep.” Factors include individual radiologist preference, on-the-fly changes to protocols, and vendor-specific naming sequences. Most departments and groups have failed to impose strict control over protocol management, and have no organized governance structure in place. Technologists often feel pressured to change protocols in spite of awareness that chaos may ensue. As a result, there are usually multiple protocols for the same study type and indication, which effect all facets of the radiology process: technologists may have a difficult time identifying which protocol to use for a given patient on a given scanner; radiologists and referring providers may view two studies performed on the same patient for the same indication that look and hang differently, making side by side comparison tedious. In addition, patients now have the opportunity to view their studies and are confused by the variability in presentation states over time and when the prep and exam instructions are inconsistent for repeat studies.
Previously described protocol management tools can offer facile ways to compare exiting protocols side-by-side [7, 9]. Direct importation of technical parameters from the modalities is currently possible, which can allow review and identification of “outliers” and serve as a catalogue of the options. However, the root causes of protocol creep are not addressed by these systems nor do these products provide a reconciliation methodology between radiologists when protocol preferences differ.
At UCHealth, we took a different approach to protocol management. This effort focused on clinical indication-driven protocol selection and standardization, with the goal of minimizing the number of protocols across all scanners and sites of practice. Indication-driven protocol standardization allows several downstream events to occur electronically (time slot and resource scheduling, accurate and more specific prep instructions sent electronically, and accurate first-time authorizations). Eventually, systems may be able to update the protocols on all devices from the central repository (reverse flow of information). As a result, a patient’s CT or MR performed anywhere in the health system will be identical in terms of sequences performed. At the same time, this simplicity will allow technical parameters to be standardized. Radiologists, referring providers, and patients will see consistency in the ordering, acquisition, and appearance of a given study no matter where it is performed. Here, we review the steps implemented to carry out this process improvement task and insure its stability over time. Although this is a work in progress, because of the urgency many feel to implement protocol standardization, we were prompted to share our process, which could allow them to begin planning for and initiating similar procedures.
Methods
Initiation of protocol standardization was structured around a body part and modality approach. We focused on CT and MR modalities first, due to their high volumes and potential positive impacts. A meeting was scheduled by PS, with invitations extended to a radiologist from each of the three regions, to review the chest CT protocols as a trial for standardizing protocols. Only two of the three regions participated, but consensus was reached on a single set of imaging protocols. However, the pace of review and change would be unacceptably slow if individual meetings had to be scheduled for each body part in CT followed by MR. Therefore, with the leadership of the radiology department’s full-time process improvement consultant (KH), two separate rapid improvement events (RIE) were scheduled: 1 day dedicated to CT, and 2 days dedicated to MR.
The RIE was attended by the section specific radiologist from each region and one to two lead technologists from each region. The radiologist can determine the appropriate clinical indications for each protocol and how many protocols are actually needed, while the technologist has insight into the technical and workflow issues that go into the actual scanning and reconstruction activities [4]. The 1-day CT RIE was divided into four segments (chest, abdomen, neuro, and MSK) with overlap of neuro and MSK to address spine imaging (a shared responsibility at the academic campus). The 2-day MR RIE used the same format, the increased time allowed for the greater complexity of MR protocols. KH and PS served as facilitators, setting ground rules at the onset of each session. The facilitators re-emphasized the purpose of the session, desired outcomes and role responsibilities, in order to gain increased buy-in from the participants. Additional facilitation techniques were used to ensure sessions were focused and productive and to encourage collaboration: the team reviewed each region’s current protocol and the facilitators emphasized areas of commonality; suggestions on how to address discrepancies were gathered; and a new or modified protocol was proposed. The facilitators checked for understanding and obtained agreement (both verbally and non-verbally) before proceeding to the next protocol.
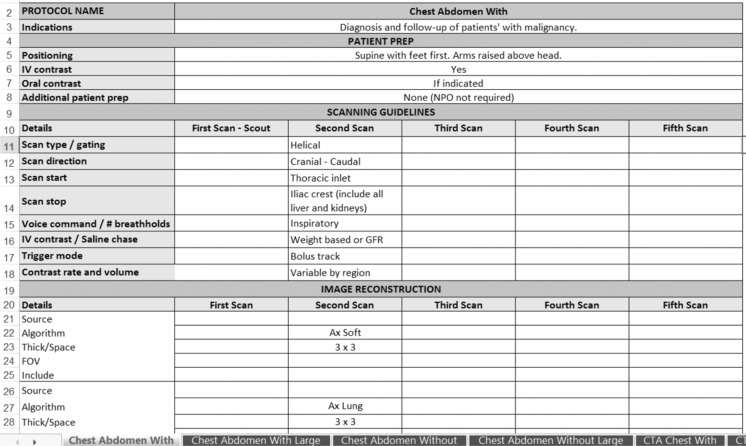
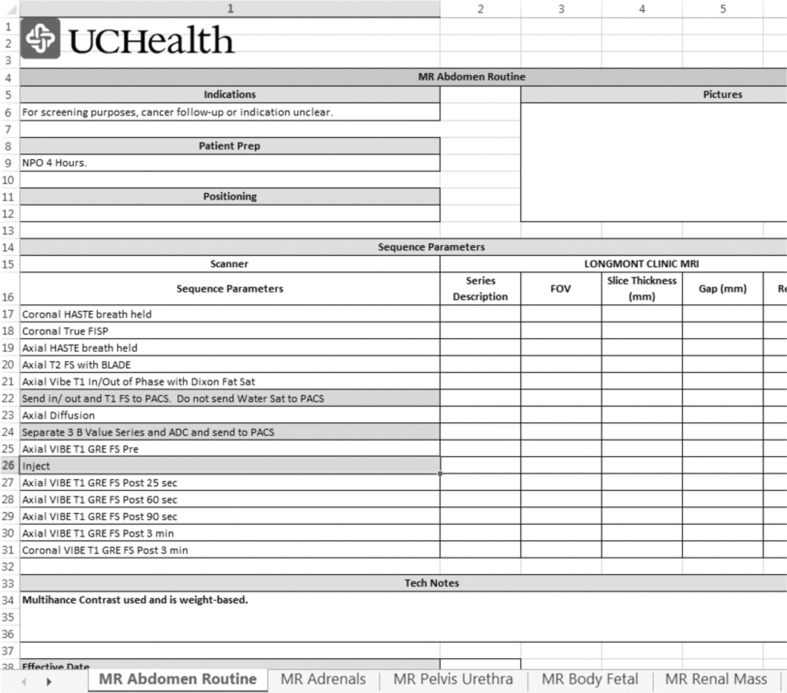
To allow for changes in electronic storage and management of protocols, a standardized protocol template was formatted in Excel for CT and MR prior to the RIE (Figs. 1 and 2). Titles from all existing protocols were collected from the three regions and placed in one spreadsheet. Prior to the RIE, similar protocols (for the same indication) were matched and reviewed, followed by the remaining protocols on the list (from any region). This protocol review list was distributed to all team members at the beginning of the RIE sessions. For each CT protocol, the team discussed and agreed upon the protocol name, required acquisitions, and reconstructions. For MR, the team discussed and gained consensus on the protocol title, indications, and series. After completion of the CT RIE, KH and PS documented the discussion details, finalized the protocols on paper, and entered the information into the CT template. For the MR RIE, the agreed upon titles, indications, and sequences were documented in real time in the MR electronic template to minimize post-session manual data entry.
Fig. 1.
A portion of an example Excel template used for standardization of CT protocols
Fig. 2.
A portion of an example Excel template used for standardization of MR protocols
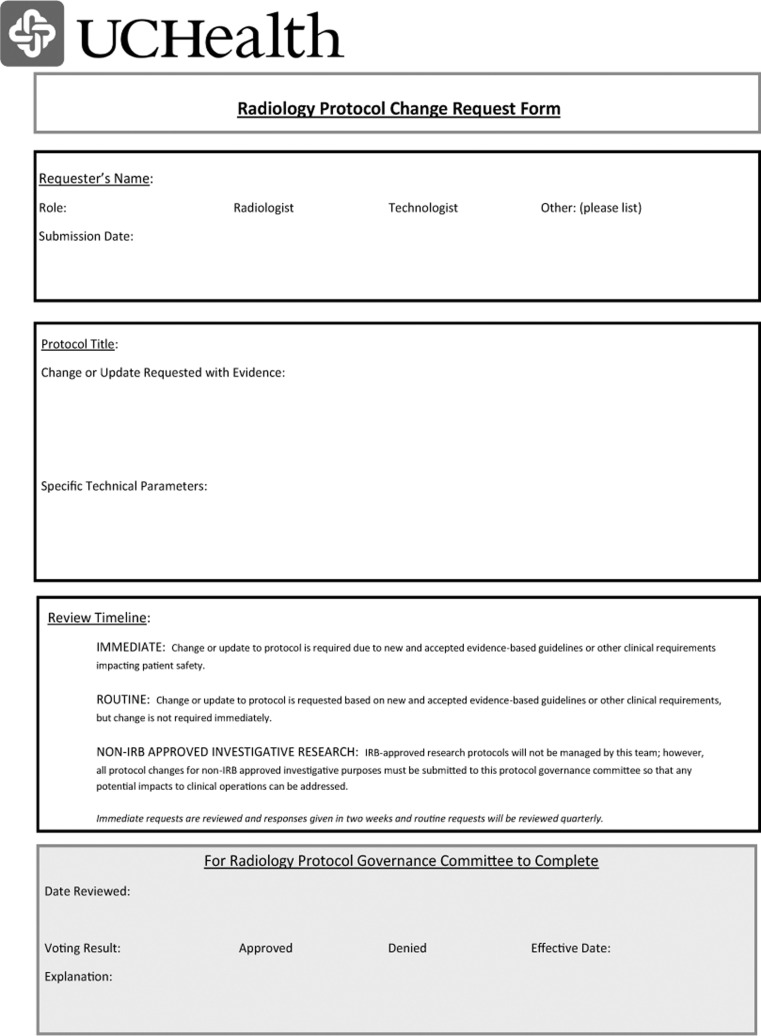
Preventing a return to the one-offs and degradation of standardization requires a transparent, yet nimble, change request and governance structure. PS and KH designed a protocol change request form (Fig. 3) and proposed the formation of a governance committee consisting of one radiologist, one CT technologist, and one MR technologist from each of the three regions as well as two ad hoc members (KH as PI lead and PBS as informatics lead). All RIE participants reviewed and edited the governance structure and change form, which was then approved by the Radiology Service Line Executive Committee and distributed to all radiologists and technologists (via their managers). The request form provides three levels of priority for protocol change: (1) if a patient is on the table and clinical needs require a change for patient safety, the radiologists and technologists are free to do so, but this cannot result in a change to the stored protocol on the machine; (2) if evidence mandates an urgent change to protocol across the enterprise, the form is submitted as level 2 and the governance committee will respond within 2 weeks; (3) if the request is non-urgent, the requesting party must submit evidence with the request and the committee will respond within 3 months [3].
Fig. 3.
Radiology Protocol Change Request Form required for any desired alteration in a CT or MR protocol
Results
During the CT RIE we reviewed 248 CT protocols, which were combined into 97 standardized protocols. On only two occasions was there significant disagreement on sequence selection, but in both cases, the facilitation process allowed consensus to be reached. The MR RIE reviewed 168 MR protocols, which were combined into 66 protocols. Conducting the RIEs and completing entry of data into the templates required 4 weeks. We anticipate all additional work will be completed in 2–3 months. The protocol manager tool will then serve as the “source-of-truth” against which any altered protocols can be compared. We also foresee using this tool to perform audits of the protocols.
Our EMR assigns imaging exam orders a unique tag, which is labeled a visit type. Currently, studies are assigned visit types based on CPT codes; for example, CT abdomen and pelvis have a single visit type assigned, although there may be several different protocols for this exam. The visit type dictates downstream events such as the performing resource (actual machine required), time slot selections, and related patient exam/prep instructions. In our new system, each protocol will be assigned its own visit type in the imaging order database. One individual visit type per protocol, per region will be needed. Therefore, the 97 CT and 66 MR protocols will require a total build of 489 visit types for the three regions. The build effort for 489 visit types will require 10 min each for a total of 80 h of actual build time. This effort will require approximately 2 months, including meetings, issues, and go-lives. As we grow as a system, building more procedures and visit types will be required, but we estimate the percentage of the total will be small (<10 %).
Discussion
Achieving consensus on protocols proved surprisingly easy. We were able to reduce the number of protocols without a significant amount of disagreement and in a reasonable period of time. Although the work effort to get the data into the templates was substantial, and the visit-type build effort is significant, each visit type can be tied to a unique performing resource (if required), and the correct time slot, protocol specific exam instructions, and prep can be sent to the patient electronically via the patient portal, email, or telephone. For these functions to work correctly, all orders will require protocolling before scheduling, which is in contrast to the current procedure where ordered exams drop to a protocol work list but may not be protocolled before scheduling. Protocolling prior to scheduling represents a change in workflow for most groups, a potential disadvantage, and is likely the key element preventing others from considering this process. Each region will have hot phones for urgent protocolling. These are already in place for ad hoc technologist questions and protocolling and have been proven to work well. A key advantage of this system, however, is preventing the need to obtain repeat insurance authorization if a protocol is changed after scheduling, a step which occurs with some frequency in the current system.
Given the complexity of the process, utilizing the RSNA Radlex playbook for naming will be included in a second project. This effort will standardize all reporting templates across the health system using these protocols as the basis for standardization, one for each unique protocol, and we anticipate implementing the playbook at that time.
Completion of protocol standardization requires a number of additional steps, which are already underway. First, the lead CT and MR technologists from each region enter machine-specific details into the standard protocol format, including kVp, Reference mAs, FOV and rotation time for CT scanners and FOV, slice thickness, and gap for MR magnets. Next, a team of physicists reviews the protocols to ensure technical parameters are correct, and dose is optimized for CT. All protocols are then uploaded to a protocol management tool (Primordial Design, Inc., San Mateo, California). Lead technologists will be responsible for updating all machines to reflect the new protocols, series names, and attached technical parameters, and deleting pre-existing protocols. Concurrently, the EMR team will update the imaging order database to reflect the new protocol names, while deleting those no longer available, and update the referring provider ordering preference lists. Finally, we will work with our vendor/developer to enable upload of protocols from the protocol manager tool back to the individual machines, eliminating the cumbersome manual updating of protocols by technologists and physicists currently required.
Conclusion
UCHealth is growing rapidly, with an expected tripling in size over the next 10 years. Standardized imaging protocols will provide a consistent image acquisition, viewing, and interpretation experience for referring providers, patients, technologists, and radiologists. These protocols will be available as a plug-and-play guide for acquisitions and new builds (and affiliations, if desired). The protocol standardization across all regions of our health system insures the continuity of these protocols and will significantly decrease the work effort for the information technology teams as expansion continues. We anticipate collecting data and reporting on a number of variables including compliance with official protocols, frequency of protocol revision, efficacy of protocolling before scheduling, and user satisfaction with the workflow.
References
- 1.Cody DD, Pfeiffer D, McNitt-Gray MF, Ruckdeschel TG, Strauss KJ, Wilcox P. CT quality control manual. Reston: ACR; 2012. [Google Scholar]
- 2.Revised requirements for diagnostic imaging services; 2013. The Joint Commission on Prepublication Requirements. Available at: http://www.jointcommission.org/assets/1/6/PREPUB-12-20-2013-DiagImaging_ HAP_CAH.pdf
- 3.Kofler JM, Cody DD, Morin RL. CT protocol review and optimization. J Am Coll Radiol. 2014;11(3):267–70. doi: 10.1016/j.jacr.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Trattner S, Pearson GDN, Chin C, et al. Standardization and Optimization of CT Protocols to Achieve Low Dose. J Am Coll Radiol. 2014;11(3):271–78. doi: 10.1016/j.jacr.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szczykutowicz TP, Bour RK, Pozniak M, Ranallo FN. Compliance with AAPM Practice Guideline 1.a: CT Protocol Management and Review - from the perspective of a university hospital. J Appl Clin Med Phys. 2015;16(2):5023. doi: 10.1120/jacmp.v16i2.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D, Savage CA, Li X, Liu B. Data-driven CT protocol review and management—experience from a large academic hospital. J Am Coll Radiol. 2015;12(3):267–72. doi: 10.1016/j.jacr.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Szczykutowicz TP, Rubert N, Belden D, et al. A Wiki Based CT Protocol Management System. Radiol Manage. 2015;37(6):25–9. [PubMed] [Google Scholar]
- 8.Szczykutowicz TP, Siegelman J. On the same page--physicist and radiologist perspectives on protocol management and review. J Am Coll Radiol. 2015;12(8):808–14. doi: 10.1016/j.jacr.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Szczykutowicz TP, Bour RK, Rubert N, Wendt G, Pozniak M, Ranallo FN. CT protocol management: simplifying the process by using a master protocol concept. J Appl Clin Med Phys. 2015;16(4):5412. doi: 10.1120/jacmp.v16i4.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]