Abstract
Objective
Clinical studies using serum cardiac biomarkers to investigate a circadian variation in acute myocardial infarct (MI) size in ST-segment elevation myocardial infarction (STEMI) patients reperfused by primary percutaneous coronary intervention (PPCI) have produced mixed results. We aimed to investigate this phenomenon using acute MI size measured by cardiovascular magnetic resonance (CMR).
Methods
Patient-level data was obtained from 4 randomized controlled trials investigating the MI-limiting effects of cardioprotective therapies in this pooled analysis. The primary analysis was performed in those patients with no pre-infarct angina; duration of ischemia > 60 min and < 360 min; Thrombolysis In Myocardial Infarction (TIMI) flow pre-PPCI ≤ 1; TIMI flow post-PPCI 3; and no collateral flow.
Results
169 out of 376 patients with CMR data met the inclusion criteria for the primary analysis. A 24-hour circadian variation in acute MI size as a % of the area-at-risk (%AAR), after adjusting for confounders, was observed with a peak and nadir MI size in patients with symptom onset between 00:00 and 01:00 and between 12:00 and 13:00 respectively (difference from the average MI size 5.2%, 95%CI 1.1–9.4%; p = 0.013). This was associated with a non-significant circadian variation in left ventricular ejection fraction (LVEF) (difference from the average LVEF 5.9%, 95%CI − 0.6–2.2%, p = 0.073). There was no circadian variation in MI size or LVEF in the whole cohort.
Conclusions
We report a circadian variation in acute MI size assessed by CMR in a subset of STEMI patients treated by PPCI, with the largest and smallest MI size occurring in patients with symptom onset between 00:00 and 01:00 and between 12:00 and 13:00 respectively.
Keywords: ST-segment elevation myocardial infarction, Circadian rhythm, Myocardial infarct size, Cardiovascular magnetic resonance
What is already known about this subject?
Several studies [1], [2], [3], [4], [5], [6], [7] have shown a circadian variation in myocardial infarct (MI) size by cardiac enzymes according to the time of onset of symptoms but the literature is divided regarding the timing of the peak MI size. Some studies have shown that peak MI size occurs in patients with symptom onset between midnight and 06:00 [3], [6], [7] while others have shown peak MI size occurring in those with symptoms onset between 06:00 and noon [1], [4], [5].
What does this study add?
In this study we used cardiac magnetic resonance (CMR), which is the gold standard method for assessing MI size, and we adjusted for the area-at-risk (AAR) and confounders. We confirmed using CMR that in a selected group of patients a circadian pattern in MI size exists and that peak MI size occurred in those with symptom onset between midnight and 06:00. This was associated with a non-significant circadian variation in acute left ventricular ejection fraction (LVEF).
How might this impact on clinical practice?
The circadian dependence in acute MI size was only observed in a subset of patients entering RCTs. This needs to be taken into account to make sure that the patients are adequately balanced according to the time of symptom onset when designing future RCTs aiming to reduce MI size. Whether this would impact on the effectiveness of the cardioprotective therapies needs to be assessed in future larger studies.
1. Introduction
The circadian rhythm has been shown to modulate cardiovascular physiology, impacting on parameters such as heart rate and blood pressure [8], [9], [10], [11], [12] via the expression of a circadian clock gene in the heart [13], [14]. A circadian oscillation has also been shown to impact on the expression of some proteins in the pro-survival pathways [15], and the susceptibility of the myocardium to acute ischemia/reperfusion injury (IRI), following myocardial infarction (MI) in mammalian hearts [16], [17].
Durgan et al. [18] reported the existence of circadian dependence in MI size according to the time of day in a murine model of acute myocardial IRI. Since then, several groups have investigated whether a circadian variation also exists in ST-segment elevation myocardial infarction (STEMI) patients but the results have been conflicting, both in terms of the timings for the peak and the nadir of acute MI size [1], [2], [3] and whether the phenomenon exists at all in humans [19]. Clinical studies investigating the circadian variation in acute MI size have assessed irreversible myocardial injury using serum cardiac biomarkers such as creatine kinase (CK) [1], [2], [3], [6], CK-MB [5], and troponin I [1], [20], and have determined the effects on left ventricular ejection fraction (LVEF) by echocardiography [5]. Cardiovascular magnetic resonance (CMR) is considered the gold standard for acute MI size quantification [21], [22], [23], the measurement of LV volumes and LVEF [23], [24], and can also provide information on the area-at-risk (AAR) [25], [26], but so far it has not been used to investigate the circadian variation in acute MI size in STEMI patients. If a circadian dependence in acute MI size is also observed by CMR, this would be an important factor to be taken into account in the design of future randomized controlled trials (RCTs) aiming to reduce MI size.
Therefore the aim of the current study was to investigate whether there is a circadian variation in acute MI size measured by CMR in STEMI patients reperfused by primary percutaneous coronary intervention (PPCI). In order to minimize the heterogeneity of the patient population and to remove potential confounders for acute MI size, the primary analysis was performed using a pre-defined subset of STEMI patients meeting specific criteria as previously described by Reiter et al. [3]
2. Methods
2.1. Study population
This was a pooled analysis of patient-level data obtained from 4 published randomized controlled trials investigating the benefit of cardioprotective therapies in STEMI patients treated by PPCI, and using acute MI size by CMR as an end-point (Table 1). The individual methods and results for each study have been published previously [27], [28], [29], [30] and all studies were conducted in accordance to the Declaration of Helsinki.
Table 1.
Details of the 4 RCTs with patient level data included in this study.
| Studies | Country/years | Intervention | Patients in study | Timing of CMR | Area-at-risk | Acute MI size | Outcome |
|---|---|---|---|---|---|---|---|
| Ludman [27] | UK/2007–2009 | Erythropoietin | 51 | 1–6 days | Endocardial surface area | 10 min after 0.2 mmol/kg Dotarem, | Erythropoietin failed to reduce MI size |
| Crimi [28] | Italy/2009–2011 | Remote ischemic conditioning of the lower limbs | 100 | 3–5 days | T2-weighted imaging | 15 min after 0.2 mmol/kg Magnevist | Reduction in enzymatic MI size in the intervention arm. Under-powered to see a difference in MI size by CMR |
| Garcia-Dorado [30] | Spain/2008–2011 | Adenosine | 201 | 2–7 days | T2-weighted imaging | 10 min after 0.2 mmol/kg of Magnevist | No difference in acute MI size in the whole cohort |
| White [29] | UK/2011–2012 | Remote ischemic conditioning of the upper limb | 197 | 3–6 days | T2 mapping | 10 min after 0.1 mmol/kg of Dotarem | 27% reduction in MI size by CMR in the intervention arm |
MI: myocardial infarct; CMR: cardiovascular magnetic resonance.
2.2. Outcomes
The main outcome of interest was acute MI size by CMR, expressed as a % of the AAR (%AAR) and LVEF. The primary exposure of interest in all analyses was the time of day, defined as the time of onset of MI symptoms and recorded to the nearest minute. The primary analysis examined the effect of time as a continuous measure, using a periodic sinusoidal function for the circadian cycle by time of symptom onset, as per the methods of Reiter et al. [3] in a selected group of patients defined by the following criteria [3]: no pre-infarct angina (which may have inadvertently preconditioned the patients and reduced MI size); duration of ischemia > 60 min and < 360 min; Thrombolysis In Myocardial Infarction (TIMI) flow pre-PPCI 0 or 1; TIMI flow post-PPCI 3; and no retrograde filling of the distal vessels (Rentrop grade 0). A secondary analysis was performed in the total unselected cohort of patients.
2.3. Statistical analysis
All analysis was performed using Stata version 12.1 (StataCorp, College Station, Texas). The period of the circadian cycle was defined in advance by converting time of onset into radians using the appropriate scale. For example, to model a 24-hour period in the circadian cycle the conversion is:
| (1) |
Then the sinusoidal function for infarct size (y) is expressed as follows:
| (2) |
The parameters to be estimated were: α representing the mean infarct size; β representing the amplitude of the rhythm; ω representing the phase of the curve, which determines when in the circadian cycle that the maximum and minimum infarct sizes occur.
The model in Eq. (2) was fitted using trigonometric linear regression as outlined by Cox [31]. A 24-hour period for circadian rhythm was assumed for the initial model and then further terms were included for sinusoidal function with a 12-hour period. Following fitting of the model using trigonometric linear regression, the delta method was used to provide Wald test p-values and 95% confidence intervals. Analysis was also performed by grouping patients into quartiles by time of day of onset of symptoms (00:00 to 05:59, 6:00 to 11:59, 12:00 to 17:59, 18:00 to 23:59).
The following confounders for MI size were adjusted for in the primary analysis: originating study, intervention (control, erythropoietin, remote ischemic conditioning upper limb or lower limb, adenosine); baseline demographic characteristics such as age and gender; onset to balloon time; risk factors (smoking; diabetes; hypertension; dyslipidemia; family history of coronary artery disease); and infarct related artery. For the secondary analysis, adjustment was made for these additional confounders: pre-infarct angina; TIMI flow pre-PPCI; presence of collateral flow; and TIMI flow post-PPCI.
3. Results
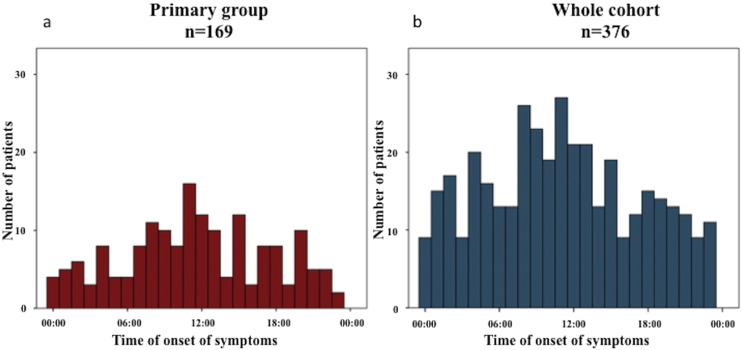
Acute CMR data were available in 376 patients and 169 patients met the inclusion criteria for the primary analysis. Fig. 1 shows the distribution of patients in the primary analysis and for the whole cohort according to the time of symptom onset. Data on the baseline demographics, angiographic and CMR findings of the whole cohort is provided in Table 2. Table 3 provides further details between those not included (n = 207) and included (n = 169) in the primary analysis. Apart from the selection criteria used, the other notable differences between patients in the primary analysis when compared to those not included in the primary analysis were: more patients from Ludman et al. [27] and White et al. [29] and less patients from Garcia-Dorado [30]; more males; less smokers; and more right coronary artery territory MI and less circumflex territory MI.
Fig. 1.
Distribution of patients according to time of onset of symptoms: (a) primary group of 169 patients; (b) whole cohort.
As expected, significantly more patients were recruited in these RCTs with time of symptom onset occurring during daytime.
Table 2.
Patients' characteristics for the whole cohort.
| Total number of patients (n = 376) |
|
|---|---|
| Trials | |
| Ludman [27] | 40 (11%) |
| Crimi [28] | 76 (20%) |
| Garcia-Dorado [30] | 177 (47%) |
| White [29] | 83 (22%) |
| Randomization | |
| Intervention | 191 (51%) |
| Placebo | 185 (49%) |
| Age | 59 ± 12 |
| Sex — male | 193 (51%) |
| Risk factors | |
| Smoking | 221 (59%) |
| Hypertension | 166 (44%) |
| Dyslipidemia | 117 (31%) |
| Diabetes Mellitus | 55 (15%) |
| Family history of CAD | 72 (19%) |
| Pre-infarct angina | 108 (29%) |
| Onset to balloon time/min | 215 ± 97 |
| Artery involved | |
| LAD | 211 (56%) |
| RCA | 71 (19%) |
| Cx | 94 (25%) |
| TIMI flow pre-PPCI | |
| 0 | 365 (97%) |
| 1 | 11 (3%) |
| Rentrop collateral flow | |
| 0 | 287 (76%) |
| 1 | 61 (16%) |
| 2 | 28 (7%) |
| TIMI flow post-PPCI | |
| 1 | 1 (1%) |
| 2 | 43 (11%) |
| 3 | 330 (88%) |
| CMR details | |
| LVEDV | 154 ± 36 |
| LVESV | 78 ± 31 |
| LVEF | 50 ± 11 |
| MI size/%LV | 22 ± 11 |
| AAR/%LV | 33 ± 12 |
| MI size/%AAR | 64 ± 21 |
| MVO/% | 111 (53%) |
CAD: coronary artery disease; LAD: left anterior descending artery; RCA: right coronary artery; Cx: circumflex artery; TIMI: Thrombolysis In Myocardial Infarction; PPCI: primary percutaneous coronary intervention; LVEDV: left ventricular end diastolic volume; LVESV: left ventricular end systolic volume; LVEF: left ventricular ejection fraction; AAR: area at risk; LV: left ventricle; MVO: microvascular obstruction.
Table 3.
Patients' characteristics for those in the primary analysis compared to those not in the primary analysis.
| Patients not in primary analysis n = 207 |
Patients in primary analysis n = 169 |
p value | |
|---|---|---|---|
| Trials | |||
| Ludman [27] | 12 (30%) | 28 (70%) | < 0.001 |
| Crimi [28] | 39 (51%) | 37 (49%) | |
| Garcia-Dorado [30] | 131 (74%) | 46 (26%) | |
| White [29] | 25 (30%) | 58 (70%) | |
| Randomization | |||
| Intervention | 107 (56%) | 84 (44%) | 0.39 |
| Placebo | 100 (54%) | 85 (46%) | |
| Age | 58 ± 12 | 59 ± 11 | 0.16 |
| Sex — male | 80 (42%) | 113 (58%) | < 0.001 |
| Risk factors | |||
| Smoking | 134 (61%) | 87 (39%) | 0.006 |
| Hypertension | 91 (55%) | 75 (45%) | 0.51 |
| Dyslipidemia | 62 (53%) | 55 (47%) | 0.33 |
| Diabetes mellitus | 30 (55%) | 25 (45%) | 0.52 |
| Family history of CAD | 41 (57%) | 31 (43%) | 0.41 |
| Onset to balloon time/min | 235 ± 118 | 192 ± 64 | < 0.001 |
| Artery involved | |||
| LAD | 114 (54%) | 97 (46%) | 0.36 |
| RCA | 31 (44%) | 40 (56%) | 0.02 |
| Cx | 62 (66%) | 32 (34%) | 0.009 |
| CMR details | |||
| LVEDV | 155 ± 39 | 153 ± 33 | 0.51 |
| LVESV | 81 ± 34 | 75 ± 25 | 0.56 |
| LVEF | 49 ± 11 | 52 ± 11 | 0.054 |
| MI size/%LV | 22 ± 12 | 22 ± 10 | 0.95 |
| AAR/%LV | 34 ± 13 | 32 ± 11 | 0.25 |
| MI size/%AAR | 63 ± 20 | 66 ± 21 | 0.08 |
| MVO/% | 111 (53%) | 98 (47%) | 0.23 |
CAD: coronary artery disease; LAD: left anterior descending artery; RCA: right coronary artery; Cx: circumflex artery; TIMI: Thrombolysis In Myocardial Infarction; PPCI: primary percutaneous coronary intervention; LVEDV: left ventricular end diastolic volume; LVESV: left ventricular end systolic volume; LVEF: left ventricular ejection fraction; AAR: area at risk; LV: left ventricle; MVO: microvascular obstruction.
3.1. AAR and circadian rhythm
In the unadjusted analysis, there was evidence of a 24-hour cycle circadian variation in AAR with the difference between natural log of average and the peak value estimated as 0.07 (95% CI 0.01 to 0.12, p = 0.027). After adjusting for confounders, there was no longer evidence of a circadian rhythm in the AAR with the difference between natural log of average and peak values of 0.03 (95% CI − 0.02 to 0.08, p = 0.27). When a 12-hour cycle of circadian dependence was considered, there was no evidence of a circadian variation in AAR.
3.2. Primary analysis
There was evidence for a 24-hour cycle in MI size as %AAR after adjusting for confounders with the maximum MI size being 5.2% larger than the average MI size (95 CI 1.1 to 9.4%, p = 0.013) (Fig. 2). The MI size was the largest for symptom onset between 00:00 and 01:00, and the smallest for symptom onset between 12:00 and 13:00. There was no evidence for a 12-hour circadian rhythm in MI size expressed as %AAR (for adjusted model: maximum MI size being 3.3% larger than the average, 95% CI − 0.6 to 7.2%, p = 0.10). When patients were grouped into quartiles by time of day of onset of symptoms (00:00 to 05:59, 6:00 to 11:59, 12:00 to 17:59, 18:00 to 23:59), the largest MI size occurred in the 00:00 to 05:59 group (Fig. 3a) and there was no significant difference in the duration of symptoms by quartiles to account for that (Fig. 3b).
Fig. 2.
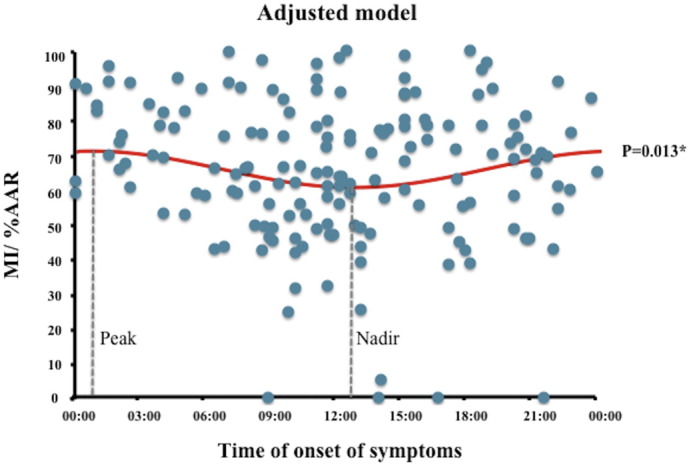
Circadian variation in MI size as a percentage of the AAR in the subset of patients in the primary analysis.
The peak MI size/%AAR occurred between 00:00 and 01:00 and the nadir occurred between 12:00 and 13:00.
* denotes statistical significant with a p value of < 0.05.
Fig. 3.
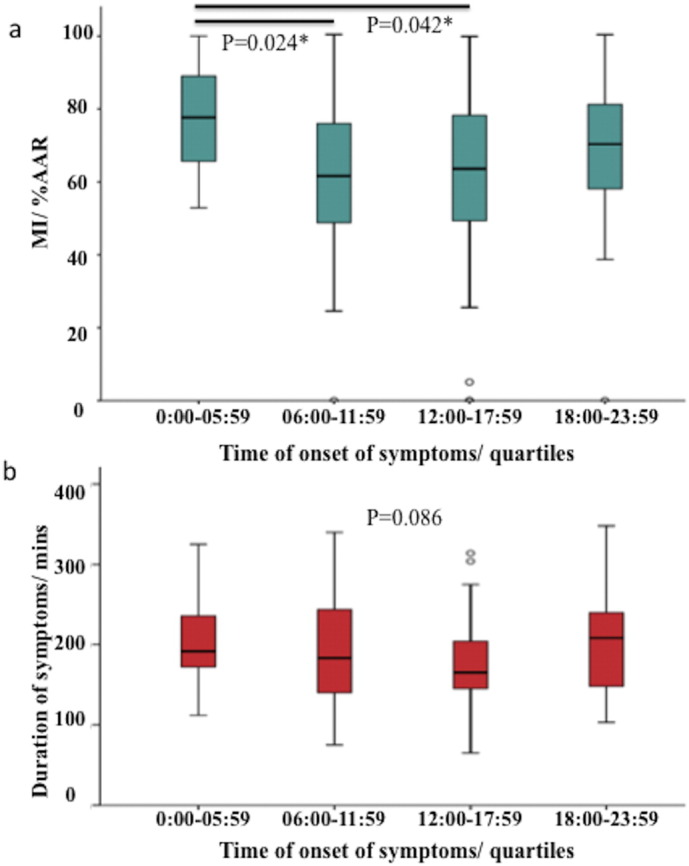
(a) MI size as a percentage of the AAR in the subset of patients in the primary analysis divided into quartiles of time of onset of symptoms; (b): comparison of duration of symptoms according to quartiles of time of onset of symptoms.
The largest MI size occurred in the 00:00 to 05:59 group and there was no difference in the duration of symptoms in the 4 quartiles.
* denotes statistical significant with a p value of < 0.05.
There was a non-significant circadian variation in LVEF in this subgroup both before and after adjusting for confounders (unadjusted model: difference between average and peak LVEF 6.0%, 95% CI − 0.6 to 12.6%, p = 0.074; adjusted model: difference between average and peak LVEF 5.9%, 95% CI − 0.6 to 12.23%, p = 0.073).
3.3. Secondary analysis — whole cohort
When MI size was expressed as %AAR, there was no evidence of a 24-hour cycle of circadian dependence for MI size (%AAR) both in the unadjusted model (difference between the average and peak value: 1.8%, 95% CI − 1.2 to 4.9%; p = 0.24) and after adjusting for confounders (difference between the average and peak value: 2.7%, 95% CI − 0.1 to 5.5%; p = 0.06). When a 12-hour cycle of circadian dependence was considered, there was no evidence of a circadian variation in MI size expressed as a percentage of the AAR.
When considering LVEF, there was no evidence of a 24-hour cycle of circadian variation both in the adjusted and unadjusted analysis. When a 12-hour circadian cycle was considered, there was evidence of a circadian variation in LVEF in the unadjusted analysis (difference between the average and the peak value was estimated as 2.2%, 95% CI 0.7 to 3.8%; p = 0.006). After adjusting for confounders, there was a non-significant difference in circadian variation in LVEF (difference between the average and peak value: 1.1%, 95% CI − 0.2 to 2.5%; p = 0.092).
4. Discussion
Our study shows that in a selected group of 169 patients in whom confounders (including duration of symptoms) for acute IRI and MI size were minimized, there was evidence of a 24-hour circadian variation in acute MI size after adjusting for the AAR. The greatest acute MI size occurred in STEMI patients with symptom onset between 00:00 and 01:00 and the nadir was in those with symptom onset between 12:00 and 13:00. The mean difference between the peak and nadir MI size (%AAR) was 10.4%, corresponding to 3.5% of the LV. When divided into quartiles, the largest MI size occurred in the 00:00 to 05:59 group. This 24-hour circadian variation in acute MI size was associated with a non-significant change in LVEF. However, there was no evidence of a circadian variation in acute MI size in the unselected cohort of 376 STEMI patients, after accounting for the AAR and confounding factors.
Following the initial work by Durgan et al. [18] who showed that acute MI size was largest in the sleep-to-wake transition and this phenomenon was abolished in cardiomyocyte-specific circadian clock mutant rodents [18], the clinical studies have shown inconsistent results as summarized in Table 4. Our findings are in keeping with Reiter et al. [3] that showed a peak MI size occurring at 01:00. Furthermore, 2 multicentre studies (Seneviratna et al. [6] and Mahmoud et al. [7]) showed that the peak MI size occurred in those with symptom onset between midnight and 06:00 and this was confirmed in our study. On the other hand, 3 other studies (Suarez-Barrientos et al. [1], Fournier et al. [4] and Ari et al. [5]) have shown that the peak MI size occurred in those with symptom onset between 06:00 and noon. However, Suarez-Barrientos et al. [1] used a 12-hour cycle and a second peak was also observed in the 18:00 to midnight group. Fournier et al. [2] subsequently reported in a multi-centre Swiss study of 6233 patients [2] that the peak CK occurred in patients with symptom onset at 23:00 and the risk of death was the highest for those with symptom onset at 00:00. In terms of clinical outcomes, Seneviratna et al. [6] also showed a similar pattern in the incidence of acute heart failure and 1-year mortality rate whereas Mahmoud et al. [7] did not find the time of symptom onset to be a significant predictor of 1-year mortality in an equally large number of patients. In a multicentre international collaborative study of 1099 patients from China, Scotland and Italy, Ammirati et al. [19] showed a circadian variation in STEMI incidence but there was no evidence of a circadian variation in MI size based on the time of symptom onset measured by CK but half of the patients in this study received thrombolysis or were not revascularized.
Table 4.
Summary of clinical studies investigating onset of symptoms and circadian variation of MI size in STEMI.
| Studies | Country/years | Patients in study | Surrogate for MI size | Outcome |
|---|---|---|---|---|
| Suarez-Barrientos [1] | Single centre — Spain | 811 | CK TnI |
Peak MI size between 06:00 and noon |
| Reiter [3] | Single centre — United States | 165 | CK | Peak MI size 01:00 onset of ischemia and 05:00 onset of reperfusion |
| Arroyo-Ucar [20] | Single centre — Spain | 108 | TnI | Peak MI size between 00:00 and 12:00 |
| Fournier [4] | Single centre — Switzerland | 353 | CK | Peak MI size between 00:00 and 05:59. |
| Ammirati [19] | Multicentre — Italy, Scotland, and China | 1099 | CK | Peak MI incidence from 06:00 to noon. No clear-cut circadian dependence of MI size |
| Fournier [2] | Multicentre — Switzerland | 6223 | CK | Peak MI size at 23:00, whereas the nadir MI size was at 11:00. Risk of death from STEMI was the highest at 00:00 and lowest at 12:00 |
| Seneviratna [6] | Multicentre — Singapore | 6710 | CK | Peak MI size and incidence of acute heart failure from midnight to 06:00 and nadir from 06:00 to noon |
| Mahmoud [7] | Multicentre — Netherlands | 6799 | CK | Peak MI size around 03:00 and nadir around 11:00 |
| Ari [5] | Single centre — Turkey | 252 | CK-MB | Peak MI size and poor LV function by echocardiography occurred in the 06:00–noon period |
CK: creatine kinase; TnI: troponin I; CK-MB: creatine kinase-myocardial band; MI: myocardial infarction; LV: left ventricle.
All the above previous clinical studies have the limitations of using cardiac enzymes to measure peak MI size, with 6 out of 9 studies using creatine kinase (CK). CK has a high sensitivity to detect myocardial necrosis but has a low specificity [32]. Furthermore, cardiac enzyme levels in general are dependent on the timing of performing the test and some of these studies were retrospective studies [2], [6], [7] and therefore it was highly unlikely that the cardiac enzymes were performed at pre-specified times. Furthermore the choice (thrombolysis versus PPCI) [33] and the success of reperfusion strategy can influence the release of cardiac enzymes into the blood stream. CMR is considered the gold standard for the accurate quantification of acute MI size [21], [22], [23]. CMR provides additional information on the AAR, and it was difficult to account for that in the previous studies using cardiac enzymes alone. Reiter et al. [3] only included a subgroup of patients with CMR (n = 45) and CK was used to assess for a circadian variation in MI size for their whole cohort. Our study is the first to use CMR to measure acute MI size as a percentage of the AAR and explore the circadian rhythm according to the time of onset of symptoms and therefore we believe that our findings are more robust that previous studies. We have found that there was a 24-hour circadian variation in acute MI size depending on time of symptom onset in a subset of patients entering RCTs in the current era in a three-centre European (England, Italy and Spain) collaborative study. We did not find any significant circadian variation in a 12-hour cycle as previously shown [1] after adjusting for confounders. Our findings are consistent with the current belief that the circadian clocks are composed of proteins that generate self-sustaining, transcriptionally-based mechanisms of positive and negative feedback loops with a free-running period of approximately 24 h [34] rather than 12 h. There are several other factors that can affect MI size in the clinical setting (pre-infarct angina, onset to balloon time, co-morbidities, TIMI flow pre- and post-PCI and collateral flow) and failure to carefully adjust for these confounders may explain the conflicting findings between those from our study, Reiter et al. [3], Seneviratna et al. [6] and Mahmoud et al. [7] and those from Suarez-Barrientos et al. [1], Fournier et al. [4]and Ari et al. [5]. Furthermore, as a result of these multiple confounders of MI size, the circadian variation in MI size may not be clinically relevant for most patients. The recent large study by Mahmoud et al. [6] showed no impact on 1-year mortality. Our study showed that the extent of variation in MI size by CMR did not significantly influence acute LVEF and the likely explanation is that the difference between the peak and nadir MI size of 10.4% of the AAR was too small to affect the LV volumes significantly for the cohort as a whole but would likely be important for those with a large AAR. Our sample size was too small to only look at the circadian variation of LVEF in those patients with a large AAR and warrants further investigation in future studies AAR.
4.1. Limitations
This was a retrospective study and patient level data was obtained from 4 RCTs and therefore may not be a true representation of the STEMI cohort presenting to hospital. However, this is representative of patients entering RCTs in the current era. The sample size in the primary analysis group was < 50% of the original cohort but this was similar to the sample reported by Reiter et al. (n = 165) [3]. Patients were randomized to an intervention arm or placebo but we have adjusted for these factors in our analysis. We only included patients from one continent unlike the study by Ammirati et al. [19] but despite this, the time zone geographically may have some subtle differences among these 3 countries and we did not account for that. The modality used to quantify the AAR by CMR was different in the 4 studies and the contrast agents used for late gadolinium enhancement were varied as shown in Table 1. We did not have clinical outcomes on these patients. Our sample size was small and the cardioprotective therapies in the 4 RCTs were different. Therefore we were not able to assess whether the effectiveness of cardioprotective therapies also had a circadian dependence in this subset of patients depending on the time of day and this needs to be assessed in future larger studies.
5. Conclusions
We have shown that a circadian variation in acute MI size assessed by CMR according to the time of symptom onset exists in a specific group of patients, after adjusting for confounders of MI size. The largest and smallest MI size occurred in those patients with symptom onset between 00:00 to 01:00 and 12:00 to 13:00, respectively. This was associated with a non-significant circadian variation in acute LVEF. The circadian dependence in acute MI size was only observed in a subset of patients entering RCTs. This needs to be taken into account to make sure that the patients are adequately balanced in the 4 quartiles of time of onset of symptoms when designing future RCTs aiming to reduce MI size. Whether this would impact on the effectiveness of the cardioprotective therapies needs to be assessed in future larger studies.
Conflicts of interest
None.
Acknowledgements
Part of this work was supported by the British Heart Foundation (FS/10/039/28270), the Rosetrees Trust, and the National Institute for Health Research University College London Hospitals Biomedical Research Centre. HACF is funded by a Startup Grant of the “Excellence Cluster Cardio-Pulmonary System” (ECCPS) from the German Research Foundation (DFG, Bonn, Germany) and the “Peter und Traudl Engelhorn-Stiftung” (Weilheim, Germany).
References
- 1.Suarez-Barrientos A., Lopez-Romero P., Vivas D., Castro-Ferreira F., Nunez-Gil I., Franco E. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011;97:970–976. doi: 10.1136/hrt.2010.212621. [DOI] [PubMed] [Google Scholar]
- 2.Fournier S., Taffe P., Radovanovic D., Von Elm E., Morawiec B., Stauffer J.C. Myocardial infarct size and mortality depend on the time of day — a large multicenter study. PLoS One. 2015;10:e0119157. doi: 10.1371/journal.pone.0119157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiter R., Swingen C., Moore L., Henry T.D., Traverse J.H. Circadian dependence of infarct size and left ventricular function after ST elevation myocardial infarction. Circ. Res. 2012;110:105–110. doi: 10.1161/CIRCRESAHA.111.254284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fournier S., Eeckhout E., Mangiacapra F., Trana C., Lauriers N., Beggah A.T. Circadian variations of ischemic burden among patients with myocardial infarction undergoing primary percutaneous coronary intervention. Am. Heart J. 2012;163:208–213. doi: 10.1016/j.ahj.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Ari H., Sonmez O., Koc F., Demir K., Alihanoglu Y., Ozdemir K. Circadian rhythm of infarct size and left ventricular function evaluated with tissue Doppler echocardiography in ST elevation myocardial infarction. Heart Lung Circ. 2016;25:250–256. doi: 10.1016/j.hlc.2015.06.833. [DOI] [PubMed] [Google Scholar]
- 6.Seneviratna A., Lim G.H., Devi A., Carvalho L.P., Chua T., Koh T.H. Circadian dependence of infarct size and acute heart failure in ST elevation myocardial infarction. PLoS One. 2015;10:e0128526. doi: 10.1371/journal.pone.0128526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmoud K.D., Nijsten M.W., Wieringa W.G., Ottervanger J.P., Holmes D.R., Jr., Hillege H.L. Independent association between symptom onset time and infarct size in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Chronobiol. Int. 2015;32:468–477. doi: 10.3109/07420528.2014.992527. [DOI] [PubMed] [Google Scholar]
- 8.Scheer F.A., van Doornen L.J., Buijs R.M. Light and diurnal cycle affect human heart rate: possible role for the circadian pacemaker. J. Biol. Rhythm. 1999;14:202–212. doi: 10.1177/074873099129000614. [DOI] [PubMed] [Google Scholar]
- 9.Krauchi K., Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am. J. Phys. 1994;267:R819–R829. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- 10.Hu K., Ivanov P., Hilton M.F., Chen Z., Ayers R.T., Stanley H.E. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc. Natl. Acad. Sci. U. S. A. 2004;101:18223–18227. doi: 10.1073/pnas.0408243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degaute J.P., van de Borne P., Linkowski P., Van Cauter E. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension. 1991;18:199–210. doi: 10.1161/01.hyp.18.2.199. [DOI] [PubMed] [Google Scholar]
- 12.Clark L.A., Denby L., Pregibon D., Harshfield G.A., Pickering T.G., Blank S. A quantitative analysis of the effects of activity and time of day on the diurnal variations of blood pressure. J. Chronic Dis. 1987;40:671–681. doi: 10.1016/0021-9681(87)90103-2. [DOI] [PubMed] [Google Scholar]
- 13.Curtis A.M., Seo S.B., Westgate E.J., Rudic R.D., Smyth E.M., Chakravarti D. Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J. Biol. Chem. 2004;279:7091–7097. doi: 10.1074/jbc.M311973200. [DOI] [PubMed] [Google Scholar]
- 14.Young M.E., Razeghi P., Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ. Res. 2001;88:1142–1150. doi: 10.1161/hh1101.091190. [DOI] [PubMed] [Google Scholar]
- 15.Hausenloy D.J., Yellon D.M. New directions for protecting the heart against ischaemia–reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc. Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Miura T., Miki T. GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ. J. 2009;73:1184–1192. doi: 10.1253/circj.cj-09-0284. [DOI] [PubMed] [Google Scholar]
- 17.Iitaka C., Miyazaki K., Akaike T., Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J. Biol. Chem. 2005;280:29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- 18.Durgan D.J., Pulinilkunnil T., Villegas-Montoya C., Garvey M.E., Frangogiannis N.G., Michael L.H. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ. Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ammirati E., Cristell N., Cianflone D., Vermi A.C., Marenzi G., De Metrio M. Questing for circadian dependence in ST-segment-elevation acute myocardial infarction: a multicentric and multiethnic study. Circ. Res. 2013;112:e110–e114. doi: 10.1161/CIRCRESAHA.112.300778. [DOI] [PubMed] [Google Scholar]
- 20.Arroyo Ucar E., Dominguez-Rodriguez A., Abreu-Gonzalez P. Influence of diurnal variation in the size of acute myocardial infarction. Med. Intensiva. 2012;36:11–14. doi: 10.1016/j.medin.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Wagner A., Mahrholdt H., Holly T.A., Elliott M.D., Regenfus M., Parker M. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 22.Thiele H., Kappl M.J., Conradi S., Niebauer J., Hambrecht R., Schuler G. Reproducibility of chronic and acute infarct size measurement by delayed enhancement-magnetic resonance imaging. J. Am. Coll. Cardiol. 2006;47:1641–1645. doi: 10.1016/j.jacc.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 23.Schulz-Menger J., Bluemke D.A., Bremerich J., Flamm S.D., Fogel M.A., Friedrich M.G. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J. Cardiovasc. Magn. Reson. 2013;15(35) doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grothues F., Smith G.C., Moon J.C., Bellenger N.G., Collins P., Klein H.U. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 25.Bulluck H., White S.K., Rosmini S., Bhuva A., Treibel T.A., Fontana M. T1 mapping and T2 mapping at 3 T for quantifying the area-at-risk in reperfused STEMI patients. J. Cardiovasc. Magn. Reson. 2015;17(73) doi: 10.1186/s12968-015-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedrich M.G., Abdel-Aty H., Taylor A., Schulz-Menger J., Messroghli D., Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2008;51:1581–1587. doi: 10.1016/j.jacc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Ludman A.J., Yellon D.M., Hasleton J., Ariti C., Babu G.G., Boston-Griffiths E. Effect of erythropoietin as an adjunct to primary percutaneous coronary intervention: a randomised controlled clinical trial. Heart. 2011;97:1560–1565. doi: 10.1136/hrt.2011.223867. [DOI] [PubMed] [Google Scholar]
- 28.Crimi G., Pica S., Raineri C., Bramucci E., De Ferrari G.M., Klersy C. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. JACC Cardiovasc. Interv. 2013;6:1055–1063. doi: 10.1016/j.jcin.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 29.White S.K., Frohlich G.M., Sado D.M., Maestrini V., Fontana M., Treibel T.A. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2015;8:178–188. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Dorado D., Garcia-Del-Blanco B., Otaegui I., Rodriguez-Palomares J., Pineda V., Gimeno F. Intracoronary injection of adenosine before reperfusion in patients with ST-segment elevation myocardial infarction: a randomized controlled clinical trial. Int. J. Cardiol. 2014;177:935–941. doi: 10.1016/j.ijcard.2014.09.203. [DOI] [PubMed] [Google Scholar]
- 31.Cox N.J. Speaking Stata: in praise of trigonometric predictors. Stata J. 2006;6(19) [Google Scholar]
- 32.Danese E., Montagnana M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann. Transl. Med. 2016;4(194) doi: 10.21037/atm.2016.05.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apple F.S., Henry T.D., Berger C.R., Landt Y.A. Early monitoring of serum cardiac troponin I for assessment of coronary reperfusion following thrombolytic therapy. Am. J. Clin. Pathol. 1996;105:6–10. doi: 10.1093/ajcp/105.1.6. [DOI] [PubMed] [Google Scholar]
- 34.Edery I. Circadian rhythms in a nutshell. Physiol. Genomics. 2000;3:59–74. doi: 10.1152/physiolgenomics.2000.3.2.59. [DOI] [PubMed] [Google Scholar]